Abstract
Background. No previous randomized controlled studies have been reported examining de novo, once every 4 weeks (Q4W) administration of erythropoiesis-stimulating agents in chronic kidney disease (CKD) patients. We report results from a randomized multinational study that compared continuous erythropoietin receptor activator (C.E.R.A.) Q4W with darbepoetin alfa once weekly (QW) or every 2 weeks (Q2W) for the correction of anaemia in non-dialysis CKD patients.
Methods. Patients were randomized (1:1) to receive either 1.2 μg/kg C.E.R.A. Q4W or darbepoetin alfa QW/Q2W during a 20-week correction period and an 8-week evaluation period. Two primary end points were assessed: the haemoglobin (Hb) response rate and the change in average Hb concentration between baseline and evaluation.
Results. The Hb response rate for C.E.R.A. was 94.1%, significantly higher than the protocol-specified 60% response rate [95% confidence interval (CI): 89.1, 97.3; P < 0.0001] and comparable with darbepoetin alfa (93.5%; 95% CI: 88.4, 96.8; P < 0.0001). C.E.R.A. Q4W was non-inferior to darbepoetin alfa QW/Q2W, with similar mean Hb changes from baseline of 1.62 g/dL and 1.66 g/dL, respectively. Patients receiving C.E.R.A. showed a steady rise in Hb, with fewer patients above the target range during the first 8 weeks compared with darbepoetin alfa [39 patients (25.8%) versus 72 patients (47.7%); P < 0.0001]. Adverse event rates were comparable between the treatment groups.
Conclusion. C.E.R.A. Q4W successfully corrects anaemia and maintains stable Hb levels within the recommended target range in non-dialysis CKD patients.
Keywords: anaemia, C.E.R.A., chronic kidney disease, darbepoetin alfa, haemoglobin
Introduction
The development of erythropoiesis-stimulating agents (ESAs) has been beneficial in treating the anaemia of chronic kidney disease (CKD) by maintaining haemoglobin (Hb) levels, so reducing the need for blood transfusions and improving quality of life [1, 2]. Improvements in anaemia management have been the subject of clinical trials with new agents and treatment regimens which attempt to demonstrate potential advantages to patients and health care providers without compromising efficacy or safety, particularly in regard to Hb levels and Hb increase over time. An ideal agent would provide a smooth correction in Hb levels and then maintain Hb within the target range at a reduced dose frequency.
Methoxy polyethylene glycol-epoetin beta (MIRCERA®; F. Hoffmann-La Roche, Basel, Switzerland) is a continuous erythropoietin receptor activator (C.E.R.A.) with pharmacological properties distinct from other currently available ESAs [3–6]. The efficacy of C.E.R.A. in correcting anaemia [as therapy every 2 weeks (Q2W)] and maintaining Hb levels [as therapy every 4 weeks (Q4W)] has been established in a series of trials, with data from the extension phase of the administration of C.E.R.A. in CKD patients to treat anaemia with a Q2W schedule (ARCTOS) study demonstrating that C.E.R.A. Q4W maintained stable Hb levels within the recommended range for up to 52 weeks in CKD patients not on dialysis, with efficacy and safety comparable with that of darbepoetin alfa administered once weekly (QW) or Q2W [7, 8].
The correction of renal anaemia in CKD patients with subcutaneous therapy (CORDATUS) study is the first randomized study to compare the efficacy and safety of de novo C.E.R.A. Q4W and darbepoetin alfa QW or Q2W for the correction of anaemia in ESA-naive patients with CKD not on dialysis.
Materials and methods
Study participants
The CORDATUS study was an open-label, randomized, controlled, multicentre, parallel-group study in patients with CKD not on dialysis. ESA-naive adults (≥18 years) with CKD Stage 3 or 4 and an estimated glomerular filtration rate of 15–59 mL/min/1.73 m2, with a baseline Hb concentration <10.5 g/dL and adequate iron status defined as serum ferritin ≥100 μg/L or transferrin saturation (TSAT) ≥20% or <10% hypochromic red blood cells (RBCs), were screened for up to 2 weeks before being randomized into two treatment groups. Exclusion criteria included previous treatment with any ESA within 12 weeks before the screening period, overt gastrointestinal bleeding or RBC transfusions within 8 weeks before or during screening, a non-renal cause of anaemia, likelihood of early withdrawal or life expectancy of <12 months.
The study was approved by local ethics committees and carried out according to the Declaration of Helsinki and local regulations. All patients provided written, informed consent before the start of the study.
Procedures
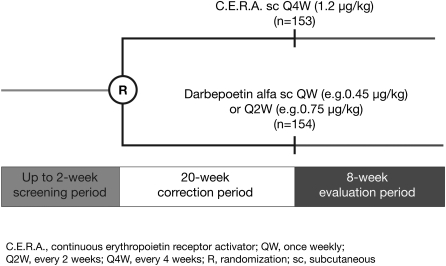
Eligible patients were randomized (1:1) following the screening period to receive a starting dose of 1.2 μg/kg subcutaneous C.E.R.A. Q4W or darbepoetin alfa according to product labelling specifications (e.g. 0.45 μg/kg QW or 0.75 μg/kg Q2W), over a 20-week correction period, followed by an 8-week evaluation period (Figure 1). Patients randomized to the darbepoetin alfa group were treated at the QW or Q2W interval at the investigator's discretion.
Fig. 1.
Study design.
The C.E.R.A. dose was adjusted by ∼25% during the correction phase until the response level (an Hb increase of ≥1 g/dL above the baseline and at a value ≥10 g/dL) was reached. Subsequent to anaemia correction, the Hb levels were to be maintained within 1 g/dL of the response Hb value and in the range of 10–12 g/dL by implementing a ∼25% dose increase or decrease. If Hb levels exceeded 12 g/dL, treatment was temporarily interrupted in both treatment arms. When Hb levels fell <12 g/dL, treatment with C.E.R.A. was restarted at a dose ∼25% below that previously administered. Darbepoetin alfa treatment was adjusted according to approved labelling and to achieve the same Hb targets. Dosing intervals remained unchanged throughout the study, and dose adjustments were made during scheduled visits, no more frequently than Q4W and were based on the most current Hb value measured within 9 days prior to the planned dose administration.
In cases of iron deficiency (serum ferritin <100 μg/L, TSAT <20% or hypochromic RBCs ≥10%), the protocol specified that iron supplementation should be administered orally or intravenously during the screening and treatment periods according to centre practice and should be discontinued in patients who had serum ferritin levels>800 μg/L or TSAT >50%.
Study outcomes
The two primary end points of this study were the response rate until the end of the evaluation period and the group difference in the mean change in Hb concentration between the baseline and evaluation periods.
The secondary end points of this study were: the changes in Hb values over time; the time to Hb response assessed by Kaplan–Meier methods; the percentage of patients with at least one Hb value>12 g/dL in the first 8 weeks of the study; the proportion of patients who achieved a stable response; the total number of dose adjustments associated with stable response and the incidence of RBC transfusions.
Assessments
All patients were scheduled for assessments Q2W. Hb concentrations and vital signs were measured at each study visit, while samples for iron parameters, proteinuria, intact parathyroid hormone and a panel of standard laboratory parameters were collected five times during the 28-week study. Serum creatinine and C-reactive protein levels were determined four times during the study. Anti-erythropoietin antibody testing was carried out at baseline, Week 17 and the final visit. Iron administration and RBC transfusions were recorded throughout the study, as were adverse events (AEs).
Statistical analyses
The first primary efficacy end point evaluated Hb response rate, defined as an increase in Hb ≥1 g/dL compared with baseline and an Hb concentration ≥10 g/dL. The Clopper–Pearson confidence limits were used for analysis of response to demonstrate that the lower limit of the confidence interval (CI) was >60%. Baseline Hb concentration was estimated by a time-adjusted mean of all values recorded between the day the first study dose was administered and the previous 20 days. The Hb value on the day of the first dose was included in the baseline calculation, as this assessment was performed before administration of the first dose. To correct for any increase in Hb caused by RBC transfusions, values measured within 3 weeks after a transfusion were replaced by the Hb value measured immediately before the transfusion.
The second primary efficacy end point evaluated the group difference in the mean change in Hb levels between the baseline and evaluation periods (Weeks 21–29). Using an analysis of covariance by treatment, a two-sided 95% CI was calculated for the mean difference between groups, and treatment with C.E.R.A. was regarded as non-inferior to treatment with darbepoetin alfa if the lower limit of the CI was greater than −0.75 g/dL, with a t-test used to calculate the P-value.
To detect both primary end points with 90% power in all analysis populations [the intent-to-treat (ITT) and per protocol populations], 150 patients were required. For the analysis of response, at least 126 patients were required in each arm to demonstrate a response rate of ≥60% with a 90% power, assuming a true response rate of 75%. For the non-inferiority test, 105 patients per arm were required to show that the lower limit of the 95% CI was greater than −0.75 g/dL, on the assumption of 90% power, a two-sided significance level of 5% and a true difference of no >0.3 g/dL between the two arms.
The safety population included all randomized patients who received at least one dose of C.E.R.A. or darbepoetin alfa. Group summary statistics were recorded for all safety parameters. Frequencies and incidence rates of AEs were tabulated by treatment group and calculated on a per patient basis. All vital sign measurements and laboratory data were assessed for clinically relevant abnormalities and any changes from baseline.
Results
Study population
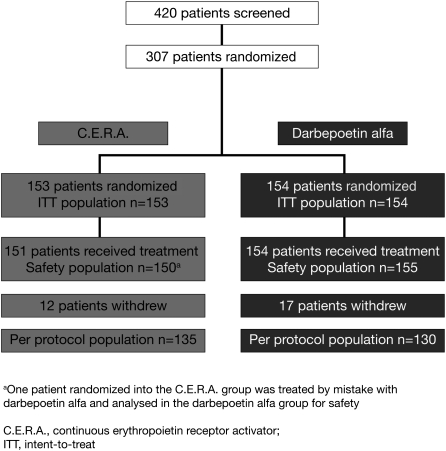
A total of 420 patients from 64 centres in 16 countries were screened, of whom 307 were randomized into two treatment groups (153 patients to C.E.R.A. and 154 patients to darbepoetin alfa, 60 at the QW interval and 94 at the Q2W interval) (Figure 2). Of these, 305 patients received at least one dose of the study drug, while two patients in the C.E.R.A. group were randomized but did not receive study medication as consent was withdrawn before the first dose of the study drug was administered. One other patient assigned to C.E.R.A. was administered darbepoetin alfa in error and maintained this treatment throughout the study. Twenty-nine patients withdrew from the study; 12 (8%) in the C.E.R.A. arm and 17 (11%) in the darbepoetin alfa arm. Six patients (4%) in the C.E.R.A. group and nine patients (6%) in the darbepoetin alfa group withdrew for safety reasons. In each treatment group, two patients withdrew due to an AE. In the C.E.R.A. group, one patient reported ulcerative keratitis and one patient reported pregnancy. In the darbepoetin alfa group, one patient reported recurrent lymphocytic leukaemia and one patient reported metastatic renal cancer. Eleven patients ceased participation in the study due to death; four in the C.E.R.A. group and seven in the darbepoetin alfa group.
Fig. 2.
Patient disposition.
Fourteen patients withdrew due to non-safety-related reasons, which included refusal of treatment, lack of co-operation and withdrawal of consent.
Patient baseline characteristics and risk factors for vascular events and haemorrhage were well balanced across both treatment groups (Table 1). The most frequent aetiologies of CKD in patients receiving C.E.R.A. or darbepoetin alfa in this study were: diabetes (46 and 45%), hypertension/large vessel disease (49 and 41%), glomerulonephritis (12 and 10%) and interstitial nephritis/pyelonephritis (13 and 8%). Multiple attributions were possible.
Table 1.
Baseline patient characteristics and risk factorsa
| C.E.R.A., n = 153 | Darbepoetin alfa, n = 154 | |
| Male, n (%) | 67 (44) | 67 (44) |
| Race, n (%) | ||
| Caucasian | 92 (60) | 97 (63) |
| Oriental | 61 (40) | 57 (37) |
| Mean (SD) age, year | 65.4 (14.3) | 67.4 (13.4) |
| Mean (SD) weight, kg | 69.6 (15.3) | 70.0 (16.8) |
| Mean (SD) Hb, g/dL | 9.53 (0.74) | 9.53 (0.65) |
| Mean (SD) GFR, mL/min/1.73 m2 | 27.5 (14.1) | 27.1 (12.8) |
| Median (IQR) ferritin, μg/L | 186 (118–331) | 207 (130–336) |
| Median (IQR) TSAT, % | 24.0 (19.0–31.5) | 23.8 (18.3–30.4) |
| Risk factors for vascular events and haemorrhage, n (%) | ||
| Arterial hypertension | 150 (98) | 147 (95) |
| Hyperlipidaemia | 106 (69) | 107 (69) |
| Diabetes | 86 (56) | 81 (53) |
| Ischaemic heart disease | 43 (28) | 48 (31) |
| Congestive heart failure | 25 (16) | 23 (15) |
GFR, glomerular filtration rate; IQR, interquartile range.
Efficacy
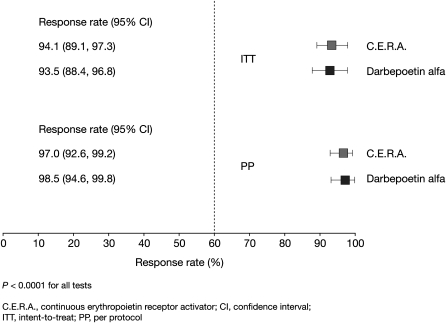
The Hb response rate for C.E.R.A. was statistically significant >60% in the ITT population, and rates were comparable between C.E.R.A. and darbepoetin alfa: 144/153 responders [94.1% (95% CI: 89.1, 97.3)] versus 144/154 responders [93.5% (95% CI: 88.4, 96.8)]. Similar findings were observed in the per protocol population, confirming results from the ITT population (Figure 3).
Fig. 3.
Hb response rate in the ITT and per protocol populations.
The second primary end point analysis demonstrated a between-group treatment difference of −0.036 g/dL (1.62 g/dL in the C.E.R.A. arm versus 1.66 g/dL in the darbepoetin alfa arm) in Hb change from baseline to evaluation period in the ITT population. The lower limit of the 95% CI for the group difference was −0.25 g/dL, which was above the protocol-specified non-inferiority limit of −0.75, demonstrating that C.E.R.A. was statistically non-inferior to darbepoetin alfa (P < 0.0001). Analysis in the per protocol population confirmed these results.
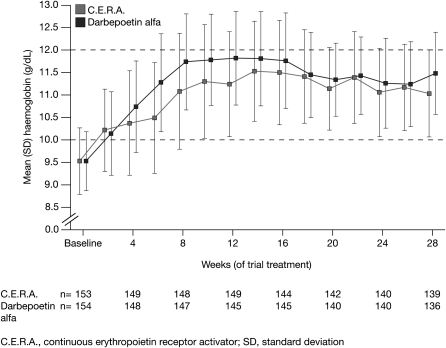
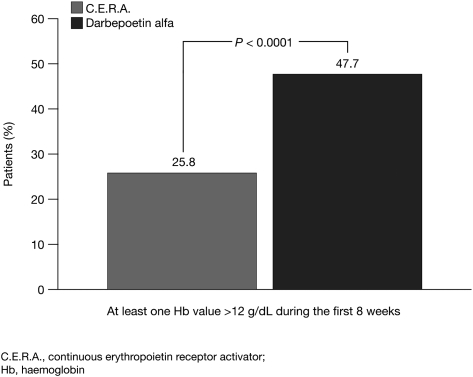
Analysis of the secondary end points of the study showed that the patients treated with C.E.R.A. had a controlled rise in Hb concentrations (Figure 4), with a mean increase of 0.20 g/dL/week during the initial 8 weeks of the study compared with 0.27 g/dL/week for patients in the darbepoetin alfa group. The median time to Hb response was 43 days in the C.E.R.A. group and 29 days in the darbepoetin alfa group, reflecting the more gradual increase in Hb concentrations associated with C.E.R.A. Fewer patients treated with C.E.R.A. had Hb values exceeding 12 g/dL in the first 8 weeks of treatment compared with those receiving darbepoetin alfa [39 patients (25.8%) versus 72 patients (47.7%), respectively; P < 0.0001] (Figure 5).
Fig. 4.
Hb increase over time in the ITT population.
Fig. 5.
Percentage of patients with Hb levels >12 g/dL.
Stable response (defined as ≥75% of the scheduled Hb values within the range 10–12 and ≥1 g/dL from baseline for any 8-week time period, regardless of the requirement for dose adjustment) was found in 105 patients (68.6%) treated with C.E.R.A. compared with 112 patients (72.7%) treated with darbepoetin alfa (not significant). The total number of patients requiring any dose change to achieve a stable Hb response under this definition was similar in the C.E.R.A. and darbepoetin alfa treatment groups [65 patients (61.9%) versus 64 patients (57.1%); not significant]. The mean number of dose changes per patient associated with stable response was very similar between treatment groups (1.12 for C.E.R.A. and 1.10 for darbepoetin alfa). Multiple dose adjustments (≥5 adjustments) were required by fewer patients treated with C.E.R.A. compared with those receiving darbepoetin alfa during the study (10.7 versus 21.3%, respectively). Correspondingly, the change in median dose from baseline to evaluation with C.E.R.A. was very small compared with the dose change in patients treated with darbepoetin alfa (6.6 versus 35.6%, respectively) (Figure 6).
Fig. 6.
Comparison of median equivalent 4-week study drug dose over time.
Ten patients (6.5%) in the darbepoetin alfa group received one or more transfusions compared with five patients (3.3%) in the C.E.R.A. group (not significant). Reasons given for transfusions were similar in the two treatment groups. Patients in the C.E.R.A. treatment arm received RBC transfusions in association with anaemia of unknown aetiology, renal neoplasm, surgery for intestinal perforation and concurrent infections or inflammations. Patients in the darbepoetin alfa treatment arm received RBC transfusions associated with anaemia of unknown aetiology, haemorrhage, infections, surgery, renal neoplasm, anaemia of malignant disease and acute cardiac failure.
Safety
Of 307 patients randomized, 305 received at least one dose of the study medication (150 patients in the C.E.R.A. arm and 155 patients in the darbepoetin alfa arm). In general, both treatments were well tolerated. Most AEs experienced by patients in each study arm were reported to be mild to moderate in intensity, and rates were comparable between the two groups. One hundred and nine patients (72.7%) in the C.E.R.A. arm experienced AEs compared with 123 patients (79.4%) in the darbepoetin alfa arm; this difference was not statistically significant. The most frequently reported AEs in the study for C.E.R.A. and darbepoetin alfa were hypertension (16.0 versus 23.9%), renal impairment (6.0 versus 10.3%), hyperkalaemia (8.7 versus 5.2%), upper respiratory tract infection (6.0 versus 7.1%) and constipation (3.3 versus 7.7%) (Table 2). Ten patients (6.7%) in the C.E.R.A. group and 17 patients (11.0%) in the darbepoetin alfa group experienced at least one AE that was assessed as treatment related by the investigator. Of these, the most common AE was hypertension, experienced by 4.7% of patients treated with C.E.R.A. and 10.3% of patients treated with darbepoetin alfa.
Table 2.
Frequently reported AEs (>5% in either group)
| C.E.R.A., n = 150 (%) | Darbepoetin alfa, n = 155 (%) | |
| Hypertension | 24 (16.0) | 37 (23.9) |
| Renal impairment | 9 (6.0) | 16 (10.3) |
| Hyperkalaemia | 13 (8.7) | 8 (5.2) |
| Upper respiratory tract infection | 9 (6.0) | 11 (7.1) |
| Constipation | 5 (3.3) | 12 (7.7) |
| Diarrhoea | 10 (6.7) | 6 (3.9) |
| Urinary tract infection | 6 (4.0) | 8 (5.2) |
| Hypotension | 5 (3.3) | 8 (5.2) |
| Nasopharyngitis | 5 (3.3) | 8 (5.2) |
| Pneumonia | 2 (1.3) | 8 (5.2) |
Numerically, more patients in the darbepoetin alfa arm experienced serious AEs compared with patients in the C.E.R.A. arm (12 versus 6%, respectively). No patient had a serious AE that was judged to be treatment related. Eleven deaths [four patients (3%) in the C.E.R.A. group and seven patients (5%) in the darbepoetin alfa group] were reported during the study, none of which was considered to be related to the treatment. No single cause of death was reported in more than one case in either treatment arm. No antibodies to study drugs were detected.
Discussion
Approximately 50% of CKD patients not on dialysis are anaemic [9]. Treatment with short-acting ESAs in this population may require frequent dosing via subcutaneous injections, which can affect patient compliance. The extended dosing interval associated with C.E.R.A. reduces the number of injections, while decreased injection site pain has been demonstrated in healthy subjects [10]. All of these characteristics may help to improve anaemia management in this population.
This study demonstrates that C.E.R.A. Q4W administered subcutaneously was as effective as darbepoetin alfa given more frequently in the correction of anaemia in ESA-naive patients with CKD not on dialysis. Both primary end points of the study were met, as patients in the C.E.R.A. Q4W arm exhibited a 94.1% response rate and statistically verified non-inferiority to darbepoetin alfa in Hb change over time. Patients in the C.E.R.A. Q4W group exhibited a steady increase in Hb concentrations, with a median time to response of 43 days. As a result of the slower Hb increase, fewer patients in the C.E.R.A. Q4W group had Hb values that exceeded 12 g/dL, the upper limit of the target range, while median C.E.R.A. doses changed very slightly over time and fewer patients receiving C.E.R.A. required multiple dose adjustments. It is possible that these differences result from the starting dose of darbepoetin alfa specified by the approved prescribing information. Nonetheless, the slight change in the C.E.R.A. dose and the limited requirement for dose adjustment over the 28-week study period suggest that the starting dose of 1.2 μg/kg/Q4W is appropriate in this clinical setting. However, the initial dosing of darbepoetin alfa to correct anaemia may need to be revised in the current more conservative climate of anaemia management.
C.E.R.A. was well tolerated; the most common AEs reported in both treatment groups were characteristic of the population under study and included hypertension, renal impairment, hyperkalaemia, upper respiratory tract infection and constipation. The safety data from this study confirmed the results from a recently published pooled analysis investigating the safety profile of C.E.R.A. compared with other ESAs in patients with CKD [11].
The results from this study add to published data from the C.E.R.A. phase III studies, which have shown that C.E.R.A. Q4W can effectively maintain Hb levels within the target range in CKD patients on dialysis and those not on dialysis without compromising efficacy or safety [7, 12, 13]. Another large, randomized study has shown that significantly more dialysis patients treated with once-monthly C.E.R.A. maintained Hb levels within the target range than those treated with darbepoetin alfa at the same dosing interval [14]. The data from the current study support the use of C.E.R.A. Q4W to correct anaemia and then maintain Hb levels in patients with CKD not on dialysis through a simplified, convenient and practical dosing regimen with benefits for both patients and healthcare providers. These studies demonstrate that C.E.R.A. is the only ESA proven to manage anaemia with a Q4W schedule in patients with all stages of CKD.
In conclusion, the CORDATUS study is the first clinical trial to prove that C.E.R.A. Q4W successfully corrects anaemia in CKD patients not on dialysis, with fewer dose changes and with a safety profile comparable to that of darbepoetin alfa administered according to approved labelling.
Acknowledgments
Contributions: S.D.R. is the lead author and was responsible for the final draft and submission of the paper. U.B. and F.C.D. contributed to the study concept, design, implementation, management and data analysis. All steering committee members contributed to the study design. All authors had full access to the data and participated in reviewing and interpreting the data and in drafting and reviewing the paper. Editorial support was provided by Tanya Chaudry from Complete HealthVizion, UK, supported by F. Hoffmann-La Roche Ltd. Primary responsibility for opinions, conclusions and interpretation of data lies with the authors. All authors read and approved the final version of this paper.
Role of funding source: This study was sponsored by F. Hoffmann-La Roche Ltd, Basel, Switzerland. U.B. and F.C.D., who are employees of F. Hoffmann-La Roche Ltd, provided clinical and statistical analysis of the data and the study report. Members of an independent steering committee provided input into the study design and interpretation of the results and reviewed the final manuscript for submission.
Transparency declarations: S.D.R. has been a scientific advisor for and has received honoraria from Amgen, F. Hoffmann-La Roche, Sandoz and Vifor. F.L. has served as an advisor for Affymax, Amgen, Jansen-Cilag and F. Hoffmann-La Roche and is a member of a Sandoz and Vifor safety committee. M.L. has received research funding from F. Hoffmann-La Roche. S.W.T. has received research funding from Novartis, Amgen and Merck and has been a member of speaker bureaux for Abbott, AstraZeneca, BMS, Merck, Pfizer and Sanofi-Aventis. T.A.G. has received honoraria from F. Hoffmann-La Roche and has served on a MIRCERA data and safety monitoring board for another study. A.M.-C. has been a scientific advisor for Abbott, Amgen, Genzyme, Shire and F. Hoffmann-La Roche and has received honoraria from Abbott, Amgen, Genzyme, Novartis and F. Hoffmann-La Roche. R.P. has served on advisory boards for F. Hoffmann-La Roche, Affymax and Fibrogen. U.B. and F.C.D. are employees of F. Hoffmann-La Roche. R.P.W., P.R., H.Y.L., M.N., A.L. and K.-D.W. declare that they have no conflict of interest.
CORDATUS study investigators: Alberto Albertazzi, Sydney Ben-Shitrit, Waclaw Bentkowski, Kazimier Ciechanowski, Marie Colomina, Olga Costero, Alexander Dreval, Vladimir Dobronravov, Magdalena Durlik, Valentine Ermolenko, Mariano Feriano, Danilo Fliser, Nuria Areste Fosaba, Julio E Marco Franco, Ulrich Frei, José Luis Gorriz, Dimitrios Grekas, Audrey Gurevich, Suchart Jenkriangkrai, Chong Myung Kang, Hyoung Kyu Kim, Chagriya Kitiyakara, Marian Klinger, Elena Kolmakova, Alice Pik Shang Kong, Udom Krairittichai, Miroslaw Kroczak, Agnes Ladanyi, Maurice Laville, Major Lajos, Francesco Locatelli, Ho Yung Lee, Bart Maes, Alberto Martínez-Castelao, Marta Molnar, Michal Nowicki, David Packham, José Portoles Perez, Natalia Petrova, Vincent Pichette, Michael Rambausek, Maria A Riscos-Guerrero, Enrique Andres Rives, Simon Roger, Prajej Ruangkanchanasetr, Boleslaw Rutkowski, Shing-Chung Siu, Marina Shestakova, Gregory Shostka, Kuo Hsiung Shu, Antoni Sokalski, Suchai Sritippayawan, Ioannis Stefanidis, Wladyslaw Sulowicz, Sheldon Tobe, Supachai Thitiarchakul, Bruno Van Vlem, Giuseppe Villa, Rowan Walker, Andrzej Wiecek, Rainer Woitas, Kwan-Dun Wu, Chun-Yip Yeung and Marianna Zsom.
Conflict of interest statement. None declared.
References
- 1.Eschbach JW, Abdulhadi MH, Browne JK, et al. Recombinant human erythropoietin in anemic patients with end-stage renal disease. Results of a phase III multicenter clinical trial. Ann Intern Med. 1989;111:992–1000. doi: 10.7326/0003-4819-111-12-992. [DOI] [PubMed] [Google Scholar]
- 2.Lefebvre P, Vekeman F, Sarokhan B, et al. Relationship between hemoglobin level and quality of life in anemic patients with chronic kidney disease receiving epoetin alfa. Curr Med Res Opin. 2006;22:1929–1937. doi: 10.1185/030079906X132541. [DOI] [PubMed] [Google Scholar]
- 3.Halstenson CE, Macres M, Katz SA, et al. Comparative pharmacokinetics and pharmacodynamics of epoetin alfa and epoetin beta. Clin Pharmacol Ther. 1991;50:702–712. doi: 10.1038/clpt.1991.210. [DOI] [PubMed] [Google Scholar]
- 4.Jarsch M, Brandt M, Lanzendörfer M, et al. Comparative erythropoietin receptor binding kinetics of C.E.R.A. and epoetin-beta determined by surface plasmon resonance and competition binding assay. Pharmacology. 2008;81:63–69. doi: 10.1159/000109166. [DOI] [PubMed] [Google Scholar]
- 5.Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–2395. doi: 10.1681/ASN.V10112392. [DOI] [PubMed] [Google Scholar]
- 6.Macdougall IC, Robson R, Opatrna S, et al. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215. doi: 10.2215/CJN.00730306. [DOI] [PubMed] [Google Scholar]
- 7.Kessler M, Martínez-Castelao A, Siamopoulos KC, et al. C.E.R.A. once every 4 weeks in patients with chronic kidney disease not on dialysis: The ARCTOS extension study. Hemodial Int. 2009;14:233–239. doi: 10.1111/j.1542-4758.2009.00421.x. [DOI] [PubMed] [Google Scholar]
- 8.Macdougall IC, Walker R, Provenzano R, et al. C.E.R.A. corrects anemia in patients with chronic kidney disease not on dialysis: results of a randomized clinical trial. Clin J Am Soc Nephrol. 2008;3:337–347. doi: 10.2215/CJN.00480107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McClellan W, Aronoff SL, Bolton WK, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20:1501–1510. doi: 10.1185/030079904X2763. [DOI] [PubMed] [Google Scholar]
- 10.Pannier A, Jordan P, Dougherty FC, et al. Subcutaneous injection pain with C.E.R.A., a continuous erythropoietin receptor activator, compared with darbepoetin alfa. Curr Med Res Opin. 2007;23:3025–3032. doi: 10.1185/030079907X242700. [DOI] [PubMed] [Google Scholar]
- 11.Locatelli F, Mann JFE, Aldigier J-C, et al. C.E.R.A. safety profile: a pooled analysis in patients with chronic kidney disease. Clin Nephrol. 2010;73:94–103. doi: 10.5414/cnp73094. [DOI] [PubMed] [Google Scholar]
- 12.Levin NW, Fishbane S, Valdés Cañedo F, et al. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA) Lancet. 2007;370:1415–1421. doi: 10.1016/S0140-6736(07)61599-2. [DOI] [PubMed] [Google Scholar]
- 13.Sulowicz W, Locatelli F, Ryckelynck J-P, et al. Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007;2:637–646. doi: 10.2215/CJN.03631006. [DOI] [PubMed] [Google Scholar]
- 14.Carrera F, Lok CE, de Francisco A, et al. Maintenance treatment of renal anaemia in haemodialysis patients with methoxy polyethylene glycol-epoetin beta versus darbepoetin alfa administered monthly: a randomized comparative trial. Nephrol Dial Transplant. 2010;25:4009–4017. doi: 10.1093/ndt/gfq305. [DOI] [PMC free article] [PubMed] [Google Scholar]