Abstract
Classic galactosemia is a rare human disease associated with the accumulation of a toxic level of galactose-1-phosphate (gal-1P) caused by the inherited deficiency of galactose-1-phosphate uridyltransferase (GALT) activity. To reduce the toxic level of gal-1P in patients, we have identified, via high-throughput screening, over 200 small molecule GALK inhibitors. We selected a 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile scaffold for further structure–activity relationships characterization, lead optimization with regards to potency, and efficacy to reduce gal-1P accumulation in patient cells.
Keywords: Galactokinase, galactose-1-phosphate, dihydrothiazinone, GHMP kinases, galactosemia
Classic galactosemia (CG) is an inherited metabolic condition caused by deficiency of galactose-1-phosphate uridyltransferase (GALT, EC 2.7.7.12) activity.1 GALT is the second enzyme in the evolutionarily conserved Leloir pathway of galactose metabolism and facilitates the simultaneous conversion of uridine diphosphoglucose (UDP-glucose) and galactose-1-phosphate (gal-1P) to uridine diphosphogalactose (UDP-galactose) and glucose-1-phosphate.2 Consequently, GALT deficiency leads to the accumulation of gal-1P and a deficiency of UDP-galactose in patient cells.3,4 If untreated, CG can result in a lethal disease in the affected newborn.5 Ever since most states in the United States included this disorder in the newborn screening panel, neonatal morbidity and mortality have decreased considerably. The current mainstay of treatment is the withdrawal of (ga-)lactose from the diet.5 However, it has become clear that despite optimal dietary management, chronic complications such as IQ deficits, ataxia, speech dyspraxia, premature ovarian insufficiency, and decreased bone mineralization persist in many affected adults.6,7 In the past few years alone, at least six groups of investigators reported that the health-related quality of life consequences of galactosemic patients and their parents were worse than generally thought.8−13 Such an outcry of concerns for a relatively rare disease suggested that the stressful conditions suffered by the patients and their family members have been underestimated for too long, and swift actions are required to improve the current situation. Moreover, little can we deny that the failure of galactose-restricted diet to prevent secondary complications has slowly eroded the early success of newborn screening of this genetic disorder, and the medical community is yearning for a more effective therapy. To develop a more effective therapy, one must elucidate the pathogenic mechanisms of the disease and identify useful therapeutic targets. Although the precise pathophysiology of CG remains unexplained, decades of clinical observations,14,15 confirmed recently by prospective outcome study,16 showed that patients with galactokinase (GALK) deficiency rarely manifest the chronic complications seen in GALT-deficient patients. Note that GALK-deficient patients do NOT accumulate 10–20-fold increases of gal-1P over their lifetime as do GALT-deficient patients even when on galactose-restricted diets. Others and we confirmed these clinical findings in a Saccharomyces cerevisiae (baker's yeast) model for CG. While a GALT-deficient mutant yeast was sensitive to galactose in growth medium, disruption of GALK function in this mutant reversed its susceptibility to galactose toxicity.17−19 These findings support the pathological role of gal-1P in GALT deficiency in humans and yeast models for CG but also raise the question about the origin of gal-1P, the enzymatic product of GALK on galactose, in a galactosemic patient who refrains from dairy products. It has been found that galactose moieties converted to gal-1P can also come from nondairy sources, for example, galactose-containing fruits and vegetables amounting to as much as 30 mg/day.20 However, galactose moieties can also be produced endogenously from UDP-glucose via the UDP-4-galactose epimerase (GALE) reaction, as well as from the natural turnover of glycolipids and glycoproteins. In fact, using isotopic labeling, Berry et al. elegantly demonstrated that a 50 kg adult male could produce up to 1.2 g of galactose per day, which is many times the amount of exogenous galactose potentially present in galactose-restricted diets.21 Therefore, endogenous synthesis of galactose is likely to undermine the efficacy of dietary management as a standard therapy. Because endogenous galactose production is not amenable to dietary manipulation, there is a need for innovative, nondietary therapy. Because gal-1P, an enzymatic product of GALK, is a major culprit for the complications seen in CG patients and GALK deficiency is more manageable than GALT deficiency, a few investigators have advocated the inhibition of human GALK as an innovative approach to treat CG.16,22
Previously, we initiated a campaign to identify small molecule inhibitors for the human GALK enzyme.23,24 We hypothesized that GALK deficiency induced pharmacologically in GALT-deficient patients would significantly reduce gal-1P accumulation and prevent the chronic outcomes. Lastly, as for the uniqueness of the therapeutic target, human GALK is unique because even though it phosphorylates galactose, it does not belong to the same family of other sugar kinases such as glucokinase (E.C. 2.7.1.2) or hexokinase (E.C. 2.7.1.1). Instead, it belongs to the superfamily of small molecule kinases, also known as the GHMP (Galactose, Homoserine, Mevalonic acid, Phosphomevalonic acid) kinases family.22,25
In our previous work, we identified over 150 small molecules inhibitors of human GALK via high-throughput screening (HTS).23 We selected 34 compounds for further characterization.24 Although their IC50 values were determined as 200 nM to 33 μM, their selectivity among the individual GHMP kinases varied, and some were shown to be toxic to cells.24 Thus, these first-generation compounds will require further optimization for therapeutic use. In this study, we chose 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile (compound 1) (Figure 1) for structure–activity relationship (SAR) studies because it shares a similar aromatic core and functionality array with many of the positive hits.24 The IC50 of compound 1 in the in vitro galactokinase inhibitory assay was 12 μM; therefore, our major objective was to improve its potency. We began by exploring nine commercially available compounds with structural similarities to compound 1 (Figure 1). These compounds carried structural modifications in the A ring or the C ring with none in the central 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile core. In the in vitro GALK inhibitory assays, only compounds 2, 3, and 4 possessed inhibitory activities against GALK, while the remaining compounds were not active below 50 μM.
Figure 1.
Lead compounds selected for SAR studies.
On the basis of these results, we rationalized that the presence of hydroxyl functional group(s) is necessary on the aryl ring A, since the active compounds have either a hydroxyl or a carboxylic acid group. However, not all of the compounds with hydroxyl groups on the A ring displayed inhibitory activity as is the case with compound 7. This compound shares the same structural similarity to compound 1 except that it has a thiobutyl group instead of the aromatic C ring. Therefore, the aromatic C ring is deemed necessary for activity since it was absent in compound 7.
Another observation in our limited SAR studies revealed that compound 3 having 2-hydroxy aryl substituent in the A ring was active, whereas compound 10 with the same thiomethyl substituent at the sixth position having 2,4-dichloro substituents in the A ring was inactive. This clearly suggests that electronic-withdrawing hydrophobic groups at the 2- and 4-positions may not be tolerated in the A ring for activity. Compound 9, however, has a spiroketal carboxyl group instead of an aryl carboxyl group that exhibited no activity. Similarly, compounds 5, 6, and 8 that do not have aromatic A ring did not display any inhibitory activity. This further underscores the preference of an aromatic ring as the A ring adjacent to the 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile core.
Through the above SAR studies, the IC50 of compound 2 with two aryl hydroxyl groups from this selection was determined to be in the low micromolar range (1.4 ± 0.7 μM) for GALK in contrast to the original compound 1, which has an IC50 of 12 μM.
To modify compound 2 for further optimization of potency, it was considered necessary to identify the functional groups that are needed for potency. As a result, new compounds with different positions of the hydroxyl groups in the A ring were designed and synthesized by a procedure described by Yokoyama26,27 where many commercially available aldehydes such as aldehyde 11 were treated with mercaptoacrylamide 12(27) in the presence of catalytic HCl in MeOH under refluxing conditions and afforded solid compound 2 in 61% yield (Scheme 1).
Scheme 1. General Synthesis of 4-Oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile by Refluxing with Acidic MeOH.
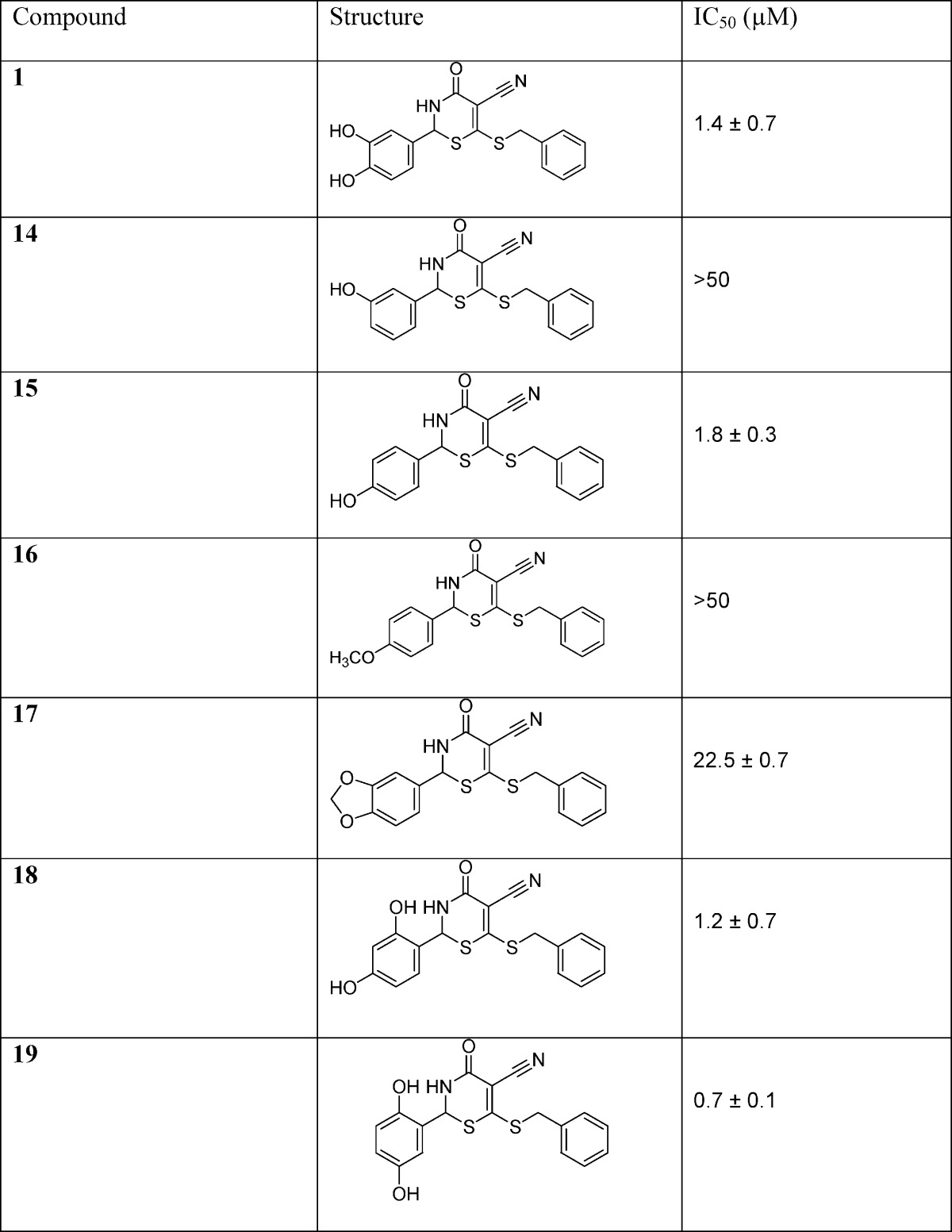
The IC50 values of these compounds were subsequently determined and used to assess the efficacy of the positions of the aryl hydroxyl groups in the newly designed inhibitors (Table 1). Results showed that compound 14 with the meta-hydroxyl group exhibited a very high IC50 of 50 μM. When the hydroxyl group was changed to the para position for compound 15, the potency of this compound dramatically improved to an IC50 of 1.8 μM. When this aryl hydroxyl group was substituted with −OCH3 functionality in compound 16, the GALK inhibitory activity was abolished (IC50 > 50 μM).
Table 1. IC50 Values of Synthesized Thiazinones Derivatives of Compound 1a.
Experimental conditions for IC50 determination are provided in the Supporting Information. Standard deviations were calculated from three replicate measurements.
Similarly, in compound 17, the substitution for benzo[d][1,3]dioxole group caused an increase in the IC50 (23 μM). The thiazinone compounds with 2,4- and 2,5-aryl hydroxyl substitutions were assayed for inhibitory activities, and compound 19 with the 2,5-dihydroxy functional group was more potent than the 2,4-dihydroxy thiazione derivative 18.
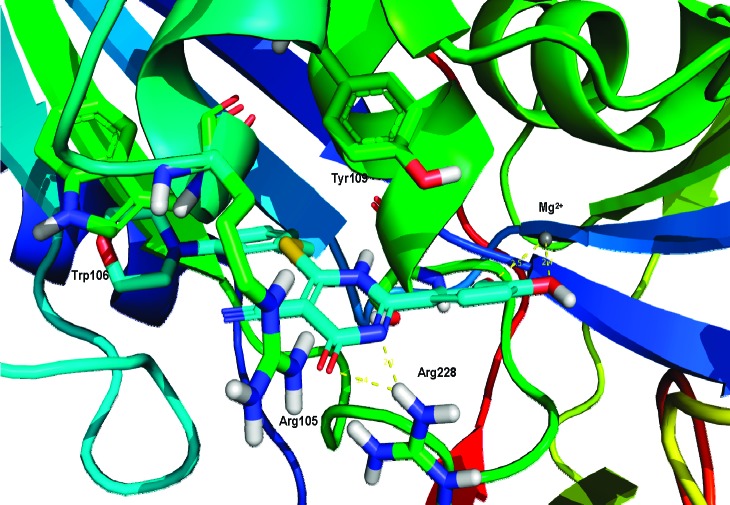
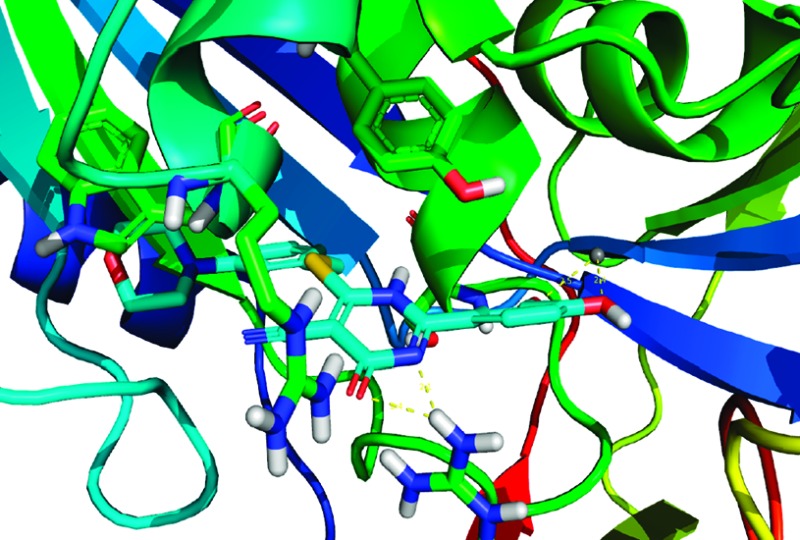
It was previously realized through computational modeling studies that the aromatic C ring of the 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile core mimics the adenine moiety of ATP within the active site of human GALK crystal structure as shown in Figure 2. Reference protein coordinates used for structure-based lead optimization were taken from the X-ray structure of the human GALK in complex with the nonhydrolyzable ATP analog, AMP-PNP (PDB: 1WUU),28 and were used in all computational experiments using the ICM suite. The active site for the GALK protein was defined as being within 8 Å of AMP-PNP in the X-ray cocrystal structure. Energy grids representing the active site (van der Waals, hydrogen bonding, electrostatics, and hydrophobic interactions) were calculated with 0.5 Å grid spacing, and docking experiments were performed using the defined AMP-PNP binding pocket with the application of our docking workflow.
Figure 2.
Mode of binding of compound 25 in complex with GALK. The active site ATP pocket is shown with critical residues that are in contact with central 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile. The endocyclic amide–Arg228 interactions are depicted as dashed yellow lines. Similarly, the dihydroxy-substituted aryl ring is positioned to γ-phosphate binding site and positioned within the distance to Mg2+. The Tyr109 is also within the distance for wedge-face ππ interactions with morpholine-substituted aryl ring.
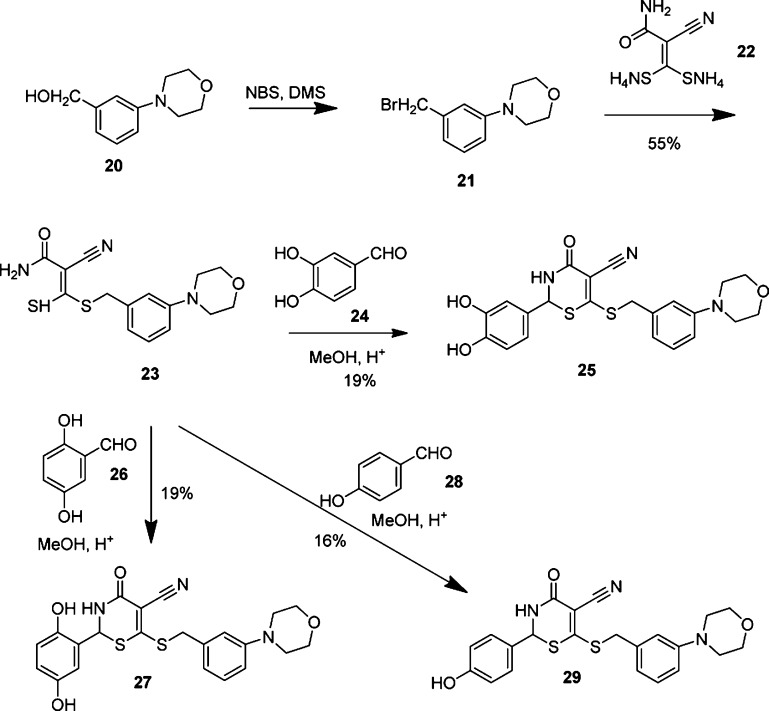
The meta position to the thiobenzyl group of the C ring could be modified with a morpholine group to further stabilize the binding of the molecule to the adenine binding site. Computational studies suggested that this group was well tolerated within the 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile scaffold and its binding mode within the ATP site of GALK. Hence, morpholine derivatives of compounds 2, 15, and 19 were synthesized with the aim to further improve the potency (Scheme 2). We chose the 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile scaffold 2, 15, and 19 (Table 1) to prepare new morpholine derivatives 25, 27, and 29 (Scheme 2) because we had previously determined that the compounds 2, 15, and 19 had IC50 values of 1.4, 1.8, and 0.7 μM, respectively (Table 1), which were among the lowest IC50 values in our in vitro GALK assays. As a result, the preparation of their morpholine derivatives was pursued by the condensation of acrylamide 23 with aldehydes 24, 26, and 28 (Scheme 2).
Scheme 2. Synthesis of Substituted Morpholine Derivatives of Compounds 2, 15, and 19.
The synthesis of morpholine 25 (Scheme 2) began with the transformation of benzylic alcohol 20 to bromide 21 by the addition of NBS/DMS cocktails.29 A slow addition of bromide 21 to the solution of bis(ammoniothio)-2-cyanoacrylamide 22 selectively afforded acrylamide 23 in 55% from alcohol 20. The synthesis of morpholine 25 was completed by the treatment of acrylamide 23 with aldehyde 24 under acid-catalyzed conditions in MeOH with a fair yield of 19%. We determined the IC50 values of the morpholine derivatives, and the results are shown in Table 2.
Table 2. IC50 Values of Synthesized Morpholine Derivatives 25, 27, and 29a.
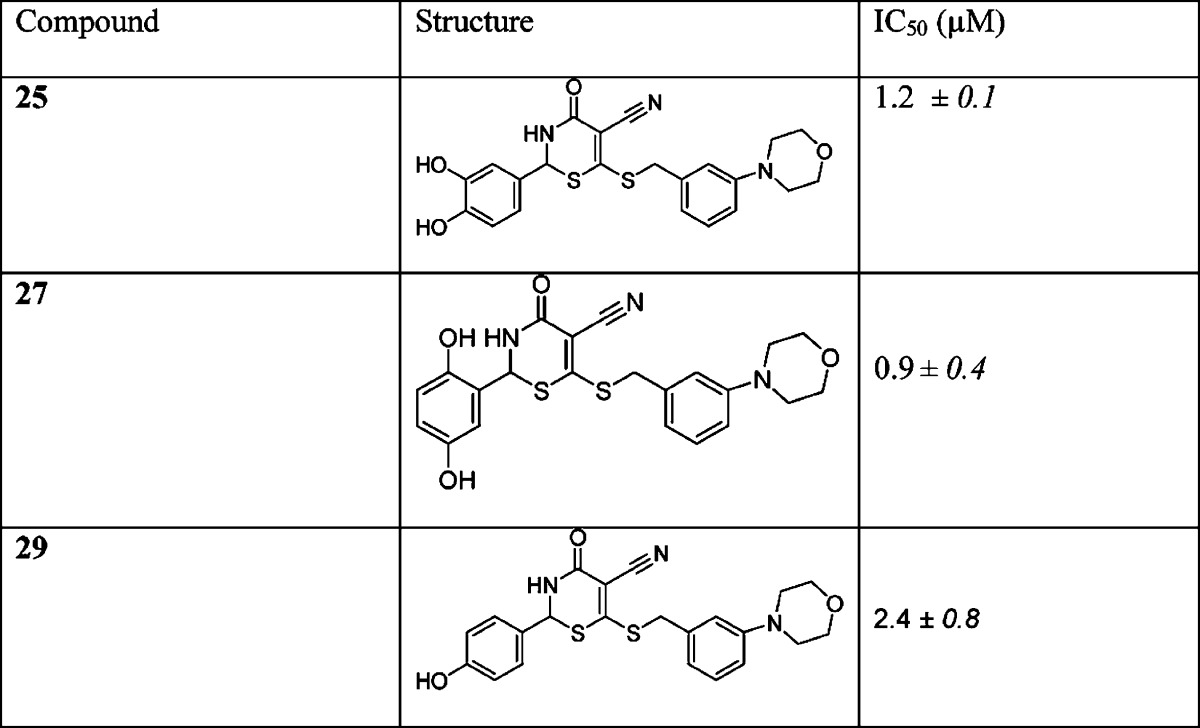
Experimental conditions for IC50 determination are provided in the Supporting Information. Standard deviations were calculated from three replicate measurements.
Initially, the intracellular inhibition of GALK and the subsequent lowering of gal-1P for compound 2 with an IC50 value of 1.4 μM could not be determined in patient cells because of its toxicity. In the subsequent cell-based studies, compound 2 in its protected form as diacetate 13 (Scheme 1) continued to be toxic to the human fibroblast cells at either 50 μM or higher concentrations and resulted in total cell lethality within hours. However, compound 25 with an IC50 value of 1.2 μM (Table 2) was tolerated at 50 μM by the cells without visible signs of lethality. Therefore, we proceeded to determine the intracellular inhibitory activity of morpholine derivative 25 against GALK by measuring the reduction of gal-1P accumulation. This compound inhibited GALK in patient fibroblast cells and lowered cellular gal-1P by 16% at 50 μM.
Additionally, morpholine derivative 27 was also well tolerated in cells at up to 100 μM and reduced cellular gal-1P by 16% at 100 μM without gross cell lethality, while morpholine derivative 29 was proved to be lethal to cells at 100 μM or higher. However, at 50 μM, this compound was well-tolerated. Unlike compound 25, neither 27 nor 29 was able to inhibit GALK or lowered gal-1P at 50 μM.
Unknown cellular side reactions with 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile scaffold might have limited the inhibitory potential of these morpholine derivatives to further lower the cellular gal-1P below 16% at 50 or 100 μM. On the basis of this observation, we have considered the design and synthesis of new 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile scaffold containing compounds that might be much less prone to cellular side reactions, and our ongoing efforts will be reported in the near future.
In summary, we have conducted limited SAR studies and optimization on a selected 4-oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile chemotype identified previously as a GALK inhibitor and was initially toxic. The new series of compounds showed improved in vitro potency, improved cell tolerance, and efficacy to partially inhibit GALK in cell-based assays. Because the accumulation of gal-1P plays a pathological role in the disorder CG, the discovery of the oxo-3,4-dihydro-2H-1,3-thiazine-5-carbonitrile scaffold substituted with morpholine is first in the class of new compounds that can be explored as therapeutic agents to lower cellular gal-1P in patient cells.
Supporting Information Available
Experimental procedures and spectral data for the test compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by U.S. National Institutes of Health Grants 5R01HD054744-05 and 3R01HD054744-04S1.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Isselbacher K. J.; Anderson E. P.; Kurahashi K.; Kalckar H. M. Congenital galactosemia, a single enzymatic block in galactose metabolism. Science 1956, 123, 635–636. [DOI] [PubMed] [Google Scholar]
- Leloir L. F. The enzymatic transformation of uridine diphosphate glucose into a galactose derivative. Arch. Biochem. 1951, 33, 186–190. [DOI] [PubMed] [Google Scholar]
- Gitzelmann R.; Curtius H. C.; Schneller I. Galactitol and galactose-1-phosphate in the lens of a galactosemic infant. Exp. Eye Res. 1967, 6, 1–3. [DOI] [PubMed] [Google Scholar]
- Lai K.; Langley S. D.; Khwaja F. W.; Schmitt E. W.; Elsas L. J. GALT deficiency causes UDP-hexose deficit in human galactosemic cells. Glycobiology 2003, 13, 285–294. [DOI] [PubMed] [Google Scholar]
- Segal S.; Berry G. T.. Disorders of galactose metabolism. In The Metabolic Basis of Inherited Diseases; Scriver C. R., et al. , Eds.; McGraw-Hill: New York, 1995; pp 967–1000. [Google Scholar]
- Waggoner D. D.; Buist N. R. M.; Donnell G. N. Long-term prognosis in Galactosemia: Results of a survey of 350 cases. J. Inherited Metab. Dis. 1990, 13, 802–818. [DOI] [PubMed] [Google Scholar]
- Waggoner D.; Buist N. R. M. Long-term complications in treated galactosemia—175 U.S. cases. Int. Pediatr. 1993, 8, 97–100. [Google Scholar]
- Antshel K. M.; Epstein I. O.; Waisbren S. E. Cognitive strengths and weaknesses in children and adolescents homozygous for the galactosemia Q188R mutation: A descriptive study. Neuropsychology 2004, 18, 658–664. [DOI] [PubMed] [Google Scholar]
- Bosch A. M.; Grootenhuis M. A.; Bakker H. D.; Heijmans H. S. A.; Wijburg F. A.; Last B. F. Living with classical galactosemia: health-related quality of life consequences. Pediatrics 2004, 113, e423–428. [DOI] [PubMed] [Google Scholar]
- Lambert C.; Boneh A. The impact of galactosaemia on quality of life—A pilot study. J. Inherited Metab. Dis. 2004, 27, 601–608. [DOI] [PubMed] [Google Scholar]
- Ridel K. R.; Leslie N. D.; Gilbert D. L. An updated review of the long-term neurological effects of galactosemia. Pediatr. Neurol. 2005, 33, 153–161. [DOI] [PubMed] [Google Scholar]
- Waisbren S. E.; Albers S.; Amato S.; Ampola M.; Brewster T. G.; Demmer L.; Eaton R. B.; Greenstein R.; Korson M.; Larson C.; Marsden D.; Msall M.; Naylor E. W.; Pueschel S.; Seashore M.; Shih V. E.; Levy H. L. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. J. Am. Med. Assoc. 2003, 290, 2564–2572. [DOI] [PubMed] [Google Scholar]
- Waisbren S. E.; Rones M.; Read C. Y.; Marsden D.; Levy H. L. Brief report: Predictors of parenting stress among parents of children with biochemical genetic disorders. J. Pediatr. Psychol. 2004, 29, 565–570. [DOI] [PubMed] [Google Scholar]
- Bosch A. M.; Bakker H. D.; van Gennip A. H.; van Kempen J. V.; Wanders R. J.; Wijburg F. A. Clinical features of galactokinase deficiency: A review of the literature. J. Inherited Metab. Dis. 2002, 25, 629–634. [DOI] [PubMed] [Google Scholar]
- Gitzelmann R.; Wells H. J.; Segal S. Galactose metabolism in a patient with hereditary galactokinase deficiency. Eur. J. Clin. Invest. 1974, 4, 79–84. [DOI] [PubMed] [Google Scholar]
- Hennermann J. B.; Schadewaldt P.; Vetter B.; Shin Y. S.; Mönch E.; Klein J. Features and outcome of galactokinase deficiency in children diagnosed by newborn screening. J. Inherited Metab. Dis. 2011, 34, 399–407. [DOI] [PubMed] [Google Scholar]
- Douglas H. C.; Condie F. The genetic control of galactose utilization in Saccharomyces. J. Bacteriol. 1954, 68, 662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K. L.; Davis C. N.; Fridovich-Keil J. L. Differential roles of the Leloir pathway enzymes and metabolites in defining galactose sensitivity in yeast. Mol. Genet. Metab. 2004, 83, 103–116. [DOI] [PubMed] [Google Scholar]
- Slepak T.; Tang M.; Addo F.; Lai K. Intracellular galactose-1-phosphate accumulation leads to environmental stress response in yeast model. Mol. Genet. Metab. 2005, 86, 360–371. [DOI] [PubMed] [Google Scholar]
- Acosta P. B.; Gross K. C. Hidden sources of galactose in the environment. Eur. J. Pediatr. 1995, 154, S87–92. [DOI] [PubMed] [Google Scholar]
- Berry G. T.; Nissim I.; Lin Z.; Mazur A. T.; Gibson J. B.; Segal S. Endogenous synthesis of galactose in normal men and patients with hereditary galactosaemia. Lancet 1995, 346, 1073–1074. [DOI] [PubMed] [Google Scholar]
- Timson D. J. GHMP Kinases—Structures, Mechanisms and Potential for Therapeutically Relevant Inhibition. Curr. Enzyme Inhib. 2007, 3, 77–94. [Google Scholar]
- Wierenga K. J.; Lai K.; Buchwald P.; Tang M. High-throughput screening for human galactokinase inhibitors. J. Biomol. Screening 2008, 13, 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M.; Wierenga K.; Elsas L. J.; Lai K. Molecular and biochemical characterization of human galactokinase and its small molecule inhibitors. Chem.-Biol. Interact. 2010, 188, 376–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork P.; Sander C; Valencia A. Convergent evolution of similar enzymatic function on different protein folds: the hexokinase, ribokinase, and galactokinase families of sugar kinases. Protein Sci. 1993, 2, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama M. A Novel Synthesis of 4H-1,3-Thiazin-4-one Derivatives. Bull. Chem. Soc. Jpn. 1971, 44, 1610–1613. [Google Scholar]
- Yokoyama M.; Nakamura M.; Ohteki H.; Imamoto T.; Yamaguchi K. S, N Double Rearrangement. 2. X-ray Crystal Structures of Rearranged Products. J. Org. Chem. 1982, 47, 1090–1094. [Google Scholar]
- Thoden J. B.; Timson D. J.; Reece R. J.; Holden H. M. Molecular structure of human galactokinase: implications for type II galactosemia. J. Biol. Chem. 2005, 280, 9662–70. [DOI] [PubMed] [Google Scholar]
- Odejinmi S. I.; Wiemer D. F. Synthesis of Arieianal, a Prenylated Benzoic Acid from Piper arieianum. J. Nat. Prod. 2005, 68, 1375–1379. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.