Abstract
Purpose
The adoption of a chemotherapeutic regimen in oncologic practice is a function of both its clinical and its economic impacts on cancer management. For breast cancer, U.S. Oncology trial 9735 reported significant improvements in disease-free and overall survival favoring adjuvant tc (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles) compared with ac (doxorubicin 60 mg/ m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles). We carried out an economic evaluation to examine the cost–utility of adjuvant tc relative to ac, in terms of cost per quality-adjusted life year (qaly) gained, given the improved breast cancer outcomes and higher costs associated with the tc regimen.
Methods
A Markov model was developed to calculate the cumulative costs and qalys gained over a 10-year horizon for hypothetical cohorts of women with breast cancer treated with ac or with tc. Event rates, costs, and utilities were derived from the literature and local resources. Efficacy and adverse events were based on results reported from U.S. Oncology trial 9735. The model takes a third-party direct payer perspective and reports its results in 2008 Canadian dollars. Costs and benefits were both discounted at 3%.
Results
At a 10-year horizon, tc was associated with $3,960 incremental costs and a 0.24 qaly gain compared with ac, for a favorable cost–utility of $16,753 per qaly gained. Results were robust to model assumptions and input parameters.
Conclusions
Relative to ac, tc is a cost-effective adjuvant chemotherapy regimen, with a cost-effectiveness ratio well below commonly applied thresholds.
Keywords: Breast cancer; adjuvant therapy; chemotherapy; tc chemotherapy; ac chemotherapy, cost; utility analysis
1. INTRODUCTION
The adoption of a chemotherapeutic regimen into oncologic practice is a function of both its clinical and its economic impacts on cancer management 1–4. Randomized clinical trials examine potential improvements in cancer-related endpoints such as disease-free survival (dfs) and overall survival (os), or toxicity differences between two equally effective therapies. From an economic perspective, the costs associated with the delivery of different regimens can vary considerably as a function of the systemic agents involved and the costs of toxicity management. Through cost-effectiveness or cost–utility analyses, those incremental costs should be considered in the context of the observed clinical benefits demonstrated in clinical trials 1–3.
Anthracycline- and taxane-based regimens are the backbones of most adjuvant chemotherapy strategies for breast cancer 5. The ac regimen (doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles) has been a standard chemotherapy option since 1975 6, and it has often been considered for those with low- to moderate-risk disease who could potentially benefit from adjuvant chemotherapy and for whom more intense regimens may not be appropriate 5. The U.S. Oncology trial 9735 recently reported significant improvements in dfs and os favoring adjuvant tc (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 every 3 weeks for 4 cycles) compared with ac for breast cancer 7,8. However, the tc regimen is more costly than ac because of the incremental costs of docetaxel (Taxotere: Sanofi–Aventis, Laval, QC). We therefore undertook an economic evaluation to examine the cost–utility of adjuvant chemotherapy with tc relative to ac in terms of cost per quality-adjusted life year (qaly) gained.
2. METHODS
We developed a Markov model 9–11 to evaluate the cost–utility of tc relative to ac based on two hypothetical cohorts of 1000 women of median age 51 years undergoing adjuvant chemotherapy for breast cancer. The model incorporated transition probabilities, costs, and utility values to estimate the cumulative costs and qalys associated with each chemotherapy strategy. The efficacy outcomes were based on the reported results of U.S. Oncology trial 9735 7,8.
2.1 U.S. Oncology Trial 9735
Between June 1997 and December 1999, Jones et al. 7,8 randomized 1016 patients with node-negative and -positive breast cancer to 4 cycles of adjuvant ac or tc administered every 3 weeks. The patients (median age: 51 years) in both treatment arms were well balanced with respect to major prognostic factors. Compared with ac, tc was associated with a statistically significant improvement in dfs at a median follow up of 5.5 years [86% vs. 80%; hazard ratio (hr): 0.67; 95% confidence interval (ci): 0.50 to 0.94; p = 0.015] and 7 years (81% vs. 75%; hr = 0.74; 95% ci: 0.56 to 0.98; p = 0.033), and also improved os at the 7-year median follow up (87% vs. 82%; hr: 0.69; 95% ci: 0.50 to 0.97, p = 0.032). Both regimens were reasonably well tolerated. More febrile neutropenic (fn) episodes (5% vs. 2.5%, p = 0.07) and fewer grade 3 or 4 chemotherapy-induced nausea and vomiting (cinv) episodes (8% vs. 3%, p value not reported) were associated with tc than with ac. A few treatmentrelated deaths were observed in the ac arm, including 1 case of congestive heart failure (chf) and 3 cases of myelodysplasia (mds) or acute myeloid leukemia (aml).
2.2 Markov Model
Our analysis took a Markov approach, defining a fixed number of possible health states 9–11 (Figure 1). Each health state was assigned a utility value 12–18 and cost 14,19–22 (Table I). All patients entered the model in the Chemotherapy state and transitioned to the Disease-Free state after completion of chemotherapy treatment. Patients could move to other states according to event rates derived from the literature 23–26,28–30 and the relevant study 7,8 (Table II). The costs (in Canadian dollars) and health consequences (in qalys) of occupying a particular health state were computed over a defined number of monthly cycles reflecting the analysis horizon examined. The overall cumulative costs and qalys associated with each chemotherapy strategy were examined to determine the incremental cost per qaly gained.
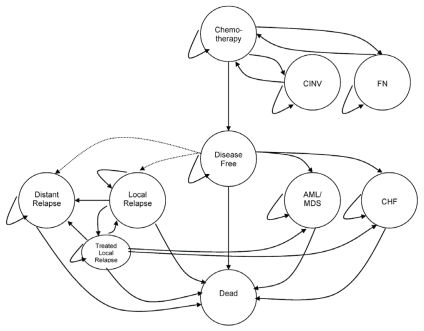
FIGURE 1.
Model schema. Health states incorporated into the model are shown in circles, and possible transitions between health states are depicted by arrows. All patients enter the model in the Chemotherapy state (docetaxel–cyclophosphamide or doxorubicin–cyclophosphamide) and move to Disease-Free state after completion of chemotherapy treatment. Chemotherapy-related adverse events could occur during Chemotherapy state [that is, chemotherapy-induced nausea and vomiting (cinv) or febrile neutropenia (fn)] or after transition to Disease-Free state [that is, acute myeloid leukemia (aml) or myelodysplasia (mds), or congestive heart failure (chf)]. Patients in Disease-Free state could develop Local Relapse or Distant Relapse. Death might occur with or without relapse or as a result of chemotherapy adverse events.
TABLE I.
Costs and utilities used in the model
| Health states | Cost (CA$)a | Utility | Duration (months) |
|---|---|---|---|
| Disease-Free | 42/month 20,23 | 0.90 12,13 | |
| Life on Chemotherapy | Δ5,299/patientb | 0.74 12,13,18 | 3 |
| Local Relapse | 11,535/event 20,23 | ||
| First relapse | 0.70 12,13 4c | ||
| Second relapse | 0.50 12,13 4c | ||
| Treated relapse | 0.90 12,13 | Life | |
| Distant Relapse | 35,230/event 20,23 | 0.60 12,13 | 21 23 |
| Early adverse eventsd | |||
| cinv | 61/event 16 | 0.85 16 | 3c |
| Febrile neutropenia | 17,236/event 19,23 | 0.47 15 | 1c |
| Late adverse eventsd | |||
| aml/mds | 66,015/event 22 | 0.26 14 | 9 24 |
| chf | 19,008/event 21 | 0.64 17 | 12 25,26 |
| Death | — | 0.00 12,13 | — |
Inflated to 2008 Canadian dollars using the Consumer Price Index 27.
Including costs of managing febrile neutropenia and growth factor support for subsequent cycles: $6,597 for docetaxel– cyclophosphamide; $1,298 for doxorubicin–cyclophosphamide.
Assumptions.
Febrile neutropenia or cinv might develop during the 3 months of chemotherapy; aml/mds and chf might develop any time during the 7 years after chemotherapy.
cinv = chemotherapy-induced nausea and vomiting; aml/mds = acute myeloid leukemia or myelodysplastic syndrome (or both); chf = congestive heart failure.
TABLE II.
Model assumptions
| Median age of cohort at entry into the model was 51 years 7,8. |
| The baseline doxorubicin–cyclophosphamide (ac) arm was constructed to reflect the relative benefits of first-generation adjuvant chemotherapy regimensa by menopausal status 28, applied to a varying baseline cancer recurrence risk of 25%–75% without adjuvant chemotherapy at 10 years (reflecting the range of nodal states encountered in practice 28). |
| In the primary analysis, 10-year recurrence rates for the ac strategy were based on a hypothetical cohort with a 52% node-positive rate, based on U.S. Oncology trial 9735 7,8. |
| The relative efficacy of docetaxel–cyclophosphamide (tc) compared with ac (that is, hazard ratio for disease-free survival) reported by U.S. Oncology trial 9735 7,8 can be applied to this hypothetical cohort of patients. |
| No carry-over benefit for tc relative to ac (that is, hazard ratio is 1) was assumed beyond the median follow-up of U.S. Oncology trial 9735. |
| Hormonal therapy and radiation treatment were similar for both strategies. |
| Febrile neutropenia or cinv could develop during the 3 months of chemotherapy; aml/mds or chf could develop at any time during the 7 years after chemotherapy. |
| A mortality risk was associated with chf and aml/mds24,25,26. |
| The distribution of breast cancer recurrences for years 1–5 and 6–10 were 75% and 25% respectively 23,28. |
| The local:distant ratio for recurrence was 1:4 in the baseline analysis 23. |
| Patients with local recurrence were treated for 4 months and then entered the Treated Local Relapse state. |
| Patients with local recurrence had a 20% instant risk of distant relapse and double the risk of subsequent recurrence events 23. |
| Patients could experience only two local recurrences. Subsequent recurrences were distant. |
| Patients with distant recurrences had a median survival of 21 months 23. |
| Survival after relapse, and costs associated with treating relapse, were similar for both strategies. |
First-generation chemotherapy regimens were assumed to be associated with reductions of one third and one fifth in the relative risk of cancer recurrence (compared with no chemotherapy) for pre- and postmenopausal women respectively 28.
cinv = chemotherapy-induced nausea and vomiting; aml/mds = acute myeloid leukemia or myelodysplastic syndrome (or both); chf = congestive heart failure.
The Markov model was developed in MS Excel (Microsoft Corporation, Redmond, WA, U.S.A.). The model used primarily a deterministic approach rather than a probabilistic one to avoid the compounded uncertainty associated with simultaneously defining arbitrary ranges for many of the input parameters in the model that were not available in the literature; however, some probabilistic modeling was used in the sensitivity analyses 9–11.
2.3 Event Rates
For the ac strategy, the baseline dfs rates used in the model were derived by combining two rates:
the general mortality rate (death without recurrence) for women with a median age of 51 years, as derived from Canadian life tables 30, and
the breast cancer recurrence rate for patients treated with ac.
The recurrence rate was derived by applying the expected relative benefits of first-generation anthracycline-based regimens such as ac to a range of baseline 10-year recurrence risks of 25%–75% in the absence of adjuvant chemotherapy according to menopausal status 28, representing the risks posed by nodal status (that is, from node-negative to high-burden node-positive disease). The model structure therefore allows for an assessment of various baseline relapse risks and nodal states for patients treated with adjuvant chemotherapy. In the base case scenario, the 10-year recurrence rates for the ac strategy were based on a hypothetical cohort with a 52% node-positive rate (that is, a 10-year relapse risk of 38% in the absence of adjuvant chemotherapy), as observed in U.S. Oncology trial 9735 7,8. The sensitivity analysis also examined cohorts with lower and higher relapse risks (25%–75%).
The corresponding recurrence rates for the tc strategy were derived by applying the relative risk for dfs at 7 years’ median follow-up from U.S. Oncology trial 9735 (hr: 0.74; 95% ci: 0.56 to 0.98; p = 0.033) to the recurrence rates used in the ac arm for the initial 7 years after chemotherapy. No carryover benefit for tc relative to ac (that is, hr: 1) beyond the reported median follow-up period was assumed in the base case scenario. Table II lists other event rates and assumptions used in the model.
We incorporated a number of early and delayed chemotherapy-related adverse events based on reported toxicities from U.S. Oncology trial 9735 7,8. The analysis considered the greater risk of grade 3 or 4 cinv associated with ac (8.0% vs. 3.0%; relative risk: 2.6; range in sensitivity analyses: 1–4) and the greater risk of fn associated with tc (5.0% vs. 2.5%; relative risk: 2.0; range in sensitivity analyses: 1–4) 7,8. The base case scenario incorporated secondary prophylaxis with granulocyte colony–stimulating factor (g-csf) after fn events and assumed no chemotherapy dose adjustments. However, outside of clinical trials, use of tc appears to be associated with higher fn rates of approximately 26% (range: 10%–46%) without and 6% (range: 0%–7%) with primary g-csf prophylaxis 27,31,32,33. Primary prophylaxis with g-csf is also sometimes used with the tc regimen in clinical practice, because it is recommended for chemotherapeutic regimens associated with a fn risk greater than 20% 34. We therefore examined two additional scenarios:
20% fn rate, with secondary g-csf prophylaxis after fn events, and
primary g-csf prophylaxis for all patients, with a 6% breakthrough fn rate despite the prophylaxis.
The analysis also assumed that ac is associated with an increased risk of aml or mds (0.4%; range in sensitivity analyses: 0.0%–1.0%) 24,29 and chf (0.4%; range in sensitivity analyses: 0.0%–1.0%) 21,25,26 over the 7-year median follow-up.
2.4 Utilities and Costs
Each health state was assigned a utility weight derived from the literature to permit an estimation of the qaly gains 12–18 (Table I). A utility weight of 1 represents perfect health; lower utilities denote worse quality of life. A utility of 0 represents death. During treatment, the base case scenario assumed comparable utilities between tc and ac (0.74 vs. 0.74) in the absence of the adverse events modelled. However, in the sensitivity analyses, we also examined the effect of varying the utilities (±20%) between tc and ac.
The upfront costs associated with adjuvant chemotherapy and the downstream costs associated with adverse events (cinv, fn, chf, and aml or mds), follow-up, and relapses (Table I) were all considered 14,19,20–22. Upfront costs were derived from local unit costs at the QEII Health Sciences Centre, Halifax, Nova Scotia, Canada, as per our previous work 23. Included were the costs associated with
chemotherapy drug (ac or tc) acquisition per the recommended dose and schedule for an average person with a body surface area of 1.7 m2;
supportive care medications;
diagnostics, including laboratory tests and pre-chemotherapy cardiac evaluations; and
health resource utilization.
All costs were converted into monthly costs or one-time event-driven costs. We also examined the effects of varying the costs after relapse (±20%) for the ac and tc regimens. For example, the costs of treating relapses may be lower after tc than after ac because palliative chemotherapy treatment with taxanes may not be indicated after adjuvant tc. A third-party direct payer perspective was considered. Using the Consumer Price Index–Health Care Component 35, costs were adjusted to 2008 Canadian dollars. Costs and benefits were both discounted at 3% annually 9,11.
2.5 Validation and Sensitivity Analyses
The dfs and os rates generated by the model in the base case analysis (with a 52% node-positive rate) were compared with those reported by U.S. Oncology trial 9735 7,8. To test the plausibility of the cost–utility results over a reasonable range of uncertainty, the robustness of the model to changes in key parameters was examined primarily in a series of one-way sensitivity analyses and alternative scenarios. We examined a number of scenarios that are more aligned with clinical practice than with the clinical trial setting: for example, node-negative status (that is, lower relapse risk), older age (60 years), lower utility during tc treatment compared with ac (–20%), a high fn rate after tc treatment (+10% or +20%), and primary g-csf prophylaxis for the tc regimen. A probabilistic sensitivity analysis with a cost-effectiveness acceptability curve 9–11 based on plausible arbitrary ranges for input parameters was also conducted. The impact of the analysis horizon on the cost–utility estimate was examined for comparison with other studies that considered a longer horizon.
3. RESULTS
Adjuvant tc was associated with an estimated upfront cost of $6,597 compared with $1,298 for ac, for an incremental cost difference of $5,299 per patient. This incremental cost reflects primarily the higher drug acquisition cost associated with tc ($5,850 vs. $269), because the costs of supportive care medications ($76 vs. $192), diagnostic investigations ($223 vs. $417), and human resources utilization ($448 vs. $421) were not substantially different for the treatments. At a 10- year horizon, the net incremental cost was $3,960 per patient when the effects of recurrences avoided and the various adverse event profiles and rates associated with the two strategies had been accounted for.
At a 10-year horizon, tc was associated, relative to ac, with an incremental gain of 0.24 qalys per patient. Survival outcomes estimated by the model were consistent with the results of U.S. Oncology trial 9735 7,8. The model predicted incremental absolute dfs and os benefits of 6% and 4% for tc compared with ac (82% vs. 76% and 88% vs. 84% respectively) compared with the 6% and 5% differences observed in the clinical trial (81% vs. 75% and 87% vs. 82% respectively). The slightly more conservative survival estimates from the model partly reflect assumptions in base case recurrence risk and background mortality that differed slightly from those in the actual clinical trial.
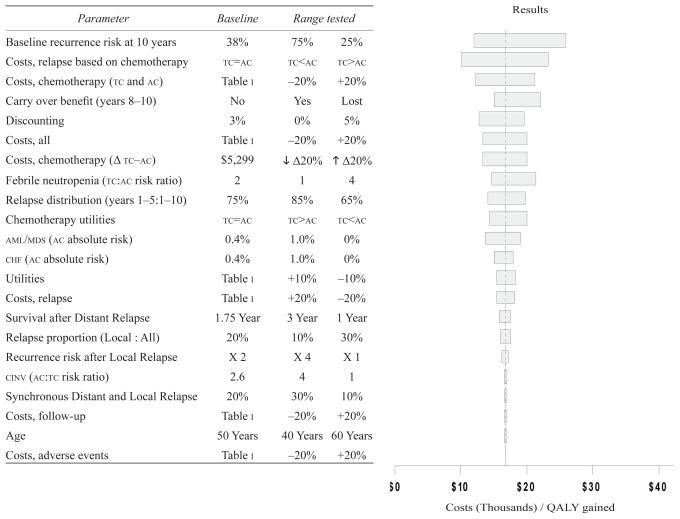
At a 10-year horizon, the cost–utility of tc relative to ac was $16,753 per qaly gained. These cost–utility results were robust to the key assumptions and input data used in the model (Figure 2). A lower upfront cost for tc (that is, a lower acquisition cost for docetaxel) resulted in increasingly favorable cost–utility estimates. Conversely, a lower base case recurrence risk (that is, node-negative disease) was associated with higher cost–utility of $26,047 per qaly gained. The higher fn rates associated with the tc regimen resulted in less favorable cost–utility estimates, although still within commonly acceptable cost–utility thresholds 36,37. Those cost–utilities were $21,333 and $33,510 per qaly gained at fn rates of 10% (that is, 4 times the fn risk with ac) and 20% (that is, the recommended fn threshold for primary g-csf prophylaxis) respectively, and $43,693 per qaly if primary g-csf was administered in all patients, assuming a 6% breakthrough fn rate. Other chemotherapy-related adverse events and variations in the utilities for tc and ac during treatment had little impact on the cost–utility results.
FIGURE 2.
Sensitivity analysis. The y axis shows the parameters and the ranges tested in the sensitivity analysis; the x axis reflects the resulting cost–utility value in thousands of Canadian dollars per quality-adjusted life year (qaly) gained. The dashed vertical line represents the mean cost–utility result (CA$16,753/qaly gained), and each bar shows the range of the cost–utility estimate for each parameter tested. For each parameter tested, the lower and higher cost–utility values in the bar respectively reflect the cost–utility estimates for the first and second columns of the range rested. The order of variables from top to bottom, with corresponding longer-to-shorter bars in the tornado plot, reflects the variables with more-to-less impact on the cost–utility results. tc = docetaxel–cyclophosphamide; ac = doxorubicin–cyclophosphamide; aml/mds = acute myeloid leukemia or myelodysplastic syndrome; chf = congestive heart failure; cinv = chemotherapy-induced nausea and vomiting. Parameter Baseline Range tested
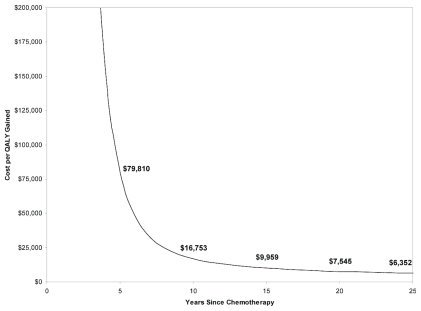
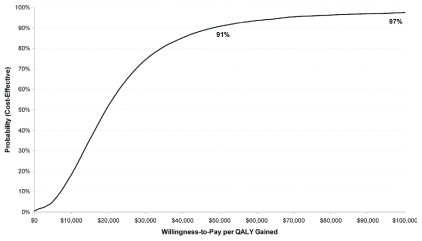
The cost–utility results were also more favorable at longer horizons, with a cost–utility of $6,352 per qaly gained at a 25-year horizon (Figure 3). Limited probabilistic sensitivity analysis suggested that the likelihoods of tc being cost-effective relative to ac at the commonly used $50,000 and $100,000 per qaly gained willingness-to-pay thresholds were 91% and 97% respectively (Figure 4).
FIGURE 3.
Cost-effectiveness by analysis horizon. The y axis shows the cost per quality-adjusted life year (qaly) gained in Canadian dollars; the x axis shows the analysis horizon. The graph shows cost per qaly gained as a function of the time horizon from adjuvant chemotherapy treatment.
FIGURE 4.
Cost-effectiveness acceptability curve. The x axis shows willingness-to-pay per quality-adjusted life year (qaly) gained in Canadian dollars; the y axis shows the probability that docetaxel– cyclophosphamide (tc) is cost-effective. The likelihood that tc is cost-effective is shown at various thresholds of willingness-to-pay per qaly gained; the probabilities are, respectively, 91% and 97% that tc is cost-effective at the commonly applied thresholds of $50,000 and $100,000 per qaly gained.
4. DISCUSSION AND CONCLUSIONS
Economic analyses—including cost-effectiveness and cost–utility studies—have become an integral component in the evaluation of new interventions or treatments, including adjuvant chemotherapy for breast cancer 2–4. The World Health Organization defines favorable cost-effectiveness based on the Gross Domestic Product (GDP) per capita in various jurisdictions 38:
Highly cost effective: <GDP/capita
Cost-effective: 1–3 times GDP/capita
Not cost effective: >3 times GDP/capita
The cost–utility of $16,753 estimated in this analysis per qaly gained for tc relative to ac compares favorably with other oncology interventions 3 and falls well below the Canadian GDP-per-capita threshold of $38,975 39 and the commonly reported thresholds of $50,000–100,000 per qaly in the United States and Canada, and of £20,000–£30,000 per qaly in the United Kingdom 36,37.
The cost–utility of tc relative to ac has been examined in two other studies, although neither examined the effects of primary g-csf prophylaxis or of high fn rates for the tc regimen. Verma et al. 40, in an abstract presentation also from a Canadian perspective, reported a 0.516 qaly gain and a $4,260 higher cost for tc relative to ac for a cost–utility of $8,251 per qaly gained at a lifetime horizon, compared with our estimate of $6,352 per qaly gained at a 25-year horizon. As in our study, their results were sensitive to the horizon examined, with a cost–utility of $43,248 per qaly gained at a 7-year horizon. In a recent publication from a Chinese perspective, Liubao et al. 41 reported an incremental gain of 0.41 qalys and 10,116 Chinese yuan (approximately CA$1,547) in higher costs associated with tc relative to ac at a lifetime horizon (40 years), with an incremental cost-effectiveness ratio of 24,305 yuan (approximately CA$3,716) per qaly gained. At a willingness-to-pay threshold of 86,514 yuan (approximately CA$13,173) per qaly, the probability of tc being cost-effective was 90%. The most sensitive parameter in the model was the cost of primary chemotherapy treatment in the tc arm. However, comparisons to international results must be made cautiously, given the potential for substantial structural differences between health care systems.
Economic evaluations for other docetaxel-based adjuvant chemotherapy regimens for breast cancer have also been conducted 23,42–46. Compared with fac (5-fluorouracil–doxorubicin–cyclophosphamide), tac (docetaxel–doxorubicin–cyclophosphamide) administered with and without prophylactic g-csf was found to be a cost-effective strategy in a number of jurisdictions 36,37,39,40. From a Canadian health care system perspective, Au et al. 42 reported cost–utility ratios of $46,003 and $18,506 per qaly gained with and without prophylactic g-csf at a 10-year horizon, and Mittmann et al. 43 reported cost–utility ratios of $13,044 and $6,848 per qaly gained at a lifetime horizon. From a U.K. National Health Service perspective, Wolowacz et al. 44 reported cost–utilities of £20,432 and £18,188 per qaly gained, with and without prophylactic filgrastim, at a 10-year horizon. From a Korean perspective, Lee et al. 45 reported cost–utilities of 12,119,561 Korean won (approximately €9,926) and 8,885,794 won (approximately €7,277) per qaly gained, with and without prophylactic g-csf, at a lifetime horizon.
Compared with fec100 (5-fluorouracil–epirubicin– cyclophosphamide), fec-d (5-fluorouracil–epirubicin– cyclophosphamide–docetaxel) was also found to be a cost-effective strategy 23,46. From a Canadian health care perspective, Younis et al. 23 reported a cost–utility of $14,612 per qaly at a 10-year horizon, and from a French hospital perspective, Marino et al. 46 reported a cost–utility of €9,665 per qaly at a 5-year horizon.
Collectively, the consistent favorable results observed in the foregoing economic evaluations from various health care jurisdictions provide compelling evidence that adjuvant chemotherapy regimens incorporating docetaxel—fec-d, tac, tc—are cost-effective strategies for women with breast cancer.
The cost–utility estimate for tc relative to ac depends on the incremental upfront cost difference between the two chemotherapeutic regimes. In our evaluation, tc was associated with higher estimated upfront costs relative to ac ($6,597 vs. $1,298), with an incremental cost difference of $5,299 per patient, primarily reflecting higher drug acquisition costs ($5,850 vs. $269). Should docetaxel become generic, lower upfront tc costs would result in an even more favorable cost–utility estimate, as observed in our study and the study by Liubao et al. 41. The cost–utility estimate of tc relative to ac is also affected by the horizon examined (that is, the analysis timeframe). Economic analyses attempt to capture all clinical benefits by modeling clinical outcomes beyond the relatively short duration of follow-up in most clinical trials. Although cost–utility estimates generally improve with a longer analytic horizon as the cumulative benefits (that is, the qaly gains) accrue for patients without recurrences, longer horizons also involve more uncertainty with regard to the magnitude of clinical benefit. In our study, and in the study by Verma et al. 40, the cost–utility estimate for tc was within commonly accepted thresholds 36,37 at a 7-year horizon (corresponding to the median follow-up reported in U.S. Oncology trial 9735 7,8).
In our primary scenario, the cost–utility estimate of $16,753 per qaly gained was based primarily on outcomes from U.S. Oncology trial 9735. We examined cohorts with 52% node-positive rates and a median age of 51 years. We assumed twice the fn risk with tc than with ac and comparable base case utilities for both regimes during the treatment period. However, in clinical practice, tc chemotherapy is perhaps more commonly used in older patients and in those with node-negative disease, and it is associated with higher fn rates and possibly with lower treatment-related utility 27,31–33. Our cost–utility estimates in those practical scenarios, and in circumstances in which primary g-csf prophylaxis is considered for all patients, were less favorable than those in the primary analysis based on clinical trial data, although they remained within commonly used cost–utility thresholds 36,37.
Our study has limitations. As with all economic analyses, the results may not be generalizable to all other health care jurisdictions because of variation in upfront or downstream costs (or both) for chemotherapeutic drugs and cancer management. However, the very favorable cost–utility estimates observed in our study and in the study by Liubao et al. 41 from the perspectives of the Canadian and the Chinese health care systems respectively, make it unlikely that tc would not be a cost-effective strategy in other jurisdictions. To test the robustness of our model, we performed mainly one-way sensitivity analyses (as opposed to probabilistic sensitivity analyses) because of a lack of evidence-based probability distributions for many of the parameters and because of the difficulties arising from defining arbitrary distributions 9–11. Our results were nevertheless robust to a wide range of uncertainty around the point estimates for key parameters examined in the model.
To summarize, adjuvant chemotherapy with tc is both more effective and more costly than ac, with favorable cost–utility estimates relative to commonly applied cost-effectiveness thresholds. For women with breast cancer, tc is an acceptable standard adjuvant chemotherapy option based on its favourable clinical and economic evaluations.
5. ACKNOWLEDGMENTS
The authors thank Marlene Sellon for her help with drug costs, and two anonymous reviewers for helpful suggestions. This study was presented, in part, as a poster at the 2008 San Antonio Breast Cancer Symposium.
Footnotes
6. CONFLICT OF INTEREST DISCLOSURES
This study was supported by a research grant from the Canadian Breast Cancer Foundation, Atlantic Chapter. The authors have no financial conflicts of interest pertinent to the study to declare.
7. REFERENCES
- 1.Shih YC, Halpern MT. Economic evaluations of medical care interventions for cancer patients: how, why, and what does it mean? CA Cancer J Clin. 2008;58:231–44. doi: 10.3322/CA.2008.0008. [DOI] [PubMed] [Google Scholar]
- 2.Meropol NJ, Schrag D, Smith TJ, et al. on behalf of American Society of Clinical Oncology. American Society of Clinical Oncology guidance statement: the cost of cancer care. J Clin Oncol. 2009;27:3868–74. doi: 10.1200/JCO.2009.23.1183. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg D, Earle C, Fang CH, Eldar–Lissai A, Neumann PJ. When is cancer care cost-effective? A systematic overview of cost–utility analyses in oncology. J Natl Cancer Inst. 2010;102:82–8. doi: 10.1093/jnci/djp472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sridhara R, Johnson JR, Justice R, Keegan P, Chakravarty A, Pazdur R. Review of oncology and hematology drug product approvals at the U.S. Food and Drug Administration between July 2005 and December 2007. J Natl Cancer Inst. 2010;102:230–43. doi: 10.1093/jnci/djp515. [DOI] [PubMed] [Google Scholar]
- 5.National Comprehensive Cancer Network (NCCN) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Ver. 2.2011. Fort Washington, PA: NCCN; [cited October 21, 2011]. [Available online at: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf; (no-cost registration required) [Google Scholar]
- 6.Jones SE, Durie BG, Salmon SE. Combination chemotherapy with Adriamycin and cyclophosphamide for advanced breast cancer. Cancer. 1975;36:90–7. doi: 10.1002/1097-0142(197507)36:1<90::AID-CNCR2820360104>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 7.Jones SE, Savin MA, Holmes FA, et al. Phase iii trial comparing doxorubicin plus cyclophosphamide with docetaxel plus cyclophosphamide as adjuvant therapy for operable breast cancer. J Clin Oncol. 2006;24:5381–7. doi: 10.1200/JCO.2006.06.5391. [Erratum in: J Clin Oncol 2007;25:1819] [DOI] [PubMed] [Google Scholar]
- 8.Jones S, Holmes FA, O’Shaughnessy J, et al. Docetaxel with cyclophosphamide is associated with an overall survival benefit compared with doxorubicin and cyclophosphamide: 7-year follow-up of U.S. Oncology research trial 9735. J Clin Oncol. 2009;27:1177–83. doi: 10.1200/JCO.2008.18.4028. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenberg FA, Beck JR. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–38. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien BJ, Briggs AH. Analysis of uncertainty in health care cost-effectiveness studies: an introduction to statistical issues and methods. Stat Methods Med Res. 2002;11:455–68. doi: 10.1191/0962280202sm304ra. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein MC, O’Brien B, Hornberger J, et al. Principles of good practice of decision analytic modeling in health care evaluation: report of the ispor task force on good research practices—modeling studies. Value Health. 2003;6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x. [DOI] [PubMed] [Google Scholar]
- 12.Tufts–New England Medical Center, Institute for Clinical Research and Health Policy Studies. Cost-Effectiveness Analysis Registry > Searching the CEA Registry > Search the CEA Registry [Web resource for utility weights] Boston, MA: Center for the Evaluation of Value and Risk in Health; n.d.. [cited October 21, 2011]. [Available at: https://research.tufts-nemc.org/cear4/SearchingtheCEARegistry/SearchtheCEARegistry.aspx. [Google Scholar]
- 13.Tengs TO, Wallace A. One thousand health-related quality-of-life estimates. Med Care. 2000;38:583–637. doi: 10.1097/00005650-200006000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Barr R, Furlong W, Henwood J, et al. Economic evaluation of allogeneic bone marrow transplantation: a rudimentary model to generate estimates for the timely formulation of clinical policy. J Clin Oncol. 1996;14:1413–20. doi: 10.1200/JCO.1996.14.5.1413. [DOI] [PubMed] [Google Scholar]
- 15.Launois R, Reboul–Marty J, Henry B, Bonneterre J. A cost– utility analysis of second-line chemotherapy in metastatic breast cancer. Docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics. 1996;10:504–21. doi: 10.2165/00019053-199610050-00008. [DOI] [PubMed] [Google Scholar]
- 16.Lachaine J, Yelle L, Kaizer L, Dufour A, Hopkins S, Deuson R. Chemotherapy-induced emesis: quality of life and economic impact in the context of current practice in Canada. Support Cancer Ther. 2005;2:181–7. doi: 10.3816/SCT.2005.n.011. [DOI] [PubMed] [Google Scholar]
- 17.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–20. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward S, Simpson E, Davis S, Hind D, Rees A, Wilkinson A. Taxanes for the adjuvant treatment of early breast cancer: systematic review and economic evaluation. Health Technol Assess. 2007;11:1–144. doi: 10.3310/hta11400. [DOI] [PubMed] [Google Scholar]
- 19.Dranitsaris G, Tran TM, McGeer A, Narine L. Pharmacoeconomic analysis of empirical therapy with ceftazidime alone or combination antibiotics for febrile neutropenia in cancer patients. Pharmacoeconomics. 1995;7:49–62. doi: 10.2165/00019053-199507010-00006. [DOI] [PubMed] [Google Scholar]
- 20.Will BP, Berthelot JM, Le Petit C, Tomiak EM, Verma S, Evans WK. Estimates of the lifetime costs of breast cancer treatment in Canada. Eur J Cancer. 2000;6:724–35. doi: 10.1016/S0959-8049(99)00340-8. [DOI] [PubMed] [Google Scholar]
- 21.Levy AR, Briggs AH, Demers C, O’Brien BJ. Cost-effectiveness of beta-blocker therapy with metoprolol or with carvedilol for treatment of heart failure in Canada. Am Heart J. 2001;142:537–43. doi: 10.1067/mhj.2001.116479. [DOI] [PubMed] [Google Scholar]
- 22.Kasteng F, Sobocki P, Svedman C, Lundkvist J. Economic evaluations of leukemia: a review of the literature. Int J Technol Assess Health Care. 2007;23:43–53. doi: 10.1017/S0266462307051562. [DOI] [PubMed] [Google Scholar]
- 23.Younis T, Rayson D, Sellon M, Skedgel C. Adjuvant chemotherapy for breast cancer: a cost-utility analysis of fec-d vs. fec100. Breast Cancer Res Treat. 2008;111:261–7. doi: 10.1007/s10549-007-9770-x. [DOI] [PubMed] [Google Scholar]
- 24.Diamandidou E, Buzdar AU, Smith TL, Frye D, Witjaksono M, Hortobagyi GN. Treatment-related leukemia in breast cancer patients treated with fluorouracil–doxorubicin– cyclophosphamide combination adjuvant chemotherapy: the University of Texas MD Anderson Cancer Center experience. J Clin Oncol. 1996;14:2722–30. doi: 10.1200/JCO.1996.14.10.2722. [DOI] [PubMed] [Google Scholar]
- 25.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–79. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 26.Towns K, Bedard PL, Verma S. Matters of the heart: cardiac toxicity of adjuvant systemic therapy for early-stage breast cancer. Curr Oncol. 2008;15(suppl 1):S16–29. doi: 10.3747/co.2008.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takabatake D, Taira N, Hara F, et al. Feasibility study of docetaxel with cyclophosphamide as adjuvant chemotherapy for Japanese breast cancer patients. Jpn J Clin Oncol. 2009;39:478–83. doi: 10.1093/jjco/hyp050. [DOI] [PubMed] [Google Scholar]
- 28.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 29.Smith RE, Bryant J, DeCillis A, Anderson S on behalf of the National Surgical Adjuvant Breast and Bowel Project. Acute myeloid leukemia and myelodysplastic syndrome after doxorubicin–cyclophosphamide adjuvant therapy for operable breast cancer: the National Surgical Adjuvant Breast and Bowel Project experience. J Clin Oncol. 2003;21:1195–204. doi: 10.1200/JCO.2003.03.114. [DOI] [PubMed] [Google Scholar]
- 30.Statistics Canada. Catalogue no. 84-537-XIE. Ottawa, ON: Statistics Canada; 2003. Life tables—Canada, provinces and territories, 1995–1997. [Google Scholar]
- 31.Chan A, Fu WH, Shih V, Coyuco JC, Tan SH, Ng R. Impact of colony-stimulating factors to reduce febrile neutropenic events in breast cancer patients receiving docetaxel plus cyclophosphamide chemotherapy. Support Care Cancer. 2011;19:497–504. doi: 10.1007/s00520-010-0843-8. [DOI] [PubMed] [Google Scholar]
- 32.Vandenberg T, Younus J, Al-Khayyat S. Febrile neutropenia rates with adjuvant docetaxel and cyclophosphamide chemotherapy in early breast cancer: discrepancy between published reports and community practice—a retrospective analysis. Curr Oncol. 2010;17:2–3. doi: 10.3747/co.v17i2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIlroy P, Lumsden G, Haslett K, Macpherson IR. Docetaxel/ cyclophosphamide (tc) chemotherapy for early breast cancer: is primary g-csf prophylaxis necessary? [abstract P5-10-25] [cited October 6, 2011];Cancer Res. 2010 70(suppl 2) doi: 10.1158/0008-5472.CAN-09-3713. [Available online at: http://www.abstracts2view.com/sabcs10/view.php?nu=SABCS10L_947. [DOI] [PubMed] [Google Scholar]
- 34.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 35.Statistics Canada. Consumer Price Index—Table 5: The Consumer Price Index for Canada, All-Items CPI, Not Seasonally Adjusted, Historical Data [Web page] Ottawa, ON: Statistics Canada; 2011. [cited October 21, 2011]. [Available at: http://www.statcan.gc.ca/pub/62-001-x/2011009/t040-eng.htm. [Google Scholar]
- 36.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to pay for a quality-adjusted life year: in search of a standard. Med Decis Making. 2000;20:332–42. doi: 10.1177/0272989X0002000310. [DOI] [PubMed] [Google Scholar]
- 37.Mason H, Baker R, Donaldson C. Willingness to pay for a qaly: past, present and future. Expert Rev Pharmacoecon Outcomes Res. 2008;8:575–82. doi: 10.1586/14737167.8.6.575. [DOI] [PubMed] [Google Scholar]
- 38.Murray CJ, Evans DB, Acharya A, Baltussen RM. Development of who guidelines on generalized cost-effectiveness analysis. Health Econ. 2000;9:235–51. doi: 10.1002/(SICI)1099-1050(200004)9:3<235::AID-HEC502>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 39.Organisation for Economic Cooperation and Development (OECD) Country Statistical Profiles 2010: Canada [Web resource] Paris, France: OECD; n.d. [cited August 17, 2010]. [Available online at: http://stats.oecd.org/index.aspx?queryid=23063. [Google Scholar]
- 40.Verma S, Mittmann N, Bernard LM, et al. Docetaxel plus cyclophosphamide is cost-effective compared to doxorubicin plus cyclophosphamide, based on an economic analysis of U.S. Oncology trial 9735: additional rationale to avoid anthracyclines in the adjuvant treatment of operable breast cancer? [abstract 6105] Cancer Res. 2009;69(suppl 2) doi: 10.1158/0008-5472.SABCS-6105. [DOI] [Google Scholar]
- 41.Liubao P, Xiaomin W, Chongqing T, et al. Cost-effectiveness analysis of adjuvant therapy for operable breast cancer from a Chinese perspective: doxorubicin plus cyclophosphamide versus docetaxel plus cyclophosphamide. Pharmacoeconomics. 2009;27:873–86. doi: 10.2165/11314750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 42.Au HJ, Golmohammadi K, Younis T, et al. Cost-effectiveness analysis of adjuvant docetaxel, doxorubicin, and cyclophosphamide (tac) for node-positive breast cancer: modeling the downstream effects. Breast Cancer Res Treat. 2009;114:579–87. doi: 10.1007/s10549-008-0034-1. [DOI] [PubMed] [Google Scholar]
- 43.Mittmann N, Verma S, Koo M, Alloul K, Trudeau M. Cost effectiveness of tac versus fac in adjuvant treatment of node-positive breast cancer. Curr Oncol. 2010;17:7–16. doi: 10.3916/C34-2010-01-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolowacz SE, Cameron DA, Tate HC, Bagust A. Docetaxel in combination with doxorubicin and cyclophosphamide as adjuvant treatment for early node-positive breast cancer: a cost-effectiveness and cost-utility analysis. J Clin Oncol. 2008;26:925–33. doi: 10.1200/JCO.2006.10.4190. [DOI] [PubMed] [Google Scholar]
- 45.Lee SG, Jee YG, Chung HC, et al. Cost-effectiveness analysis of adjuvant therapy for node positive breast cancer in Korea: docetaxel, doxorubicin and cyclophosphamide (tac) versus fluorouracil, doxorubicin and cyclophosphamide (fac) Breast Cancer Res Treat. 2009;114:589–95. doi: 10.1007/s10549-008-0035-0. [DOI] [PubMed] [Google Scholar]
- 46.Marino P, Siani C, Roché H, et al. Cost-effectiveness of adjuvant docetaxel for node positive breast cancer patients: results of the pacs 01 economic study. Ann Oncol. 2010;21:1448–54. doi: 10.1093/annonc/mdp561. [DOI] [PubMed] [Google Scholar]