Abstract
A portable, small footprint, light, general purpose analyzer (processor) to control the flow in immunoassay cassettes and to facilitate the detection of test results is described. The durable analyzer accepts disposable cassettes that contain pouches and reaction chambers for various unit operations such as hydration of dry reagents, stirring, and incubation. The analyzer includes individually controlled, linear actuators to compress the pouches in the cassette, which facilitates the pumping and mixing of sample and reagents, and to close diaphragm-based valves for flow control. The same types of actuators are used to compress pouches and actuate valves. The analyzer also houses a compact OEM scanner/reader to excite fluorescence and detect emission from labels. The analyzer is hydraulically isolated from the cassette, reducing the possibility of cross-contamination. The analyzer facilitates programmable, automated execution of a sequence of operations such as pumping and valving in a timely fashion, reducing the level of expertise required from the operator and the possibility for errors. The analyzer’s design is modular and expandable to accommodate cassettes of various complexities and additional functionalities. In this paper, the utility of the analyzer has been demonstrated with the execution of a simple, consecutive, lateral flow assay of a model biological system and the test results were detected with up converting phosphor labels that are excited at infrared frequencies and emit in the visible spectrum.
Keywords: Point of Care Test, Immunoassay, Lateral Flow (LF), Microfluidic Cassette, Pouch, Portable Analyzer, Processor, Linear Actuator
1. Introduction
In recent years, there has been a growing interest in carrying out various diagnostic procedures outside the lab at the point of testing. This trend has been motivated by, among other things, the desire (i) to bring sophisticated diagnostic capabilities to remote areas, where both clinical laboratories and trained personnel are in short supply or non-existent; (ii) to reduce the time delay from test to answer to enable health care providers and other authorities to make informed and timely decisions; (iii) to reduce cost; (iv) to facilitate personalized medicine and administer care based on individual needs rather than according to rigid protocols; (v) to enhance privacy; and (vi) to improve public health and safety. As a result, various “kits” for field tests have evolved, ranging from pregnancy tests to HIV antibody detection.
Lateral flow strips (dipsticks) are often used to detect the presence of antigens and antibodies to various pathogens [1, 2]. The popularity of lateral flow assays stems from their simplicity, reliability, low cost, and ability to operate without any instrumentation. Briefly, the lateral flow strip (made of a porous substrate) is functionalized with one or more capture lines of immobilized proteins (ligands) designed to bind with target molecules in the sample and a control line used to assure that the sample has, indeed, migrated up the strip and that the labels (reporters) have not degraded. The user brings the lateral flow strip in contact with the raw sample or a mixture of sample and buffer (diluent). The sample or mixture then is wicked along the lateral flow strip by capillary forces. Along its path, the sample hydrates labels (i.e., reporter particles such as gold) that are dry-stored on the lateral flow strip, and it interacts with the immobilized ligands at the test line(s) and the control line. The lateral flow test can operate either as a sandwich assay [3] or a competitive assay [4]. In some cases, gold conjugate labels are used, and the test results can be read by eye without any instrument [5]. In other cases, labels such as fluorophores, quantum dots, and phosphor particles, which require optical excitation at specific wavelengths and whose signal is detected with either a photomultiplier or with a camera, are used [6]. Instrument-based detection often provides higher sensitivity and consistency of the signal, facilitates quantification, and enables one to maintain permanent records [7].
The main advantage of the lateral flow assay is that it can operate without any external pumps and/or flow control. Lateral flow assays suffer, however, from certain disadvantages [2, 8–10], such as non-specific binding, high background signal, inability to undergo stringent washes to remove unbound material, lack of control of the sample’s volume, and low binding capacity at the test line(s). As a result, the performance of lateral flow immunoassays falls short of that of centralized laboratory equipment, and lateral flow strip immunoassays are generally limited to screening applications [8, 9].
In an effort to improve the performance of point of care immunoassays, a few researchers modified the classical lateral flow protocol and employed a consecutive flow format [11]. To improve sensitivity, the consecutive flow format employs a wash step. Additionally, to facilitate multiplexing and reduce cost, non-specific reporter particles are occasionally used. As a result, the sample cannot be premixed with reporter particles as is done in the standard lateral flow assay. Instead, the sample must migrate up the strip without interacting with the reporter particles. The target analytes are captured at the test line(s). The strip is then loaded with wash buffer to remove any non-specific bound proteins, and then it is finally loaded with the labeling reagents [12]. These operations either must be carried out manually, which makes them prone to operator error, or executed with an instrument capable of judiciously flowing the reagents needed for the consecutive flow format at the appropriate sequence and times.
As an alternative to lateral flow assays, several groups have opted to use microbeads as the immobilization medium. Bead - based assays are attractive due to the availability of a rich library of beads with various functionalizations. In one embodiment, many differently functionalized, bar-coded beads were distributed randomly in an array and used to concurrently detect multiple analytes [13, 14]. In another embodiment, beads with various functionalizations were placed at predetermined positions to allow for multiplexing [15, 16]. Porous beads, such as agarose beads, are particularly attractive since they have a large surface area to volume ratio and provide a high density of binding sites, which improves assay sensitivity [15, 16]. In contrast to the lateral flow assays, most, if not all, of the bead-based assays require means to induce fluid flow and effectuate flow control.
One commonly used technique to facilitate flow control in immunoassay cassettes is through the use of pouches (blisters) and diaphragm-based valves [14, 17]. The pouches can be filled either with the buffers needed for the process at hand or with air. In the latter case, in operation, the air-filled pouches are connected to liquid storage chambers. When compressed, the pouches displace buffer from a storage compartment. A pouch-based fluid propulsion strategy is attractive for point of care devices since it allows one to store all the reagents in the disposable cassette and avoids any liquid contact between the cassette and the analyzer. In practice, this means that a generic analyzer can drive multiple types of cassettes, and there is no possibility of cross-contamination. Moreover, a single analyzer can operate with different types of cassettes designed to detect different targets.
In simple cases, the pouches can be actuated manually. Qiu et al [14] describe a manually-controlled immunoassay cassette in which the pouches are compressed by finger. To prevent an operator’s error and improve test consistency, however, it may be desirable to automate the process. To this end, Liu et al. [18] describe a spring-loaded, mechanically-driven, timer actuator capable of actuating pouches sequentially. The timer actuator does not require any electrical power. Unfortunately, the timer-actuator can actuate only a relatively small number of pouches, and the sequence of operations is implemented in hardware and cannot be readily changed. To reduce the limitation on the number of actuators and to increase device flexibility (by allowing software-control of the sequence of operations), we describe here a portable, fully automated analyzer (processor) comprised of an array of linear actuators that serve to compress pouches and diaphragm valves. The sequence and timing of the various operations are controlled with software and can be altered from one type of cassette to another. Thus, the same analyzer can serve to process various types of cassettes and diagnose a variety of target molecules.
To make the ideas involved more concrete, we demonstrate the use of the analyzer in one relatively simple, specific application of a consecutive flow assay. The consecutive flow protocol requires sample premixing with lateral flow buffer, wash, and reporter loading steps. The wash step removes non-specific bound substances from the detection zone of the lateral flow strip. The reporter particles are loaded only in the last step. In our assay, we utilize upconverting phosphor conjugates (UCP) [19–22] as reporter particles. UCPs are excited by long wavelength radiation and emit at shorter wavelengths. In contrast, almost all natural phosphorescence phenomena emit at a wavelength larger than the excitation wavelength. As a result, UCPs are free of background emission. The UCP labels do not bleach, and they provide a permanent record. Since the UCP labels are excited in the infra red (IR), we equipped our analyzer with a reader that can excite the labels at the appropriate wavelengths and detect the resulting emission. The reader transverses along the lateral flow strip to obtain a scan of the emitted signal as a function of position.
2. Analyzer Design
To better understand the tasks required of the analyzer, we briefly introduce the various operations carried out by the immunoassay cassette. In the interest of space, we describe a relatively simple immunoassay cassette with lateral flow detection operating in a consecutive flow format. For additional details on various pouch actuated devices, see Liu et al [18], Qiu et al [14], and Chen et al. [17]. We then provide a brief description of the analyzer.
2.1 The Microfluidic Cassette and Immunoassay
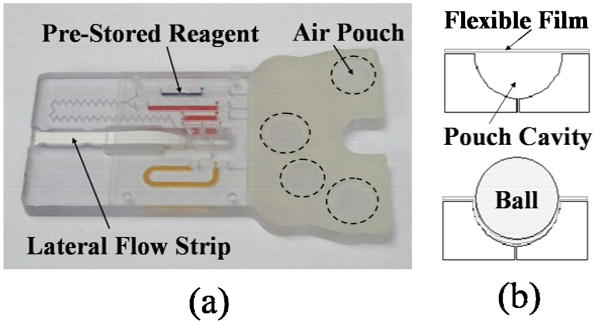
The immunoassay cassettes (94 mm × 59 mm × 6 mm) are prototyped in a polycarbonate (PC) sheet with a computer-numerical controlled (CNC) milling machine (Haas Milling OM-2A, Haas Automation Inc., Oxnard, CA). The assembled cassette is featured in Fig. 1 [18]. The cassette incorporates air pouches that when depressed provide a pneumatic force to drive the pre-stored liquid reagents through the microfluidic circuit. A pouch is depressed by applying force through a ball actuator to a flexible film that covers the pouch, such that air inside the pouch is expelled into the channels of the microfluidic circuit to propel a liquid charge (Fig. 1, (b)). The air pouch wells are formed by machining hemispherical cavities (using a ball-end mill) into the topside of the polycarbonate substrate that hosts the microfluidic circuit. A 100 μm thick, flexible, natural latex rubber film (McMaster-Carr, New Brunswick, NJ) cap layer is then attached to the cassette body with double-sided adhesive tape (McMaster-Carr, New Brunswick, NJ) to seal the well [18]. Alternatively, pouches can be capped by thermally bonding a thin (~100 μm), flexible film (TPS-4049B, Tolas Inc., Feasterville, PA) to the surface of the polyethylene [14].
Fig. 1.
(a) Microfluidic immunoassay cassette loaded with colored dyes representing the various reagents. (b) Depressible air pouches actuate the on-board fluid as indicated in the cross-section schematics of a pouch compartment (top: pre-actuation and bottom: post-actuation).
The force needed to compress the pouches was measured by placing weights on the pouches. We estimate that a force of about 3 N is needed to completely compress each latex rubber film pouch. The flow rate of the fluids in the cassette was adjusted by controlling the speed of the actuator (which, in turn, controlled the speed of the pouch’s compression). As a result, the flow rates of the reagents were independent of their rheological properties.
The timed sequence of operations in the cassette consists of:
Sample loading
Mixing of the sample with a lateral flow buffer and ejection of the mixture onto the lateral flow strip
After a two minute wait (to avoid flooding the lateral flow strip), ejection of wash solution onto the lateral flow strip
After another two minute wait, ejection of solution, laden with UCP labels, onto the strip
After about a 10–30 minute wait, reading of the signal emitted by the labels
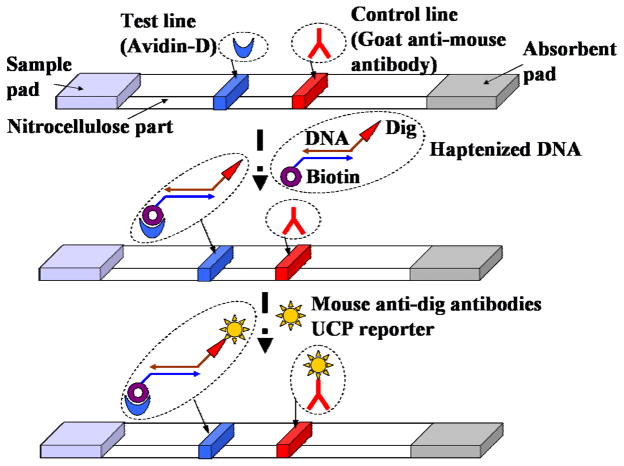
Fig. 2 is a schematic of the consecutive flow, lateral flow assay. The lateral flow strip is made with nitrocellulose. The strip is mated with a sample loading pad at its upstream end and an absorbent pad on the downstream end. Ligands are immobilized along lines transverse to the strip to form a “test line” and a “control line.” The test line contains immobilized ligands (avidin in our case) specific to the target analyte. The control line is made with ligands that bind to the reporter particles.
Fig. 2.
A schematic depiction of the sequence of operations carried out in the lateral flow (LF) assay
The operation and sensitivity of the analyzer were assessed using a dilution series of 305-bp haptenized PCR amplicon from B. Cereus that served as the test target. As is evident, in addition to assaying antigens and antibodies, the lateral flow assay can also be used to detect conjugated PCR amplicons. The PCR products that serve as the target analyte were functionalized, via the primers, at one end with biotin and at the opposite end with Digoxigenin (DIG) hapten. The nitrocellulose lateral flow strip is striped with a test line of immobilized avidin to capture biotin-labeled target analyte and with a control line of immobilized goat anti-mouse IgG antibody to capture any unbound, anti-DIG coated, UCP reporter particles.
The sequence of operations of the consecutive flow strip assay is as follows (see Fig. 2). The diluted sample with the labeled amplicon is loaded on the strip, where it is captured at the test line. The strip is then washed by loading it with buffer. Next the strip is loaded with a suspension of anti-DIG-coated, UCP particles. The UCP particles migrate up the strip and bind to the DNA target molecules functionalized with DIG and captured at the test line. UCP particles which were not captured by the target molecules at the test line continue to the control line, where they are immobilized through the anti-DIG binding with the striped anti-mouse IgG antibody.
2.2 Instrument Design and Operation
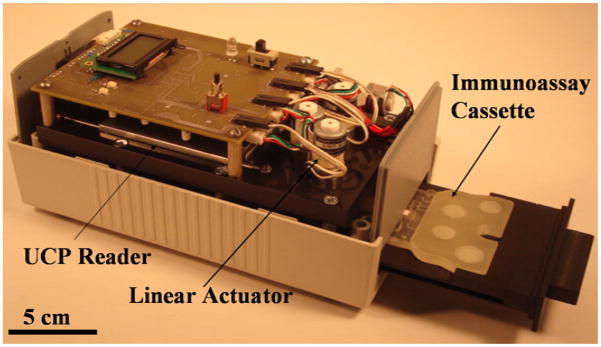
Fig. 3 shows the portable, custom-made analyzer (190 mm × 100 mm × 80 mm) with its cover removed and an immunoassay cassette inserted in the analyzer’s drawer. The portable analyzer is controlled with a PIC microcontroller (PIC18F45K20-I/PT, Microchip Technology Inc, Chandler, Arizona). The analyzer houses four low-voltage, stepper motor drivers (A3906, Allegro MicroSystems Inc., Massachusetts), which power three linear actuators (15000 series linear stepper motors, Haydon Kerk, CT) that perform the operations required for the lateral flow assay. Each actuator is capable of providing up to 7 N of force, which far exceeds the 3 N of force that is needed to compress the pouch.
Fig. 3.
Custom-made, immunoassay actuator/analyzer instrument with an immunoassay cassette inserted in the pulled-out drawer.
A compact, OEM type, UCP reader (72 mm × 47 mm × 18 mm, FLUO SENS integrated, ESE GmbH, Germany), driven by a linear actuator (15000 series linear stepper motors, Haydon Kerk, CT), is used to scan the strip. A USB to serial UART interfacing component (FT232R, Future Technology Devices International Limited, Glasgow, United Kingdom) enables the analyzer to communicate with a computer via a USB port.
The analyzer contains a pull-out drawer that houses the cassette (Fig. 3). The drawer is shaped to position and self-align the cassette to assure that, when closed, the actuators will be positioned directly over their corresponding pouches and the lateral flow strip is aligned with the reader’s path. Once the analyzer is powered on and the “start button” pressed, a small LCD module lights up and displays instructions for the user.
Figure 4 depicts schematically the linear actuator with an affixed actuation ball used to depress the pouch membranes of the chip. Once the actuation ball moves down, it is programmed to stay down during the entire duration of the assay to prevent any reagents from being sucked back into the channel and pouch. At the conclusion of the test, all the actuators return to their home positions. The linear actuator consists of a miniature, 15mm diameter stepper motor with a linear step increment of 0.00079″ (0.02 mm) per step. The compact size of the actuators (installed in an upright position in the instrument) allows considerable latitude in positioning the pouches on the cassette. When needed, an array of many actuators can be accommodated with a relatively small footprint. The flow rate is regulated by the speed of movement of the actuator.
Fig. 4.
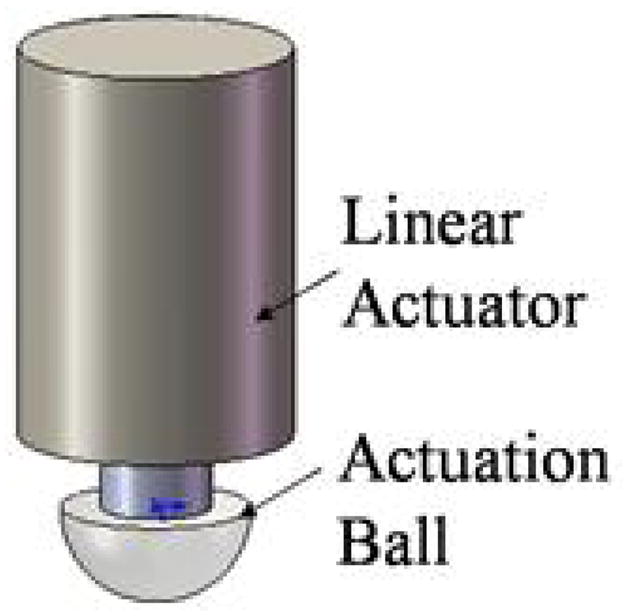
Linear actuator for depression pouch membranes
Each actuator is controlled in an open-loop mode. A custom “home position” sensor, consisting of an electric switch, was constructed to identify the position of the motor. See Fig. 5. The switch is triggered by the linear motor itself. Once the linear motor returns to its home position, the switch closes and grounds the resistor (R). The resulting signal is sent to the microcontroller as an interruption signal. This interruption signal clues the microcontroller to turn off the power to the linear actuator.
Fig. 5.
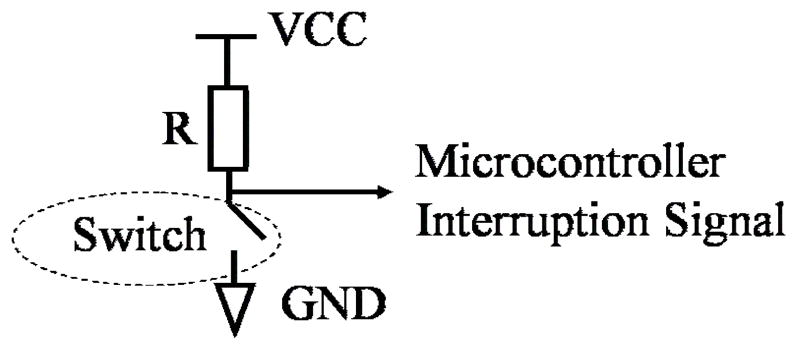
Home position reset sensor
To facilitate mixing, the analyzer compresses two pouches simultaneously. One pouch controls the sample flow and the other pouch controls the lateral buffer flow. The two fluids flow concurrently through the zigzag path machined in the cassette (Fig. 1a). The bends in the zigzag conduit induce secondary flows that enhance mixing. The performance of the mixer was evaluated in Li et al [18]. The principles of operation of mixers that utilize bends to induce secondary flows are described in Yi et al [23].
After the various reagents have been discharged onto the lateral flow strip, the system waits about thirty minutes to allow for the liquids and reagents to wick up the strip and interact with the test and control lines and for the UCP labels to dry. Then, the optical reader scans the strip. A linear actuator drives the reader at a constant speed along the lateral flow strip at a velocity of 0.67 mm/s. To minimize the scanning time, only the part of the strip’s length (~7 mm) that includes both the test line and the control line is scanned. The optical reader (FLUO SENS) uses the Modbus ASCII communication protocol. During the scan, the detection data (1500 data points) is stored in the reader’s flash memory.
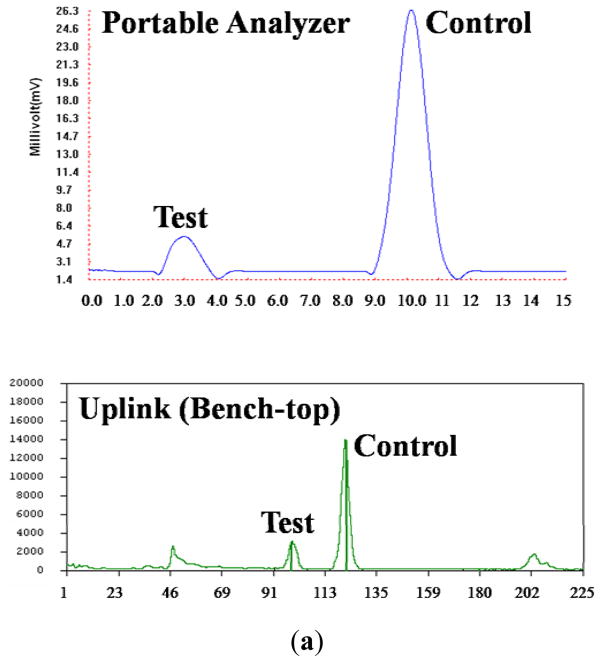
Custom software was written with Visual C++ 6.0 (Microsoft) to retrieve and process the collected data from the reader. A Butterworth low-pass filter was used to eliminate high frequency noise and to smooth the signal. The cut-off frequency of the low-pass filter was determined based on a Fast Fourier Transform of the unprocessed data. The correlation between the signal and the reader’s position along the strip was determined based on the scanning range and the sequential position of the data points in the data stream. The signal intensity data is displayed on the computer screen as a function of position along the strip. The areas (Fig. 6) under the test peak and the control peak are calculated and their ratio is displayed.
Fig. 6.
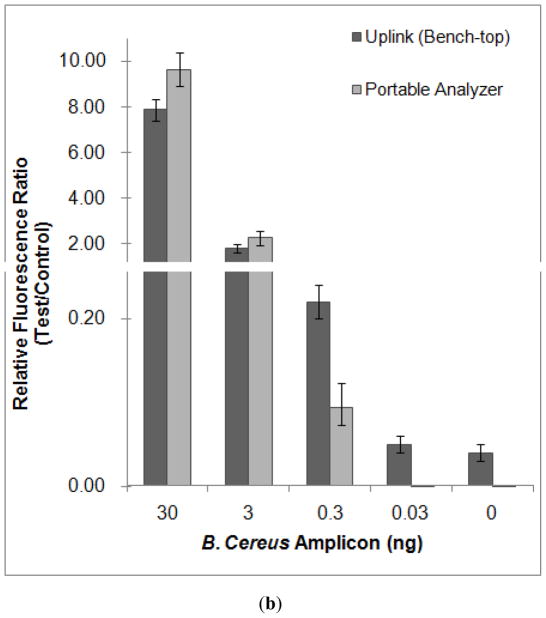
(a) Emission intensity, as a function of position along the strip (scan result), of a 0.3 ng haptenized DNA sample obtained with the portable analyzer (top) and with the benchtop UPLink reader (bottom). (b) The ratio between the area of the test peak and the area of the control peak as a function of the target analyte’s mass. The dark black and gray bars correspond, respectively, to results obtained with the benchtop UPLink instrument and with the portable analyzer. The error bars indicate the scatter of the data (n=3).
The analyzer’s power consumption is less than 1W. This power is needed only for relatively short amounts of time. We estimate that the analyzer’s energy consumption is about 0.01 W-h per assay. If desired, the analyzer can be operated with a standard 9 V battery, which typically stores over 5 W-h of energy.
3. Procedure and Materials
Experiments were carried out in parallel with our instrument and manually (for comparison) on a bench top. The bench top, LF-immunoassay protocol consisted of three steps: (i) 10 μL of sample was mixed with 40 μL of LF buffer, and then blotted onto the LF strip; (ii) after two minutes, 20 μL of LF buffer was blotted onto the strip to carry away any unbound analyte; and (iii) two minutes later, 60 μL of LF buffer laden with 60 ng of UCP conjugate [18] were added to the LF strip to label the immunoassay. After thirty minutes of incubation, the lateral flow strip was scanned with an UCP optical reader (OraSure UPLink, OraSure Technologies, Bethlehem, PA). The same protocol was implemented in the automated microfluidic assay. In the latter case, all the reagents were pre-stored in the cassette in liquid form.
The nitrocellulose LF strips were supplied by OraSure Technologies, Inc., (Bethlehem, PA). The LF buffer consisted of 100 mM HEPES (pH 7.2), 270 mM NaCl, 0.5% (v/v) Tween-20, and 1% (w/v) BSA. The immunolabeling buffer was a mixture of LF buffer with mouse anti-dig antibodies-conjugated UCP reporter particles for haptenized DNA.
The DNA samples were produced by lysing B. cereus cells (ATTC, Culture Type 6464, designation NRS 210), isolating the (genomic) DNA with a QIAGEN DNeasy™ Tissue Kit (QIAGEN Inc., Valencia, CA 91355), and using the eluted genomic DNA as the PCR template. The PCR reagents were 50 mM Tris-HCl (pH 9.0), 1.5–3.5 mM MgCl2, 200 μM dNTP, and 0.1 μg/μL BSA. The forward and reverse primers used at 0.3 μM were, respectively, (5′-TCT CGC TTC ACT ATT CCC AAG T-3′) and (5′-AAG GTT CAA AAG ATG GTA TTC AGG-3′). At the 5′-end, the forward and reverse primers were, respectively, provided with a Biotin (Bio) and a Digoxinenin (DIG) hapten. The primers targeted a 305 bp DNA fragment with 5′-digoxigenin (DIG) hapten on one strand and a 5′-biotin (Bio) hapten on the opposite strand [22]. The haptenized DNA concentration of the initial PCR products was determined using an Infinite® 200 PRO series plate reader (Tecan Group Ltd., Switzerland) with a Quant-iT™ PicoGreen assay (Invitrogen Corporation, Carlsbad, California). The test samples contained 0 ng, 0.03 ng, 0.3 ng, 3 ng, and 30 ng of B. cereus DNA amplicon in 10 μl of water.
4. Results
First, to evaluate the sensitivity of the detector and to compare the performances of the detector with that of a commercially available machine, the lateral flow assay was carried out manually on a benchtop and the lateral flow strip was scanned both with our portable analyzer and a commercial Uplink IR (OraSure) laser reader.
Fig. 6 shows representative chromatograms of the detection results as obtained with our detector (top) and the UPLink analyzer (bottom). Fig. 6a depicts the signal intensities (in arbitrary units) as functions of position along the strip. The words “Test” and “Control” in Fig. 6a denote, respectively, the test and control peaks. The area under the test peak is proportional to the amount of captured target analyte. The presence of a control peak indicates that the labels have migrated up the lateral flow strip. The sample in Fig. 6a contains 0.3 ng of DNA. In both cases, the test peak is clearly visible above the background level. In the case of our analyzer, the test peak is about 2.5 times the background level.
The tests were repeated with various analyte concentrations. Fig. 6b depicts the ratio of the areas under the test and the control peaks as a function of target concentration. The dark black and gray bars correspond, respectively, to results obtained with the UpLink (OraSure) scanner and with our analyzer. The error bars indicate the scatter of the data (n=3). In the case of our analyzer, all the operations were carried out by the analyzer. As the mass of the DNA in the sample increases, the area of the test peak increased and the area of the control peak decreased. As the DNA mass increase, more labels are consumed to form the sandwich at the test line and fewer labels are available to bind at the control line. When the sample’s mass was smaller than 0.3 ng, our analyzer did not detect any test peak. Although the UpLink detected a test peak even when the mass of the target molecules was smaller than 0.3 ng, the area of the peak was insensitive to the target analyte concentration. In fact, the UpLink provided similar ratios of test to control peaks in the presence of 0.03 ng DNA and in the absence of DNA. Thus, one may conclude that the limits of detection of our analyzer and that of the OraSure scanner are approximately 0.3 ng of target DNA.
When the mass of the DNA in the sample was greater than 0.3 ng, the ratios of the test peak area and the control peak area detected with our anaylyzer were comparable to those obtained with the UpLink device. When the DNA mass is between 0.3 and 30 ng, the ratio of the test and control peak areas is nearly proportional to the analyte concentration.
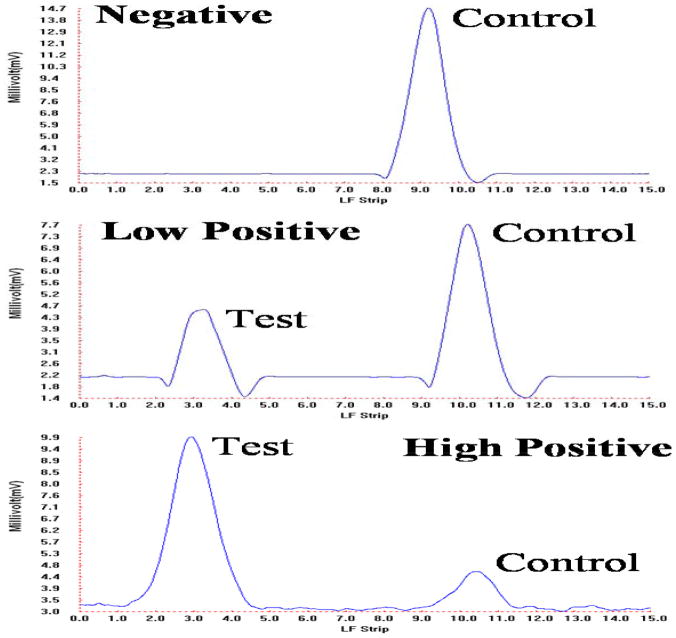
Next, the lateral flow assay was carried out in the microfluidic cassettes with our portable analyzer. Fig. 7 depicts representative chromatograms of negative (no target), low positive (0.3 ng) and high positive (30 ng) samples. The negative signal yielded no test peak and a large control peak. The low positive sample yielded moderately high test and control peaks. The high positive sample yielded a high test peak and a low control peak. The tests were repeated several times (n=3) with similar results. The results indicate that target analyte as small as 0.3 ng can be detected with our microfluidic diagnostics system and that the ratio of the areas of the test and control peaks can provide a rough estimate of target analyte concentration.
Fig. 7.
Scan result for the negative (0 ng), low positive (0.3 ng), and high positive (30 ng) samples carried out with our portable analyzer
In our previous work, we used a timer actuator to control the processes in the cassette [18]. The timer actuator has the advantage that it does not require any electric power and is less expensive than the electronic processor. However, in the timer actuator, the sequence and timing of operations are implemented in hardware during the manufacturing process and cannot be changed. In contrast, the electronic actuator/processor described in this paper can be reprogrammed at any time. Thus, the electronic device has much greater flexibility compared to the mechanical device described in Liu et al. [18].
6. Conclusions and Outlook
A new integrated, portable analyzer/processor for operating microfluidic immunoassay cassettes utilizing up-converting phosphor reporters has been developed. The portable analyzer was realized by combining miniature, independently-controlled actuators with a compact optical reader. The actuators’ sequence and timing of operations are controlled by software. Thus, the actuators’ array can be configured to accommodate different types of microfluidic chips. This ability to conveniently alter the sequence and timing of operations was not available in our earlier mechanical processor, in which the “program” was cast in hardware [18].
The pouch-based cassette design (with pre-loaded reagents) and the portable analyzer described here facilitate fully automated operation. To demonstrate the analyzer’s operation, we augmented the performance of the lateral flow immunoassay by adding the capability of a consecutive flow format. The architecture of the analyzer is flexible and can be expanded to include additional functions. Similar analyzers can be used to carry out immunoassays with bead-based cassettes of the types described in Qiu et al [14] and Thompson et al [16]. Similar design rules can be applied to construct significantly more complex processor. In fact, in subsequent work, using similar design concepts as the ones described in this paper, we developed a device for nucleic acid processing capable, among other things, of isolating and purifying nucleic acids from samples laden with bacteria and viruses and carrying out thermal cycling – based enzymatic amplification (PCR) [24].
The compact actuator/analyzer unit is portable and can be powered, if desired, by a standard 9 V battery. The analyzer can be interfaced with a laptop computer, cell phone, GPS unit, and a barcode reader to identify the cassette and customize operations as appropriate for the target(s) coded in the barcode. By this means, test data can be automatically matched with time and date of test, geographic location of the test, patient identification, and demographic data. Test results and other data can be analyzed in situ as well as communicated over a network or wirelessly.
The analyzer provided performance comparable to that of benchtop procedures. The ability to carry out relatively sophisticated diagnostics with devices that can be operated by unskilled personnel in the field as part of an interconnected healthcare network may assist in tracking and stemming epidemics, especially in resource-poor areas lacking laboratory facilities. Similar devices can also be used in the clinic to facilitate individualized medicine and at home to enable one to adjust medication regimens according to individual needs rather than follow rigid protocols.
Acknowledgments
The work was supported by NIH/NIDCR Grant U01DE017855.
Biographies
Xianbo Qiu received his PhD degree in Electrical Engineering from the Department of Automation, Shanghai Jiao Tong University, Shanghai, China in 2006. He was a Research Fellow at the University of Pennsylvania from 2006 to 2011. He is currently an Associate Professor at the National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University. His main research interests include biomedical instrumentation and microfluidic chips for point-of-care diagnostics.
Changchun Liu received his PhD degree in Electrical Engineering from the Institute of Electronics, Chinese Academy of Sciences (IECAS), Beijing, China in 2005. Currently, he is a Research Associate at the Department of Mechanical Engineering and Applied Mechanics, University of Pennsylvania. His research interest focuses on the microfluidic chips, biosensors and BioMEMS devices for point-of-care diagnostics.
Michael G. Mauk has a PhD in Electrical Engineering from the University of Delaware (USA). He is a Researcher at the Univ. of Pennsylvania with an interest in developing microfluidic point of care diagnostics devices for infectious diseases.
Robert W. Hart studied biomedical engineering at Drexel University, USA, and received his PhD in 2010. At the time of publication, he was carrying out postdoctoral training at the University of Pennsylvania. He is currently a principal scientist at Optofluidics, Inc., (Philadelphia PA) where he develops microfluidic and photonic devices.
Dafeng Chen received his PhD in Mechanical Engineering from Nanyang Technological University, Singapore in 2007. He was a Research Fellow at the University of Pennsylvania from 2007 to 2010. His main research interests include microfluidics devices, BioMEMS, and Point of Care diagnostics.
Jing Qiu studied biology at New York University and received her M.S. degree in 2010. She is currently a researcher at the Cold Spring Harbor Laboratory studying breast cancer focusing on the influence of chemotherapy on tumor-associated myeloid cells in the tumor microenvironment.
Terry Kientz received his B.S. in Electrical Engineering from the University of Connecticut in 1989. He is currently a Control Systems Engineer that specializes in the development of automation systems for the water filtration industry.
Jonathan Fiene received his B.S. in Mechanical Engineering from the University of Nevada, Las Vegas in 2001 and his Master and Ph.D. in Mechanical Engineering from Stanford University in 2003 and 2007, respectively. He is currently a Senior Lecturer in the Mechanical Engineering and Applied Mechanics Department at the University of Pennsylvania where he focuses on mechanical design, fabrication, robotics, and mechatronics.
Haim H. Bau received his PhD in Mechanical Engineering from Cornell University, Ithaca, NY, in 1980. He is currently a Professor of Mechanical Engineering and Applied Mechanics at the University of Pennsylvania. His research interests are micro and nanofluidics and point of care diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liu L, Peng C, Jin Z, Xu C. Development and evaluation of a rapid lateral flow immunochromatographic strip assay for screening 19-nortestosterone. Biomed Chromatogr. 2007;21:861–866. doi: 10.1002/bmc.832. [DOI] [PubMed] [Google Scholar]
- 2.Posthuma-Trumpie GA, Korf J, Amerongen Av. Lateral flow (immuno) assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- 3.Qian S, Bau HH. A mathematical model of lateral flow bio-reactions applied to sandwich assays. Anal Biochem. 2003;322:89–98. doi: 10.1016/j.ab.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Qian S, Bau HH. Analysis of lateral flow bio-detectors: competitive format. Anal Biochem. 2004;326:211–224. doi: 10.1016/j.ab.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Wang S, Zhang C, Wang J, Zhang Y. Development of colloidal gold-based flow-through and lateral-flow immunoassays for the rapid detection of the insecticide carbaryl. Anal Chim Acta. 2005;546:161–166. [Google Scholar]
- 6.Li Z, Wang Y, Wang J, Tang Z, Pounds JG, Lin Y. Rapid and sensitive detection of protein biomarker using a portable fluorescence biosensor based on quantum dots and a lateral flow test strip. Anal Chem. 2010;82:7008–7014. doi: 10.1021/ac101405a. [DOI] [PubMed] [Google Scholar]
- 7.Kim S, Park JK. Development of a test strip reader for a lateral flow membrane-based immunochromatographic assay. Biotech and Biopro Eng. 2004;9:127–131. [Google Scholar]
- 8.Bonenberger J, Doumanas M. Overcoming sensitivity limitations of lateral-flow immunoassays with a novel labeling technique. IVD Tech. 2006 May; [Google Scholar]
- 9.O’Farrell B, Bauer J. Developing highly sensitive, more reproducible lateral-flow assays, part 2: new challenges with new approaches. IVD Tech. 2006 July; [Google Scholar]
- 10.Peruski AH, Peruski LF., Jr Immunological methods for detection and identification of infectious agents and biological warfare agents. Clin and Diag Lab Immunol. 2003;10:506–513. doi: 10.1128/CDLI.10.4.506-513.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corstjens PLAM, Chen Z, Zuiderwijk M, Bau HH, Abrams WR, Malamud D, Niedbala RS, Tanke HJ. Rapid assay format for multiplex detection of humoral immune responses to infectious disease pathogens (HIV, HCV, and TB) Ann N Y Acad Sci. 2007;1098:437–445. doi: 10.1196/annals.1384.016. [DOI] [PubMed] [Google Scholar]
- 12.Schubert-Ullrich P, Rudolf J, Ansari P, Galler B, Führer M, Molinelli A, Baumgartner S. Commercialized rapid immunoanalytical tests for determination of allergenic food proteins: an overview. Anal Bioanal Chem. 2009;395:69–81. doi: 10.1007/s00216-009-2715-y. [DOI] [PubMed] [Google Scholar]
- 13.Walt DR. Chemistry miniature analytical methods for medical diagnostics. Sci. 2005;308:217–219. doi: 10.1126/science.1108161. [DOI] [PubMed] [Google Scholar]
- 14.Qiu X, Thompson JA, Chen Z, Liu C, Chen D, Ramprasad S, Mauk MG, Ongagna S, Barber C, Abrams WR, Malamud D, Corstjens PLAM, Bau HH. Finger-actuated, self-contained immunoassay cassettes. Biomed Microdevices. 2009;11:1175–1186. doi: 10.1007/s10544-009-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirby R, Cho EJ, Gehrke B, Bayer T, Park YS, Neikirk DP, McDevitt JT, Ellington AD. Aptamer-based sensor arrays for the detection and quantitation of proteins. Anal Chem. 2004;76:4066–4075. doi: 10.1021/ac049858n. [DOI] [PubMed] [Google Scholar]
- 16.Thompson JA, Bau HH. Microfluidic, bead-based assay: theory and experiments. J of Chromatogr B. 2010;878:228–236. doi: 10.1016/j.jchromb.2009.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen D, Mauk MG, Qiu X, Liu C, Kim J, Ramprasad S, Ongagna S, Abrams WR, Malamud D, Corstjens PLAM, Bau HH. An integrated microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomed Microdevices. 2010;12:705–19. doi: 10.1007/s10544-010-9423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu C, Qiu X, Ongagna S, Chen D, Chen Z, Abrams WR, Corstjens PL, Bau HH. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab Chip. 2009;9:768–776. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corstjens PLAM, Zuiderwijk M, Tanke HJ, van der Ploeg-Schip J, Ottenhoff THM, Geluk A. A user-friendly, highly sensitive assay to detect the IFN-γ secretion by T cells. Clin Biochem. 2008;6:440–444. doi: 10.1016/j.clinbiochem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rijke Fvd, Zijlmans H, Li S, Vail T, Raap AK, Niedbala RS, Tanke HJ. Up-converting phosphor reporters for nucleic acid microarrays. Nat Biotech. 2001;19:273–276. doi: 10.1038/85734. [DOI] [PubMed] [Google Scholar]
- 21.Li JJ, Ouellette AL, Giovangrandi L, Cooper DE, Ricco AJ, Kovacs GTA. Optical scanner for immunoassays with up-converting phosphorescent labels. IEEE Trans on Biomed Eng. 2008;55:1560–1571. doi: 10.1109/TBME.2007.914674. [DOI] [PubMed] [Google Scholar]
- 22.Wang J, Chen Z, Corstjens PLAM, Mauk MG, Bau HH. A disposable microfluidic cassette for DNA amplification and detection. Lab Chip. 2006;6:46–53. doi: 10.1039/b511494b. [DOI] [PubMed] [Google Scholar]
- 23.Yi M, Bau HH. The Kinematics of Bend-Induced Mixing in Micro-Conduits. International Journal of Heat and Fluid Flow. 2003;24:645–656. [Google Scholar]
- 24.Qiu X, Chen D, Liu C, Mauk MG, Kientz T, Bau HH. A Portable, Integrated Analyzer for Microfluidic - Based Molecular Analysis. accepted for publication in Biomedical Microdevices. 2011 doi: 10.1007/s10544-011-9551–5. [DOI] [PubMed] [Google Scholar]