Abstract
Reflecting on the 2009 H1N1 pandemic, we summarize lessons regarding influenza vaccines that can be applied in the future. The two major challenges to vaccination during the 2009 H1N1 pandemic were timing and availability of vaccine. Vaccines were, however, well-tolerated and immunogenic, with inactivated vaccines containing 15μg of HA generally inducing antibody titers ≥1:40 in adults within 2 weeks of the administration of a single dose. Moreover, the use of oil-in-water adjuvants in Europe permitted dose- reduction, with vaccines containing as little as 3.75 or 7.5μg HA being immunogenic. Case-control studies demonstrated that monovalent 2009 H1N1 vaccines were effective in preventing infection with the 2009 H1N1 virus, but preliminary data suggests that it is important for individuals to be re-immunized annually.
Keywords: 2009 H1N1, pandemic, influenza, vaccines
Introduction
Influenza viruses are enveloped RNA viruses belonging to the Orthomyxoviridae family. There are three types of influenza viruses that cause disease in people: A, B and C. Type A viruses infect a wide range of hosts and pose the most significant public health risk. They are further subtyped based on the antigenicity of the hemagglutinin (HA) and neuraminidase (NA) surface proteins. Sixteen HA and nine NA subtypes have been identified in the natural hosts, waterfowl (order Anseriformes) and shorebirds (order Charadriformes) and several subtypes have transmitted to domestic poultry and mammalian species including pigs, horses, and humans [1,2].
The genome is comprised of 8 segments of negative-stranded RNA that encode at least 11 proteins. Reassortment of gene segments between influenza viruses is a frequent occurrence in nature. This may lead to the emergence of a novel subtype of influenza capable of infecting humans. There have been three influenza pandemics in the 20th century. The first, caused by an H1N1 virus, occurred in 1918. The virus was maintained in humans until reassortment events led to the emergence of an H2N2 pandemic virus in 1957, which circulated until it was replaced by an H3N2 virus in 1968 [1,2]. In 1977, H1N1 viruses re-emerged in humans and co-circulated with H3N2 viruses, causing seasonal influenza epidemics until 2009, when a new H1N1 virus emerged (2009 H1N1) (reviewed by [3]). In addition to these pandemics, people have also been infected with H5, H7 and H9 viruses. Since 2003, almost 500 cases of human infection with highly pathogenic avian influenza (HPAI) H5N1 viruses have been reported, mostly due to direct transmission from poultry to humans. Although there has not yet been sustained transmission from person to person, there is concern that continued transmission from birds to humans may increase the probability of an H5N1 virus emerging with the capacity to transmit efficiently.
Vaccination remains the primary strategy for the prevention and control of influenza [4,5] and both inactivated and live attenuated vaccines are licensed. Vaccine production begins with the generation of vaccine reference strains, reassortant viruses with the HA and NA derived from the vaccine target, combined with internal protein genes from a strain which, for inactivated vaccines, specifies high yield in eggs (A/Puerto Rico/8/34; PR8) [6] and, for live attenuated influenza vaccines (LAIV), confers temperature sensitivity, cold-adaptation (ca) and attenuation that limits viral replication to the cooler, upper respiratory tract (A/Ann Arbor/6/60 ca; A/Leningrad/47/57 ca) [7].
We are now in the post-pandemic period following the 2009 H1N1 virus outbreaks. Reflecting on the pandemic, this review summarizes lessons regarding influenza vaccines that can be applied in the future.
The 2009 H1N1 Pandemic
The 2009 pandemic H1N1 virus first emerged in Mexico and California in April and quickly spread globally. On June 11, 2009, a pandemic was declared by the World Health Organization [8]. The virus was antigenically unrelated to previously circulating seasonal H1N1 viruses, and molecular studies revealed that it had gene segments from several viruses that were circulating in pigs: the North American H3N2 triple-reassortant and the Eurasian ‘avian-like’ swine H1N1 viruses [9–11].
The majority of cases occurred in younger age-groups, with the median age estimated to be 12–17 years in Canada, USA, Chile, Japan and the UK [3]. In most individuals, infection led to a mild, self-limiting, upper respiratory tract illness, but 2–5% of cases required hospitalization [12] and pregnant women were at a higher risk for severe disease [13–15]. The overall case-fatality rate was 0.15–0.25%, and deaths mostly occurred in middle-aged adults (median age 40–50 years) [3]. Deaths amongst hospitalized patients mostly occurred in those with underlying health conditions, but nearly one third who died were previously healthy [3].
National authorities identified the following high-priority groups for vaccination: health care workers, pregnant women, young children, and individuals with underlying cardiovascular or respiratory medical conditions, autoimmune disorders and diabetes. Production of seasonal influenza vaccine for the 2009–2010 season was, however, already under way (reviewed in [16]) and the decision was made to produce a separate monovalent pandemic H1N1 vaccine.
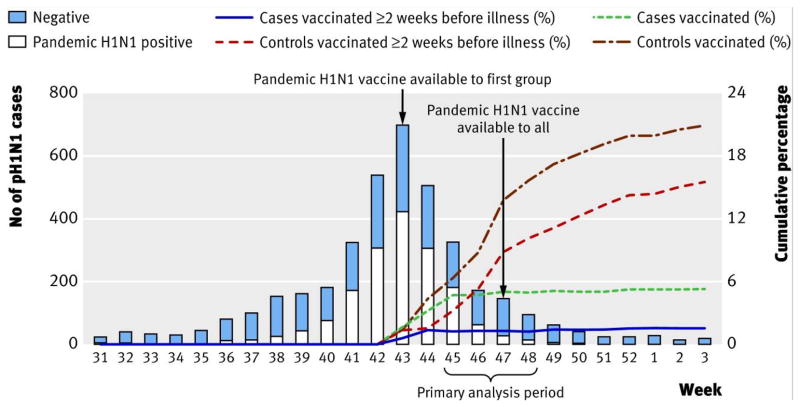
A major challenge to pandemic vaccine production was timing. In spite of a rapid response, vaccines were neither available for widespread use during the 2009 winter season in the Southern Hemisphere [3], nor until after the autumn/winter wave had nearly peaked in the Northern Hemisphere [17] (Figure 1). Another challenge was availability. As of June 2009, the global capacity for seasonal vaccine production was only 876 million doses a year, with 7 manufacturers providing 64% of this capacity [3]. In addition, pandemic vaccine virus yields in eggs and cell culture were lower than expected (reviewed by [16,18]), the optimal quantity of HA per dose was unknown, nor was it clear whether adjuvants should be utilized for dose sparing, as had been demonstrated for H5N1 vaccines (reviewed in [19]). It was also assumed that two doses of vaccine would be needed to induce protection, as clinical trials conducted in 1977 indicated that two doses of vaccine were needed to successfully immunize naive individuals against a novel virus [20–24].
Figure 1.
The number of cases in the 2009 H1N1 pandemic in Canada with respect to availability of vaccine (adapted from Skowronski et al., 2011)
The number of cases in the 2009 H1N1 pandemic peaked at week 43, coinciding with the first vaccine availability
Since then, new manufactures have emerged in China, India, Thailand and South America and there are now 26 manufacturers making pandemic 2009 H1N1 vaccines [3] using various platforms in many countries, but challenges remain for vaccinating people living in under-resourced countries that lack the infrastructure for mass vaccination.
Immunogenicity
All registered pandemic H1N1 vaccines were evaluated for safety and immunogenicity in clinical trials and met criteria required for licensure of seasonal influenza vaccines by regulatory authorities, including the Committee for Medicinal Products for Human Use (CHMP) in Europe and the Food and Drug Administration (FDA) in the United States [25]. This includes an assessment of the proportion of participants with either seroconversion or significant increase in antibody titer, the fold-increase in geometric mean antibody titer (GMT) and the proportion of participants with antibody titers of 1: 40 or greater, which is associated with a 50% reduction in the risk of illness in a susceptible adult population [26]. Typically hemagglutination inhibition (HAI) antibody titers are determined as a surrogate for neutralizing antibodies.
The results of monovalent 2009 H1N1 vaccine trials are summarized in Table 1. A single dose of unadjuvanted inactivated split-virion 2009 H1N1 vaccine containing 15μg HA led to antibody titers ≥1:40 in 95–98% of healthy adults [27–30]. In children, one study found that 92.5% of 6 month-9 year olds had antibody titers ≥1:40 after a single dose of 15μg HA in an inactivated split-virion unadjuvanted 2009 H1N1 monovalent vaccine [31], however another study found that only 47–50% of 6–35 month-olds and 65–75% of 3–9 year olds achieved these titers [32]. After two doses, however, 90–100% children had titers ≥1:40 in both studies [31,32] (Table 1). Therefore, a single dose of monovalent 2009 H1N1 vaccine was recommended in adults, but young children were recommended to receive 2 doses (reviewed by [3]). It is likely that a single dose was sufficient to induce immunity in adults because prior exposure to seasonal H1N1 viruses had immunologically primed the population. This hypothesis is supported by studies in a mouse model of infection, where prior infection with seasonal influenza virus or seasonal LAIV primed mice for a robust response to a single dose of pandemic LAIV while unprimed mice were only partially protected against 2009 H1N1 infection with a single dose of pandemic LAIV [33].
Table 1.
Immunogenicity of monovalent 2009 H1N1 Vaccines
| Type of Vaccine | Country | Dose of HA (μg) | Subject age | n | Following 1 dose | Following 2 doses | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % with HAI antibody titers ≥1:40 (95% CI) | % sero-conversion (95% CI) | GMT** ratio (95% CI) | % with HAI antibody titers ≥1:40 (95% CI) | % sero-conversion (95% CI) | GMT ratio (95% CI) | ||||||
| Inactivated, split-virus, unadjuvanted | Australia | 15 | 18–64 years | 240 | 95.0 (89.4–98.1) | 74.2 (65.4–81.7) | 11.8 (8.9–15.7) | 98.3 (94.0–99.8) | 82.1 (73.9–88.5) | 16.0 (12.4–20.7) | Greenberg et al., 2009 |
| Inactivated, split-virus, non-adjuvanted | Australia | 15 | 6 months-9 years | 370 | 92.5 (87.6–95.6) | 86.8 (80.9–91.0) | 13.6 (11.8–15.6) | 100 (97.7–100) | 97.5 (93.7–99.0) | 44.6 (36.3–54.8) | Nolan et al., 2010 |
| Inactivated, split-virus, unadjuvanted | USA | 7.5 | 18–64 years | 141 | 95 (90–98) | 92 (86–96) | 38.9 (30.4–49.7) | No data | No data | No data | Plennevaux et al., 2010 |
| 15 | 18–64 years | 145 | 98 (94–100) | 96 (91–99) | 64.3 (50.9–81.2) | No data | No data | No data | |||
| Inactivated, split-virus, unadjuvanted | USA | 7.5 | 6–35 months | 92 | 47 (36–57) | 46 (35–56) | 3.33 (2.54–4.35) | 91 (84–96) | 90 (82–95) | 32.2 (24.1–43.1) | Plennevaux et al., 2011 |
| 3–9 years | 98 | 65 (55–75) | 63 (53–73) | 6.82 (5.09–9.15) | 97 (91–99) | 96 (90–99) | 40.3 (30.5–53.2) | ||||
| 15 | 6–35 months | 87 | 48 (40–61) | 48 (37–59) | 4.45 (3.42–5.81) | 99 (94–100) | 99 (94–100) | 54.6 (42.3–70.4) | |||
| 3–9 years | 91 | 75 (65–83) | 75 (65–83) | 9.86 (7.46–13.0) | 99 (94–100) | 99 94–100) | 55.0 (42.7–70.7) | ||||
| Inactivated, split-virus, unadjuvanted | China | 15 | 3–11 years | 110 | 74.5 (65.1–82.5) | No data | 64.1/5.8 | 82.7 (74.0–89.4) | No data | 11.4† | Zhu et al., 2009 |
| 12–17 years | 110 | 97.1 (91.9–99.4) | No data | 430.7/7.3 | 97.0 (91.6–99.4) | No data | 93.2† | ||||
| 18–60 years | 110 | 97.1 (91.9–99.4) | No data | 237.8/6.9 | 92.6 (85.9–96.8) | No data | 53.8† | ||||
| 61+ years | 110 | 79.1 (70.0–86.4) | No data | 122.1/6.3 | 84.1 (75.8–90.5) | No data | 28.3† | ||||
| Inactivated, split-virus, unadjuvanted | China | 7.5 | 3– <12 years | 232 | 76.7 (70.7–82.0) | No data | No data | 97.7 (94.8–99.3) | No data | No data | Liang et al., 2010 |
| 12–<18 years | 218 | 96.8 (93.5–98.7) | No data | No data | 100 (98.1–100) | No data | No data | ||||
| 18–<60 years | 323 | 89.5 (85.6–92.6) | No data | No data | 98.4 (96.3–99.5) | No data | No data | ||||
| 60+ years | 147 | 80.3 (72.9–86.4) | No data | No data | 88.3 (81.7–93.2) | No data | No data | ||||
| 15 | 3–12 years | 113 | 85.1 (78.5–92.,3) | No data | No data | 98.4 (97.3–99.1) | No data | No data | |||
| 12–18 years | 1091 | 97.3 (96.1–98.1) | No data | No data | 100 (99.6–100) | No data | No data | ||||
| 18–60 years | 1280 | 94.3 (92.9–95.5) | No data | No data | 97.2 (96.1–98.1) | No data | No data | ||||
| 60+ years | 842 | 84.4 (81.8–86.8) | No data | No data | 96.6 (95.0–97.8) | No data | No data | ||||
| Inactivated, split-virus, MF59-adjuvanted (Novartis) | Italy | 7.5 | 6–23 months (pre-terms ranging from <32 to 42 weeks) | 105 | 94.1–100 | 94.1–100 | 9–39.7 | 100 | 100 | 13.6–88.9 | Esposito et al., 2011 |
| Inactivated, surface antigen, MF59- adjuvanted | UK | 3.75 | 20–48 years | 25 | 92 (74–99) | 88 (69–98) | 29.7 (14.4–61.2) | 100 (86–100) | 92 (74–99) | 45.6 (26.9–77.5) | Clark et al., 2009 |
| 7.5 | 18–50 years | 26 | 77 (56–91) | 73 (52–88) | 25.9 (11.5–58.9) | 92 (73–99) | 92 (73–99) | 53.0 (29.5–95.5) | |||
| Inactivated, surface antigen, non-adjuvanted | 7.5 | 23–49 years | 25 | 72 (51–88) | 72 (51–88) | 18.9 (8.8–40.4) | 79 (58–93) | 79 (58–93) | 22.9 (12.7–41.2) | ||
| 15 | 24–49 years | 25 | 63 (41–81) | 52 (31–73) | 13.5 (5.6–32.7) | 74 (52–90) | 74 (52–90) | 27.4 (12.2–61.9) | |||
| Inactivated, split-virus, AS03B #-adjuvanted | UK | 1.875 | 6 months-13 years | 435 | No data | No data | No data | 99.3 * (97.9–99.8) | 98.7* (97.1–996) | 89.5 (81.9–97.8) | Waddington et al., 2010 |
| Whole virion, inactivated, non-adjuvanted | 7.5 | 6 months-13 years | 456 | No data | No data | No data | 88.5 (85.1–91.3) | 88.4 (73.8–85.5) | 15.0 (13.2–17.2) | ||
| Inactivated, split-virus, AS03A #-adjuvanted | UK | 3.75 | 18–44 years | 70 | 94 (85–98) | 88 (78–95) | 60.1 (39.6–91.3) | 100 (94–100) | 95 (87–99) | 94.1 (66.3–133.5) | Nicholson et al., 2011 |
| 45–64 years | 68 | 77 (65–87) | 74 (62–84) | 20.2 (13.3–30.5) | 91 (81–97) | 88 (77–95) | 32.3 (22.8–45.8) | ||||
| ≥65 years | 37 | 78 (71–84) | 75 (68–91) | 25.3 (19.1–33.6) | 91 (86–95) | 87 (81–92) | 36.2 (28.5–46.0) | ||||
| Whole virion, inactivated, unadjuvanted | 7.5 | 18–44 years | 70 | 51 (43–59) | 46 (38–53) | 8.3 (6.3–11.0) | 54 (46–62) | 49 (42–57) | 8.2 (6.2–10.8) | ||
| 45–64 years | 68 | 71 (59–82) | 36 (25–49) | 4.6 (2.9–7.3) | 44 (32–57) | 41 (29–54) | 5.9 (3.9–9.0) | ||||
| ≥65 years | 34 | 32 (17–51) | 29 (15–48) | 4.1 (2.5–6.7) | 36 (20–55) | 33 (18–52) | 3.6 (2.2–6.0) | ||||
| Inactivated, split-virus, AS03A #-adjuvanted | Belgium | 3.75 | 18–60 years | 116 | 96 (86.3–995) | 94 (83.5–98.7) | 45.3 (30.3–67.7) | 94 (83.5–98.7) | 92.0 (80.8–97.8) | 38.6 (26.0–57.5) | Roman et al., 2010 |
| 60+ years | 116 | 85.7 (72.8–94.1) | 77.6 (63.4–88.2) | 13.3 (9.0–19.6) | 98.5 (92.0–100) | 94.0 (85.4–98.3) | 33.6 (24.9–45.3) | ||||
| Inactivated, split virus, AS03A # adjuvanted | Spain | 3.75 | 6–35 months | 53 | 100 (92.6–100) | 97.9 (88.7–99.9) | 46.3 (35.4–60.5) | 100 (92.9–100) | 100 (92.6–100) | 323 (231–449) | Carmona et al., 2010 |
| Inactivated, split virus, AS03B # adjuvanted | 1.9 | 6–35 months | 104 | 100 (96.4–100) | 99.0 (94.6–100) | 54.5 (46.4–64.0) | 100 (96.3–100) | 100 (96.3–100) | 347 (288–418) | ||
| Inactivated, whole-virion, alum-adjuvanted | Hungary | 6 | 18–60 years | 103 | No data | 74.3 (64.6–82.4) | 9.1 (7.7–10.7) | No data | No data | No data | Vajo et al., 2010 |
| 60+ years | 75 | No data | 61.3 (49.4–72.4) | 6.3 (5.2–7.6) | No data | No data | No data | ||||
| Live attenuated 2009 H1N1, non-adjuvanted | USA | 107.5 FFU of live vius per 0.5ml | 2–17 years | 261 | No data | 11.1 | 3.53/2.81 | No data (26.4% had antibody titers ≥1:32) | 32.0 | 2.71† | Mallory et al., 2010 |
| 18–49 years | 240 | No data | 6.1 | 3.44/3.00 | No data (13.5% had antibody titers ≥1:32) | 14.9 | 1.62† | ||||
GMT: geometric mean titer
antibody titer determined by microneutralization
AS03A contains 11.86mg tocopherol oil-in-water adjuvant; AS03B contains 5.93mg tocopherol
calculated using figures from original reference
The use of oil-in-water adjuvants, MF59 (Focetria, Novartis Vaccines, Italy) and AS03 (GlaxoSmithKline (GSK) Biologicals, Dresden, Germany) in 2009 H1N1 vaccines was approved by the European Medicines Agency (EMEA). The adjuvants had previously been approved in Europe for use in seasonal influenza vaccines and adjuvanted inactivated H5N1 vaccines had enabled dose sparing and induced broad cross-clade humoral responses [34,35]. Their mechanism of action remains incompletely understood, however they may amplify immune responses by enhancing antigen presentation and recruiting inflammatory cells to the area of antigen deposition (reviewed by [36]).
Adjuvanted monovalent 2009 H1N1 vaccines permitted dose sparing (Table 1). Antibody titers ≥1:40 were induced in 92% of adults given a single dose of MF59-adjuvanted vaccine [37] and 94–96% given the AS03-adjuvanted vaccine each containing only 3.75μg HA [38,39]. In children, antibody titers ≥1:40 were induced in 100% of 6–35 month olds given 2 doses of 7.5μg or 3.75μg HA [40,41] and 99.3% of 6 month-13 year olds given 1.875μg HA in adjuvanted vaccine [42]. Moreover, in the UK, adjuvanted split-virion vaccines induced seroconversion and antibody titers ≥1:40 in a larger percentage of adults and children than non-adjuvanted whole virion vaccines [38,42]. Adjuvanted influenza vaccines are, however, not licensed in the USA [43]. Fortunately, adjuvants were not necessary to achieve adequate immunity to the 2009 H1N1 virus, though they may prove to be important in the future.
Live attenuated influenza vaccine (LAIV) viruses were also licensed for use against the 2009 H1N1 virus, however, it was necessary to introduce point mutations in the HA to optimize the yield in eggs [44]. Intranasal vaccination with 2 doses of 107 fluorescent focus units of virus, 28 days apart, led to antibody titers ≥1:32 in 26.4% children 2–17 years old and 13.5% of adults 18–49 years old (Table 1) [45]. While these responses were modest, HAI titers are not predictive of protective efficacy of LAIV and vaccine efficacy is seen in the absence of seroconversion [46,47]. Determining meaningful immune correlates of protection for LAIV is an active area of research.
Safety
The safety of the monovalent 2009 H1N1 vaccines was assessed in clinical trials (Table 1). All vaccines were well-tolerated and elicited only mild or moderate transient side-effects, such as local pain, swelling and redness at the injection site, temporary fever, headache, fatigue or myalgia. Moreover, in the USA, data from the Vaccine Adverse Event Reporting System (VAERS) indicated that the adverse event profile was consistent with that of seasonal influenza vaccines and Guillian-Barre syndrome, anaphylaxis and death were rare [48]. However, there are reports of narcolepsy being a rare adverse event following vaccination with an AS03-adjuvanted 2009 H1N1 influenza vaccine, Pandemrix [49–51]. At the time of writing, this is under investigation [52].
In the 2010–2011 influenza season, trivalent vaccines incorporated an A/California/07/2009 H1N1-like virus, an A/Perth/16/2009 (H3N2)-like virus and a B/Brisbane/60/2008-like virus. While the majority of these vaccines are well tolerated, in Australia, an increased risk of febrile convulsions following immunization with an inactivated, split-virion, unadjuvanted vaccine, Fluvax, led to the suspension of seasonal influenza immunization of healthy children in April 2010. The cause of this association remains unknown, and an investigation is underway. A similar vaccine was utilized in the UK, until the Department of Health advised against its use in younger children and enhanced surveillance for febrile convulsions. Other vaccine products were, however, not associated with an increased risk of febrile convulsions [53].
Effectiveness
Robust immunogenicity does not always result in greater vaccine effectiveness (VE) [54]. This may be, in part, because the 1:40 HAI titer used to measure immunogenicity is extrapolated to all influenza viruses. It is, therefore, important to estimate the effectiveness of vaccines at the population level. Published effectiveness data from 2009–2010 is available from studies conducted in Europe, UK, Canada, China and Korea (Table 2), with VEs ranging from 66.0% to 93.0% [17,54–57]. These studies demonstrate that the monovalent 2009 H1N1 vaccines were effective in preventing infection with 2009 H1N1 virus, in contrast to the 2009–2010 trivalent vaccines, which did not contain the pandemic H1N1 vaccine antigen and showed no evidence of effectiveness against 2009 H1N1 infection, with an adjusted VE of only 9.9% (95% CI, −65.2–50.9%) ([17,54–57]).
Table 2.
Effectiveness of monovalent 2009 H1N1 vaccines against confirmed 2009 H1N1 virus infection
| Type of Vaccine | Country | Time of analysis | Number of ILI patients | Adjusted Vaccine Effectiveness (95% CI) | Reference |
|---|---|---|---|---|---|
| Inactivated, unadjuvanted, 15μg HA; Inactivated, MF59 –adjuvanted, 3.75μg HA | Korea | December 2009–March 2010 | 416 | 73.4 (49.1–86.1) | Song et al., 2011 |
| Inactivated, split virion AS03 adjuvanted, 3.75μg HA; whole virion, non-adjuvanted, 7.5μg HA | UK | November 2009–January 2010 | 5,808 | 72.0 (21–90) | Hardelid et al., 2011 |
| Inactivated, AS03-adjuvanted, 3.75μg HA | Canada | 8 November – 5 December 2009 | 552 | 93 .0 (69–98) | Skowronski et al., 2011 |
| Inactivated, split-virion, non-adjuvanted, 15μg HA | China | September 2009–January 2010 | 95,244 | 87.3 (75.4–93.4) | Wu et al., 2010 |
| Unspecified | France, Hungary, Ireland, Italy, Romania, Portugal, Spain | November 2009–February 2010 | 2,902 | 66.0 (69.9–93.2) | Valenciano et al., 2011 |
Vaccine efficacy studies for the 2010/2011 trivalent influenza vaccines based on mid-season data analysis (Table 3) show evidence of some protection against confirmed 2009 H1N1, however the VEs are lower than the monovalent vaccines administered in 2009/10 (44.1 to 52%) [58–60]. One study also reported that if individuals were vaccinated with monovalent vaccine in the 2009/2010 season, but not with seasonal vaccine in 2010/2011, the VE against current circulating H1N1 viruses was 34%, compared to 46% if individuals were only vaccinated in the 2010/11 season, and 63% if individuals were vaccinated with both monovalent vaccine in the 2009/2010 and the trivalent vaccine in 2010/11 [59]. This suggests that the longevity of protection is poor and that repeat vaccination boosted protective immune responses, reinforcing the recommendation for annual re-immunization.
Table 3.
Effectiveness of 2010/11 trivalent inactivated vaccines against confirmed 2009 H1N1 virus infection
| Country | Time of analysis | Number of ILI patients | Adjusted VE in preventing 2009 H1N1 (95% CI) | Reference |
|---|---|---|---|---|
| UK | September 2010–January 2011 | 3,480 | 46 (7–69) | Pebody et al., 2011 |
| Spain | 12 December 2010–12 February 2011 | 1,061 | 52 (6–75) | Savulescu et al., 2011 |
| France, Hungary, Ireland, Italy, Romania, Poland, Portugal, Spain | November 2010–January 2011 | 1,671 | 44.1 (14.3–72.7) | Kissling et al., 2011 |
Concluding remarks
The two major challenges to vaccination during the 2009 H1N1 pandemic were timing and availability of vaccine. The vaccines were well tolerated and surprisingly immunogenic, with inactivated vaccines containing 15μg of HA generally inducing antibody titers ≥1:40 in adults within 2 weeks of the administration of a single dose. This suggests that exposure to H1N1 viruses ‘primed’ the majority of the population, such that only one dose of 2009 H1N1 vaccine was needed for protection. Moreover, the use of an oil-in-water adjuvant permitted dose-reduction, with vaccines containing as little as 3.75 or 7.5μg HA being immunogenic. These factors enabled more vaccine doses to be distributed than was first expected. In future pandemics, we may not be so lucky, and priorities have been set for accelerating vaccine production, including a shift towards cell-based vaccines (Reviewed by [16]). Additionally, the use of adjuvanted vaccines may become more widespread in the future. Case-control studies demonstrated that monovalent 2009 H1N1 vaccines were effective in preventing infection with the 2009 H1N1 virus, but preliminary data suggests that it is important for individuals to be re-immunized annually.
Highlights.
We summarize lessons regarding 2009 H1N1 influenza vaccines
1 dose of inactivated vaccine (15μg HA) was well tolerated & immunogenic in adults
Oil-in-water adjuvanted vaccines licensed in Europe permitted dose- sparing
2009 H1N1 monovalent vaccines were effective in preventing 2009 H1N1 virus infection
Vaccination with 2009 H1N1 and 2010/11 vaccines boosted protective immunity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Palese P, Shaw M. Orthomyxoviridae: The Viruses and Their Replication. In: Knipe D, Griffin D, Lamb R, Straus S, Howley P, Martin M, Roizman B, editors. Fields Viroloy. Vol. 2 Wolters Kluwer: Lippincott Williams & Wilkins; 2007. pp. 1647–1689. [Google Scholar]
- 2.Wright P, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe D, Griffin D, Lamb R, Straus S, Howley P, Martin M, Roizman B, editors. Fields Viroloy. Vol. 2 Wolters Kluwer: Lippincott Williams & Wilkins; 2007. pp. 1691–1740. [Google Scholar]
- **3.Girard MP, Tam JS, Assossou OM, Kieny MP. The 2009 A (H1N1) influenza virus pandemic: A review. Vaccine. 28:4895–4902. doi: 10.1016/j.vaccine.2010.05.031. A comprehensive review article from the World Health Organization giving an overview of the 2009 H1N1 influenza pandemic. Etiology, epidemiology, clinical observations, and control efforts are discussed. [DOI] [PubMed] [Google Scholar]
- 4.Cox NJ, Subbarao K. Influenza. Lancet. 1999;354:1277–1282. doi: 10.1016/S0140-6736(99)01241-6. [DOI] [PubMed] [Google Scholar]
- 5.Nichol KL, Treanor JJ. Vaccines for seasonal and pandemic influenza. J Infect Dis. 2006;194 (Suppl 2):S111–118. doi: 10.1086/507544. [DOI] [PubMed] [Google Scholar]
- 6.Kilbourne ED. Future influenza vaccines and the use of genetic recombinants. Bull World Health Organ. 1969;41:643–645. [PMC free article] [PubMed] [Google Scholar]
- 7.Maassab HF. Adaptation and growth characteristics of influenza virus at 25 degrees c. Nature. 1967;213:612–614. doi: 10.1038/213612a0. [DOI] [PubMed] [Google Scholar]
- 8.World now at the start of 2009 influenza pandemic on World Wide Web URL. http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html
- 9.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 10.Garten RJ, Davis CT, Russell CA, Shu B, Lindstrom S, Balish A, Sessions WM, Xu X, Skepner E, Deyde V, et al. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325:197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, Ma SK, Cheung CL, Raghwani J, Bhatt S, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. doi: 10.1038/nature08182. [DOI] [PubMed] [Google Scholar]
- 12.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, et al. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 13.Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009;374:451–458. doi: 10.1016/S0140-6736(09)61304-0. [DOI] [PubMed] [Google Scholar]
- 14.Lapinsky SE. Critical illness as a result of influenza A/H1N1 infection in pregnancy. BMJ. 340:c1235. doi: 10.1136/bmj.c1235. [DOI] [PubMed] [Google Scholar]
- 15.Peiris JS, Poon LL, Guan Y. Emergence of a novel swine-origin influenza A virus (S-OIV) H1N1 virus in humans. J Clin Virol. 2009;45:169–173. doi: 10.1016/j.jcv.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 363:2036–2044. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 17.Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, Crowcroft N, Kwindt TL, Tang P, Charest H, Fonseca K, et al. Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ. 342:c7297. doi: 10.1136/bmj.c7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leung GM, Nicoll A. Reflections on pandemic (H1N1) 2009 and the international response. PLoS Med. :7. doi: 10.1371/journal.pmed.1000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prieto-Lara E, Llanos-Mendez A. Safety and immunogenicity of prepandemic H5N1 influenza vaccines: a systematic review of the literature. Vaccine. 28:4328–4334. doi: 10.1016/j.vaccine.2010.03.068. [DOI] [PubMed] [Google Scholar]
- 20.Boyer KM, Cherry JD, Wright PF, Lerman SJ, Gross PA, Foy HM, Taylor JW, Noble GR. Clinical reactions and serologic responses in healthy children aged six to 35 months after two-dose regimens of inactivated A/New Jersey/76 influenza virus vaccines. J Infect Dis. 1977;136 (Suppl):S579–583. doi: 10.1093/infdis/136.supplement_3.s579. [DOI] [PubMed] [Google Scholar]
- 21.Dolin R, Wise TG, Mazur MH, Tuazon CU, Ennis FA. Immunogenicity and reactogenicity of influenza A/New Jersey/76 virus vaccines in normal adults. J Infect Dis. 1977;136 (Suppl):S435–442. doi: 10.1093/infdis/136.supplement_3.s435. [DOI] [PubMed] [Google Scholar]
- 22.Gutman LT, Wilfert CM, Idriss ZH, Schmidt E, Andrews S, Katz SL. Single-dose trials of monovalent A/New Jersey/76 (Hsw1N1) influenza virus vaccine in children in Durham, North Carolina. J Infect Dis. 1977;136 (Suppl):S575–578. doi: 10.1093/infdis/136.supplement_3.s575. [DOI] [PubMed] [Google Scholar]
- 23.Levine MM, Hughes TP, Simon P, O’Donnell S, Grauel S, Levine SG. Monovalent inactivated A/New Jersey/8/76 (Hsw1N1) vaccine in healthy children aged three to five years. J Infect Dis. 1977;136 (Suppl):S571–574. doi: 10.1093/infdis/136.supplement_3.s571. [DOI] [PubMed] [Google Scholar]
- 24.Meiklejohn G, Eickhoff TC, Graves P. Antibody response of young adults to experimental influenza A/New Jersey/76 virus vaccines. J Infect Dis. 1977;136 (Suppl):S456–459. doi: 10.1093/infdis/136.supplement_3.s456. [DOI] [PubMed] [Google Scholar]
- 25.FDA; DHHS. Guidance for Industry Clinical Data Needed to Support the Licensure of Pandemic Influenza Vaccines. Rockville: 2007. pp. 1–18. [Google Scholar]
- 26.Hobson D, Curry RL, Beare AS, Ward-Gardner A. The role of serum haemagglutination-inhibiting antibody in protection against challenge infection with influenza A2 and B viruses. J Hyg (Lond) 1972;70:767–777. doi: 10.1017/s0022172400022610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Greenberg ME, Lai MH, Hartel GF, Wichems CH, Gittleson C, Bennet J, Dawson G, Hu W, Leggio C, Washington D, et al. Response to a monovalent 2009 influenza A (H1N1) vaccine. N Engl J Med. 2009;361:2405–2413. doi: 10.1056/NEJMoa0907413. The authors conducted a randomized, observer-blind, parallel-group trial evaluating two doses of an inactivated, split-virus 2009 pandemic H1N1 vaccine in healthy adults between the ages of 18 and 64 years at a single site in Australia. This is one of the first reports that a single 15-microgram dose of monovalent pandemic 2009 H1N1 vaccine was immunogenic in adults. [DOI] [PubMed] [Google Scholar]
- 28.Liang XF, Wang HQ, Wang JZ, Fang HH, Wu J, Zhu FC, Li RC, Xia SL, Zhao YL, Li FJ, et al. Safety and immunogenicity of 2009 pandemic influenza A H1N1 vaccines in China: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 375:56–66. doi: 10.1016/S0140-6736(09)62003-1. [DOI] [PubMed] [Google Scholar]
- 29.Plennevaux E, Sheldon E, Blatter M, Reeves-Hoche MK, Denis M. Immune response after a single vaccination against 2009 influenza A H1N1 in USA: a preliminary report of two randomised controlled phase 2 trials. Lancet. 375:41–48. doi: 10.1016/S0140-6736(09)62026-2. [DOI] [PubMed] [Google Scholar]
- 30.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, Zhang XF, Pan HX, Meng FY, Hu YM, et al. A novel influenza A (H1N1) vaccine in various age groups. N Engl J Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 31.Nolan T, McVernon J, Skeljo M, Richmond P, Wadia U, Lambert S, Nissen M, Marshall H, Booy R, Heron L, et al. Immunogenicity of a monovalent 2009 influenza A(H1N1) vaccine in infants and children: a randomized trial. JAMA. 303:37–46. doi: 10.1001/jama.2009.1911. [DOI] [PubMed] [Google Scholar]
- *32.Plennevaux E, Blatter M, Cornish MJ, Go K, Kirby D, Wali M, Reeves-Hoche MK, Denis M. Influenza A (H1N1) 2009 two-dose immunization of US children: an observer-blinded, randomized, placebo-controlled trial. Vaccine. 29:1569–1575. The authors conducted a randomized, observer-blinded, multicenter phase 2 study in the US assessing the safety and immunogenicity of 2 doses of a monovalent, inactivated, split-virion pandemic 2009 H1N1 influenza vaccine in children aged 6–35 months or 3–9 years. A second dose of vaccine was required to obtain antibody titers of ≥1:40 and seroconversion rates of 90–99% in both age groups. The vaccine elicited similar rates of solicited and unsolicited injection site and systemic reactions as the placebo. This is one of the first reports of monovalent pandemic H1N1 vaccine in children indicating that 2 doses should be recommended. [Google Scholar]
- 33.Chen GL, Lau YF, Lamirande EW, McCall AW, Subbarao K. Seasonal influenza infection and live vaccine prime for a response to the 2009 pandemic H1N1 vaccine. Proc Natl Acad Sci U S A. 108:1140–1145. doi: 10.1073/pnas.1009908108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leroux-Roels I, Bernhard R, Gerard P, Drame M, Hanon E, Leroux-Roels G. Broad Clade 2 cross-reactive immunity induced by an adjuvanted clade 1 rH5N1 pandemic influenza vaccine. PLoS One. 2008;3:e1665. doi: 10.1371/journal.pone.0001665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, Hons E, Devaster JM, Leroux-Roels G. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 36.Walker WT, Faust SN. Monovalent inactivated split-virion AS03-adjuvanted pandemic influenza A (H1N1) vaccine. Expert Rev Vaccines. 9:1385–1398. doi: 10.1586/erv.10.141. [DOI] [PubMed] [Google Scholar]
- *37.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. The authors conducted a single-center study in the UK in adults aged 18 to 50 years, to test the monovalent pandemic H1N1 surface-antigen vaccine with and without MF59 adjuvant. Both the geometric mean antibody titers and the percentage of individuals with antibody titers ≥1:40 were higher among subjects who received MF59-adjuvanted vaccine than among those who had received nonadjuvanted vaccine. This is one of the first reports comparing adjuvanted and non-adjuvanted monovalent pandemic H1N1 influenza vaccines and showed that adjuvants could have a dose-sparing effect. [DOI] [PubMed] [Google Scholar]
- 38.Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, Medina MJ, Nguyen-Van-Tam JS, Read RC, Warren FC, et al. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: a randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect Dis. 11:91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 39.Roman F, Vaman T, Kafeja F, Hanon E, Van Damme P. AS03(A)-Adjuvanted influenza A (H1N1) 2009 vaccine for adults up to 85 years of age. Clin Infect Dis. 51:668–677. doi: 10.1086/655830. [DOI] [PubMed] [Google Scholar]
- 40.Carmona A, Omenaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, Gillard P, Aristegui J. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine. 28:5837–5844. doi: 10.1016/j.vaccine.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 41.Esposito S, Pugni L, Daleno C, Ronchi A, Valzano A, Serra D, Mosca F, Principi N. Influenza A/H1N1 MF59-Adjuvanted Vaccine in Preterm and Term Children Aged 6 to 23 Months. Pediatrics. 127:e1161–1168. doi: 10.1542/peds.2010-1920. [DOI] [PubMed] [Google Scholar]
- 42.Waddington CS, Walker WT, Oeser C, Reiner A, John T, Wilkins S, Casey M, Eccleston PE, Allen RJ, Okike I, et al. Safety and immunogenicity of AS03B adjuvanted split virion versus non-adjuvanted whole virion H1N1 influenza vaccine in UK children aged 6 months-12 years: open label, randomised, parallel group, multicentre study. BMJ. 340:c2649. doi: 10.1136/bmj.c2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Butler D. Regulators face tough flu-jab choices. Nature. 2009;460:446. doi: 10.1038/460446a. [DOI] [PubMed] [Google Scholar]
- 44.Chen Z, Wang W, Zhou H, Suguitan AL, Jr, Shambaugh C, Kim L, Zhao J, Kemble G, Jin H. Generation of live attenuated novel influenza virus A/California/7/09 (H1N1) vaccines with high yield in embryonated chicken eggs. J Virol. 84:44–51. doi: 10.1128/JVI.02106-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *45.Mallory RM, Malkin E, Ambrose CS, Bellamy T, Shi L, Yi T, Jones T, Kemble G, Dubovsky F. Safety and immunogenicity following administration of a live, attenuated monovalent 2009 H1N1 influenza vaccine to children and adults in two randomized controlled trials. PLoS One. 5:e13755. doi: 10.1371/journal.pone.0013755. The authors conducted 2 randomized, double-blind, placebo-controlled studies in children aged 2–17 years and adults aged 18–49 years in order to evaluate the safety, tolerability, and immunogenicity of a monovalent intranasal pandemic H1N1 live attenuated influenza vaccine (LAIV). This is the first report of a pandemic H1N1 LAIV, which had a safety and immunogenicity profile similar to other previously studied and efficacious formulations of seasonal trivalent LAIV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Edwards KM, Dupont WD, Westrich MK, Plummer WD, Jr, Palmer PS, Wright PF. A randomized controlled trial of cold-adapted and inactivated vaccines for the prevention of influenza A disease. J Infect Dis. 1994;169:68–76. doi: 10.1093/infdis/169.1.68. [DOI] [PubMed] [Google Scholar]
- 47.Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine. 1999;18:899–906. doi: 10.1016/s0264-410x(99)00334-5. [DOI] [PubMed] [Google Scholar]
- 48.Vellozzi C, Broder KR, Haber P, Guh A, Nguyen M, Cano M, Lewis P, McNeil MM, Bryant M, Singleton J, et al. Adverse events following influenza A (H1N1) 2009 monovalent vaccines reported to the Vaccine Adverse Event Reporting System, United States, October 1, 2009–January 31, 2010. Vaccine. 28:7248–7255. doi: 10.1016/j.vaccine.2010.09.021. [DOI] [PubMed] [Google Scholar]
- 49.Dauvilliers Y, Montplaisir J, Cochen V, Desautels A, Einen M, Lin L, Kawashima M, Bayard S, Monaca C, Tiberge M, et al. Post-H1N1 narcolepsy-cataplexy. Sleep. 33:1428–1430. doi: 10.1093/sleep/33.11.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Montastruc JL, Durrieu G, Rascol O. Pandemrix degrees, (H1N1)v influenza and reported cases of narcolepsy. Vaccine. 2010;29 doi: 10.1016/j.vaccine.2010.12.092. [DOI] [PubMed] [Google Scholar]
- 51.Zarocostas J. WHO backs further probes into possible link between H1N1 vaccine and narcolepsy in children. BMJ. 342:d909. doi: 10.1136/bmj.d909. [DOI] [PubMed] [Google Scholar]
- 52.Tsai TF, Crucitti A, Nacci P, Nicolay U, Cioppa GD, Ferguson J, Clemens R. Explorations of clinical trials and pharmacovigilance databases of MF59((R))-adjuvanted influenza vaccines for associated cases of narcolepsy. Scand J Infect Dis. doi: 10.3109/00365548.2011.580777. [DOI] [PubMed] [Google Scholar]
- 53.Bryan P, Seabroke S. No increased risk of febrile convulsions after seasonal influenza immunisation in UK. Lancet. 377:904. doi: 10.1016/S0140-6736(11)60352-8. [DOI] [PubMed] [Google Scholar]
- *54.Valenciano M, Kissling E, Cohen JM, Oroszi B, Barret AS, Rizzo C, Nunes B, Pitigoi D, Larrauri Camara A, Mosnier A, et al. Estimates of pandemic influenza vaccine effectiveness in Europe, 2009–2010: results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) multicentre case-control study. PLoS Med. 8:e1000388. A multicenter case-control study based on sentinel practitioner surveillance networks from 7 European countries was undertaken to estimate the vaccine effectiveness (VE) of 2009–2010 pandemic and seasonal influenza vaccines. The adjusted pandemic H1N1 influenza VE was 66.0% (95% CI 23.9–84.8) and the adjusted 2009–2010 seasonal influenza VE was 9.9% (95% CI −65.2 to 50.9), suggesting good protection by pandemic monovalent vaccines against medically attended pandemic H1N1 influenza infection and no effect of the 2009–2010 seasonal influenza vaccine. [Google Scholar]
- 55.Hardelid P, Fleming DM, McMenamin J, Andrews N, Robertson C, SebastianPillai P, Ellis J, Carman W, Wreghitt T, Watson JM, et al. Effectiveness of pandemic and seasonal influenza vaccine in preventing pandemic influenza A(H1N1)2009 infection in England and Scotland 2009–2010. Euro Surveill. :16. [PubMed] [Google Scholar]
- 56.Song JY, Cheong HJ, Heo JY, Noh JY, Choi WS, Park DW, Lee J, Jeong HW, Kee SY, Kim WJ. Effectiveness of the pandemic influenza A/H1N1 2009 monovalent vaccine in Korea. Vaccine. 29:1395–1398. doi: 10.1016/j.vaccine.2010.12.050. [DOI] [PubMed] [Google Scholar]
- 57.Wu J, Xu F, Lu L, Lu M, Miao L, Gao T, Ji W, Suo L, Liu D, Ma R, et al. Safety and effectiveness of a 2009 H1N1 vaccine in Beijing. N Engl J Med. 363:2416–2423. doi: 10.1056/NEJMoa1006736. [DOI] [PubMed] [Google Scholar]
- 58.Kissling E, Valenciano M. Early estimates of seasonal influenza vaccine effectiveness in Europe, 2010/11: I-MOVE, a multicentre case-control study. Euro Surveill. :16. doi: 10.2807/ese.16.11.19818-en. [DOI] [PubMed] [Google Scholar]
- *59.Pebody R, Hardelid P, Fleming D, McMenamin J, Andrews N, Robertson C, Thomas D, Sebastianpillai P, Ellis J, Carman W, et al. Effectiveness of seasonal 2010/11 and pandemic influenza A(H1N1)2009 vaccines in preventing influenza infection in the United Kingdom: mid-season analysis 2010/11. Euro Surveill. :16. This was the first study to provide mid-season estimates of the effectiveness of the 2010/11 trivalent influenza vaccine and previous vaccination with the monovalent pandemic H1N1 vaccine in preventing confirmed pandemic influenza H1N1 infection in the UK in the 2010/11 season. The adjusted vaccine effectiveness (VE) was 34% (95% CI: −10 – 60%) if subjects were vaccinated only with monovalent vaccine in the 2009/10 season; 46% (95% CI: 7 – 69%) if vaccinated only with trivalent influenza vaccine in the 2010/11 season and 63% (95% CI: 37 – 78%) if vaccinated in both seasons. [Google Scholar]
- 60.Savulescu C, Jimenez-Jorge S, de Mateo S, Ledesma J, Pozo F, Casas I, Larrauri A. Effectiveness of the 2010/11 seasonal trivalent influenza vaccine in Spain: preliminary results of a case-control study. Euro Surveill. :16. doi: 10.2807/ese.16.11.19820-en. [DOI] [PubMed] [Google Scholar]