Abstract
Background
Although arthralgia is a known adverse effect of aromatase inhibitor (ai) treatment in postmenopausal breast cancer patients, few studies have carried out a comprehensive evaluation of the nature, onset, and incidence of musculoskeletal (msk) pain in these patients. We therefore used a pilot study to identify conditions or markers predictive of pain.
Methods
For 24 weeks, we monitored 30 eligible postmenopausal women starting ai therapy. Pre-existing and incident msk conditions and pain were assessed clinically and with ultrasonography of the hands and wrists. In addition, patient questionnaires were used to assess pain before and during ai therapy. Biochemical markers were measured at baseline and at regular intervals after anastrozole therapy began. Gene profiling studies were carried out before and 48 hours after the initial ai administration.
Results
Over the 24-week study period, 20 participants (67%) showed no pain symptoms; 5 (17%) experienced low or moderate pain at baseline, which did not increase with ai treatment; and during therapy, 5 (17%) showed exacerbation of pain attributable to osteoarthritis of the hand and to finger flexor tenosynovitis. Although all 30 participants had some degree of msk conditions before anastrozole therapy started, the pre-existing conditions did not necessarily predispose the women to increased pain during anastrozole treatment. Higher levels of urinary N-telopeptides of type i collagen were associated with the groups presenting pain, suggesting a higher extent of pre-existing bone resorption, without significant evolution over the 24-week treatment period. Slightly higher levels of 1,25(OH)2 vitamin D3 were observed at baseline in patients with pain increase, but did not significantly change during treatment; however, average levels of 25(OH) vitamin D3 increased, likely because of supplementation. Although biochemical markers did not discriminate efficiently between pain groups, a signature of 166 genes in peripheral blood mononuclear cells was identified that could stratify patients into the various groups observed in this pilot study. The gene signature was enriched in components of inflammatory signalling and chemokine expression, of antitumoural immunity pathways, and of metabolic response to hormones and xenobiotics, although no clinically significant association could be made in the present study, considering the small number of patients. Nevertheless, the observed trend suggests the feasibility of developing surrogate predictive markers of msk pain. Patient compliance was high in this study and was not affected by pain exacerbation.
Conclusions
Baseline msk assessment showed pre-existing causes for pain in most of the study patients before initiation of the ai. Exacerbation of existing osteoarthritis pain and tenosynovial symptoms was the primary cause of pain increase. Musculoskeletal pain assessment at baseline and prompt treatment of pain symptoms may help to optimize adherence to ai therapy. The value of routinely assessing inflammatory markers such as C-reactive protein and erythrocyte sedimentation rate was not supported by our pilot study. Gene expression profiles in peripheral blood mononuclear cells may be further explored in larger-scale studies as stratification markers to identify patients at risk of developing arthralgia.
Keywords: Breast cancer, ai, aromatase inhibitor, arthralgia, musculoskeletal pain
1. INTRODUCTION
Compared with tamoxifen, aromatase inhibitors (ais) such as anastrozole, letrozole, and exemestane show increased disease-free survival benefits; they have therefore become the standard of care for adjuvant endocrine treatment of postmenopausal women with hormone receptor–positive early breast cancer 1. Adverse effects of ais include a higher risk for bone fractures and arthralgia, the latter being a clinical symptom defined as nonspecific pain in the joints. In one of the first reports of arthralgia associated with anastrozole treatment, 16% of women with metastatic breast cancer developed joint pain within 2 months of starting treatment 2.
The incidence of musculoskeletal (msk) symptoms in phase iii clinical trials of anastrozole, letrozole, and exemestane has been recently reviewed 3, and women on those ais have shown significantly higher rates of arthralgia than are seen with tamoxifen (Table I). In a specific study investigating arthralgia in 200 patients on ais, 47% of patients reported ai-related joint pain, and 44% reported stiffness 8. Typically, patients on ais experience stiffness, achiness, or pain that is frequently symmetric, occurring in the hands, arms, knees, feet, and pelvic and hip bones 9,10. In addition, patients on ais may develop tenosynovial changes, including fluid in the tendon sheath, increased tendon thickness, trigger finger, and carpal tunnel syndrome (cts) 11–13.
TABLE I.
Arthralgia rates in major clinical trials of aromatase inhibitors
| Reference (trial name) | Follow-up (months) | Rate of arthralgia (%) with | p Value | Comments | |||
|---|---|---|---|---|---|---|---|
| Tamoxifen | Anastrozole | Letrozole | Exemestane | ||||
| Coombes et al., 2004 4 (ies 30) | 6 | 3.6 | 5.4 | 0.01 | Women were switched to exemestane after 2–3 years of tamoxifen | ||
| Goss et al., 2005 5 (ma.17) | 30 | 21 | 25 | 0.001 | Women were switched to letrozole after 5 years of tamoxifen More women experienced myalgia than experienced arthritis |
||
| Howell et al., 2005 6 (atac) | 60 | 29.4 | 35.6 | 0.0001 | |||
| Coates et al., 2007 7 (big 1–98) | 51 | 13.5 | 20 | <0.001 | |||
ies = International Exemestane Study; atac = Arimidex, Tamoxifen, Alone or in Combination; big = Breast International Group.
In ai trials in which the intensity of pain was reported (Breast International Group 1-98, for example), pain was usually mild, with 58% of women on letrozole experiencing pain categorized as grade 1, and 33%, grade 2 14. The Arimidex, Tamoxifen, Alone or in Combination (atac) trial reported that pain symptoms resolved within 6–18 months (50% and 75% of patients respectively) 15. Nevertheless, pain has a considerable impact on quality of life in women on ai therapy. In a prospective study of 100 patients on either letrozole or exemestane, 45.4% developed joint symptoms meeting criteria for rheumatology referral 11. Median time to development of symptoms was 1.6 months, and 13% of patients discontinued therapy after a median period of 6.1 months 11. Discontinuation rates on account of pain have not been reported in large clinical trials, but rates as high as 20% have been noted in studies outside of such trials 16,17.
Few studies have set out to determine the risk factors that may be associated with onset of arthralgia in women on ai therapy. In the atac trial, risk factors for arthralgia (regardless of whether patients were on anastrozole or tamoxifen) included previous chemotherapy, previous hormone replacement therapy, hormone receptor positivity, and obesity 18. In a study by Crew et al. specifically investigating the prevalence of pain in women with early-stage breast cancer on ai therapy, those who had received prior taxane therapy had a likelihood of experiencing pain that was increased by a factor of 4 8.
Because joint pain is perceived by the nociceptive fibres innervating articular structures, and because estrogen has a protective effect on those fibres 19,20, it has also been suggested that ai-associated arthralgia may be triggered by the ability of ais to swiftly and effectively cause estrogen depletion 21. In a survey carried out to assess the perceived onset, characteristics, and risk factors for arthralgia among 300 postmenopausal women receiving ai therapy, multivariate analysis showed that time since the last menstrual period was the only significant predictor for arthralgia and that women within 5 years of their last menstrual period had a tripled age-adjusted risk compared with women who were within 10 years of their last menstrual period 22.
Because arthralgia and other msk symptoms appear to be a common side effect of ai therapy that may lead to discontinuation of treatment if symptoms persist, and because very little is known about this specific type of pain, we sought to determine the time of onset and the nature of pain symptoms in a prospective study of women in which a rheumatology examination was performed for all eligible patients before anastrazole start. We also wanted to evaluate the frequency with which a clinical rheumatology evaluation would have to be performed in a future larger-sample study. To minimize variability between patients, we confined our study to women on anastrozole because it is not known whether there are differences in the ability of the various ais to induce arthralgia and because our pilot study was small. Another secondary goal was to identify markers associated with msk pain symptoms. Within a multidisciplinary group of clinicians, we first attempted to select a series of markers that would help to identify patients susceptible to developing pain symptoms during ai administration. Because potential markers are numerous, and because we wanted to better select the markers, we designed an exploratory pilot study to include a wide variety of potential markers and to minimize the required number of patients before we embarked on a larger trial using specific markers.
Biologic markers have not thus far been predictive of arthralgia, but some associations have been noted. Patients on letrozole showed an increase in Creactive protein, a biomarker of inflammation, within the first 6 months after treatment start; however, that increase was not specifically correlated with arthralgia 23. Urinary N-telopeptide of type i collagen is a marker of bone resorption and distinguishes normal, osteopenic, and osteoporotic bone mineral density levels 24. Our rationale for selection of biomarkers was to test the potential effects on pain increase of the rate of estrogen depletion, of initial vitamin D status, and of pre-existing onset of bone loss.
We also conducted gene expression profiling studies and assessed signatures of inflammatory signalling and antitumoural activity to determine if certain expression signatures might predict the onset of pain. We turned to gene expression profiling because genomics has proven very useful at stratifying patients according to tumour type or response to therapy. Gene expression signatures have proved to have value in the stratification of breast patients into tumour subclasses with different associated prognoses 25. In addition, expression signatures in peripheral blood mononuclear cells (pbmcs) have shown usefulness in the early detection of breast cancer 26. Here, we explored the potential of gene expression profiling studies in pbmcs to stratify patients according to pain groups; our aim was to assess the potential value of profiling as a predictor of arthralgia risk. It has been proposed that women who develop ai-related arthralgia may have an anergic cytokine phenotype with lower baseline levels of some interleukins and colony- stimulating factors 3. Furthermore, to accurately determine the effect of pain on patient compliance, we measured serum estradiol levels as an indicator of patient adherence to ai treatment.
2. METHODS
2.1 Patients
Over a period of 3 months from 2008 to 2009, we recruited 30 postmenopausal women with breast cancer eligible for adjuvant hormonal therapy with anastrozole at a single medical centre. Menopause was defined as age 56 and older with no spontaneous menses for at least 12 months before study entry, or age 55 or younger with no spontaneous menses for at least 12 months before study entry (spontaneous or secondary to a prior hysterectomy or bilateral oophorectomy) and with an estradiol level in the postmenopausal range. Table II shows patient demographics and baseline characteristics.
TABLE II.
Demographics and baseline characteristics of the study patients
| Variable | Value |
|---|---|
| Age (years) | |
| Median | 67 |
| Range | 52–79 |
| Body mass index | |
| Median | 27.7 |
| Range | 21.5–54.8 |
| Surgery [n (%)] | |
| Lumpectomy | |
| With sentinel node biopsy | 17 (57) |
| With axillary dissection | 8 (27) |
| Mastectomy | 5 (17) |
| Chemotherapy [n (%)] | 13 (43) |
| Time from completion of surgery or chemotherapy to ai start (weeks) | |
| Median | 20 |
| Range | 5–52 |
| Conditions experienced [n (%)] | |
| Musculoskeletal symptoms | 5 (17) |
| Osteoarthritis (arthrosis) | 19 (63) |
| Carpal tunnel syndrome (mild) | 1 (3) |
| Osteoporosis | 5 (17) |
| Other arthropathy | 4 (13) |
| Conditions experienced per patient (n) | |
| 1 | 20 |
| 2 | 8 |
| 3 | 2 |
Patients were followed for a period of 24 weeks, with assessments for pain carried out at specific intervals as shown in Table III.
TABLE III.
Assessment schedule
| Time point | |||||||
|---|---|---|---|---|---|---|---|
| Week | |||||||
| Test | Baseline | 1 | 3 | 5 | 7 | 12 | 24 |
| Rheumatology exam | X | X | X | X | X | ||
| Ultrasonography (hand/wrist) | X | X | |||||
| Pain intensity and qualitya | X | X | X | X | X | X | X |
| Depressionb | X | X | X | ||||
| Functional assessmentc | X | X | X | X | X | X | X |
| Estradiol (lc-mc/ms and ria) | X | X | X | X | X | X | |
| Vitamin D | X | X | X | X | X | X | |
| Urinary N-telopeptides of type i collagen | X | X | X | X | X | X | |
| C-Reactive protein | X | X | X | X | X | X | |
| Erythrocyte sedimentation rate | X | X | X | X | X | X | |
| Bone mineral densitometry | X | ||||||
| Blood samples for gene expression | Taken immediately before and 48 hours after initial administration of the aromatase inhibitor | ||||||
Determined using the Brief Pain Inventory.
Determined using the Geriatric Depression Scale.
Determined using the Health Assessment Questionnaire.
lc-mc/ms = liquid chromatography mass spectrometry; ria = radioimmunoassay.
2.2 Drug Treatment
Women were treated with oral anastrozole 1 mg and with vitamin D 800 IU and calcium 1000 mg supplementation daily.
2.3 Pain Assessment and Rheumatology Evaluation
Patients completed the Brief Pain Inventory Short Form (bpi) 27, a self-directed questionnaire used to determine the presence, localization, and intensity of pain. A functional assessment was also carried out using the Health Assessment Questionnaire 28. Questionnaires were completed at baseline before treatment start and were repeated after treatment start (Table III). At each visit, patients were questioned by the study staff about their pain and whether they had taken pain medication.
Clinical assessment by a rheumatologist was performed at baseline and was repeated over the course of the 24 weeks if patients had a pain score greater than 4 or twice the degree of pain demonstrated at baseline [as determined by the numeric score (0–10) for the question on general pain in the bpi questionnaire]. Included in the assessment by a rheumatologist was the localization and probable cause of msk and joint pain sites on physical examination.
2.4 Ultrasonography
Ultrasonography examination of the hands and wrists was performed to evaluate the occurrence of tenosynovial changes and cts, because ultrasonography is a useful imaging tool for assessing tenosynovial changes, trigger finger, and cts, and it is well tolerated by patients 29–31. Static and dynamic ultrasonography was performed at baseline and at 12 weeks by a msk r adiologist u sing a 7–12 MHz or 9–15 MHz linear transducer (iU22 xMatrix: Philips Healthcare, Andover, MA, U.S.A.). The dorsal and volar aspects of both hands and wrists were systematically scanned in transverse and longitudinal planes, and measurements were taken of the cross-sectional area of the median nerve at the level of the carpal tunnel and of the thickness of the A1 pulley of each finger. The flexor and extensor tendons were assessed for the presence of fluid in the tendon sheath and for tendon thickness.
2.5 Quality of Life Assessment
The Geriatric Depression Scale 32, a self-administered questionnaire, was used to determine if patients were depressed.
2.6 Biomarker Tests
The inflammatory biomarkers C-reactive protein and erythrocyte sedimentation rate were measured using standard laboratory techniques, as were vitamin D levels. Urinary cross-linked N-telopeptides of type i collagen were measured using Osteomark (Wampole Laboratories, Princeton, NJ, U.S.A.), an enzyme-linked immunosorbent assay. Serum estradiol was measured by both liquid chromatography mass spectrometry (lc-ms/ms) and radioimmunoassay. The limit of quantification (intra- and inter-assay variabilities) for the lc-ms/ms was less than 15%. Table III presents the assessment schedule for each biochemical test. We performed analysis of variance and Tukey tests using the “aov” and “TukeyHSD” functions of the R language (http://www.R-project.org).
2.7 Gene Expression Profiles
Blood samples treated with the anticoagulant ethylenediaminetetraacetic acid were taken from each patient before and 48 hours after the initial ai administration. Peripheral blood mononuclear cells were isolated on Ficoll–Paque Plus gradient (GE Healthcare, Baie d’Urfe, QC), inspected visually for elimination of red blood cells, and lyzed in trizol. Extracted rna w as q uantified u sing a N anoDrop spectrophotometer (Thermo Scientific, Wilmington, DE, U.S.A.), and quality was assessed by Bioanalyzer (Agilent Technologies, Santa Clara, CA, U.S.A.). Gene expression analysis (29 patients, 2 time points) was performed on Human Whole Genome BeadArray Technology (Illumina, San Diego, CA, U.S.A.). Microarrays were normalized using the Lumi package (Bioconductor, Seattle, WA, U.S.A.) with the quantile normalization and the vst variance stabilizing transform methods 33,34. Moderated t statistics from the Limma package (Bioconductor) 35 were used to identify statistically significant changes in gene expression. Genes with a p value of less than 0.05 between patient pain groups or for the ai within a group were used for classification. The 166-gene classifier was identified by the nearest shrunken centroid method 36.
3. RESULTS
3.1 Patient Characteristics
The 30 women enrolled in the study had been postmenopausal at the time of their breast cancer diagnosis. All women who had undergone lumpectomy received radiation. Chemotherapy was administered to 13 women (43%) after surgery and radiation at the discretion of the treating physician. In 11 of the patients, body mass index exceeded 30.
Osteoarthritis was the most common condition at baseline, affecting 63% of patients, and osteoporosis was present in 5 women (17%, Table II). One of the major findings of this pilot study is that the rheumatology assessment showed that all patients had a history of msk conditions before commencement of ai therapy. Every patient was thoroughly examined by a rheumatologist, which is unusual in the context of breast oncology and ai administration. Given the median age of this patient population (67 years), it was anticipated that, based on rigorous examination by an expert, all patients would be found to have msk conditions.
3.2 Pain Symptoms and MSK Conditions During AI Treatment
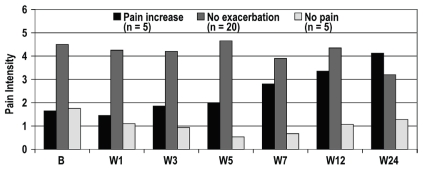
Despite the existing msk conditions at baseline, most patients (20 of 30, 67%) showed no pain symptoms (as determined by the bpi questionnaire) requiring any intervention during the course of ai treatment (Table IV). At baseline, 14 of the 30 patients (47%) claimed to take pain medication occasionally when required. It should be noted that 2 patients in this group had pain related to breast cancer surgery, but did not have joint or msk pain. Low or moderate pain was present in 5 women (17%) at baseline, with no exacerbation over the 24-week study period. An increase in pain during treatment was reported by 5 women (17%). Of those 5 women, 4 experienced exacerbation of pain from pre-existing osteoarthritis, all localized in the hand joints; 1 woman experienced exacerbation of pain from knee osteoarthritis. Pain symptoms attributable to a tenosynovial phenomenon (finger flexor tenosynovitis) was experienced by 2 patients. In patients who experienced increased pain during treatment, the onset of pain occurred 7 weeks after treatment initiation. Over the 24-week period, the pain gradually increased to levels similar to those experienced by patients whose pain was not exacerbated, but remained below an intensity score of 5 on the bpi pain intensity score (Figure 1). The higher pain intensity was reached at 24 weeks, at which time data collection had been stopped per the study design. The patients are still taking ais; however, no data on their pain intensity are available. Patients whose pain was not exacerbated during the course of treatment experienced pain intensity levels that were greater than those in patients whose pain increased with ai treatment; however, their level of pain over the 24- week treatment period was similar to that at baseline.
TABLE IV.
Pain symptoms during treatment with aromatase inhibitora
| Condition | Patients [n/N (%)] |
|---|---|
| No pain symptoms | 20/30 (67) |
| No exacerbation of pain | 5/30 (17) |
| Increase in pain symptoms during treatment | 5/30 (17) |
| Exacerbation of pain from osteoarthritis | 4b/5c (80) |
| New pain symptoms from a tenosynovial phenomenon | 2b/5c (40) |
Based on examination by a rheumatologist.
One patient presented with both these patterns.
Of the original 30 patients.
FIGURE 1.
Pain profile of the patients during the 24-week study period. Of the 30 study patients, 20 experienced no exacerbation of pain; 5 experienced low to moderate pain at baseline, but no exacerbation; and 5 experienced an increase in pain with aromatase inhibitor treatment. B = baseline; W = week.
In 3 patients, ultrasonography at 12 weeks demonstrated an increase in the cross-sectional area of the median nerve of both wrists, but those patients remained asymptomatic for cts. Fluid developed in 2 patients, and a 3rd patient showed an increased amount of fluid within the tendon sheath surrounding the flexor tendons in 1 digit, but without associated clinical symptoms.
Patients were encouraged to take pain medication as needed. In total, 17 of 30 patients (57%) received nonsteroidal anti-inflammatory drugs, mainly naproxen, for pain relief either regularly or intermittently during the 24-week study period. Patients on regular pain medication were those who presented with pain at baseline and those who developed pain during treatment. A few patients took pain medication when required, but not regularly. Among the 5 patients who developed pain during treatment, 2 received steroid injections (one for plantar fasciitis, and the other for finger flexor tenosynovitis) in addition to medication for pain relief. The level of pain was tolerable after treatment, although it did not completely resolve. After the initial baseline evaluation by a rheumatologist, referral to a rheumatologist was required for 9 of the 30 patients (30%) during the subsequent weeks. No correlation was observed between pain from ai use and functionality or depression as measured by the Health Assessment Questionnaire and the Geriatric Depression Scale.
3.3 Biologic Markers
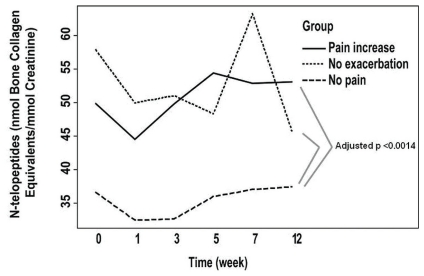
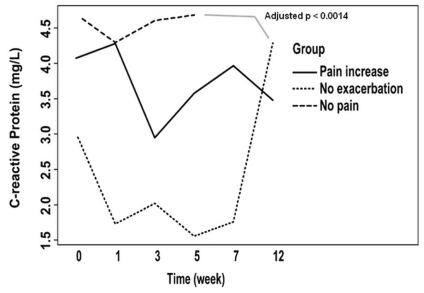
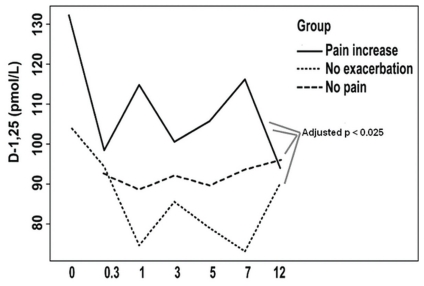
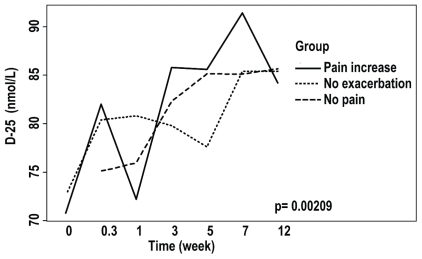
Regardless of the time of observation, differences in biologic markers observed between the patient groups included N-telopeptides of type i collagen (higher in the no-pain-exacerbation and pain-increase groups than in the no-pain group, adjusted p < 0.006, Figure 2), C-reactive protein (lower in the no-exacerbation group than in the no-pain group, adjusted p < 0.0014, Figure 3), and levels of 1,25(OH)2 vitamin D3 (higher in the pain-increase group than in the no-pain and no-pain-exacerbation groups, adjusted p < 0.025, Figure 4). Levels of 25(OH) vitamin D3 increased significantly from an average of 69.7 nmol/L to 85.4 nmol/L over the course of the study (analysis of variance p = 0.00209), without significant difference between the groups, probably reflecting the benefit of vitamin D supplementation during the study period (Figure 5). There is no clear explanation for these data, because the number of patients was too small for conclusions to be drawn.
FIGURE 2.
Levels of urinary N-telopeptides of type i collagen in the patient groups during the 24-week study period.
FIGURE 3.
Levels of C-reactive protein in the patient groups during the 24-week study period.
FIGURE 4.
Levels of 1,25(OH)2 vitamin D3 in the patient groups during the 24-week study period.
FIGURE 5.
Levels of 25(OH) vitamin D3 in the patient groups during the 24-week study period.
Mean estradiol levels, as assessed by lc-ms/ms, were low at baseline (2.7 ± 1.4 pg/mL). Of the 30 patients, 14 (47%) showed no detectable levels of estradiol within 48 hours of commencing anastrozole treatment; 14 showed levels of below 0.9 pg/mL. In 2 patients, levels reached 1–2 pg/mL after 48 hours of treatment, but levels had been higher at baseline (6–7 pg/mL) in those patients than in the remainder of the cohort. Estradiol levels were non-detectable in all patients after 1 week of anastrozole treatment. Interestingly, levels increased suddenly in 2 patients (one at 12 days, one at 4 weeks), suggesting that those patients had failed to take their dose of anastrozole around those times. Estradiol levels could not be assessed by radioimmunoassay, because the level of sensitivity of the assay was insufficient.
3.4 Gene Expression Profiles
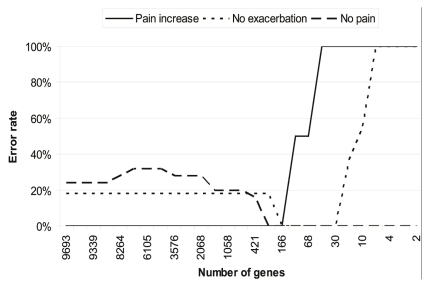
Gene expression profiles were generated using pbmcs purified from patient blood samples obtained immediately before and 48 hours after the initial administration of anastrozole. Genes with significant differences in expression level (Figure 6) at the two time points (4454 genes) or at baseline between the various groups (6731 genes) were identified so that their capacity to stratify patients according to pain group could be investigated. It was possible to reduce the gene set to a signature set of 166 genes that presented optimal patient classification (Figure 7). Top canonical pathways found by Ingenuity Pathway Analysis (Ingenuity Systems, Redwood City, CA, U.S.A.) to be enriched in this group of genes included lipopolysaccharide-mediated and interleukin 1–mediated inhibition of rxr (retinoid X receptor) function, ox40 signalling pathway, aryl hydrocarbon receptor signalling, sulphur metabolism, and xenobiotic metabolism signalling (Table V).
FIGURE 6.
Differential expression of genes in peripheral mononuclear cells in the study pain groups or in response to treatment.
FIGURE 7.
Classification error rate.
TABLE V.
Canonical pathways enriched in 166 gene signatures by Ingenuity Pathway Analysisa
| Name | p Value | Ratio |
|---|---|---|
| lps/il-1 mediated inhibition of rxr function | 7.02−07 | 10/216 (0.046) |
| ox40 signalling pathway | 2.67−04 | 5195 (0.053) |
| Aryl hydrocarbon receptor signalling | 2.69−04 | 61154 (0.039) |
| Sulfur metabolism | 4.01−04 | 3/61 (0.049) |
| Xenobiotic metabolism signalling | 5.94−04 | 8/239 (0.027) |
Ingenuity Systems, Redwood City, CA, U.S.A.
lps = lipopolysaccharide; il-1 = interleukin 1; rxr = retinoid X receptor.
We found it possible to identify and reduce gene signatures such that low error rates for classification of the group with pain increase were achieved. In the absence of a larger cohort, it was impossible to validate this gene signature, but our results support the feasibility of such an approach.
4. DISCUSSION
Although the phenomenon of pain symptoms in women on adjuvant hormonal treatment for early breast cancer is well documented, few studies have examined the factors predisposing those women to pain and, in particular, whether pain exists before initiation of treatment. Furthermore, no other study has reported rheumatology examination before initiation of ai therapy. In the present study, we found that all the participating women had msk conditions at baseline, but that those conditions did not necessarily predispose the patients to increased pain during anastrozole treatment. Of the 30 women, 5 (17%) were already experiencing pain before initiation of treatment, but did not experience pain exacerbation once treatment was initiated. Only 5 of the 30 women (17%) showed an increase in pain symptoms during treatment. Exacerbation of osteoarthritis (mainly in the hands) and finger flexor tenosynovitis were the clinical manifestations arising during ai treatment. Those conditions were diagnosed at clinical evaluation, but were not corroborated by ultrasonography.
Large clinical trials evaluating ais—anastrozole in atac 6, letrozole in Breast International Group 1-98 7, and exemestane in the International Exemestane Study 4—have reported arthralgia rates ranging from 5.4% to 35.6% (Table I). This broad range likely reflects a variety of definitions of arthralgia, arthritis, and bone pain, and the difficulty of categorizing nonspecific bone symptoms 9. Small studies carried out before the routine use of ais as adjuvant treatment reported results similar to ours, with the incidence of pain ranging from 10% to 15% 2. However, more recent studies of women receiving ais as adjuvant treatment have reported rates of 47% 8 and as high as 61% 16.
Regardless of the incidence, it is clear that ai treatment is associated with a degree of arthralgia requiring medical attention, and this aspect of ai treatment is being evaluated in a number of ongoing studies 3. In our study, awareness that all women had msk conditions at baseline and regular monitoring of pain symptoms made it possible to keep the symptoms under control. The 5 women who experienced pain during the study received medication, and although the pain continued, it is possible that the intervention improved symptoms such that the women were able to continue taking anastrozole. Furthermore, study participants were encouraged to take pain relief medication if required, and those who presented with pain at baseline were kept on pain medication.
We observed no significant changes in biologic markers in our patient cohort over the study period apart from an increase in the average level of 25(OH) vitamin D3, regardless of pain group, probably reflecting daily oral supplementation. Levels of circulating 1,25(OH)2 vitamin D3 were slightly elevated (about 20%) in patients with increased pain compared with the other two groups, possibly reflecting higher levels of 1 α-hydroxylase enzyme in some vitamin D target tissues in those patients; however, those levels did not increase over time. Levels of urinary N-telopeptides of type i collagen, a useful marker of osteopenia or osteoporosis 24, were elevated in the groups that presented with pain during ai administration (the no-pain-exacerbation and pain-increase groups), suggesting pre-existing bone resorption. Inflammatory markers were not associated with pain groups, given that no significant differences were observed in erythrocyte sedimentation rates, and levels of C-reactive protein were in fact slightly lower in the group with no exacerbation of pain than in the group with no pain. Our findings confirm that none of the biochemical markers tested are predictive of pain increase.
Carpal tunnel syndrome and tenosynovial changes in the hands have been reported in association with ai treatment and have been postulated to be a cause of joint stiffness and pain 37. In the present study, 3 women developed a subclinical increased amount of tenosynovial fluid in flexor tendons of the hands, and 3 women showed an increase in the cross-sectional area of the median nerve at 12 weeks, without clinical symptoms of cts. It would have been interesting to see if pain developed at a later time in those patients.
Gene expression profiles from pbmcs in the study patients at baseline and 48 hours after the initial administration of anastrozole indicate that it was possible in this pilot study to identify a gene signature that correctly classified the patients into pain categories. That signature was enriched in components of inflammatory signalling and chemokine expression 38,39, of antitumoural immunity 40 pathways, and of metabolic response to hormones and xenobiotics 41,42. Although larger patient groups are required to further evaluate the identified gene sets, our results suggest that pbmc expression signatures represent a potential, readily available source of biomarkers for identifying patients at risk of developing arthralgia. These markers prognostic of pain increase, if validated in larger cohorts, could allow for identification of patients at risk of pain increase early in treatment.
Discontinuation of adjuvant hormone treatment has been noted in several studies, and some concern about that discontinuation is justified, because a recent study showed that women on tamoxifen with an adherence rate of less than 80% (determined by prescription records) had an increased risk of mortality at a median duration of 2.4 years 43. In a study of anastrozole, only 69% of women were adherent over a median duration of 16.6 months 44. Although there may be a variety of reasons for drug discontinuation, a Canadian study based on chart reviews showed that 22% of women ceased taking adjuvant ai because of toxicity, and in that study, arthralgia and arthritis were experienced by 20% and 10% of women respectively 45. All the patients in our study were compliant with their medication, with only 2 patients (one experiencing no pain symptoms, and one experiencing no exacerbation of pain symptoms during ai therapy) intermittently forgetting as evidenced by a short spike in their estradiol levels. The lc-ms/ms method was shown to be effective, and superior to the radioimmunoassay method, in monitoring patient compliance, as evidenced by the fact that 2 patients were revealed to be nonadherent. This level of adherence is considerably higher than levels reported in other studies, albeit over a shorter period of time.
The fact that interventions for pain relief were applied immediately upon presentation of symptoms could account for the high level of adherence to treatment in our study. Furthermore, patients had access to physician and nursing care in the event of pain symptoms, which may have contributed to their treatment adherence. Effective and timely attention to pain symptoms in patients on ai treatment may allow them to continue their medication rather switch to another class of drug.
5. CONCLUSIONS
Identification of pre-existing msk conditions allows for effective treatment of pain, possibly increasing adherence to therapy. In the present study, baseline msk assessment showed pre-existing causes for pain in most patients before initiation of the ai. Assessment of msk pain at baseline and prompt treatment of pain symptoms may help to optimize adherence to ai therapy. Among the 30 study participants, only 5 (17%) showed an increase in pain symptoms during treatment. Exacerbation of osteoarthritis, mainly in the hands, and finger flexor tenosynovitis were the two clinical manifestations arising during ai treatment.
Given that patients begin to experience pain only about 7 weeks after initiation of ai treatment, the most appropriate time to begin evaluating pain in those patients is within 2–3 months after treatment initiation. Access to and frequent contact with physicians and nursing care play an important role in patient compliance with treatment.
Although the limitation of sample size precluded firm conclusions with respect to the stratifying power of any of the markers, identification of predictive markers of arthralgia based on gene expression profiles in pbmcs may further facilitate follow-up of patients at higher risk for msk pain. In this pilot study, the main goals were hypothesis generation and preliminary assessment; the data presented here need to be confirmed in a larger study.
6. ACKNOWLEDGMENTS
Data from this study were presented at the San Antonio Breast Cancer Symposium in 2009.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
AstraZeneca Canada sponsored this paper. The authors thank Science and Medicine Canada for their contribution and editorial support.
8. REFERENCES
- 1.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnellan PP, Douglas SL, Cameron DA, Leonard RC. Aromatase inhibitors and arthralgia. J Clin Oncol. 2001;19:2767. [PubMed] [Google Scholar]
- 3.Din OS, Dodwell D, Wakefield RJ, Coleman RE. Aromatase inhibitor-induced arthralgia in early breast cancer: what do we know and how can we find out more? Breast Cancer Res Treat. 2010;120:525–38. doi: 10.1007/s10549-010-0757-7. [DOI] [PubMed] [Google Scholar]
- 4.Coombes RC, Hall E, Gibson LJ, et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med. 2004;350:1081–92. doi: 10.1056/NEJMoa040331. [Erratum in: N Engl J Med 2006;355:1746] [DOI] [PubMed] [Google Scholar]
- 5.Goss PE, Ingle JN, Martino S, et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from ncic ctg ma.17. J Natl Cancer Inst. 2005;97:1262–71. doi: 10.1093/jnci/dji250. [DOI] [PubMed] [Google Scholar]
- 6.Howell A, Cuzick J, Baum M, et al. Results of the atac (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet. 2005;365:60–2. doi: 10.1016/S0140-6736(05)74803-0. [DOI] [PubMed] [Google Scholar]
- 7.Coates AS, Keshaviah A, Thürlimann B, et al. Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study big 1-98. J Clin Oncol. 2007;25:486–92. doi: 10.1200/JCO.2006.08.8617. [DOI] [PubMed] [Google Scholar]
- 8.Crew KD, Greenlee H, Capodice J, et al. Prevalence of joint symptoms in postmenopausal women taking aromatase inhibitors for early-stage breast cancer. J Clin Oncol. 2007;25:3877–83. doi: 10.1200/JCO.2007.10.7573. [DOI] [PubMed] [Google Scholar]
- 9.Burstein HJ, Winer EP. Aromatase inhibitors and arthralgias: a new frontier in symptom management for breast cancer survivors. J Clin Oncol. 2007;25:3797–9. doi: 10.1200/JCO.2007.11.9529. [DOI] [PubMed] [Google Scholar]
- 10.Dizdar O, Ozçakar L, Malas FU, et al. Sonographic and electrodiagnostic evaluations in patients with aromatase inhibitorrelated arthralgia. J Clin Oncol. 2009;27:4955–60. doi: 10.1200/JCO.2008.20.5435. [DOI] [PubMed] [Google Scholar]
- 11.Henry NL, Giles JT, Ang D, et al. Prospective characterization of musculoskeletal symptoms in early stage breast cancer patients treated with aromatase inhibitors. Breast Cancer Res Treat. 2008;111:365–72. doi: 10.1007/s10549-007-9774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morales L, Pans S, Paridaens R, et al. Debilitating musculoskeletal pain and stiffness with letrozole and exemestane: associated tenosynovial changes on magnetic resonance imaging. Breast Cancer Res Treat. 2007;104:87–91. doi: 10.1007/s10549-006-9394-6. [DOI] [PubMed] [Google Scholar]
- 13.Sestak I, Sapunar F, Cuzick J. Aromatase inhibitor-induced carpal tunnel syndrome: results from the atac trial. J Clin Oncol. 2009;27:4961–5. doi: 10.1200/JCO.2009.22.0236. [DOI] [PubMed] [Google Scholar]
- 14.Breast International Group (BIG) 1-98 Collaborative Group. Thürlimann B, Keshaviah A, Coates AS, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353:2747–57. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 15.Buzdar AU on behalf of the ATAC Trialists Group. Clinical features of joint symptoms observed in the Arimidex, Tamoxifen, Alone or in Combination (atac) trial [abstract 551] [cited October 12, 2011];J Clin Oncol. 2006 24 doi: 10.1200/JCO.2006.05.9113. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=40&abstractID=32588. [DOI] [PubMed] [Google Scholar]
- 16.Presant CA, Bosserman L, Young T, et al. Aromatase inhibitorassociated arthralgia and/or bone pain: frequency and characterization in non-clinical trial patients. Clin Breast Cancer. 2007;7:775–8. doi: 10.3816/CBC.2007.n.038. [DOI] [PubMed] [Google Scholar]
- 17.Fontaine C, Meulemans A, Huizing M, et al. Tolerance of adjuvant letrozole outside of clinical trials. Breast. 2008;17:376–81. doi: 10.1016/j.breast.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 18.Sestak I, Cuzick J, Sapunar F, et al. Risk factors for joint symptoms in patients enrolled in the atac trial: a retrospective, exploratory analysis. Lancet Oncol. 2008;9:866–72. doi: 10.1016/S1470-2045(08)70182-7. [DOI] [PubMed] [Google Scholar]
- 19.Kuba T, Quinones–Jenab V. The role of female gonadal hormones in behavioral sex differences in persistent and chronic pain: clinical versus preclinical studies. Brain Res Bull. 2005;66:179–88. doi: 10.1016/j.brainresbull.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 20.Pandya N, Morris GJ. Toxicity of aromatase inhibitors. Semin Oncol. 2006;33:688–95. doi: 10.1053/j.seminoncol.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Felson DT, Cummings SR. Aromatase inhibitors and the syndrome of arthralgias with estrogen deprivation. Arthritis Rheum. 2005;52:2594–8. doi: 10.1002/art.21364. [DOI] [PubMed] [Google Scholar]
- 22.Mao JJ, Stricker C, Bruner D, et al. Patterns and risk factors associated with aromatase inhibitor–related arthralgia among breast cancer survivors. Cancer. 2009;115:3631–9. doi: 10.1002/cncr.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Azria D, Lamy P, Belkacemi Y, et al. Letrozole induced arthralgia: results of a multicentric prospective trial exploring clinical parameters and plasma biomarkers [abstract 228]. Presented at the ASCO Breast Cancer Symposium; June 1–5, 2007; Chicago, IL. [cited October 12, 2011]. [Available online at: http://www.asco.org/ASCOv2/Meetings/Abstracts?&vmview=abst_detail_view&confID=52&abstractID=40265. [Google Scholar]
- 24.Schneider DL, Barrett–Connor EL. Urinary N-telopeptide levels discriminate normal, osteopenic, and osteoporotic bone mineral density. Arch Intern Med. 1997;157:1241–5. doi: 10.1001/archinte.157.11.1241. [DOI] [PubMed] [Google Scholar]
- 25.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aarøe J, Lindahl T, Dumeaux V, et al. Gene expression profiling of peripheral blood cells for early detection of breast cancer. Breast Cancer Res. 2010;12:R7. doi: 10.1186/bcr2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–38. [PubMed] [Google Scholar]
- 28.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 29.Iagnocco A, Perella C, Naredo E, et al. Etanercept in the treatment of rheumatoid arthritis: clinical follow-up over one year by ultrasonography. Clin Rheumatol. 2008;27:491–6. doi: 10.1007/s10067-007-0738-3. [DOI] [PubMed] [Google Scholar]
- 30.Boutry N, Titécat M, Demondion X, Glaude E, Fontaine C, Cotten A. High-frequency ultrasonographic examination of the finger pulley system. J Ultrasound Med. 2005;24:1333–9. doi: 10.7863/jum.2005.24.10.1333. [DOI] [PubMed] [Google Scholar]
- 31.Sernik RA, Abicalaf CA, Pimentel BF, Braga–Baiak A, Braga L, Cerri GG. Ultrasound features of carpal tunnel syndrome: a prospective case–control study. Skeletal Radiol. 2008;37:49–53. doi: 10.1007/s00256-007-0372-9. [DOI] [PubMed] [Google Scholar]
- 32.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 33.Du P, Kibbe WA, Lin SM. Lumi: a pipeline for processing Illumina microarray. Bioinformatics. 2008;24:1547–8. doi: 10.1093/bioinformatics/btn224. [DOI] [PubMed] [Google Scholar]
- 34.Lin SM, Du P, Huber W, Kibbe WA. Model-based variance-stabilizing transformation for Illumina microarray data. Nucleic Acids Res. 2008;36:e11. doi: 10.1093/nar/gkm1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 36.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99:6567–72. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morales L, Pans S, Verschueren K, et al. Prospective study to assess short-term intra-articular and tenosynovial changes in the aromatase inhibitor-associated arthralgia syndrome. J Clin Oncol. 2008;26:3147–52. doi: 10.1200/JCO.2007.15.4005. [DOI] [PubMed] [Google Scholar]
- 38.Fantuzzi G, Dinarello CA. The inflammatory response in interleukin- 1beta–deficient mice: comparison with other cytokine-related knock-out mice. J Leukoc Biol. 1996;59:489–93. doi: 10.1002/jlb.59.4.489. [DOI] [PubMed] [Google Scholar]
- 39.Núñez V, Alameda D, Rico D, et al. Retinoid X receptor alpha controls innate inflammatory responses through the up-regulation of chemokine expression. Proc Natl Acad Sci U S A. 2010;107:10626–31. doi: 10.1073/pnas.0913545107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen SM, Maston LD, Gough MJ, et al. Signaling through ox40 enhances antitumour immunity. Semin Oncol. 2010;37:524–32. doi: 10.1053/j.seminoncol.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pasqualini JR. Estrogen sulfotransferases in breast and endometrial cancers. Ann N Y Acad Sci. 2009;1155:88–98. doi: 10.1111/j.1749-6632.2009.04113.x. [DOI] [PubMed] [Google Scholar]
- 42.Ramadoss P, Marcus C, Perdew GH. Role of the aryl hydrocarbon receptor in drug metabolism. Expert Opin Drug Metab Toxicol. 2005;1:9–21. doi: 10.1517/17425255.1.1.9. [DOI] [PubMed] [Google Scholar]
- 43.McCowan C, Shearer J, Donnan PT, et al. Cohort study examining tamoxifen adherence and its relationship to mortality in women with breast cancer. Br J Cancer. 2008;99:1763–8. doi: 10.1038/sj.bjc.6604758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziller V, Kalder M, Albert US, et al. Adherence to adjuvant endocrine therapy in postmenopausal women with breast cancer. Ann Oncol. 2009;20:431–6. doi: 10.1093/annonc/mdn646. [DOI] [PubMed] [Google Scholar]
- 45.Dent S, DiValentin T, Vandermeer L, et al. Long term toxicities in women with early stage breast cancer treated with aromatase inhibitors: data from a tertiary care center [abstract 4057] Breast Cancer Res Treat. 2006;100(suppl 1) [Google Scholar]