Abstract
Introduction
In non-small-cell lung cancer (nsclc), invasive mediastinal staging is typically used to guide treatment decision-making. Here, we present clinical practice guideline recommendations for invasive mediastinal staging in nsclc patients who have been staged T1–4, N0–3, with no distant metastases.
Methods
Draft recommendations were formulated based on the best available evidence gathered by a systematic review and a consensus of expert opinion. The draft recommendations underwent an internal review by clinical and methodology experts, and an external review by clinical practitioners through a survey assessing the clinical relevance and overall quality of the guideline. Feedback from the internal and external reviews was integrated into the clinical practice guideline.
Results
In general, most clinical experts agreed with the guideline, approving it for methodologic rigour. More than 80% of the surveyed practitioners gave it a high quality rating. The expert reviewers also provided written comments, with some of the suggested changes being incorporated into the final version of the guideline.
Conclusions
In the clinical practice guideline, invasive mediastinal staging of nsclc is recommended in all cases except those involving patients with normal-sized lymph nodes, negative combine positron-emission tomography and computed tomography, and peripheral clinical stage 1A tumour. When performing mediastinoscopy, 5 nodal stations (2R/L, 4R/L, and 7) should routinely be examined.
Keywords: Non-small-cell lung cancer, nsclc, clinical practice guideline, mediastinal staging
1. INTRODUCTION
Lung cancer is the leading cause of cancer death in Ontario, with almost 8000 new cases being diagnosed annually 1, most of which are non-small-cell lung cancer (nsclc). The main factor in treatment decision-making is the nature and extent of disease, represented by stage according to the TNM (tumour size, lymph node, metastases) classification. The methods available for staging lymph nodes (lns) range from noninvasive techniques such as computed tomography (ct) and positron-emission tomography (pet), to less-invasive methods such as needle aspiration with endobronchial ultrasonography (ebus) or endoscopic ultrasonography (eus), and to invasive techniques such as mediastinoscopy.
With the incidence of nsclc rising and invasive mediastinal staging techniques to guide treatment decision-making evolving, a clinical practice guideline is warranted. The recommendations on invasive mediastinal staging that follow were developed with consideration for the results of noninvasive staging techniques such as ct and pet–ct imaging, which were assumed to be standards of care. Although pet has a higher sensitivity and specificity for the evaluation of mediastinal lns (mlns) than ct does, the accuracy of pet depends on the size of the mlns. Up to 25% of lns identified by pet as malignant are falsely positive. Hence, pathology confirmation of malignancy is required so that the patient is not denied potentially curative therapy. By contrast, for normal-sized lns, pet has lower sensitivity (82%–93%). The estimated false-negative rate for pet imaging in the setting of normal-sized lns is 20%. In a clinical setting in which the probability of mln metastases is increased, pathology confirmation that the mlns are negative is required to avoid subjecting patients to futile (noncurative) lung resection 2.
The present clinical practice guideline serves to answer the primary question of when mediastinal staging would be indicated in nsclc patients who have been clinically staged T1–4, N0–3, with no distant metastases. Secondary questions addressed during guideline development include the proper technique for invasive mediastinal staging and the lns that should be biopsied. The intended users of this guideline are thoracic surgeons, respirologists, and medical and radiation oncologists who treat lung cancer.
2. METHODS
In this clinical practice guideline, recommendations are subdivided by patient group:
A1: Normal-sized mlns on ct, and negative pet–ct scan in the mediastinum
A2: Normal-sized mlns, and positive pet–ct in the mediastinum
B1: Enlarged (≥1-cm diameter) discrete ipsilateral (N2) or contralateral (N3) mlns on ct, and negative pet–ct in the mediastinum
B2: Enlarged mlns, and positive pet–ct in the mediastinum
Recommendations for group A1 depend on the location of the primary tumour and the tumour stage. Patients with central tumours were grouped with those having N1 disease because of the difficulty in assessing N1 nodes separately in such cases.
This guideline was developed by the Invasive Mediastinal Staging Working Group and Expert Panel of Cancer Care Ontario’s Surgical Oncology Program and the Program in Evidence-Based Care (pebc) using the methods of the practice guidelines development cycle 3,4. The recommendations were formulated using a systematic review of existing guidelines and primary studies, consensus by members of the Invasive Mediastinal Staging Working Group and Expert Panel concerning the interpretation of the evidence, an internal review by the Report Approval Panel of the pebc, and an external review by clinical experts and practitioners. This guideline is a convenient and up-to-date source of the best available evidence on invasive staging of mlns. Higher-level evidence is not expected to emerge in the near future.
2.1 Systematic Review
Existing guidelines were identified by scanning the U.S. National Guidelines Clearinghouse, the Canadian Medical Association InfoBase, the Physician Data Query database of the U.S. National Cancer Institute, and the Cochrane database of systematic reviews. A guideline from the American Association of Chest Physicians (accp) on invasive staging of lung cancer 5, which provided the evidence base up to 2006, was found. Two other guidelines from the European Society of Thoracic Surgeons 6 and the Scottish Intercollegiate Guidelines Network (sign) 7 were also referenced. The primary literature in medline, embase, and the Cochrane Library (all from 2006 to August 11, 2010) were systematically searched to identify other studies on invasive staging techniques. The secondary questions were answered based on consensus of expert opinion. More details on the systematic review methodology and the evidentiary base are available on the Web site of Cancer Care Ontario’s pebc.
2.2 Internal Review
Before submission of the guideline for external review, the draft report was reviewed by the Invasive Mediastinal Staging Expert Panel, Cancer Care Ontario’s Lung Cancer Disease Site Group, and the pebc Report Approval Panel. The Report Approval Panel consisted of 2 members, including an oncologist with expertise in clinical and methodology issues.
2.3 External Review
The external review by clinical practitioners used two processes:
targeted peer review, and
professional consultation.
In the targeted peer review, 8 reviewers from Ontario, Quebec, Nova Scotia, Alberta, and British Columbia (considered to be clinical or methodology experts on the topic) were contacted by e-mail. The 5 who agreed to participate reviewed a draft report and answered a questionnaire evaluating the methods, results, and interpretive summary used to inform the draft recommendations. They also indicated whether the draft recommendations should be approved as a guideline. Written comments were invited. Followup reminders were sent by e-mail at 2 and 4 weeks.
In the professional consultation, feedback was obtained through a brief online survey of thoracic surgeons, radiation oncologists, medical oncologists, radiologists, and respirologists from Ontario. Participants were asked to rate the overall quality of the guideline and whether they would use it themselves or recommend it to others. Written comments were invited.
3. RESULTS AND DISCUSSION
3.1 Internal Review
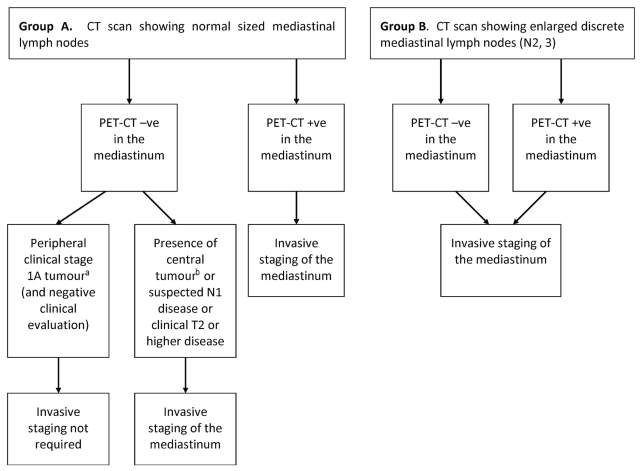
Responses were received from 10 members of the Invasive Mediastinal Staging Expert Panel and the Lung Cancer Disease Site Group and from both members of the Report Approval Panel. The guideline was approved for its methodologic rigour, and in general, panel members agreed with the guideline. One concern was raised regarding the algorithm illustrating the recommendations (Figure 1), in which the role of pet when mlns are enlarged on ct seemed uncertain because biopsy was recommended regardless of pet results. In response, the Working Group affirmed that the algorithm was purposely presented in that manner to emphasize the insufficiency of pet–ct for mediastinal staging in patients with enlarged lns. However, for further clarification, an explicit statement was added to the effect that “pet–ct +ve” and “−ve” refers to the mediastinum only.
FIGURE 1.
Invasive mediastinal staging recommendations. This algorithm applies to the target population of non-small-cell lung cancer patients in Ontario who have been clinically staged T1–4, N0–3, with no distant metastases. a Stage 1A: T1N0M0 (T1: primary tumour diameter 3 cm or smaller and surrounded by lung or visceral pleura, or endobronchial tumour distal to the lobar bronchus; N0: no lymph nodes involved; M0: no metastases). b For the purposes of this guideline, a tumour in the central third of the hemithorax is considered central. A tumour in the distal two thirds of the hemithorax is considered peripheral. ct = computed tomography; pet = positron-emission tomography.
3.2 External Review
The targeted peer review elicited 3 responses (Table I), and the professional consultation, 43 responses (Table II). More than 80% of practitioners provided a rating of “high quality” for the guideline. The main points made in the written comments from the external review, together with the responses and actions from the Working Group, were these:
TABLE I.
Responses (n = 3) to items on the targeted peer-reviewer questionnaire
| Quality (n respondents) | |||||
|---|---|---|---|---|---|
| Lowest | Highest | ||||
| Item | 1 | 2 | 3 | 4 | 5 |
| Guideline development methods | 1 | 2 | |||
| Guideline presentation | 3 | ||||
| Guideline recommendations | 1 | 2 | |||
| Completeness of reporting | 1 | 2 | |||
| Does this document provide sufficient information to inform your decisions? | 1 | 1 | 1 | ||
| Overall quality of the guideline | 2 | 1 | |||
| Strongly disagree | Neutral | Strongly agree | |||
| 1 | 2 | 3 | 4 | 5 | |
| I would use this guideline in my professional decisions | 1 | 1 | 1 | ||
| I would recommend this guideline for use in practice | 2 | 1 | |||
TABLE II.
Responses to items on the professional consultation survey
| Quality (% respondents) | |||||
|---|---|---|---|---|---|
| Lowest | Highest | ||||
| Item | 1 | 2 | 3 | 4 | 5 |
| Overall quality of the guideline | 0 | 2 | 16 | 45 | 36 |
| Strongly disagree | Neutral | Strongly Agree | |||
| 1 | 2 | 3 | 4 | 5 | |
| I would use this guideline in my professional decisions | 2 | 7 | 11 | 27 | 52 |
| I would recommend this guideline for use in practice | 2 | 5 | 14 | 23 | 57 |
-
Include more about the role of ebus and eus, and who should be doing them (physicians or surgeons). Is ebus recommended for N1 staging? Or will it not affect treatment?
Response: More studies about ebus and eus are needed before recommendations can be made. Mediastinoscopy is the standard technique for invasive staging of the mediastinum. With no clear evidence supporting induction treatment, N1 disease is treated surgically, and so preoperative diagnosis of N1 involvement would generally not change management.
-
Update the guideline to the new 7th edition staging system. In the new system, a 5–7 cm N0 tumour is stage iia and therefore out of step with the recommendation for invasive staging of clinical stage 1B.
Response: Edits were made to reflect the T category instead of tumour stage. Clinical stage 1B tumour was edited to T2 tumour or higher.
-
The recommendation to conduct mediastinoscopy in everyone other than those with peripheral T1 lesions waters down the absolute need to do one for a positive pet scan to ensure that it is not falsely positive and potentially denying curative treatment.
Response: A bullet point was added to emphasize that invasive staging is important to confirm pet findings.
-
Sample other lns—that is, stations 5 and 6 to detect cancer in the left upper lobe or left hilum, and station 3.
Response: No randomized trials have examined whether stations 5 and 6 should be staged before resection, and they are not easily accessible by mediastinoscopy. Station 3 is not routinely biopsied. However, a bullet point was added to indicate that any enlarged or suspicious lns should be biopsied.
-
There is no recommendation regarding when node sampling (that is, biopsy) versus node dissection (that is, complete removal) is indicated.
Response: This guideline deals with preoperative invasive mediastinal staging, and therefore recommendations regarding intraoperative ln sampling or dissection are beyond its scope.
-
The accp guideline did not differentiate between T1 and T2 tumours. On the other hand, this guideline, which is partly based on the accp evidence, recommends on invasive staging of T2 tumours despite normal ct and negative pet–ct. The supporting evidence should be mentioned.
Response: A qualifying statement was added to state that the evidence was inferred from the adverse prognosis for larger tumours shown by the International Association for the Study of Lung Cancer staging project, which advised the revision of the TNM staging system to upstage larger tumours.
-
The combination of ct-negative, pet-positive in the mediastinum would require invasive mediastinal staging and is not specifically mentioned.
Response: A bullet point was added to make this statement in the recommendations.
-
One reviewer disagreed with the requirement for invasive mediastinal staging for all 1B tumours— for example, a 1B peripheral tumour that is 1B by virtue of touching the visceral pleura, but smaller than 3 cm, should not require invasive mediastinal staging if ct and pet are negative in the mediastinum.
Response: Although these tumours probably do not require invasive staging, they do have a worse prognosis. Furthermore, stage T2, by virtue of visceral pleural invasion, is a pathology (that is, postoperative) diagnosis, and therefore generally does not influence preoperative decision-making regarding invasive mediastinal staging.
4. CLINICAL PRACTICE GUIDELINE
Evidence from the accp guideline 5, primary literature from a systematic search, consensus of expert opinion, and feedback from internal and external reviews collectively form the basis of this clinical practice guideline on invasive mediastinal staging of nsclc. After the external review, the literature search was updated to August 2010, but the new studies that were located corroborated the existing evidence and did not change the recommendations. The recommendations, key evidence, and qualifying statements follow. Figure 1 shows the corresponding algorithm.
4.1 Recommendations
Based on evidence from the accp guideline and a primary literature search,
invasive mediastinal staging is not needed when, in conjunction with a peripheral clinical stage 1A tumour, mlns are of normal size on ct, a pet–ct scan is negative, and a clinical evaluation is negative.
-
A clinical stage 1A tumour is defined as T1N0M0.
○ T1: primary tumour diameter is 3 cm or smaller, and tumour is surrounded by lung or visceral pleura, or endobronchial tumour is distal to the lobar bronchus
○ N0: no lns are involved
○ M0: no metastases were observed
invasive staging is recommended when
-
mlns are of normal size on ct, with negative mediastinal pet–ct, and
○ a central tumour is present (tumour in the central third of the hemithorax), or
○ N1 disease is suspected (enlarged N1 nodes, or positive N1 nodes on pet–ct, or both), or
○ T2 or greater tumours are present.
mlns are enlarged and discrete on ct (N2, N3), with a negative or positive pet–ct.
ct is negative and pet is positive in the mediastinum.
Invasive staging is important to confirm pet findings.
Based on a consensus of expert opinion,
5 nodal stations (2R/L, 4R/L, and 7) should routinely be examined when performing invasive mediastinal staging, with at least 1 node sampled from each station unless none are present after actual dissection in the region of a particular nodal station.
any enlarged or suspicious lns should be biopsied.
mediastinoscopy is the standard technique for invasive staging of the mediastinum. Endobronchial ultrasonography–guided transbronchial needle aspiration may be useful, but more data are required before that procedure can be considered equivalent.
4.2 Key Evidence
4.2.1 Invasive Staging Not Required
For normal ct, negative pet–ct, and a peripheral clinical stage 1A tumour,
the accp systematic review found that the false-negative rate for ct imaging in patients with T1 tumours (that is, clinical stage 1A) is approximately 9% 5.
sign recommends that patients with small peripheral tumours and negative ct imaging of the mediastinum require no further investigation because the false negative rate in all categories of patients with lung cancer is 13% 7. The definition of “small” was not provided, but it may be equivalent to a clinical stage 1A tumour. Based on the sign systematic review, the European Society of Thoracic Surgeons does not recommend mediastinoscopy in the case of a “T1 squamous cell tumour with N0 disease on ct” 6.
negative pet–ct imaging in the mediastinum carries a false-negative rate of approximately 5% (range: 3%–6%).
4.2.2 Invasive Staging Recommended
For normal ct, negative pet–ct, and a central tumour, N1 disease or a T2 tumour or higher,
the accp review found that false-negative rates were high for ct (20%–25%) and for pet–ct (24%–83%) imaging in the mediastinum for patients with a central tumour 5.
another systematic review found a false-negative rate of 22% for ct imaging of mlns with central tumours 8.
Cerfolio et al. 9 found that patients with clinical N1 disease suggested by integrated pet–ct/ct had a relatively high incidence of unsuspected N2 disease (17.6% after mediastinoscopy and 23.5% after eus-guided fine-needle aspiration).
For enlarged lns on ct, and pet–ct positive or negative,
the pet–ct false-negative rate is (according to two meta-analyses reported in the accp review) estimated to be 13%–25% in patients with nodal enlargement detected by ct imaging 5. These estimates were based on indirect data and patient groups that were not clearly defined. Direct data from studies in patients with mln or hilar node enlargement show a pet–ct false-negative rate of 20%–28% for N2/3 involvement.
imaging by pet–ct has been shown to falsely identify malignancy in approximately one quarter of patients with nodes that are enlarged for other reasons (usually inflammation or infection) 2.
4.3 Qualifying Statements
Based on the International Association for the Study of Lung Cancer staging project showing an adverse prognosis for larger tumours, the Working Group believes that T2 tumours should undergo invasive staging.
In addition to tumour location (that is, central compared with peripheral), several other factors have been noted in the literature to potentially affect the likelihood of N2 disease, including maximum standardized uptake value of the primary tumour (non-fluorodeoxyglucose-avid primary tumours), tumour histology, degree of differentiation, size, and bronchoalveolar cell carcinoma. Those factors should be taken into account when deciding whether to perform invasive staging.
Mediastinoscopy continues to be the standard technique for invasive mediastinal staging, but newer techniques such as ebus-guided transbronchial needle aspiration and eus-guided fine-needle aspiration have shown promise.
5. SUMMARY
This guideline on invasive mediastinal staging in nsclc patients is based on a systematic review of the literature and consensus of expert opinion, together with integration of feedback obtained through the external review process, with final approval by the Invasive Mediastinal Staging Expert Panel and the Report Approval Panel of the pebc. Updates of the report will be conducted as new evidence emerges.
6. ACKNOWLEDGMENTS
The authors thank the Invasive Mediastinal Staging Expert Panel of Cancer Care Ontario’s Surgical Oncology Program for reviewing drafts of the manuscript. The Invasive Mediastinal Staging Expert Panel members are Julius Toth (Southlake Regional Health Centre); Ken Gehman (Thunder Bay Regional Health Sciences Centre); Ken Reid and Shafeequr Salahudeen (Kingston General Hospital); Donna Maziak (Ottawa Hospital); Paul Chiasson (William Osler Health Centre); Matt Kilmurry (St. Mary’s General Hospital); Yee Ung (Odette Cancer Centre); Bill Evans (Juravinski Cancer Centre); Marisa Finlay (Credit Valley Hospital); Karen Gulenchyn (Hamilton Health Sciences); Michael Sanatani (London Health Sciences Centre); John Vlasschaert (Peterborough Regional Health Centre); and Leigh McKnight, Amber Hunter, and Robin McLeod (Cancer Care Ontario).
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors have no conflicts of interest to declare. The work reported here is supported by the Ontario Ministry of Health and Long-Term Care through Cancer Care Ontario and is editorially independent of its funding source.
8. REFERENCES
- 1.Canadian Cancer Society and the National Cancer Institute of Canada. Canadian Cancer Statistics 2009. Toronto, ON: Canadian Cancer Society; 2009. [Google Scholar]
- 2.Silvestri GA, Gould MK, Margolis ML, et al. Noninvasive staging of non-small cell lung cancer: accp evidenced-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl 3):178S–201S. doi: 10.1378/chest.07-1360. [DOI] [PubMed] [Google Scholar]
- 3.Browman GP, Levine MN, Mohide EA, et al. The practice guidelines development cycle: a conceptual tool for practice guidelines development and implementation. J Clin Oncol. 1995;13:502–12. doi: 10.1200/JCO.1995.13.2.502. [DOI] [PubMed] [Google Scholar]
- 4.Browman GP, Newman TE, Mohide EA, et al. Progress of clinical oncology guidelines development using the practice guidelines development cycle: the role of practitioner feedback. J Clin Oncol. 1998;16:1226–31. doi: 10.1200/JCO.1998.16.3.1226. [DOI] [PubMed] [Google Scholar]
- 5.Detterbeck FC, Jantz MA, Wallace M, Vansteenkiste J, Silvestri GA on behalf of the American College of Chest Physicians. Invasive mediastinal staging of lung cancer: accp evidence-based clinical practice guidelines (2nd edition) Chest. 2007;132(suppl 3):202S–20S. doi: 10.1378/chest.07-1362. [DOI] [PubMed] [Google Scholar]
- 6.De Leyn P, Lardinois D, Van Schil PE, et al. ests guidelines for preoperative lymph node staging for non-small cell lung cancer. Eur J Cardiothorac Surg. 2007;32:1–8. doi: 10.1016/j.ejcts.2007.01.075. [DOI] [PubMed] [Google Scholar]
- 7.Scottish Intercollegiate Guidelines Network. Management of Patients with Lung Cancer A National Clinical Guideline. Edinburgh, Scotland: Scottish Intercollegiate Guidelines Network; 2005. [Google Scholar]
- 8.Ghosh S, Nanjiah P, Dunning J. Should all patients with nonsmall cell lung cancer who are surgical candidates have cervical mediastinoscopy preoperatively? Interact Cardiovasc Thorac Surg. 2006;5:20–4. doi: 10.1510/icvts.2005.122838. [DOI] [PubMed] [Google Scholar]
- 9.Cerfolio RJ, Bryant AS, Eloubeidi MA. Routine mediastinoscopy and esophageal ultrasound fine-needle aspiration in patients with non-small cell lung cancer who are clinically N2 negative: a prospective study. Chest. 2006;130:1791–5. doi: 10.1378/chest.130.6.1791. [DOI] [PubMed] [Google Scholar]