Abstract
Adenosine is produced during cellular hypoxia and apoptosis, resulting in elevated tissue levels at sites of injury. Adenosine is also known to regulate a number of cellular responses to injury, but its role in hepatic stellate cell (HSC) biology and liver fibrosis is poorly understood. We tested the effect of adenosine on the cytosolic Ca2+ concentration, chemotaxis, and upregulation of activation markers in HSCs. We showed that adenosine did not induce an increase in the cytosolic Ca2+ concentration in LX-2 cells and, in addition, inhibited increases in the cytosolic Ca2+ concentration in response to ATP and PDGF. Using a Transwell system, we showed that adenosine strongly inhibited PDGF-induced HSC chemotaxis in a dose-dependent manner. This inhibition was mediated via the A2a receptor, was reversible, was reproduced by forskolin, and was blocked by the adenylate cyclase inhibitor 2,5-dideoxyadenosine. Adenosine also upregulated the production of TGF-β and collagen I mRNA. In conclusion, adenosine reversibly inhibits Ca2+ fluxes and chemotaxis of HSCs and upregulates TGF-β and collagen I mRNA. We propose that adenosine provides 1) a “stop” signal to HSCs when they reach sites of tissue injury with high adenosine concentrations and 2) stimulates transdifferentiation of HSCs by upregulating collagen and TGF-β production.
Keywords: platelet-derived growth factor, Ca2+, fibrosis
Adenosine is produced extracellularly and intracellularly from the dephosphorylation of adenosine tri-, di-, and monophosphates (27). A further source of adenosine is the degradation of nucleic acids, via the uric acid pathway, during cellular injury and apoptosis (12). These sources of adenosine result in elevated levels at sites of tissue ischemia and cellular apoptosis, with concentrations increasing ≥100-fold from the 30- to 300-nM range present in health (16, 17). Adenosine is efficiently metabolized by deamination or phosphorylation to inosine and adenine, respectively. The tight association of adenosine levels with tissue injury provides a potential means to communicate tissue injury to adaptive cellular responses.
Hepatic stellate cells (HSCs) are central to the adaptive response of the liver to hepatocyte injury and apoptosis (2). Such injury results in the chemotaxis of HSCs to the sites of cellular damage and differentiation with upregulation of TGF-β and collagen in addition to many other changes. PDGF induces HSC chemotaxis and is thought to be important in localizing HSCs to sites of liver injury (26). PDGF is produced by a variety of cells, including bile duct epithelia, and is upregulated during liver injury (3). In this model, HSCs move via chemotaxis up a gradient of PDGF until they reach the point of maximal concentration. There, they are subsequently retained at this point and may receive additional signals such as TGF-β to promote further differentiation.
It is clear that chronic liver injury results in excess liver fibrosis, which can progress to cirrhosis. However, the relative contribution of the injury stimulus and the subsequent hepatocyte apoptosis in the development of liver fibrosis remain unknown. The recent demonstration that hepatocyte apoptosis, in the absence of an injury stimulus, can result in liver fibrosis has made the identity of signals generated from apoptotic cells that regulate liver fibrosis of great interest (24). The known increase in tissue adenosine at sites of cellular injury and apoptosis stimulated us to test whether adenosine is involved in the regulation of HSCs. We studied the ability of adenosine to regulate HSC cytosolic Ca2+ fluxes, chemotaxis, and collagen production.
In this study, we demonstrated that adenosine inhibits the increase in HSC cytosolic Ca2+ concentration induced by ATP and PDGF. This inhibition was associated with an inhibition of PDGF-induced HSC chemotaxis, and the effect was dose dependent, reversible, and likely mediated via the A2a receptor subtype. We further showed that adenosine upregulates TGF-β and collagen mRNA. This identifies a novel role for adenosine in providing a “stop” signal for HSCs when they have migrated to a site of cellular injury, with upregulation of collagen and TGF-β synthesis.
MATERIALS AND METHODS
Cell culture and reagents
LX-2 cells are immortalized human HSCs and were a gift from Dr. Scott Friedman (25). LX-2 cells were cultured in RPMI plus 5% FBS and 1% penicillin-streptomycin, and the media were changed every 3 days. Primary HSCs were cultured in medium 199, 10% FBS, 1% penicillin-streptomycin, 2% gentamycin, and fungizone at a 1:100 density, with a media change 24 h after cell isolation and then after every 3 days. Histodenz, DNase, collagenase, hematoxylin-eosin stain, forskolin, xanthine, inosine, adenine, hypo-xanthine, uric acid, MRS-1523 (an A3 antagonist), 8-(p-sulfophenyl)-theophylline (8-SPT; a peripheral nonselective adenosine antagonist), and 5′-(N-ethylcarboxamido)adenosine (NECA; a nonselective adenosine receptor agonist) were obtained from Sigma (St. Louis, MO). Tryphan blue, TRIzol reagent, fungizone, trypsin-EDTA, RNase-free water, PBS, medium 199, RPMI, DMEM, HBSS, and F-12 (HAM) were purchased from GIBCO-BRL Invitrogen (Carlsbad, CA). NaOH, HCl, and glutaraldehyde were bought from J. T. Baker (Mallinckrodt Baker, Phillipsburg, NJ). 8-cyclopentyl-1,3-dipropylxanthine (DPCPX; an A1 antagonist), ZM-241385 (an A2a antagonist), and MRS-1706 (an A2b antagonist) were obtained from Tocris (Ellisville, MI). Monocyte chemoattractant protein (MCP)-1 and PDGF were from Pepro-Tech (Rocky Hill, NJ). Triton X-100 was from Cole-Parmer (Vernon Hills, IL). Transwell inserts were from Corning Costar (Corning, NY). All reagents were of the highest quality grade commercially available. TGF-β concentrations were measured in the supernatant of LX-2 cells after the addition of adenosine by standard ELISA using primary BAF240 for detection and MAB1835 for capture (both from R&D Research).
Animals and HSC isolation
Mice of the C57BL/6 strain were used for HSC isolation. All experiments and animal handling were done according to Yale University Institutional Animal Care and Use Committee guidelines. HSCs were isolated by in situ pronase-collagenase perfusion followed by density gradient centrifugation, as described previously (1). Primary cells were >95% pure. Cells were grown on standard tissue culture plastic dishes in medium 199 with 10% FCS and antibiotics. Primary cells were used at 7 days after isolation.
Semiquantitative real-time RT-PCR for expression of TGF-β and collagen I mRNA
LX-2 cells were incubated with the adenosine receptor agonist NECA at a concentration of 10 μM for 24 and 48 h or incubated in media alone. After incubation, total RNA was extracted, and cDNA was prepared using murine Moloney leukemia virus reverse transcriptase (BD Biosciences, Clontech, Palo Alto, CA). Semiquantitative real-time PCR was performed for TGF-β and collagen I using commercial primer-probe sets (Applied Biosystems) and the Applied Biosystem 7500 real-time PCR system. The expression of GAPDH was used to standardize the samples, and the results were expressed as a ratio compared with untreated LX-2 cells. Nonquantitative RT-PCR was used to determine adenosine receptor mRNA expression in LX-2 cells. Specific oligonucleotide primers were made at the Yale Keck Facility, and the thermal cycles used were based on the sequences used in a previous study (10).
Measurement of changes in cytosolic Ca2+ concentration
LX-2 cells were grown on glass coverslips, loaded with the Ca2+-sensitive fluorophore fluo-4 AM (Molecular Probes), and put into a specially designed chamber for use on a confocal microscope as previously described (13). Cells were perfused initially with HEPES buffer and then with buffer containing ATP (100 μM) or PDGF (10 ng/ml). Changes in fluo-4 fluorescence were monitored using a Zeiss confocal imaging system. Fluo-4 fluorescence was excited using a Kr/Ar laser at 488 nm; emitted fluorescence >515 nm was collected. Changes in fluorescence over time were expressed as peak fluorescence (f) divided by initial fluorescence (f0).
Chemotaxis assay
The migration of LX-2 cells was studied using Transwell inserts equipped with 8-μm-pore polycarbonate-free filters as previously described (6). Briefly, 2 × 104 LX-2 cells were plated per well. Cells were treated with the appropriate adenosine receptor antagonist (8-SPT or ZM-241385) 10 min before the addition of either adenosine, NECA, or forskolin. MCP-1 or PDGF was added to the lower chamber 2 h afterward. After 24 h, the lower surface of the membrane was stained using hematoxylin-eosin, photographed, and analyzed.
All experiments were repeated in triplicate. For each experimental group, a total of 40 high-power field images were taken. Cells per high-power field were counted, with results being expressed as average numbers of cells per high-power field. Statistical analysis in the form of a Student’s t-test was performed with P ≤ 0.05 as significant.
RESULTS
Adenosine does not induce an increase in cytosolic Ca2+ concentration
Confocal video microscopy was used to quantitate changes in cytosolic Ca2+ concentrations in response to adenosine. Adenosine did not induce an increase in the cytosolic Ca2+ concentration in LX-2 cells at concentrations between 10 and 100 μM (Fig. 1, A and B).
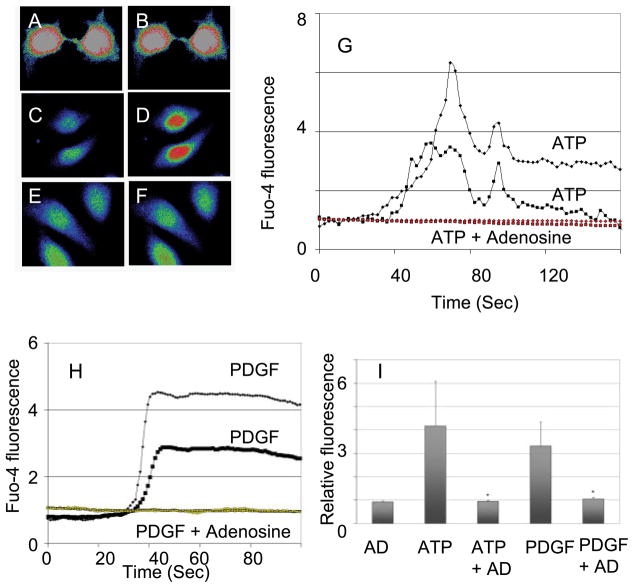
Fig. 1.
Adenosine inhibits increase in cytosolic Ca2+ induced by ATP and PDGF. A: human LX-2 hepatic stellate cell (HSC) loaded with a Ca2+-sensing dye (fluorophore fluo-4 AM) before the addition of adenosine. B: same cell as in A at 60 s after the addition of adenosine (10 μM) with no change in cytosolic Ca2+. C: untreated LX-2 cell. D: same cell as in C at 60 s after the addition of ATP at 100 μM. E: LX-2 cell treated with adenosine (10 μM) for 10 min. F: same cell as in E at 60 s after the addition of ATP at 100 μM. G: representative plots of changes in cytosolic Ca2+ after treatment of the cells with ATP alone or ATP after cells had been exposed to adenosine (10 μM for 10 min). H: representative plots of changes in cytosolic Ca2+ after treatment of the cells with PDGF alone (10 ng/ml) or PDGF after cells had been exposed to adenosine (10 μM for 10 min). I: summary of relative changes in the fluorescence of fluorophore fluo-4 AM-loaded LX-2 cells after treatment with the listed agents. In all instances, when adenosine was used, it was at 10 μM and for 10 min before the addition of ATP or PDGF.
Adenosine inhibits increases in cytosolic Ca2+ concentrations induced by ATP and PDGF
Confocal video microscopy was used to quantitate changes in cytosolic Ca2+ concentration in response to ATP and PDGF. ATP induced a rapid increase in cytosolic Ca2+ concentration in LX-2 cells (Fig. 1, C and D), and this was inhibited by pretreatment for 10 min by 10 μM adenosine (Fig. 1, E and F). Figure 1, G and H, shows the typical time course for the increase in cytosolic Ca2+ concentration upon exposure to ATP or PDGF and the effects of pretreatment with adenosine. Figure 1I shows data from a number of cells, with significant (P < 0.05) inhibition by adenosine of ATP- and PDGF-induced increases in cytosolic Ca2+ concentration. The increase in cytosolic Ca2+ concentration by ATP is mostly due to the release of Ca2+ from endoplasmic reticulum stores by activation of inositol (1,4,5)-trisphosphate receptors. This mechanism is shared by a variety of extracellular signaling molecules that are active on HSCs, including PDGF (4).
Adenosine inhibits HSC chemotaxis by MCP-1 and PDGF
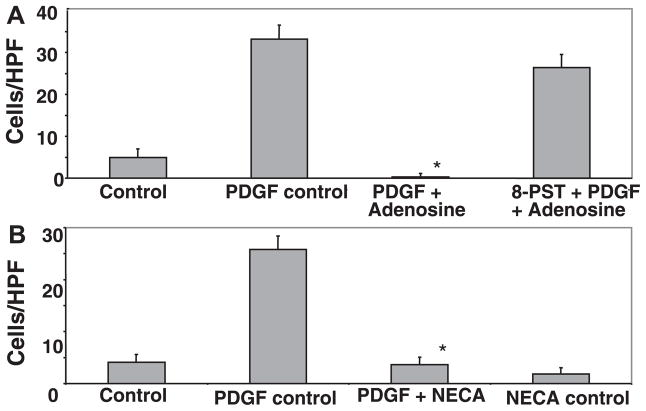
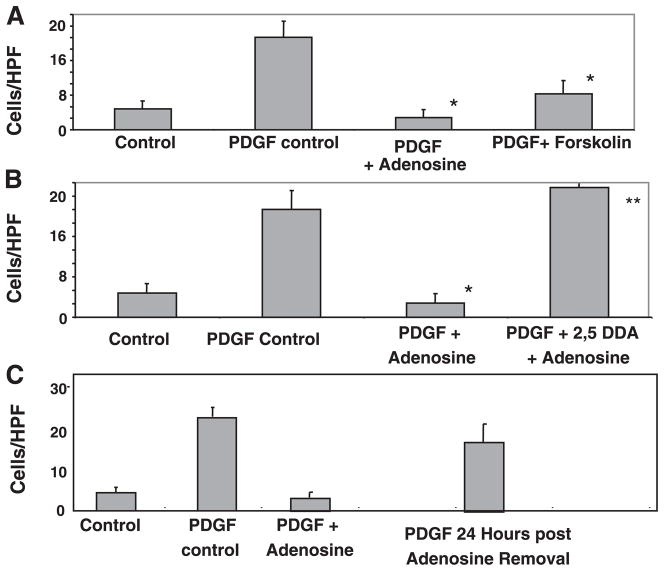
The recruitment of HSCs to sites of injury is thought to be an early step in their function in repair and matrix remodeling. PDGF induces HSC chemotaxis, and this is dependent on increases in cytosolic Ca2+ concentration. We tested whether adenosine can inhibit PDGF-induced HSC chemotaxis. Adenosine at 10 μM almost completely blocked PDGF-induced chemotaxis of LX-2 cells (Fig. 2A) and primary mouse HSCs (Fig. 2B) across 8-μm pores. The inhibition of chemotaxis was dose dependent (Fig. 2A), with adenosine concentrations below 1 μM having no effect. We established that this is a receptor-mediated phenomenon by demonstrating that it could be blocked by preadministration of the pan-adenosine receptor antagonist 8-SPT (Fig. 3A). Adenosine is metabolized to a number of molecules that may be biologically active. To determine if the inhibition of chemotaxis could be mediated via adenosine receptors, the pan-adenosine receptor agonist NECA was also shown to inhibit HSC chemotaxis to PDGF efficiently (Fig. 3B).
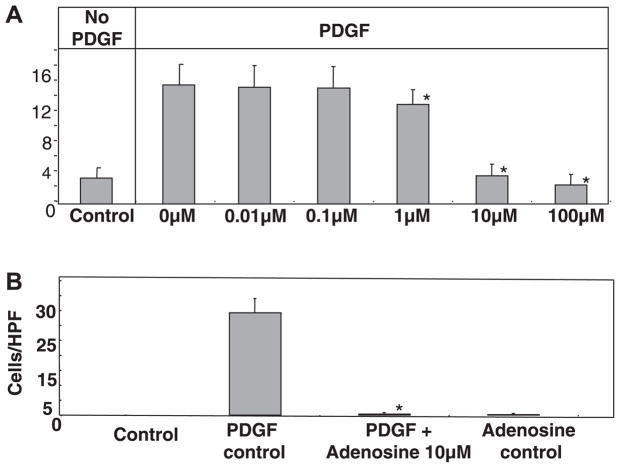
Fig. 2.
A: adenosine inhibited PDGF-induced chemotaxis in a dose-dependent manner. Human LX-2 HSCs (20,000 cells) were plated in the upper chamber of Transwell inserts with recombinant human PDGF-BB (10 ng/ml) as the chemoattractant in the lower chamber. Hematoxylin and eosin staining of the undersurface was done at the 24-h time point with migrating cells counted per high-power field (HPF). To test for the effect of adenosine on chemotaxis, cells were incubated with adenosine (at doses of 0.01, 0.1, 1, 10, and 100 μM) for 120 min before the addition of PDGF, and adenosine was maintained in the culture medium until the end of the 24-h study period (*P ≤ 0.05 vs. the PDGF control by Student’s t-test). B: primary mouse HSC chemotaxis was inhibited by adenosine. Primary mouse HSCs were isolated from B6 mice and cultured on plastic. Cells at day 5 were plated (20,000 cells) in the upper chamber of Transwell inserts, with PDGF-BB (10 ng/ml) as the chemoattractant in the lower chamber. Experiments were performed as in A with the addition of adenosine 120 min before the addition of PDGF (*P ≤ 0.05 vs. the PDGF control by Student’s t-test).
Fig. 3.
A: the pan-adenosine receptor antagonist 8-(p-sulfo-phenyl)-theophylline (8-SPT) blocked the ability of adenosine to inhibit PDGF-induced chemotaxis. Experiments were performed as in Fig. 2A with the addition of 8-SPT (10 μM) 15 min before the addition of adenosine. B: the nonhydrolysable adenosine agonist 5′-(N-ethylcarboxamido)adenosine (NECA) inhibited chemotaxis of LX-2 cells induced by PDGF. Experiments were performed as in Fig. 2A except that NECA (10 μM) was added 120 min before the addition of PDGF (*P ≤ 0.05 vs. the PDGF control by Student’s t-test).
Inhibition of chemotaxis is via the A2a receptor subtype
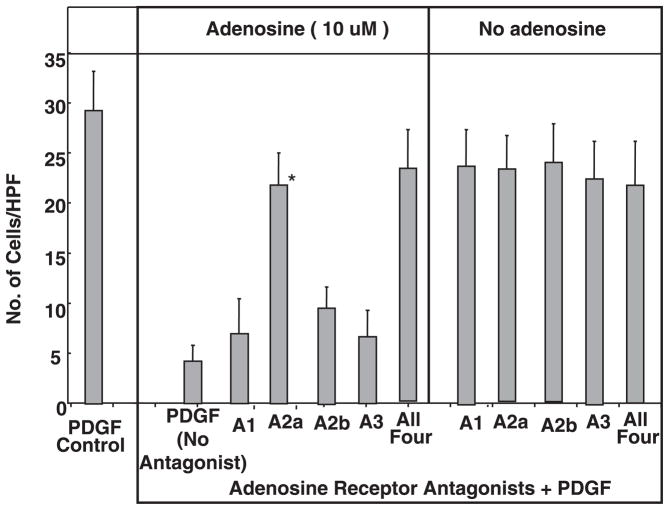
Adenosine is known to signal via four receptor subtypes: A1, A2a, A2b, and A3. These are widely expressed including in the liver and mediate their effects via coupled G proteins. A number of antagonists are selective for the receptor subtypes. The following antagonists were used to antagonize adenosine receptor subtypes: DPCPX (10 nM), A1 receptor subtype; ZM241385 (1 μM), A2a receptor subtype; MRS-1706 (10 nM), A2b receptor subtype; and MRS-1523 (5 μM), A3 receptor subtype. As shown in Fig. 4, the ability of adenosine to inhibit HSC chemotaxis to PDGF was significantly antagonized by the A2a receptor subtype antagonist but not by the A1, A2b, and A3 receptor-selective agents. The A2a antagonist was as effective at blocking adenosine-mediated inhibition of PDGF chemotaxis as the combination of all four antagonists. This strongly implicates the A2a receptor subtype as being responsible for the inhibition of HSC chemotaxis. The inability of A1, A2b, and A3 receptor antagonists to block the adenosine-induced inhibition of PDGF chemotaxis suggests that these receptor subtypes have minimal roles or no role at all. Caffeine is known to be a pan-adenosine receptor antagonist, and we found that it was also (at 10 μM) able to block the adenosine-mediated inhibition of chemotaxis (data not shown, experiments repeated in triplicate).
Fig. 4.
Inhibition of PDGF (10 ng/ml)-mediated HSC chemotaxis by adenosine was reversed by preincubation with the A2a-specific receptor antagonist ZM-241835 at 1 μM [*P ≤ 0.05 vs. the PDGF (no antagonist) + adenosine (10 μM) control by Student’s t-test], whereas the A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) at 10 nM, A2b receptor antagonist MRS-1706 at 10 nM, and the A3 receptor antagonist MRS-1523 at 5 μM had no effect. A similar antagonism of adenosine inhibition was seen with all four inhibitors combined at the above concentrations. Experiments were performed as in Fig. 3A with the addition of the receptor antagonists 15 min before the addition of adenosine.
Expression of adenosine receptors in HSCs
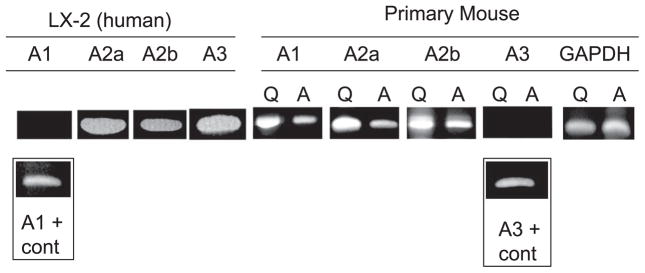
The presence of mRNA for adenosine receptor subtypes on LX-2 HSCs was assayed using RT-PCR. As shown in Fig. 5, LX-2 cells contained mRNA for A2a, A2b, and A3 (but not A1) receptor subtypes. Human peripheral blood lymphocytes were used as a positive control, and signals for all four receptor subtypes were detected.
Fig. 5.
Expression of adenosine receptor mRNA in LX-2 cells, quiescent primary mouse HSCs on day 1 of cell isolation (Q), and plastic activated primary mouse HSCs on day 5 (A). cDNA was prepared from and amplified with previously published primers and under previously published conditions. cDNA from human peripheral blood lymphocytes was used as positive control for human cells and the mouse brain for mouse primers, and gave a product for all four receptor subtypes. In LX-2 cells, mRNA for A2a, A2b, and A3 receptor subtypes was readily detected. In primary mouse HSCs, mRNA for A1, A2a, and A2b receptor subtypes was readily detected.
The A2a receptor effect is mediated via cAMP and is reversible
Signaling downstream of the A2a receptor subtype is mediated predominantly via Gs-dependent adenylate cyclase activation, resulting in an increase in cAMP. An additional Gs-independent, G12/13 pathway of Ras and ERK1/2 activation has also been documented (21, 22). Forskolin, an activator of adenylate cyclase, was used to test if elevations in cAMP could reproduce the adenosine-induced inhibition of HSC chemotaxis. As shown in Fig. 6A, forskolin also had potent inhibitory activity on HSC chemotaxis to PDGF. The importance of cAMP in the adenosine effect was further confirmed by the demonstration that pretreatment of cells with the adenylate cyclase inhibitor 2,5-DDA blocked the ability of adenosine to inhibit chemotaxis of HSCs in response to PDGF (Fig. 6B). Forskolin was found to completely block the ATP-induced increase in cytoplasmic Ca2+ concentration, further confirming the role of cAMP in this phenomenon (data not shown, experiments repeated in triplicate). It was of interest for us to know whether, at the cessation of cellular injury, when adenosine levels normalize, HSCs could be expected to recover the ability to undergo PDGF-mediated chemotaxis. As shown in Fig. 6C, 24 h after we replaced adenosine-containing with adenosine-free media, HSCs were again able to undergo chemotaxis in response to PDGF.
Fig. 6.
A: inhibition of HSC chemotaxis by adenosine (*P ≤ 0.05 vs. the PDGF control by Student’s t-test) was reproduced by the cAMP-inducing agent forskolin. Forskolin (100 μM) was added 30 min before the addition of PDGF, and the rest of the assay was performed as previously described (*P ≤ 0.05 vs. the PDGF control). B: inhibition of HSC chemotaxis by adenosine (*P ≤ 0.05 vs. the PDGF control) was blocked by the adenyl cyclase inhibitor 2,5-dideoxyadenosine (2,5-DDA). 2,5-DDA (100 μM) was added 30 min before the addition of adenosine (**P ≤ 0.05 vs. PDGF + adenosine). C: inhibition of PDGF-induced chemotaxis by adenosine was reversible. Adenosine inhibited PDGF-induced chemotaxis as shown in Fig. 2. Adenosine was removed from the media, and PDGF was added 24 h later. LX-2 cells were able to undergo chemotaxis in response to PDGF.
Adenosine upregulates collagen and TGF-β mRNA
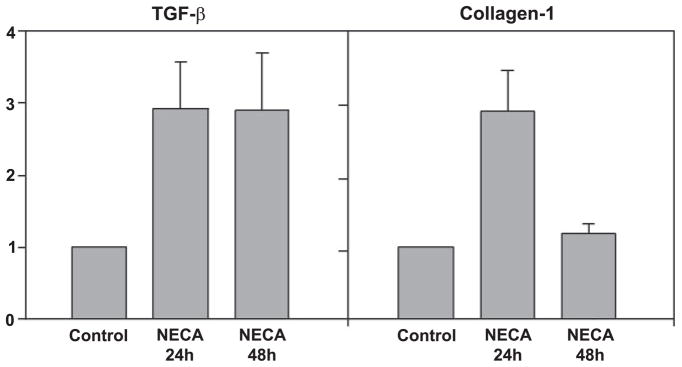
HSCs are known to upregulate TGF-β (which subsequently functions in autocrine and paracrine loops) and also to upregulate a number of matrix constituents including collagen I. We tested whether adenosine upregulated the expression of TGF-β and collagen genes in HSCs by performing semiquantitative real-time PCR. As shown in Fig. 7, adenosine at 100 μM resulted in an approximately threefold increase in TGF-β and collagen mRNA at 24 h. At 48 h, there was a decrease in the level of collagen mRNA to basal levels, but TGF-β levels were maintained. The assay of the TGF-β concentration in the supernatant by ELISA showed a significant increase between control and adenosine-treated plates at 3 and 24 h (3-h control: 3.6 ± 0.9 ng/ml, 3-h adenosine: 12.1 ± 2.0 ng/ml, 24-h control: 4.9 ± 1.0 ng/ml, 24-h adenosine: 15.1 ± 1.1 ng/ml, means ± SE; this was significant at <0.01 for both time points). This demonstrates that at sites of high adenosine concentrations, adenosine induces HSC differentiation in addition to stopping HSC chemotaxis.
Fig. 7.
The adenosine receptor agonist NECA induced upregulation of TGF-β and collagen I mRNA. LX-2 cells were cultured in the presence or absence of NECA (10 μM), and cDNA was isolated 24 and 48 h later. Semiquantitative real-time PCR was performed using commercially available primer probe combinations. Results were standardized to GAPDH and are shown relative to untreated LX-2 cells. NECA induced upregulation of TGF-β at the 24- and 48-h time points (P ≤ 0.05 vs. the control) and induced upregulation of collagen I at 24 h (P ≤ 0.05 vs. the control).
DISCUSSION
Developmental apoptosis is programmed at the level of the cell and organ, in contrast with necrosis, which is unpredictable and not programmed. Pathological apoptosis has features of developmental apoptosis, in that it uses much of the same cellular machinery but is not programmed at the level of the organ and is usually unpredictable. Pathological apoptosis, therefore, requires a complex adaptive response, which in the liver includes HSC migration, differentiation, and matrix remodeling (5). HSC numbers increase at the site of hepatocyte injury, and this local increase in HSCs is partly due to chemotaxis toward a gradient of a number of cytokines. For HSCs, PDGF is well characterized to induce chemotaxis and is produced by macrophages, activated HSCs, and injured bile duct segments (9). In such a model, HSCs are retained at the site of highest concentration of the chemotactic cytokine.
The role of hepatocyte apoptosis (as distinct from the injury stimulus and immune response) in the development of liver fibrosis has been of great interest. Clinical specimens from patients with hepatitis C infection have revealed a correlation between hepatocyte apoptosis, HSC activation, and increased stage of fibrosis (15). More convincing evidence that hepatocyte apoptosis is sufficient to stimulate liver fibrosis has been provided by a number of experimental models (23, 24). These studies bring to the forefront questions about the identity of the molecules that communicate hepatocyte apoptosis to the hepatic repair response. We chose to test whether adenosine might fulfill some of this function because it is produced during conditions of energy imbalance by the dephosphorylation of adenosine tri-, di-, and monophosphates and is also produced from the metabolism of purines during the degradation of nucleic acids of apoptosing cells. Adenosine is also rapidly metabolized to inosine by adenosine deaminase. The production of adenosine therefore does not require any new protein synthesis, and extracellular concentrations rise rapidly in tissue injury from the 0.1- to 0.3-μM range to >100 μM. The rapid metabolism limits the half-life to a few minutes. There is a very large body of literature on the regulation by adenosine of adaptive responses to tissue injury in a number of organs, but very little is known about its role in liver injury and repair (8).
Our finding that adenosine inhibits increases in cytosolic Ca2+ concentration induced by ATP and PDGF is novel, and, as predicted, adenosine resulted in inhibition of HSC chemotaxis by PDGF. Since adenosine levels are highest in the immediate microenvironment of cellular injury and apoptosis, this new function of adenosine adds an important component to the model of migration of HSCs. The previously described chemotaxis of HSC toward an increasing gradient of PDGF is still valid, but our data suggest that upon arriving at a site of cellular injury with high adenosine levels, chemotaxis will be inhibited (11). This provides a stop signal to HSCs. A system in which there is a start signal (chemokine/cytokine) and a protein synthesis-independent stop signal (adenosine) has distinct advantages. First, it stops HSCs at the site where they are most needed and keeps them there until hepatocyte apoptosis is no longer occurring and adenosine levels drop. This is necessary as PDGF and other chemokines induce chemokinesis as well as chemotaxis; thus, even when HSCs have reached the peak of the chemokine gradient, they will be actively moving in the absence of a stop signal. Another advantage is that it may allow fine localization of HSCs to the site of cellular injury. In pathological states, chemokines are produced by many tissues and cells including Kupffer cells and bile segments. Such chemokine production may function to bring HSCs into a general area (i.e., around a bile duct). The adenosine-induced stop signal may function to localize HSCs into the exact vicinity of cellular injury. The demonstration that the adenosine stop signal is reversible suggests that after the local injury has resolved, HSCs are able to respond to new chemokine gradients. In addition to stopping HSCs, we demonstrated that adenosine increases HSC differentiation by an upregulation of TGF-β and collagen I mRNA. This is consistent with increasing HSC survival and stimulating matrix remodeling once HSCs have reached the site of tissue injury.
The important role of adenosine in liver fibrosis proposed by us is consistent with a study (14) in experimental lesions in the skin, where activation of the A2a receptor accelerated wound healing. Elevated adenosine levels also result in pulmonary fibrosis. A number of studies have correlated coffee and tea consumption with a reduced risk of chronic liver disease. This was recently confirmed in a prospective study (19) of a population in the United States. Caffeine and its metabolites have a number of properties that may be responsible for this effect. These include antioxidant properties, inhibition of lipid peroxidation, and possibly an improvement of insulin sensitivity (18). However, the only known biological function of caffeine for which it has significant activity at concentrations achieved during normal human consumption (peak: 70 μM) is antagonism of A1 and A2 adenosine receptors. Our demonstration of a role for the A2a receptor in localizing HSCs to sites of tissue injury suggests that the effect of caffeine in decreasing liver fibrosis may be by A2a receptor antagonism with a consequent interference with HSC trafficking and differentiation. This is further supported by our demonstration of the ability of caffeine to antagonize the effect of adenosine to stop HSC chemotaxis.
A previous study (7) on CCl4-induced liver fibrosis in rats demonstrated a decrease in the development of fibrosis and a more rapid resolution of fibrosis when adenosine was administered. The results of this study are very surprising because adenosine was administered 3 times/wk and was not in any sustained release form. Since adenosine is rapidly degraded, this would have resulted in elevated adenosine levels for a matter of minutes for 3 times/wk. How such transient elevations in adenosine can have such significant effects is difficult to explain.
The adenosine A2a receptor is Gαs coupled and results in an increase in cAMP (20). Additional downstream pathways include PKA, CREB, and p38 activation. A separate G12/13/Ras/MEK pathway has also been identified. Adenosine has been shown to increase cAMP levels in primary rat HSCs, and our replication of the antichemotactic effects by forskolin demonstrates that elevations in cAMP are sufficient for this effect. The inability of adenosine to inhibit chemotaxis in the presence of the adenylate cyclase inhibitor 2,5-DDA, moreover, demonstrates that elevations in cAMP are required for this effect.
In summary, we demonstrated that adenosine blocks cytosolic elevations in Ca2+ concentration in HSCs and is a potent stop signal for chemotaxis, and we propose that adenosine has an important role in liver fibrosis by localizing HSCs to the site of tissue injury and inducing HSC differentiation. This identifies adenosine receptors as potential targets for modulating HSC biology and regulating the development of liver fibrosis.
Acknowledgments
We appreciate Dr. Scott Friedman’s expert advice and the use of the LX-2 cell line.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grants KO8-DK-02965-04 and P30-DK-34989 and by a research gift from Gilead Pharmaceuticals. J. A. Dranoff was supported by an American Heart Association grant-in-aid.
References
- 1.Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L. ERK (MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38 (SAPK) Cancer Res. 2003;63:1684–1695. [PubMed] [Google Scholar]
- 2.Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001;5:315–334. doi: 10.1016/s1089-3261(05)70168-9. [DOI] [PubMed] [Google Scholar]
- 3.Bonner JC. Regulation of PDGF and its receptors in fibrotic diseases. Cytokine Growth Factor Rev. 2004;15:255–273. doi: 10.1016/j.cytogfr.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Dawson AP. Calcium signalling: how do IP3 receptors work? Curr Biol. 1997;7:R544–R547. doi: 10.1016/s0960-9822(06)00277-6. [DOI] [PubMed] [Google Scholar]
- 5.Friedman SL. Mechanisms of disease: mechanisms of hepatic fibrosis and therapeutic implications. Nat Clin Pract Gastroenterol Hepatol. 2004;1:98–105. doi: 10.1038/ncpgasthep0055. [DOI] [PubMed] [Google Scholar]
- 6.Gentilini A, Marra F, Gentilini P, Pinzani M. Phosphatidylinositol 3-kinase and extracellular signal-regulated kinase mediate the chemotactic and mitogenic effects of insulin-like growth factor-I in human hepatic stellate cells. J Hepatol. 2000;32:227–234. doi: 10.1016/s0168-8278(00)80067-7. [DOI] [PubMed] [Google Scholar]
- 7.Hernandez-Munoz R, Diaz-Munoz M, Suarez-Cuenca JA, Trejo-Solis C, Lopez V, Sanchez-Sevilla L, Yanez L, De Sanchez VC. Adenosine reverses a preestablished CCl4-induced micronodular cirrhosis through enhancing collagenolytic activity and stimulating hepatocyte cell proliferation in rats. Hepatology. 2001;34:677–687. doi: 10.1053/jhep.2001.27949. [DOI] [PubMed] [Google Scholar]
- 8.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80:697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi R, Saitoh O, Nakata H. Identification of adenosine receptor subtypes expressed in the human endothelial-like ECV304 cells. Pharmacology. 2005;74:143–151. doi: 10.1159/000084547. [DOI] [PubMed] [Google Scholar]
- 11.Marra F, Gentilini A, Pinzani M, Choudhury GG, Parola M, Herbst H, Dianzani MU, Laffi G, Abboud HE, Gentilini P. Phosphatidylinositol 3-kinase is required for platelet-derived growth factor’s actions on hepatic stellate cells. Gastroenterology. 1997;112:1297–1306. doi: 10.1016/s0016-5085(97)70144-6. [DOI] [PubMed] [Google Scholar]
- 12.McGaraughty S, Cowart M, Jarvis MF, Berman RF. Anticonvulsant and antinociceptive actions of novel adenosine kinase inhibitors. Curr Top Med Chem. 2005;5:43–58. doi: 10.2174/1568026053386845. [DOI] [PubMed] [Google Scholar]
- 13.Minagawa N, Kruglov EA, Dranoff JA, Robert ME, Gores GJ, Nathanson MH. The anti-apoptotic protein Mcl-1 inhibits mitochondrial Ca2+ signals. J Biol Chem. 2005;280:33637–33644. doi: 10.1074/jbc.M503210200. [DOI] [PubMed] [Google Scholar]
- 14.Montesinos MC, Gadangi P, Longaker M, Sung J, Levine J, Nilsen D, Reibman J, Li M, Jiang CK, Hirschhorn R, Recht PA, Ostad E, Levin RI, Cronstein BN. Wound healing is accelerated by agonists of adenosine A2 (G alpha s-linked) receptors. J Exp Med. 1997;186:1615–1620. doi: 10.1084/jem.186.9.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pianko S, Patella S, Ostapowicz G, Desmond P, Sievert W. Fas-mediated hepatocyte apoptosis is increased by hepatitis C virus infection and alcohol consumption, and may be associated with hepatic fibrosis: mechanisms of liver cell injury in chronic hepatitis C virus infection. J Viral Hepat. 2001;8:406–413. doi: 10.1046/j.1365-2893.2001.00316.x. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Nunez A, Cid E, Rodriguez-Garcia J, Camina F, Rodriguez-Segade S, Castro-Gago M. Concentrations of nucleotides, nucleosides, purine bases, oxypurines, uric acid, and neuron-specific enolase in the cerebrospinal fluid of children with sepsis. J Child Neurol. 2001;16:704–706. doi: 10.1177/088307380101600918. [DOI] [PubMed] [Google Scholar]
- 17.Roth S, Park SS, Sikorski CW, Osinski J, Chan R, Loomis K. Concentrations of adenosine and its metabolites in the rat retina/choroid during reperfusion after ischemia. Curr Eye Res. 1997;16:875–885. doi: 10.1076/ceyr.16.9.875.5045. [DOI] [PubMed] [Google Scholar]
- 18.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 19.Ruhl CE, Everhart JE. Coffee and tea consumption are associated with a lower incidence of chronic liver disease in the United States. Gastroenterology. 2005;129:1928–1936. doi: 10.1053/j.gastro.2005.08.056. [DOI] [PubMed] [Google Scholar]
- 20.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cell Signal. 2003;15:813–827. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 21.Seidel MG, Klinger M, Freissmuth M, Holler C. Activation of mitogen-activated protein kinase by the A2A-adenosine receptor via a rap1-dependent and via a p21(ras)-dependent pathway. J Biol Chem. 1999;274:25833–25841. doi: 10.1074/jbc.274.36.25833. [DOI] [PubMed] [Google Scholar]
- 22.Sexl V, Mancusi G, Holler C, Gloria-Maercker E, Schutz W, Freissmuth M. Stimulation of the mitogen-activated protein kinase via the A2A-adenosine receptor in primary human endothelial cells. J Biol Chem. 1997;272:5792–5799. doi: 10.1074/jbc.272.9.5792. [DOI] [PubMed] [Google Scholar]
- 23.Sheen-Chen SM, Ho HT, Chen WJ, Eng HL. Effect of ZVAD-fmk on hepatocyte apoptosis after bile duct ligation in rat. World J Gastroenterol. 2005;11:2330–2333. doi: 10.3748/wjg.v11.i15.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takehara T, Tatsumi T, Suzuki T, Rucker EB, 3rd, Hennighausen L, Jinushi M, Miyagi T, Kanazawa Y, Hayashi N. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C, Zeisberg M, Mosterman B, Sudhakar A, Yerramalla U, Holthaus K, Xu L, Eng F, Afdhal N, Kalluri R. Liver fibrosis: insights into migration of hepatic stellate cells in response to extracellular matrix and growth factors. Gastroenterology. 2003;124:147–159. doi: 10.1053/gast.2003.50012. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann H. Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]