Abstract
Regulation of isoprenoid end-product synthesis required for normal growth and development in plants is not well understood. To investigate the extent to which specific genes for the enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) are involved in end-product regulation, we manipulated expression of the HMG1 and HMG2 genes in tomato (Lycopersicon esculentum) fruit using arachidonic acid (AA). In developing young fruit AA blocked fruit growth, inhibited HMG1, and activated HMG2 expression. These results are consistent with other reports indicating that HMG1 expression is closely correlated with growth processes requiring phytosterol production. In mature-green fruit AA strongly induced the expression of HMG2, PSY1 (the gene for phytoene synthase), and lycopene accumulation before the normal onset of carotenoid synthesis and ripening. The induction of lycopene synthesis was not blocked by inhibition of HMGR activity using mevinolin, suggesting that cytoplasmic HMGR is not required for carotenoid synthesis. Our results are consistent with the function of an alternative plastid isoprenoid pathway (the Rohmer pathway) that appears to direct the production of carotenoids during tomato fruit ripening.
Isoprenoids are a structurally diverse group of compounds that are involved in many aspects of cell development. All of these compounds are derived from a common isoprenoid precursor, IPP (McGarvey and Croteau, 1995). It is generally assumed that in eukaryotic cells the irreversible reaction that converts 3-hydroxy-3-methylglutaryl CoA into mevalonic acid, eventually leading to the synthesis of the biologically active IPP unit, is a key regulatory step in the isoprenoid pathway. This NADPH-dependent reaction is catalyzed by the enzyme HMGR (EC 1.1.1.34). In mammals HMGR activity is tightly regulated at many levels, from the expression of the only gene encoding the enzyme to the stability of the protein and the modulation of the activity by phosphorylation or allosteric effectors. This multivalent regulation responds to the levels of cholesterol, the principal end product (Goldstein and Brown, 1990).
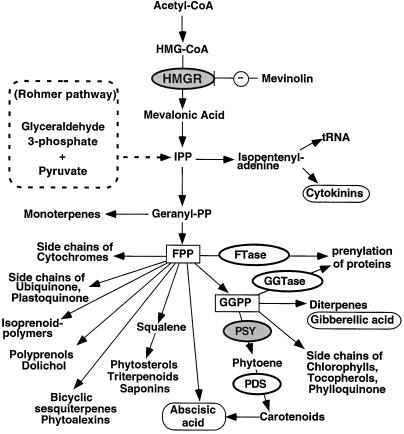
In addition to sterols, plant cells produce a large number of IPP-derived compounds, including carotenoids, phytoalexins, and other specialized terpenoids, growth hormones (gibberellins, ABA, and cytokinins), and the polyprenol substituents of dolichols, quinones, and proteins (Bach, 1995; Chappell, 1995a; McGarvey and Croteau, 1995) (Fig. 1). In contrast to what is known of mammalian cells, it is still controversial whether HMGR is a rate-limiting step in isoprenoid biosynthesis in plant cells (Chappell, 1995a, 1995b; Re et al., 1995). The recent discovery of an alternative pathway for isoprenoid production in plants (Eisenreich et al., 1996; Lange et al., 1998) also raises the question of whether HMGR activity is required for all types of isoprenoid end products. HMGR is encoded by a small multigene family in all plants that have been examined thus far, ranging from two genes in Arabidopsis (Enjuto et al., 1994) to four genes in tomato (Bach et al., 1991; S.M. Jenkins and W. Gruissem, unpublished data). Tomato (Lycopersicon esculentum) HMG1 is highly expressed during the early stages of fruit development, when sterol biosynthesis is required for membrane biogenesis during cell division and expansion (Narita and Gruissem, 1989). HMG2 expression is not detectable in young fruit, but is activated during fruit maturation and increases strongly during ripening, in parallel with the accumulation of lycopene (Gillaspy et al., 1993).
Figure 1.
Isoprenoid pathway in plants. Plant growth regulators and some enzymes of the pathway are circled. HMGR and PSY are highlighted in gray because of their possible rate-limiting roles. The steps catalyzed by PDS, FTase, and GGTase are also indicated. FPP and GGPP are the main branch points of the pathway. The alternative Rohmer pathway for the production of IPP in plastids is also indicated.
Based on the specific expression patterns of HMG genes that are correlated with end-product accumulation in tomato, it could be argued that the corresponding HMGR isozymes regulate the isoprenoid pathway to produce specific end products. Therefore, changes in the expression level of HMG1 and HMG2 should most strongly affect only the accumulation of the corresponding type of end product. We report experiments in which we have manipulated HMG1 and HMG2 expression levels in tomato fruit by using the fungal elicitor AA, which represses HMG1 and induces HMG2 expression in tomato leaves, stems, and fruit discs (J.O. Narita, M. Rodríguez-Concepción, and W. Gruissem, unpublished data). Unlike other studies in which heterologous (Chappell et al., 1995) or homologous (Re et al., 1995) HMG genes were ectopically expressed in transgenic plants, we show that AA can be used to simultaneously repress HMG1 and induce HMG2 expression in the same tissue. The results show that the lower level of HMG1 transcripts in AA-treated young fruit is correlated with growth inhibition. In mature fruit AA strongly and prematurely induced expression of HMG2 and PSY1, as well as lycopene synthesis. Although this would be consistent with a role of HMG2 in carotenoid biosynthesis, we found that concomitant inhibition of HMGR activity by mevinolin did not affect lycopene accumulation.
MATERIALS AND METHODS
Plant Material and Treatments
Tomato (Lycopersicon esculentum cv VFNT) plants were grown in the greenhouse. The stages of development were defined according to the diameter of the fruit and are referred to as young fruit (0.5–0.7 cm in diameter) or mature-green fruit (2.5–3.0 cm in diameter). For each treated fruit there was a corresponding control fruit with similar features with regard to size, position in the inflorescence, position of the inflorescence on the plant, light, etc., before the treatment. No more than two fruits per inflorescence were selected, labeled, and numbered. The rest of the fruits in the inflorescence were removed 1 d before the treatments.
AA was purchased from Sigma. The AA stock solution contained 100 mg/mL AA in ethanol. The AA working solution was freshly prepared by diluting 5 μL of the stock solution to 100 μL, and contained 5% (v/v) ethanol, 5 mg/mL (16 mm) AA, and 50 mm Tris-HCl, pH 7.0. The control solution was the same but did not contain AA. Mevinolin (a gift from Merck, Darmstadt, Germany) was prepared by dissolving 4 mg in 150 μL of ethanol. After adding 225 μL of 0.1 m NaOH and incubating at 50°C for 2 h, the pH was adjusted to pH 7.5 with HCl, and water was added to 1 mL to give a 10 mm stock solution of active mevinolin. A solution containing 9 mm active mevinolin in the AA working solution was used for fruit treatment.
Treatments were performed by injecting the same volume of the corresponding solution to plant-attached young (4 μL) or mature-green (10 μL) fruit with a 25-μL syringe (Bio-Phore, Bio-Rad) through the bottom of every fruit. After the indicated times, fruits were collected from the plant, weighed, frozen in liquid nitrogen, and stored at −70°C until used for experimental purposes.
RNA Analysis
Frozen tissue was ground to a fine powder in liquid nitrogen with a mortar and pestle. The powder was transferred to a tube containing 1.5 volumes of phenol saturated with Tris-HCl, pH 8.0, and mixed. Immediately, 3 volumes of NEST buffer (100 mm NaCl, 1 mm EDTA, 1% [w/v] SDS, 10 mm Tris-HCl, pH 7.5) and 1.5 volumes of chloroform:isoamyl alcohol (24:1) were added and the mixture was vortexed for 1 min. After centrifugation, the aqueous phase was transferred to another tube with 1 volume of 4 m LiCl. Precipitation was carried out overnight at 4°C. After centrifugation for 20 min at 15,000g and further precipitation with ethanol, the total RNA was recovered and resuspended in sterile water. The amount of RNA was estimated by measuring the A260 and by ethidium-bromide staining.
Twenty micrograms of total RNA was loaded in 1.2% agarose gels containing formaldehyde. After electrophoresis the nucleic acids were capillary transferred to nylon filters (Hybond N+, Amersham) and UV cross-linked with a Stratalinker (Stratagene). The membranes were prehybridized for at least 15 min at 65°C in PSE buffer (0.3 m sodium phosphate, pH 7.2, 7% [w/v] SDS, 1 mm EDTA). Hybrid-ization was carried out in the same solution containing probes randomly labeled with [32P]dCTP using the Stratagene Prime-It kit. Specific probes were prepared from the 3′ end cDNA fragments SpeI-HincII of HMG1 and BglII-HincII of HMG2. The probes for tomato PSY1 (Bartley et al., 1992) and FTA (Yalovsky et al., 1997) were made from the full-length cDNAs. The PDS (Giuliano et al., 1993) probe was made from a cDNA with the 3′ end truncated 140 bp upstream of the stop codon. A 1.5-kb XmnI-EcoRI fragment from pHA2 for pea 18S rRNA (Jorgensen et al., 1987) was used as a probe for tomato 18S rRNA.
After hybridization for 12 to 16 h at 65°C, membranes were washed sequentially in washing buffer (1× SSC, 0.1% [w/v] SDS), once at room temperature and three times at 65°C for 20 min each. Exposure to film (BioMax MS, Kodak) was at −80°C with intensifying screens from 1 to 6 d. Quantification of the hybridization signals was achieved after reexposing the blots using a phosphor imager (Molecular Dynamics, Sunnyvale, CA).
Lycopene Measurement
Samples of 3 g of tomato fruit tissue were ground in liquid nitrogen and extracted with 10 mL of a 2:1 acetone:hexane solution. The suspension was centrifuged at 5000g for 10 min in 50-mL Corex tubes. The upper hexane layer was removed with a Pasteur pipette, and the A505 of a 1:10 dilution of this extract was determined in a spectrophotometer (model UV-160A, Shimadzu, Columbia, MD). The amount of lycopene was calculated from these data using a specific extinction coefficient of 3400 (Davies, 1976).
HMGR Activity
The same pool of frozen fruit tissue used for RNA extraction and lycopene measurement was used for microsomal HMGR activity assays, as described previously (Chappell et al., 1995). Three to five fruits (control, treated with AA, or treated with AA and mevinolin) were ground together in liquid nitrogen to a fine powder. Part of this powder was mixed with 5 volumes of ice-cold homogenization buffer and centrifuged at 10,000g for 15 min, and the supernatant was recovered to isolate the microsomal fraction after centrifugation at 100,000g for 1.5 h. Aliquots of microsomes equivalent to 50 μg of protein were used for activity assays, as described previously (Chappell et al., 1995). Activity assays were carried out in duplicate with or without 100 μm mevinolin added to the reaction mixture. HMGR activity was determined by subtracting the value obtained with mevinolin added to the reaction mixture (in which no HMGR activity should remain) from the value obtained without added mevinolin. When mevinolin was injected directly into the fruit, HMGR activity experiments were done with fruit tissue distant from the injection site to ensure that mevinolin had been transported to all cells in the fruit.
RESULTS
AA Causes a Decrease in HMG1 mRNA Levels and Inhibits the Growth of Young Tomato Fruit
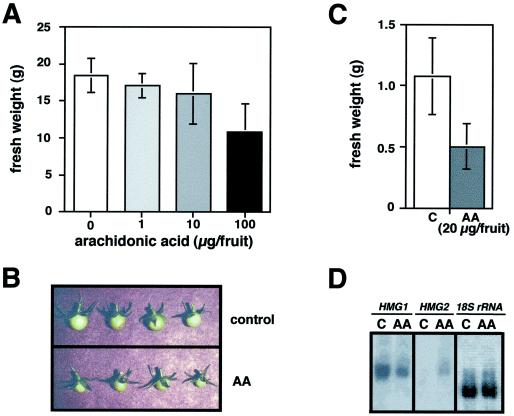
During the early stages of fruit development, HMG1 is the only member of the HMG gene family whose transcripts can be detected, suggesting that it may provide the sterols required for membrane biogenesis and fruit growth (Gillaspy et al., 1993). To determine whether altered levels of HMG1 expression would result in changes in growth, young tomato fruits were treated with AA at concentrations ranging from 0.25 to 25 μg/μL (1–100 μg of AA per fruit). The AA solution was injected into fruits using a syringe with a fine-gauge needle. We also injected the same solution without AA into control fruits (see Methods) to ensure that any difference between control and AA-treated fruits result from the presence of AA only. One month after treatment, both control and AA-injected fruit had developed into mature red fruits, but fruits treated with AA remained smaller and weighed less than control fruits in an AA-concentration-dependent manner (Fig. 2A). The difference in size between control and AA-injected fruit was visible as soon as 3 d after treatment (Fig. 2B) and was correlated with an approximately 50% reduction in fresh weight (Fig. 2C). Apart from the size and weight, fruits treated with AA showed no visible difference in color, morphology, or anatomy compared with control or untreated fruits.
Figure 2.
Effect of AA on young fruit development. Young tomato fruits attached to the plant were injected with a control or AA solution. Groups of three to five fruits were collected after 3 d or 1 month and used for experiments. A, Fruit weight 1 month after treatment with different concentrations of AA. B, Fruit samples from the experiment shown in C. C, Fruit weight 3 d after injection of 4 μL of a control solution or 5 μg/μL AA solution. Columns represent means and bars represent sd values. D, RNA blot of total RNA samples from the fruit samples shown in C. The same blot was hybridized with gene-specific HMG1, HMG2, and 18S rRNA probes.
RNA-blot analyses revealed that the level of HMG1 mRNA was decreased in AA-treated fruit compared with control fruit (Fig. 2D). AA treatment also induced the ectopic expression of HMG2 in young fruit (Fig. 2D), a stage at which HMG2 transcripts are normally absent (Gillaspy et al., 1993). Our results are consistent with earlier reports and with our own observations (Choi et al., 1992; J.O. Narita, M. Rodríguez-Concepción, and W. Gruissem, unpublished data) that HMG1 and HMG2 expression are affected differently by AA. The reduction in HMG1 transcript levels and the concomitant growth inhibition after AA treatment suggest that full HMG1 expression is required during fruit development. Apparently, the growth inhibition cannot be reversed by the ectopic expression of HMG2.
AA Induces Lycopene Accumulation in Mature-Green Fruit
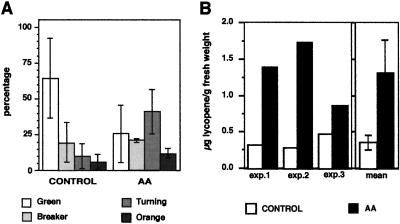
Expression of HMG2 is induced during the later stages of tomato fruit development, coincident with ripening and carotenoid biosynthesis (Gillaspy et al., 1993). To determine the potential role of HMGR2 in carotenoid accumulation, mature-green fruits (in which HMG2 expression is not yet induced) were injected with AA. The goal of this experiment was to induce HMG2 expression (and presumably enzyme activity) prematurely and to analyze the effects of such induction on carotenoid (lycopene) accumulation. A total of 120 mature-green fruits in three independent experiments were injected with either the AA (50 μg per fruit) or the control solution, as described for young fruit. Seventy-two hours after injection no significant differences in size or weight were detected between control and AA-treated fruits. However, whereas most of the control fruits remained green, about 75% of the AA-injected fruits showed visible lycopene accumulation (Fig. 3A). The amount of lycopene in the pool of AA-treated fruits was on average 3 times higher than in control fruits (Fig. 3B).
Figure 3.
Effect of AA treatment on mature tomato fruit. Mature-green fruits were injected with either 10 μL of a control solution or 5 μg/μL AA solution while attached to the plant, and collected 3 d later. A, Proportion of control and AA-treated fruits at different stages of pigmentation (Gillaspy et al., 1993). B, Lycopene concentration in control and AA-treated fruits from three different experiments. Columns represent means and bars represent sd values from three independent experiments.
Induction of HMGR Activity Is Not Required for AA-Induced Carotenoid Accumulation
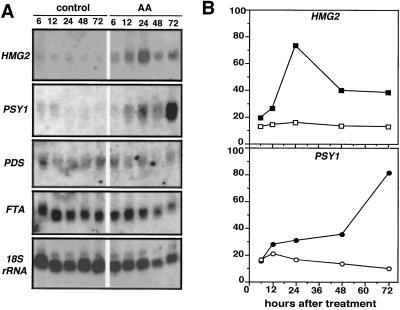
To compare the time course of HMG mRNA accumulation, HMGR activity, and lycopene synthesis, 40 mature-green fruits in two groups were injected with either AA or control solution. At 6, 12, 24, 48, and 72 h, pools of 3 to 5 control and AA-treated fruits were collected from the plants and ground together to a fine tissue powder in liquid nitrogen. This material was used for RNA extraction, HMGR activity determination, and lycopene measurements. The RNA blots probed with an HMG1-specific probe showed no detectable HMG1 mRNA (data not shown). The expression of HMG2 remained low and constant in control fruits (Fig. 4A), but was induced significantly in fruits treated with AA. At 24 h the HMG2 mRNA level in AA-treated fruits was approximately 4-fold that of control fruits (Fig. 4B). The level of HMG2 transcripts declined after 48 h, but at 72 h was still significantly higher than in the control fruits.
Figure 4.
RNA-blot analysis of gene expression in control and AA-treated mature fruit. Mature-green fruits were injected with 10 μL of a control solution or 5 μg/μL AA solution while attached to the plant. Groups of three to five fruits were collected and ground together after 6, 12, 24, 48, or 72 h. A, RNA-blot analysis of total RNA extracted from the different fruit pools. The blot was hybridized with a gene-specific HMG2 probe, and other probes from tomato PSY1, PDS, and FTA. An 18S rRNA probe was used to compare the RNA amounts loaded in each lane. B, Quantification of the steady-state levels of HMG2 and PSY1 mRNA. Open symbols, Control fruit; closed symbols, AA-treated fruit. The values shown are normalized with the 18S rRNA amounts and are expressed relative to the level detected in red, firm fruit.
HMGR activity also increased in AA-injected fruits (Fig. 5A). HMGR activity 24 h after injection with AA was about 5-fold higher compared with control fruits, but unlike the pattern of HMG2 mRNA accumulation, the highest HMGR activity was detected at 48 h. By 72 h after injection HMGR activity also decreased (Fig. 5A).
Figure 5.
HMGR activity and lycopene concentration in control and AA-treated fruit. Fruits were treated as described in Figure 4 with 10 μL of a control solution (C), with an AA solution (AA), or with an AA solution containing 9 mm mevinolin (AA+MEV), collected after 24, 48, and 72 h, and used for determination of microsomal HMGR activity (A) and measurement of lycopene concentration (B). Columns represent means and bars represent sd values from three independent fruit samples.
To determine whether the increase in lycopene formation in AA-injected fruit was a consequence of increased HMGR activity, a specific inhibitor of HMGR activity, mevinolin (Alberts et al., 1980), was coinjected with AA into mature green fruit (see Methods). The pattern of HMG2 mRNA expression in fruits injected with AA and mevinolin was identical to that observed in fruits treated only with AA (data not shown), but the microsomal HMGR activity was inhibited to undetectable levels (Fig. 5A). This inhibition of HMGR activity by mevinolin, however, did not abolish the AA-induced accumulation of lycopene. The pattern of lycopene accumulation in fruits injected with both AA and mevinolin was essentially identical to that observed in fruits injected with AA alone (Fig. 5B). Accumulation of lycopene began 24 h after injection, and after 72 h the lycopene amount in fruits injected with AA alone or with AA and mevinolin was 3-fold higher than in control fruits. We conclude from these results that the higher rate of carotenoid production in the chloroplast induced by AA does not depend on increased cytoplasmic HMGR activity.
AA Induces the Expression of PSY1, the First Committed Step in the Biosynthesis of Carotenoids
For lycopene to be produced at higher levels in AA-treated fruit, increased amounts of intermediates for its synthesis would be expected. Therefore, we analyzed the effect of AA on the expression of genes encoding enzymes that may affect the availability of such intermediates (Fig. 4). PSY is the first committed enzyme in the carotenoid-biosynthesis pathway and catalyzes the formation of phytoene from two molecules of GGPP (Fig. 1). AA treatment highly induced the expression of PSY1 (Fig. 4), which encodes the plastid isoform required for lycopene synthesis in tomato fruit (Bartley et al., 1992). The expression of PSY1 in control fruit did not increase during the same 72-h period. It is noteworthy that the pattern of AA-induced PSY1 expression (Fig. 4B) was closely correlated with the pattern of AA-induced lycopene accumulation (Fig. 5B).
The next step in carotenoid biosynthesis is catalyzed by the plastid-localized enzyme PDS, which converts phytoene into ζ-carotene (Giuliano et al., 1993). Unlike PSY1, however, PDS was expressed at similar levels in control and AA-injected fruits (Fig. 4). We also analyzed the transcript levels of the tomato FTA gene, which encodes the α-subunit that is shared by cytoplasmic FTase and GGTase (Yalovsky et al., 1997). These enzymes modify specific proteins by covalent attachment of farnesyl or geranylgeranyl to a conserved Cys at their carboxy terminus (Fig. 1). Thus, GGTase could compete for GGPP if the cytoplasmic intermediate were also required for carotenoid biosynthesis. Although FTA is expressed in both control and AA-injected fruits, mRNA accumulation was not induced by AA (Fig. 4A), as was observed for PSY1. Our results are consistent with PSY1 catalyzing the committed step for the biosynthesis of carotenoids, and show that regulation of this step of the pathway may be key in controlling the production of carotenoids in plastids during fruit development.
DISCUSSION
Expression of HMG1 and HMG2 Can Be Modulated by AA during Fruit Growth
We took advantage of the modulation of HMG1 and HMG2 expression by AA to investigate their role during tomato fruit development. The inhibitory effect of AA on fruit growth is similar to that previously reported for the HMGR inhibitor mevinolin (Narita and Gruissem, 1989). However, in contrast to mevinolin, which caused complete inhibition of HMGR enzyme activity, AA reduced HMG1 mRNA levels but induced the accumulation of HMG2 transcripts, which are normally undetectable in young fruit (Gillaspy et al., 1993). The ectopic expression of HMG2 could not reverse the AA-induced growth inhibition of young fruit, which is consistent with previous reports showing that HMG1 expression in tomato is most closely correlated with growth processes that require phytosterol production (Narita and Gruissem, 1989; Gillaspy et al., 1993; J. Jelesko and W. Gruissem, unpublished data). At present it is not possible to measure specific activities of the HMGR1 or HMGR2 enzymes and, therefore, we cannot conclude that the AA-induced ectopic expression of HMG2 also resulted in the production of an active enzyme. It is also possible that AA has a pleiotropic effect on the function of other enzymes or genes required during tomato fruit development. In potato tubers AA also inhibits squalene synthase (Tjamos and Kuc, 1982; Zook and Kuc, 1991), and a similar inhibition of this enzyme in tomato could explain the arrest of fruit development as well. However, unlike animals and yeast, the unique temporal expression patterns of tomato HMG1 and HMG2, together with their opposing response to AA, strongly suggest that the enzymes encoded by these genes have specific roles in cellular isoprenoid allocation and potential end-product accumulation.
HMGR Activity Is Not Required for Carotenoid Biosynthesis in Tomato Fruit
Because HMG2 is highly expressed during fruit ripening and carotenoid biosynthesis (Gillaspy et al., 1993), we investigated the requirement of HMGR activity for accumulation of carotenoids. For this purpose we took advantage of AA to ectopically induce both HMG2 mRNA accumulation (Fig. 4) and HMGR activity (Fig. 5A) in mature-green fruit before the onset of HMG2 expression during the normal ripening program. A strong, AA-dependent induction of HMG2 mRNA accumulation was detected 24 h after treatment with AA, followed by maximum HMGR activity 48 h after treatment. At this time the HMG2 mRNA level had already begun to decline. The increase in HMGR enzyme activity was followed by an AA-dependent accumulation of lycopene (Fig. 5B).
The in vivo inhibition of HMGR with mevinolin, however, did not prevent the AA-induced accumulation of carotenoids (Fig. 5B), suggesting that the strong induction in HMGR enzyme activity that we could assay was not necessary for carotenoid biosynthesis. Similarly, treatment of tomato fruit discs with mevinolin did not arrest the ripening and accumulation of carotenoids (J.O. Narita and W. Gruissem, unpublished data). In other experiments, inhibition of HMGR with mevinolin had little effect on the synthesis of chlorophyll and carotenoids, but prevented sterol biosynthesis and reduced growth (Bach and Lichtenthaler, 1983; Narita and Gruissem, 1989). It is possible that carotenoid synthesis in mevinolin-treated tomato fruit results from utilization of preexisting isoprenoid intermediates, or that a residual HMGR activity may be sufficient to sustain carotenoid biosynthesis. In view of the recent discovery of an alternative glyceraldehyde 3-phosphate/pyruvate pathway for IPP synthesis in the chloroplast (Rohmer pathway, Fig. 1; Eisenreich et al., 1996; Schwender et al., 1996; Lange et al., 1998; Lois et al., 1998), it is more likely that this pathway is responsible for carotenoid biosynthesis. This would explain why inhibition of HMGR activity does not affect carotenoid accumulation in tomato fruit. At the same time, it raises the interesting question of why HMG2 is induced to high levels during ripening.
Is There a Role for HMGR2 in Defense Responses?
The strong induction of HMG2 expression and HMGR activity by the fungal elicitor AA was most likely the result of a cellular defense response. HMG2 expression is induced not only by elicitors but also by direct pathogen inoculation (Cramer et al., 1993; Weissenborn et al., 1995). In addition, the pattern of AA-induced HMG2 mRNA accumulation in tomato fruit (Fig. 4) was very similar to that of the corresponding HMG2 gene in potato (Choi et al., 1992) and to that of other transcriptionally regulated defense genes in plants (Matton and Brisson, 1989; Koch et al., 1992). HMG2 activation could be a more general response to cellular disintegration, in particular during fruit ripening and in plant defense, rather than a specific regulation correlated with carotenoid biosynthesis.
Although it is known that wounding accelerates ripening and the production of carotenoids (Ulrich and Renac, 1950; Hugueney et al., 1996), we have shown that carotenoid biosynthesis can also be induced by AA, an elicitor of pathogen defense responses. Because HMGR enzyme activity is not required for lycopene accumulation, it is likely that this induction event is parallel to the activation of HMG2 expression. AA also induces PSY1 expression, suggesting that the parallel activation of the cytoplasmic isoprenoid and chloroplast carotenoid biosynthesis pathways may be part of a general defense mechanism.
AA Induces the Expression of PSY1, Resulting in Accelerated Carotenoid Accumulation
The regulation of carotenoid biosynthesis may be accomplished through one or more alternative pathways that do not require cytoplasmic HMGR enzyme activity. Other steps in the carotenoid biosynthetic pathway may be rate limiting and/or required for diversion of isoprenoid intermediates into the production of carotenoid end products. Therefore, we analyzed the expression pattern of three genes encoding enzymes (or subunits of enzymes) that use different intermediates in the isoprenoid pathway (Fig. 1): PSY (Bartley et al., 1992; Bartley and Scolnik, 1993; Giuliano et al., 1993), PDS (Giuliano et al., 1993), and protein prenyltransferases (Yalovsky et al., 1997). We found that the mRNA levels for plastid PDS and the cytoplasmic α-subunit of protein prenyltransferases (which is shared by FTase and GGTase) did not change in control or AA-injected fruit (Fig. 4). In contrast, AA induced the expression of PSY1 in a pattern that paralleled the accumulation of lycopene (Figs. 4 and 5B). The activation of PSY1 expression, therefore, could explain the AA-induced accumulation of lycopene, assuming that enzyme levels and/or activity are also increased and that GGPP is present at saturating levels.
Our results are consistent with reports for transgenic tomato plants showing that modulation of PSY1 gene expression by ectopic expression or antisense RNA inhibition of PSY1 alone is sufficient to alter carotenoid levels (Bird et al., 1991; Bramley et al., 1992; Fray and Grierson, 1993). In addition, constitutive expression of PSY1 in transgenic tomato also causes dwarfism, possibly by redirecting GGPP from the gibberellin pathway (Fig. 1) to the synthesis of carotenoids (Fray et al., 1995). In contrast, inhibition of PSY1 expression leads to the accumulation of GGPP and FPP and elevated gibberellin levels during tomato fruit development (Bird et al., 1991; Fraser et al., 1995). PSY1 is likely a branch-point enzyme whose regulation may control GGPP allocation to carotenoid biosynthesis.
CONCLUSION
The regulation of HMG1 and HMG2 expression in tomato is consistent with the theory that levels of the different HMGR isozymes in plants are modulated in response to specific developmental and stress signals. It is likely that this results in the channeling of mevalonic acid to specific end products of the isoprenoid pathway (Bach, 1995; Chappell, 1995a, 1995b). Other enzymes at important branch points in the isoprenoid pathway, such as IPP isomerase, FPP synthase, and GGPP synthase (Scolnik and Bartley, 1996), are also encoded by small gene families, although little is known about the expression of their genes. In addition to the differential regulation of genes for isozymes, compartmentation, channeling through multienzyme complexes or “metabolons” (Srere, 1987), or the regulation of the activity of other rate-determining enzymes may also operate to control the accumulation of specific isoprenoid end products. Although our results suggest that the control of cytoplasmic isoprenoid synthesis can be exerted at the level of HMG1 and HMG2 expression, the activity of their enzymes in tomato does not appear to be required for plastid carotenoid production. Instead, based on the induction of PSY1 expression before lycopene accumulation, it is likely that plastid PSY has an important role in channeling GGPP from the HMGR-independent Rohmer pathway (Schwender et al., 1996) into plastid carotenoid production. The results reported here and in other recent reports (Chappell et al., 1995; Hugueney et al., 1996; Lange et al., 1998) offer an interesting opportunity to investigate the regulation of isoprenoid synthesis in different cellular compartments in combination with genetic approaches to determine the role of specific enzymes in the pathways.
ACKNOWLEDGMENTS
We are grateful to Drs. R.L. Jones, S.M. Jenkins, and J. Keddie for critical reading of the manuscript. We also thank Drs. G.E. Bartley for the PDS and PSY1 clones, S. Yalovsky for the FTA clone, J. Jelesko and S.M. Jenkins for advice, and the departmental greenhouse staff for excellent care of plants.
Abbreviations:
- AA
arachidonic acid
- FPP
farnesyl diphosphate
- FTA
α-subunit of FTase
- FTase
farnesyl transferase
- GGPP
geranylgeranyl diphosphate
- GGTase
geranylgeranyl transferase
- HMGR
3-hydroxy-3-methylglutaryl CoA reductase
- IPP
isopentenyl pyrophosphate
- PDS
phytoene desaturase
- PSY
phytoene synthase
Footnotes
This work was supported by Department of Energy grant no. DE-FG03-85ER13375. M.R.-C. was supported by a postdoctoral fellowship from the Spanish Ministerio de Educación y Cultura.
LITERATURE CITED
- Alberts AW, Chen J, Kuron G, Hunt V, Hoffman C, Rothrock J, Lopes M, Joshua H, Harris E, Patchett A and others. Mevinolin: a highly potent competitive inhibitor of hydroxymethylglutaryl-coenzyme A reductase and a cholesterol-lowering agent. Proc Natl Acad Sci USA. 1980;77:3957–3961. doi: 10.1073/pnas.77.7.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach TJ. Some new aspects of isoprenoid biosynthesis in plants: a review. Lipids. 1995;30:191–202. doi: 10.1007/BF02537822. [DOI] [PubMed] [Google Scholar]
- Bach TJ, Lichtenthaler HK. Inhibition by mevinolin of plant growth, sterol formation and pigment accumulation. Physiol Plant. 1983;59:50–60. [Google Scholar]
- Bach TJ, Wettstein A, Boronat A, Ferrer A, Enjuto M, Gruissem W, Narita JO (1991) Properties and molecular cloning of plant HMG-CoA reductase. In GW Patterson, WD Nes, eds, Physiology and Biochemistry of Sterols. American Oil Chemists Society, Washington, DC, pp 29–49
- Bartley GE, Scolnik PA. cDNA cloning, expression during development, and genome mapping of PSY2, a second tomato gene encoding phytoene synthase. J Biol Chem. 1993;268:25718–25721. [PubMed] [Google Scholar]
- Bartley GE, Viitanen PV, Bacot KO, Scolnik PA. A tomato gene expressed during fruit ripening encodes an enzyme of the carotenoid biosynthesis pathway. J Biol Chem. 1992;267:5036–5039. [PubMed] [Google Scholar]
- Bird CR, Ray JA, Fletcher JD, Boniwell JM, Bird AS, Teulieres C, Blain I, Bramley PM, Schuch W. Using antisense RNA to study gene function: inhibition of carotenoid biosynthesis in transgenic tomatoes. Biotechnology. 1991;9:635–639. [Google Scholar]
- Bramley PM, Teulieres C, Blain I, Bird C, Schuch W. Biochemical characterisation of transgenic tomato plants in which carotenoid synthesis has been inhibited through expression of antisense RNA to pTOM5. Plant J. 1992;2:343–349. [Google Scholar]
- Chappell J. Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995a;46:521–547. [Google Scholar]
- Chappell J. The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol. 1995b;107:1–6. doi: 10.1104/pp.107.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J, Wolf F, Proulx J, Cuellar R, Saunders C. Is the reaction catalyzed by 3-hydroxy-3-methylglutaryl coenzyme A reductase a rate-limiting step for isoprenoid biosynthesis in plants? Plant Physiol. 1995;109:1337–1343. doi: 10.1104/pp.109.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D, Ward BL, Bostock RM. Differential induction and suppression of potato 3-hydroxy-3-methylglutaryl coenzyme A reductase genes in response to Phytophthora infestans and to its elicitor arachidonic acid. Plant Cell. 1992;4:1333–1344. doi: 10.1105/tpc.4.10.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer CL, Weissenborn D, Cottingham CK, Denbow CJ, Eisenback JD, Radin DN, Yu X. Regulation of defense-related gene expression during plant-pathogen interactions. J Nematol. 1993;25:507–518. [PMC free article] [PubMed] [Google Scholar]
- Davies BH (1976) Carotenoid analysis. In TH Goodwin, ed, Chemistry and Biochemistry of Plant Pigments, Ed 2, Vol 2. Academic Press, New York, pp 38–165
- Eisenreich W, Menhard B, Hylands PJ, Zenk MH, Bacher A. Studies on the biosynthesis of taxol: the taxane carbon skeleton is not of mevalonoid origin. Proc Natl Acad Sci USA. 1996;93:6431–6436. doi: 10.1073/pnas.93.13.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enjuto M, Balcells L, Campos M, Caelles C, Arro M, Boronat A. Arabidopsis thaliana contains two differentially expressed 3-hydroxy-3-methylglutaryl-CoA reductase genes, which encode microsomal forms of the enzyme. Proc Natl Acad Sci USA. 1994;91:927–931. doi: 10.1073/pnas.91.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser PD, Hedden P, Cooke DT, Bird CR, Schuch W, Bramley P. The effect of reduced activity of phytoene synthase on isoprenoid levels in tomato pericarp during fruit development and ripening. Planta. 1995;196:321–326. [Google Scholar]
- Fray R, Grierson D. Identification and genetic analysis of normal and mutant phytoene synthase genes of tomato by sequencing, complementation and co-suppression. Plant Mol Biol. 1993;22:589–602. doi: 10.1007/BF00047400. [DOI] [PubMed] [Google Scholar]
- Fray R, Wallace A, Fraser PD, Valero D, Hedden P, Bramley P, Grierson D. Constitutive expression of a fruit phytoene synthase gene in transgenic tomato causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J. 1995;8:693–701. [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. Fruits: a developmental perspective. Plant Cell. 1993;5:1439–1451. doi: 10.1105/tpc.5.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano G, Bartley GE, Scolnik PA. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Hugueney P, Bouvier F, Badillo A, Quennemet J, d'Harlingue A, Camara B. Developmental and stress regulation of gene expression for plastid and cytosolic isoprenoid pathways in pepper fruits. Plant Physiol. 1996;111:619–626. doi: 10.1104/pp.111.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen RA, Cuellar RE, Thompson WF, Kavanagh TA. Structure and variation in ribosomal RNA genes of pea. Plant Mol Biol. 1987;8:3–12. doi: 10.1007/BF00016429. [DOI] [PubMed] [Google Scholar]
- Koch E, Meier BM, Eiben HG, Slusarenko A. A lipoxygenase from leaves of tomato (Lycopersicon esculentum Mill.) is induced in response to plant pathogenic pseudomonads. Plant Physiol. 1992;99:571–576. doi: 10.1104/pp.99.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange BM, Wildung MR, McCaskill D, Croteau R. A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Natl Acad Sci USA. 1998;95:2100–2104. doi: 10.1073/pnas.95.5.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois LM, Campos N, Putra SR, Danielsen K, Rohmer M, Boronat A. Cloning and characterization of a gene from Escherichia coli encoding a transketolase-like enzyme that catalyzes the synthesis of d-1-deoxyxylulose 5-phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc Natl Acad Sci USA. 1998;95:2105–2110. doi: 10.1073/pnas.95.5.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matton DP, Brisson N. Cloning, expression, and sequence conservation of pathogenesis-related gene transcripts of potato. Mol Plant Microbe Interact. 1989;2:325–331. doi: 10.1094/mpmi-2-325. [DOI] [PubMed] [Google Scholar]
- McGarvey DJ, Croteau R. Terpenoid metabolism. Plant Cell. 1995;7:1015–1026. doi: 10.1105/tpc.7.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita JO, Gruissem W. Tomato hydroxy-methylglutaryl-CoA reductase is required early in fruit development but not during ripening. Plant Cell. 1989;1:181–190. doi: 10.1105/tpc.1.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Re EB, Jones D, Learned RM. Co-expression of native and introduced genes reveals cryptic regulation of HMG CoA reductase expression in Arabidopsis. Plant J. 1995;7:771–784. doi: 10.1046/j.1365-313x.1995.07050771.x. [DOI] [PubMed] [Google Scholar]
- Schwender J, Seemann M, Lichtenthaler HK, Rohmer M. Biosynthesis of isoprenoids (carotenoids, sterols, prenyl side-chains of chlorophylls and plastoquinone) via a novel pyruvate/glyceraldehyde 3-phosphate non-mevalonate pathway in the green alga Scenedesmus obliquus. Biochem J. 1996;316:73–80. doi: 10.1042/bj3160073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik PA, Bartley GE. A table of some cloned plant genes involved in isoprenoid biosynthesis. Plant Mol Biol Rep. 1996;14:305–319. [Google Scholar]
- Srere PA. Complexes of sequential metabolic enzymes. Annu Rev Biochem. 1987;56:89–124. doi: 10.1146/annurev.bi.56.070187.000513. [DOI] [PubMed] [Google Scholar]
- Tjamos EC, Kuc JA. Inhibition of steroid glycoalkaloid accumulation by arachidonic and eicosa pentaenoic acids. Science. 1982;217:542–544. doi: 10.1126/science.217.4559.542. [DOI] [PubMed] [Google Scholar]
- Ulrich R, Renac J. Le metabolisme des fruits de tomate et son alteration sous l'effet des blessures. CR Acad Sci Paris. 1950;230:567–569. [Google Scholar]
- Weissenborn DL, Denbow CJ, Laine M, Lang SS, Yang Z, Yu X, Cramer CL. HMG-CoA reductase and terpenoid phytoalexins: molecular specialization within a complex pathway. Physiol Plant. 1995;93:393–400. [Google Scholar]
- Yalovsky S, Trueblood CE, Callan KL, Narita JO, Jenkins SM, Rine J, Gruissem W. Plant farnesyltransferase can restore yeast ras signaling and mating. Mol Cell Biol. 1997;17:1986–1994. doi: 10.1128/mcb.17.4.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook MN, Kuc JA. Induction of sesquiterpene cyclase and suppression of squalene synthetase activity in elicitor treated or fungal infected potato tuber tissue. Physiol Mol Plant Pathol. 1991;39:377–390. [Google Scholar]