Abstract
A previously isolated parsley (Petroselinum crispum) cDNA with high sequence similarity to cinnamate 4-hydroxylase (C4H) cDNAs from several plant sources was expressed in yeast (Saccharomyces cerevisiae) containing a plant NADPH:cytochrome P450 oxidoreductase and verified as encoding a functional C4H (CYP73A10). Low genomic complexity and the occurrence of a single type of cDNA suggest the existence of only one C4H gene in parsley. The encoded mRNA and protein, in contrast to those of a functionally related NADPH:cytochrome P450 oxidoreductase, were strictly coregulated with phenylalanine ammonia-lyase mRNA and protein, respectively, as demonstrated by coinduction under various conditions and colocalization in situ in cross-sections from several different parsley tissues. These results support the hypothesis that the genes encoding the core reactions of phenylpropanoid metabolism form a tight regulatory unit.
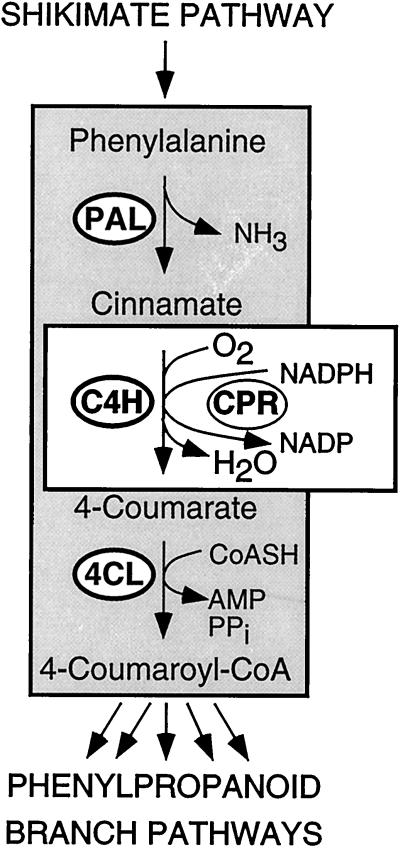
C4H (EC 1.14.13.11) constitutes the CYP73 family of the large group of Cyt P450 monooxygenases (Teutsch et al., 1993). It catalyzes the 4-hydroxylation of trans-cinnamate, the central step in the generation of Phe-derived substrates for the many branches of phenylpropanoid metabolism (Russell, 1971). The first and the last enzymes of this short sequence of closely related reactions, termed the general phenylpropanoid metabolism, are PAL (EC 4.3.1.5) and 4CL (EC 6.2.1.12), respectively (Hahlbrock and Scheel, 1989). A second metabolic link couples C4H to the membrane-localized CPR (EC 1.6.2.4; Durst and O'Keefe, 1995; Schuler, 1996). The resulting pivotal role of C4H at the interface between cytosolic phenylpropanoid pathways and membrane-localized electron-transfer reactions is schematically illustrated in Figure 1.
Figure 1.
Schematic diagram of the pivotal role of C4H as a functional link between the cytosolic enzymes of general phenylpropanoid metabolism, PAL and 4CL, and the membrane-associated electron-transfer reactions catalyzed by CPR.
The expression patterns of all three C4H-linked enzymes, PAL, 4CL, and CPR, and of the corresponding mRNAs have recently been analyzed in cell-suspension cultures and various intact tissues of parsley (Petroselinum crispum L.; Logemann et al., 1995; Reinold and Hahlbrock, 1996, 1997; Koopmann and Hahlbrock, 1997; Batz et al., 1998) and Arabidopsis (Bell-Lelong et al., 1997; Mizutani et al., 1997; Mizutani and Ohta, 1998). In our studies with parsley C4H was included to the extent that was possible, using a tentatively identified cDNA probe (Logemann et al., 1995; Koopmann and Hahlbrock, 1997; Batz et al., 1998). However, a thorough comparison required more detailed information concerning the genomic complexity of C4H in this plant, as well as the unequivocal verification of its biochemical function.
All previous results, although preliminary with respect to C4H, indicated a remarkable degree of coordination in the regulation of PAL, C4H, and 4CL at both the mRNA and protein levels (Logemann et al., 1995; Reinold and Hahlbrock, 1996, 1997). By contrast, CPR and C4H appeared to be less tightly coregulated (Koopmann and Hahlbrock, 1997), despite the essential requirement of C4H for CPR activity. Thus, the question arose as to whether C4H formed a closer regulatory unit with PAL and 4CL than with CPR, and whether it was as strictly coregulated with PAL and 4CL, as previously demonstrated for the latter two under a large variety of conditions. Here we show that this is the case, although each of the three coregulated enzymes appears to be encoded by a different number of genes.
MATERIALS AND METHODS
C4H Expression in Yeast
Primers for amplification of the C4H-coding sequence and introduction of the EcoRI and BamHI restriction sites were purchased from MWG Biotech (Ebersberg, Germany). PCR was performed using 100 ng of the template and 60 pmol of the primer in the following cycles: one reaction cycle for 5 min at 95°C and 30 cycles for 1 min at 95°C, 1 min at 50°C, and 2.5 min at 72°C. The product was sequenced and the EcoRI/BamHI fragment was cloned into the expression vector pYeDP60. The yeast (Saccharomyces cerevisiae) strains W(R) and W(At11) were transformed, the yeast cultures grown, and the microsomes prepared as described by Urban et al. (1994).
C4H Antiserum and Immunotitration
Primers for amplification of the C4H-coding sequence and introduction of the NcoI and BamHI restriction sites were from MWG Biotech and PCR was performed as described above. The PCR product was cloned into the expression vector pQE60 and the construct was used for transformation of Escherichia coli strain SG13009 containing the plasmid pUBS520. For a 100-mL culture grown at 37°C, an overnight culture of transformed cells was taken as inoculum (1:50). At an A600 of 0.8, 1 mm isopropylthiogalactopyranoside was added and the culture was grown for another 2 h.
Cells were harvested by centrifugation (2,500g for 10 min at 4°C). The pellet containing the recombinant protein in inclusion bodies was resuspended in 1 mL of lysis buffer (50 mm Tris-HCl, pH 8.0, 35% [w/v] Suc, and 1 mm EDTA), 200 μL of lysozyme (10 mg/mL lysis buffer) was added, and the solution was incubated for 30 min on ice. After 52 μL of DNase I (1 mg/mL lysis buffer), 20 mm MgCl2, and 2 mm MnCl2 were added, the solution was again incubated for 30 min on ice and then vigorously mixed with 4 to 6 mL of detergent buffer (20 mm Tris-HCl, pH 7.5, 200 mm NaCl, 1% [w/v] desoxycholate, 1% [w/v] Nonidet P40, 2 mm EDTA, and 10 mm mercaptoethanol) and centrifuged (15,000g for 10 min at 4°C). The pellet was washed several times with Triton buffer (20 mm Tris-HCl, pH 7.5, 0.5% [v/v] Triton X-100, 1 mm EDTA, and 10 mm mercaptoethanol) and centrifuged as above until the pellet appeared white.
To remove the detergent, the pellet was washed in Tris buffer (50 mm Tris-HCl, pH 7.5, and 10 mm mercaptoethanol), centrifuged, dissolved again in Tris buffer, frozen in liquid nitrogen, and stored at −80°C until use. This preparation of purified inclusion bodies was dissolved in buffer (8 m urea, 0.1 m sodium phosphate, and 10 mm Tris-HCl, pH 8.0), and the protein was purified further on a Ni-nitrilotriacetic acid column (Qiagen, Düsseldorf, Germany) according to the manufacturer's protocol. The mixture was then injected into a rabbit (4 × 60 μg) following the immunization procedure of Eurogentec (Seraing, Belgium). The final blood collection after 12 weeks was used as the C4H antiserum.
For immunoinhibition of C4H activity, 11 μg of microsomal protein from cultured parsley cells that had been treated for 15 h with fungal elicitor (Ayers et al., 1976; Kombrink and Hahlbrock, 1986) was incubated with increasing amounts of purified IgGs from the C4H antiserum or from the preimmune serum.
C4H Activity Assay
Microsomes from cultured parsley cells were prepared and C4H activity was determined as described by Vetter et al. (1992). The assay mixture contained 0.5 to 15 μg of microsomal protein from parsley or yeast cells, 50 mm potassium phosphate, pH 7.0, 2 mm DTT, 10 mm Glc-6-P, 0.5 unit of Glc-6-P dehydrogenase, and 20.8 μm [3-14C]cinnamate (53.6 mCi/mmol, Isotopchim, Ganagobie, France) in a total volume of 50 μL. The reaction was started by adding 0.5 mm NADPH and stopped with 5 μL of 4 n HCl after incubation for 7.5 min at 30°C. Tracer (1 μL of an ethanolic solution containing 5 mg/mL each of cinnamate and 4-coumarate) was added, and the mixture was extracted twice with 100 μL of ethylacetate. The organic phase was evaporated, the residue dissolved in 10 μL of ethylacetate and spotted on a silica F60 TLC plate (Merck, Darmstadt, Germany), and the chromatogram developed in diethylether:petrol ether (30°C–50°C):formic acid (70:30:1, v/v). Radioactivity was visualized and quantified using a phosphor imager (Molecular Dynamics, Krefeld, Germany).
In Situ mRNA and Protein Localization
All methods used for plant propagation, including inoculation of leaf buds with zoospores of Phytophthora sojae, tissue sectioning and fixation, probe generation, in situ RNA/RNA hybridization, immunolocalization of proteins, and the PAL probes used, have recently been described (Reinold and Hahlbrock, 1996, 1997). Sense and antisense C4H riboprobes were generated using the SnaBI/SpeI cDNA fragment cloned into the pBluescript vector.
Analytical Methods
Proteins were separated by SDS-PAGE (Laemmli, 1970) and transferred onto PVDF membranes (Millipore) according to the method of Towbin et al. (1979). C4H and PAL antisera were used at a dilution of 1:1000 in 5% (w/v) dry milk powder in TBS.
RESULTS
Functional Identification
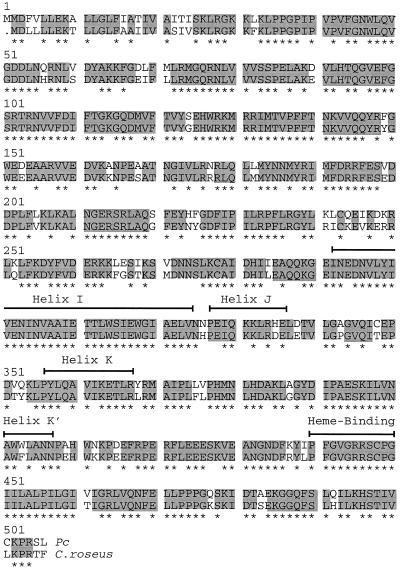
Several characteristic features, including >80% sequence identity with putative or functionally identified C4H proteins from other plants, were previously used as a basis for the tentative assignment of function to a parsley cDNA obtained by heterologous screening (Logemann et al., 1995). Among the most striking features (Fig. 2) is the combined occurrence of a putative ER membrane anchor at the N terminus (Nelson and Strobel, 1988), a Pro-rich region that may be responsible for correctly orienting the protein in the membrane (Szczesna-Skorupa et al., 1993; Yamazaki et al., 1993), the helical domains I, J, K, and K′, and the heme-binding motif PFGXGRRXCXG near the C terminus (Durst and Nelson, 1995).
Figure 2.
Comparison of deduced amino acid sequences for C4H from parsley (Pc) and a previously reported C4H from periwinkle (Hotze et al., 1995). Identical amino acids are marked in gray and putative helical and heme-binding domains (Durst and Nelson, 1995) are indicated by brackets. Asterisks highlight sequence identity among all functionally established C4H. The GAP program from the Genetics Computer Group package (Madison, WI) was used with the gap-weight penalty set at 3.0 and the gap-length penalty set at 0.1.
To verify the deduced function of the encoded protein, we expressed the cDNA in two different yeast strains. One strain, W(R), contained the endogenous CPR gene under the control of a Gal-inducible promotor. The other strain, W(At11), used the same promotor, but the endogenous CPR gene was replaced by the CPR gene ATR1 from Arabidopsis (Urban et al., 1997). Both strains, when transformed with the expression vector pYeDP60 containing the parsley cDNA, exhibited high C4H activity upon CPR induction by Gal. The specific C4H activity in microsomal fractions from these strains (1 μkat/mg protein) was 50-fold higher than that obtained with analogous preparations from elicitor-treated parsley cells (20 nkat/mg protein), probably at least in part because of the high induced level of CPR activity (approximately 5-fold higher in Gal-stimulated yeast cells than in elicitor-stimulated parsley cells; Koopmann and Hahlbrock, 1997). Yeast cells transformed with the vector alone showed no detectable C4H activity.
HPLC analysis confirmed the exclusive formation of 4-coumarate from cinnamate using microsomal fractions from the transformed yeast cells. Neither 2- nor 3-coumarate was detectable. An apparent Km value of 5 μm for cinnamate was in agreement with similar values reported for C4H from other plant sources (Gabriac et al., 1991; Mizutani et al., 1993) or from transformed yeast (Urban et al., 1994). The pH optimum was found to be slightly lower (7.0) than usual (7.5). The preference of parsley C4H for the trans-isomer of cinnamic acid has previously been demonstrated (Pfändler et al., 1977). An antiserum raised against the E. coli-expressed and purified C4H protein (Koopmann and Hahlbrock, 1997) inhibited the C4H activity of parsley microsomes by 70%, in contrast to the completely ineffective preimmune serum (data not shown).
Genomic Complexity and Definition of Probes
Similar to the results reported for Arabidopsis (Bell-Lelong et al., 1997; Mizutani et al., 1997) and pea (Frank et al., 1996), gel-blot analysis with genomic parsley DNA and 3′ or 5′ fragments from the C4H cDNA for hybridization gave very simple patterns. Because complete genomic clones were difficult to obtain and were thus not available for direct comparison of these patterns with defined fragments, we analyzed further the cDNA library from which the original clone had been isolated. The 18 additional cDNA clones hybridizing under moderately stringent conditions gave the same restriction fragmentation patterns, except for differences in length, and 12 were at least partially sequenced and found to be identical to the original cDNA by this criterion.
Although these results do not exclude with certainty the presence of an additional C4H gene(s) in parsley, they strongly suggest the existence of only one gene, in contrast to the existence and expression of four PAL genes (Logemann et al., 1995), two 4CL genes (Douglas et al., 1987), and at least two CPR genes (Koopmann and Hahlbrock, 1997). For the following comparative studies, a PAL-1 cDNA (Logemann et al., 1995) and a PAL-1 antiserum (Appert et al., 1994) were used, both of which detected all four PAL isoforms. For C4H mRNA analysis, a hybridization probe lacking the putative N-terminal membrane anchor and the highly conserved heme-binding domain was used to prevent cross-hybridization with mRNAs encoding other P450 proteins. The C4H antiserum has been described elsewhere (Koopmann and Hahlbrock, 1997).
mRNA Accumulation upon Fungal Infection
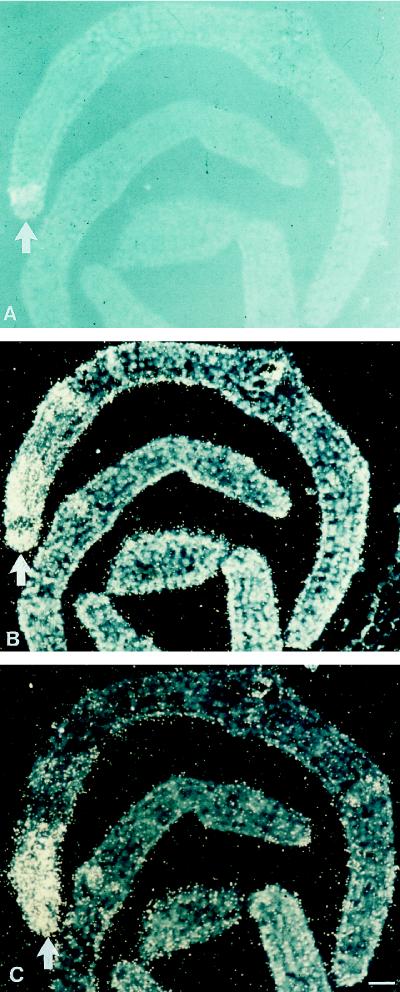
Rapid and transient accumulation of C4H mRNA in response to treatments of leaves or cultured cells of parsley with various external stimuli, including fungal elicitor, UV-containing white light, or wounding, was previously reported (Logemann et al., 1995). However, the cDNA probe used in those studies proved unsuitable for in situ mRNA localization under the stringency conditions required for hybridization in fixed tissue slices. By using the probe that lacked frequently occurring domain structures, we were able to apply this technique and used it first to test the induction of C4H mRNA by fungal attack in cross-sections of parsley leaf buds. Figure 3, A and B, demonstrates the strong accumulation of C4H mRNA around a fungal infection site, with spatial distribution patterns identical to those of PAL mRNA (Fig. 3C).
Figure 3.
In situ localization of C4H and PAL mRNAs in parsley leaf buds 6 h postinoculation with P. sojae. The fungal infection site (A, arrow) was visualized by autofluorescence of the developing necrotic spot under UV/blue light of 365 nm. The same and an adjacent cross-section were hybridized with 35S-labeled C4H (B) and PAL (C) antisense riboprobes, respectively. Magnification bar = 100 μm.
Attempts to immunolocalize the encoded C4H protein in parallel tissue sections were unsuccessful, probably because of the high preexisting level in uninfected tissue. Similar, although surmountable, difficulties had previously been encountered with PAL and 4CL protein accumulation around infection sites (Reinold and Hahlbrock, 1996).
Cell-Type-Specific Expression Patterns
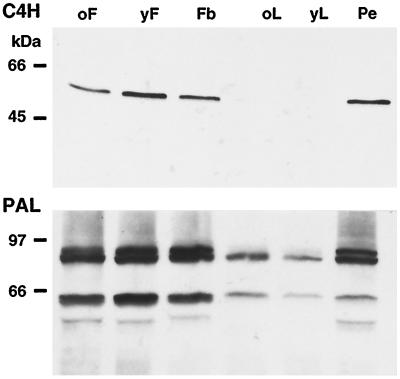
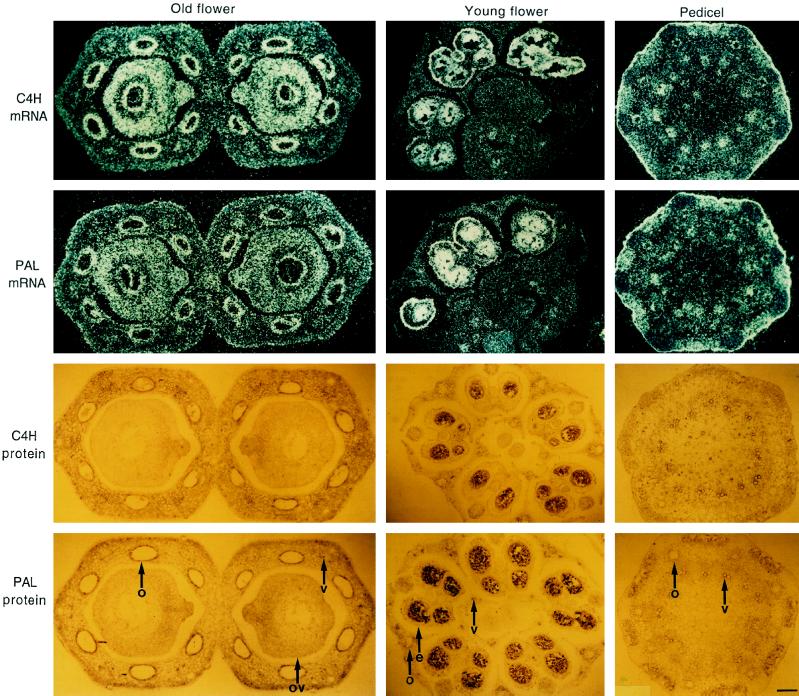
When protein extracts from three selected organs of parsley plants (flower, leaf, and pedicel) at various developmental stages were used, immunoblot analysis indicated similar overall expression patterns for C4H and PAL (Fig. 4). Both were strongly expressed in the pedicel and at all flower stages analyzed, whereas young and old leaves contained relatively small amounts of PAL protein, and C4H protein was not detectable under these conditions. Based on these and recently reported results of the tissue localization of PAL and 4CL (Reinold and Hahlbrock, 1997), we decided to use the pedicel and two different flower stages for in situ C4H mRNA and protein localization. Again, PAL was used as a reference.
Figure 4.
Comparison of total extractable C4H and PAL levels in various aerial parts of parsley plants. Protein blots on PVDF membranes (Millipore) after SDS-PAGE (10 μg of protein each, 10% polyacrylamide) were treated with C4H or PAL antiserum at a dilution of 1:1000. oF, Old flower; yF, young flower; Fb, flower bud; oL, old leaf; yL, young leaf; and Pe, pedicel.
The results revealed three major features of C4H expression (Fig. 5). First, C4H mRNA and protein occurred fairly ubiquitously throughout the tissue areas analyzed, although with distinct patterns. Second, among the most prominent accumulation sites were those known for high phenylpropanoid biosynthetic activity: epidermal and oil-duct epithelial cells, vascular tissue, and major parts of the ovule (Reinold and Hahlbrock, 1997). Third, the mRNA and protein-distribution patterns were identical within experimental error for C4H and PAL. Control experiments with sense RNA or preimmunserum as the probes gave low background levels in all cases, similar to those observed for PAL and 4CL under the same conditions as used here (Reinold and Hahlbrock, 1997).
Figure 5.
In situ localization of C4H and PAL mRNA (top) and protein (bottom), respectively, in cross-sections from selected parsley tissues as indicated. 35S-Labeled antisense riboprobes and antisera were the same as described in Figures 3 and 4, respectively. e, Epidermis; o, oil-duct epithelial cells; ov, ovary; and v, vascular tissue. Magnification bar = 100 μm.
In analogous experiments, C4H was also compared with CPR1, the CPR isoform with an apparent close metabolic relationship to C4H (Koopmann and Hahlbrock, 1997). However, CPR1 mRNA and protein showed much lower cell-type specificity than C4H in various tissues from adult parsley plants (data not shown), in contrast to the previously reported, massive accumulation of both C4H and CPR1 mRNAs in the same tissue area at fungal infection sites in primary leaf buds (Koopmann and Hahlbrock, 1997).
DISCUSSION
These results have clarified three related points: (a) the previous, tentative identification of the parsley C4H cDNA by sequence comparison was confirmed by functional data in vitro; (b) in contrast to all three associated enzymes (PAL, 4CL, and CPR), C4H appears to be encoded by a single gene; and (c) expression of this gene was regulated in tight coordination with the PAL gene family, strongly supporting its function in vivo as the predicted metabolic link between PAL and 4CL (Fig. 1).
Sequence identity of 80% or more with a functionally identified ortholog is often taken as a sufficiently strong criterion for the assignment of function to a newly isolated gene, cDNA, or protein. Although this has frequently proven to be a reliable approximation, several cases of considerable uncertainty exist. For instance, many of the numerous, structurally closely related proteins within the Cyt P450 family have widely differing functional properties, and even the exchange of only three amino acid residues can lead to a drastic change in substrate specificity (Lindberg and Negishi, 1989). At the opposite extreme, P450 proteins with only 26% sequence identity may catalyze the same biochemical reaction (Ma et al., 1994). Therefore, an important step toward the functional identification of the putative parsley C4H cDNA is the demonstration in vitro of its exclusive hydroxylation of cinnamate in the para position of the aromatic ring to give 4-coumarate.
A prerequisite for the second line of evidence supporting a specific role in phenylpropanoid metabolism was an analysis of the genomic complexity. Although an unequivocal determination of the number of genes encoding a particular enzyme in a given organism is very difficult to achieve, our results are taken as a strong indication that a single C4H gene encoded the mRNA and protein analyzed, in contrast, for example, to corn, in which at least three C4H genes seem to exist (Potter et al., 1995). This result enabled us to determine their cell-type-specific distribution patterns in vivo without interference from structurally similar gene products, the functional relatedness of which would have remained uncertain. However, we cannot definitely exclude the existence of functionally related but structurally dissimilar proteins. This latter possibility could be tested using only defined mutants. The fact that no null mutant for C4H has been reported may be taken as a hint of the functional uniqueness of this enzyme.
Both the C4H mRNA and the C4H protein occurred under all tested conditions in various parsley tissues in a highly coordinated fashion with PAL mRNA and protein. This high degree of coordination was observed not only here for the spatial distribution patterns (Figs. 4 and 5), but also in a previous study for the temporal patterns of accumulation (Logemann et al., 1995). Since analogous results have been obtained previously for PAL and 4CL (Logemann et al., 1995; Reinold and Hahlbrock, 1996, 1997), we conclude that all genes or gene families encoding the small set of three closely related enzymes of general phenylpropanoid metabolism (Fig. 1) form a tight regulatory unit. Surprisingly, this unit does not include the likewise closely related CPR1, whose mRNA accumulation patterns in parsley differ from those of C4H (Koopmann and Hahlbrock, 1997). CPR mRNA and protein were uniformly distributed throughout the tissue and appear to be more generally involved in various metabolic activities than is observed for C4H, with its apparent specific involvement in general phenylpropanoid metabolism.
These results strengthen further the hypothesis (Batz et al., 1998) that the genes or gene families encoding PAL, C4H, and 4CL, despite their different sizes, constitute an exceptional case of tightly linked regulation, possibly mediated through an unusually large structural and functional similarity of their promotors (Hahlbrock et al., 1995; Logemann et al., 1995; Bell-Lelong et al., 1997; Mizutani et al., 1997). This tight regulatory link at the gene expression level, coupled with large similarities in the apparent mRNA and protein turnover rates (Hahlbrock et al., 1976, 1981; Logemann et al., 1995), may indicate a likewise tight association at the enzyme activity level, perhaps including the formation of a true multienzyme complex. Experimental results that point in this direction come not only from previous studies (Czichi and Kindl, 1977; Stafford, 1981; Hrazdina and Wagner, 1985) but also from recent experiments in our own laboratory (E. Koopmann, unpublished results). In such a complex, C4H could serve as both a structural and metabolic anchor of cytosolic activities to the ER.
ACKNOWLEDGMENTS
We thank Daniele Werck-Reichhart, Francis Durst, and Jean-Louis Arnault for their kind help with the functional expression of C4H, Susanne Reinold for the introduction to in situ hybridization, and Paul Rushton for valuable comments concerning the manuscript.
Abbreviations:
- C4H
cinnamate 4-hydroxylase
- 4CL
4-coumarate:CoA ligase
- CPR
NADPH:Cyt P450 oxidoreductase
- PAL
Phe ammonia-lyase
LITERATURE CITED
- Appert C, Logemann E, Hahlbrock K, Schmid J, Amrhein N. Structural and catalytic properties of the four phenylalanine ammonia-lyase isoenzymes from parsley (Petroselinum crispum Nym.) Eur J Biochem. 1994;225:491–499. doi: 10.1111/j.1432-1033.1994.00491.x. [DOI] [PubMed] [Google Scholar]
- Ayers AR, Ebel J, Valent B, Albersheim P. Host-pathogen interactions. X. Fractionation and biological activity of an elicitor isolated from the mycelial walls of Phythophtora megasperma var. sojae. Plant Physiol. 1976;57:760–765. doi: 10.1104/pp.57.5.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batz O, Logemann E, Reinold S, Hahlbrock K. Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. Biol Chem. 1998;379:1127–1135. doi: 10.1515/bchm.1998.379.8-9.1127. [DOI] [PubMed] [Google Scholar]
- Bell-Lelong DA, Cusumano JC, Meyer K, Chapple C. Cinnamate-4-hydroxylase expression in Arabidopsis. Regulation in response to development and the environment. Plant Physiol. 1997;113:729–738. doi: 10.1104/pp.113.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czichi U, Kindl H. Phenylalanine ammonia-lyase and cinnamic acid hydroxylase as assembled consecutive enzymes on microsomal membranes of cucumber cotelydons. Cooperation and subcellular distribution. Planta. 1977;134:133–143. doi: 10.1007/BF00384962. [DOI] [PubMed] [Google Scholar]
- Douglas C, Hoffmann H, Schulz W, Hahlbrock K. Structure and elicitor- or UV-light-stimulated expression of two 4-coumarate coenzyme A ligase genes in parsley. EMBO J. 1987;6:1189–1196. doi: 10.1002/j.1460-2075.1987.tb02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F, Nelson DR. Diversity and evolution of plant P450 and P450-reductases. Drug Metab Drug Interact. 1995;12:189–206. doi: 10.1515/dmdi.1995.12.3-4.189. [DOI] [PubMed] [Google Scholar]
- Durst F, O'Keefe DP. Plant cytochromes P450: an overview. Drug Metab Drug Interact. 1995;12:171–187. doi: 10.1515/dmdi.1995.12.3-4.171. [DOI] [PubMed] [Google Scholar]
- Frank MR, Deyneka JM, Schuler MA. Cloning of wound-induced cytochrome P450 monooxygenases expressed in pea. Plant Physiol. 1996;110:1035–1046. doi: 10.1104/pp.110.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriac B, Werck-Reichhart D, Teutsch H, Durst F. Purification and immunocharacterization of a plant cytochrome P450: the cinnamic acid 4-hydroxylase. Arch Biochem Biophys. 1991;288:302–309. doi: 10.1016/0003-9861(91)90199-s. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Knobloch KH, Kreuzaler F, Potts JRM, Wellmann E. Coordinated induction and subsequent activity changes of two groups of metabolically interrelated systems. Light-induced synthesis of flavonoid glycosides in cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1976;61:199–206. doi: 10.1111/j.1432-1033.1976.tb10012.x. [DOI] [PubMed] [Google Scholar]
- Hahlbrock K, Lamb CL, Purwin C, Ebel J, Fautz E, Schäfer E. Rapid response of suspension-cultured parsley cells to the elicitor from Phytophthora megasperma var. sojae. Plant Physiol. 1981;67:768–773. doi: 10.1104/pp.67.4.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahlbrock K, Scheel D. Physiology and molecular biology of the phenolpropanoid metabolism. Annu Rev Plant Physiol Mol Biol. 1989;40:347–369. [Google Scholar]
- Hahlbrock K, Scheel D, Logemann E, Nuernberger T, Parniske M, Reinold S, Sacks WR, Schmelzer E. Oligopeptide elicitor-mediated defense gene activation in cultured parsley cells. Proc Natl Acad Sci USA. 1995;92:4150–4157. doi: 10.1073/pnas.92.10.4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotze M, Schroeder G, Schroeder J. Cinnamate 4-hydroxylase from Catharanthus roseus, and a strategy for the functional expression of plant cytochrome P450 proteins as translational fusions with P450 reductase in Escherichia coli. FEBS Lett. 1995;374:345–350. doi: 10.1016/0014-5793(95)01141-z. [DOI] [PubMed] [Google Scholar]
- Hrazdina G, Wagner G. Metabolic pathways as enzyme complexes. Evidence for the synthesis of phenylpropanoids and flavonoids on membrane associated enzyme complexes. Arch Biochem Biophys. 1985;237:88–100. doi: 10.1016/0003-9861(85)90257-7. [DOI] [PubMed] [Google Scholar]
- Kombrink E, Hahlbrock K. Responses of cultured parsley (Petroselinum crispum) cells to elicitors from phytopathogenic fungi. Timing and dose dependency of elicitor-induced reactions. Plant Physiol. 1986;81:216–221. doi: 10.1104/pp.81.1.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann E, Hahlbrock K. Differential regulation of NADPH:cytochrome P450 oxidoreductases in parsley. Proc Natl Acad Sci USA. 1997;94:14954–14959. doi: 10.1073/pnas.94.26.14954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindberg RL, Negishi M. Alteration of mouse cytochrome P450coh substrate specificity by mutation of a single amino-acid residue. Nature. 1989;339:632–634. doi: 10.1038/339632a0. [DOI] [PubMed] [Google Scholar]
- Logemann E, Parniske M, Hahlbrock K. Modes of expression and common structural features of the complete phenylalanine ammonia-lyase gene family in parsley. Proc Natl Acad Sci USA. 1995;92:5905–5909. doi: 10.1073/pnas.92.13.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, Cohen MB, Berenbaum MR, Schuler MA. Black swallowtail (Papilio polyxenes) alleles encode cytochrome P450s that selectively metabolize linear furanocoumarins. Arch Biochem Biophys. 1994;310:332–340. doi: 10.1006/abbi.1994.1175. [DOI] [PubMed] [Google Scholar]
- Mizutani M, Ohta D. Two isoforms of NADPH:cytochrome P450 reductase in Arabidopsis thaliana. Plant Physiol. 1998;116:357–367. doi: 10.1104/pp.116.1.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani M, Ohta D, Sato R. Purification and characterization of a cytochrome P450 (trans-cinnamic acid 4-hydroxylase) from etiolated mung bean seedlings. Plant Cell Physiol. 1993;34:481–488. [Google Scholar]
- Mizutani M, Ohta D, Sato R. Isolation of a cDNA and a genomic clone encoding cinnamate 4-hydroxylase from Arabidopsis and its expression manner in planta. Plant Physiol. 1997;113:755–763. doi: 10.1104/pp.113.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DR, Strobel HW. On the membrane topology of vertebrate cytochrome P450 proteins. J Biol Chem. 1988;263:6038–6050. [PubMed] [Google Scholar]
- Pfändler R, Scheel D, Sandermann H, Grisebach H. Stereospecificity of plant microsomal cinnamic acid 4-hydroxylase. Arch Biochem Biophys. 1977;178:315–316. doi: 10.1016/0003-9861(77)90197-7. [DOI] [PubMed] [Google Scholar]
- Potter S, Moreland DE, Kreuz K, Ward E. Induction of cytochrome P450 genes by ethanol in maize. Drug Metab Drug Interact. 1995;12:317–327. doi: 10.1515/dmdi.1995.12.3-4.317. [DOI] [PubMed] [Google Scholar]
- Reinold S, Hahlbrock K. Biphasic temporal and spatial induction patterns of defense-related mRNAs and proteins in fungus-infected parsley leaves. Plant Physiol. 1996;112:131–140. doi: 10.1104/pp.112.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinold S, Hahlbrock K. In situ localization of phenylpropanoid biosynthetic mRNAs and proteins in parsley (Petroselinum crispum) Bot Acta. 1997;110:431–443. [Google Scholar]
- Russell DW. The metabolism of aromatic compounds in higher plants. Properties of cinnamic acid 4-hydroxylase of pea seedlings and some aspects of its metabolic and developmental control. J Biol Chem. 1971;246:3870–3778. [PubMed] [Google Scholar]
- Schuler MA. Plant cytochrome P450 monooxygenases. Crit Rev Plant Sci. 1996;15:235–284. [Google Scholar]
- Stafford HA (1981) Phenylalanine ammonia-lyase. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants. Academic Press, New York, pp 117–137
- Szczesna-Skorupa E, Straub P, Kemper B. Deletion of a conserved tetrapeptide, PPGP, in P450 2C2 results in a loss of enzymatic activity without a change in cellular localization. Arch Biochem Biophys. 1993;304:170–175. doi: 10.1006/abbi.1993.1335. [DOI] [PubMed] [Google Scholar]
- Teutsch HG, Hasenfratz M-P, Lesot A, Stoltz C, Garnier J-M, Jeltsch J-M, Durst F, Werck-Reichhart D. Isolation and sequence of a cDNA encoding the Jerusalem artichoke cinnamate-4-hydroxylase, a major plant cytochrome P450 involved in the general phenylpropanoid pathway. Proc Natl Acad Sci USA. 1993;90:4102–4107. doi: 10.1073/pnas.90.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P, Mignotte C, Kazmaier M, Delorme F, Pompon D. Cloning, yeast expression, and characterization of the coupling of two distantly related Arabidopsis thaliana NADPH-cytochrome P450 reductases with P450 CYP73A5. J Biol Chem. 1997;272:19176–19186. doi: 10.1074/jbc.272.31.19176. [DOI] [PubMed] [Google Scholar]
- Urban P, Werck-Reichhart D, Teutsch HG, Durst F, Regnier S, Kazmaier M, Pompon D. Characterization of recombinant plant cinnamate 4-hydroxylase produced in yeast. Kinetic and spectral properties of the major plant P450 of the phenylpropanoid pathway. Eur J Biochem. 1994;222:843–850. doi: 10.1111/j.1432-1033.1994.tb18931.x. [DOI] [PubMed] [Google Scholar]
- Vetter H-P, Mangold U, Schröder G, Marner F-J, Werck-Reichhart D, Schröder J. Molecular analysis and heterologous expression of an inducible cytochrome P-450 protein from periwinkle (Catharanthus roseus L.) Plant Physiol. 1992;100:998–1007. doi: 10.1104/pp.100.2.998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Sato K, Suhara K, Sakaguchi M, Mihara K, Omura T. Importance of a proline-rich region following signal-anchor sequence in the formation of correct conformation of microsomal cytochrome P-450s. J Biochem. 1993;114:652–657. doi: 10.1093/oxfordjournals.jbchem.a124232. [DOI] [PubMed] [Google Scholar]