Abstract
γδ T cells function between the innate and adaptive immune responses, promoting antigen-presenting cell function, and manifesting cytolytic activity. Their numbers often increase during infections, such as HIV, and at sites of chronic inflammation. However, the turnover dynamics of human γδ T cells are poorly understood. Here we find that despite more rapid proliferation in vitro by human Lyme arthritis synovial γδ T cells of the Vδ1 subset, they have reduced surviving cell numbers compared to αβ T cells due to increased cell death by the γδ T cells. Because caspases are involved in cell proliferation and death, and signaling is more efficient through TCR-γδ than TCR-αβ, we examined the levels of active caspases during cell cycling and following TCR restimulation. We observed higher overall caspase activity in Borrelia-reactive γδ T cells than comparable αβ T cells. This was paralleled by greater spontaneous cell death and TCR restimulation-induced cell death of the γδ T cells, which was caspase dependent. Our current findings thus are consistent with a model where human γδ T cells evolved to function quickly and transiently, in an innate fashion.
Keywords: caspase, apoptosis, T lymphocytes, Lyme disease, cell growth
1. Introduction
The role of γδ T cells in human immune responses remains poorly understood. Previous studies have suggested that γδ T cells link the innate and adaptive immune responses by promoting the function of antigen-presenting cells, potentially presenting antigen themselves, rapidly producing inflammatory cytokines, and manifesting cytolytic activity [1-3]. Although they account for only 1-5% of the total T cells in peripheral blood and lymphoid tissues, γδ T cell numbers increase during certain infections such as HIV [4, 5]. Furthermore, γδ T cells rapidly migrate to and accumulate at sites of chronic inflammation such as the synovium in rheumatoid arthritis [6-9] and Lyme arthritis [10], the intestine in celiac disease [11], and the lung in sarcoidosis [12, 13]. In vivo turnover studies of murine γδ T cells using BrdU labeling have shown that γδ T cells have a high rate of proliferation and subsequent turnover [14]. However, a potential explanation for this high turnover has not been provided.
Because caspases are involved in both cell proliferation and death, we considered the possibility that the level of caspase activity may contribute to the turnover of γδ T cells. Caspases are cysteine aspartic acid proteases that are crucial for the initiation (e.g. caspase-8, -9, -10) and execution (e.g. caspase-3, -6, -7) of apoptosis [15] through cleavage of death substrates, such as the BH3 domain-only protein BID by caspase-8 [16] and inhibitor of caspase-activated deoxyribonuclease (ICAD) by caspase-3 [17, 18]. It has become appreciated more recently that caspase activity, specifically caspase-8, is also required for T cell growth [19-21], and that the level of active caspases within cells may be a key determinant of survival or death [22, 23]. We have previously observed that murine αβ T cells bearing high levels of caspase activity manifest increased rates of both cell growth and cell death [24]. However, the amount of caspase activity in human γδ versus αβ T cells has not been studied.
We therefore examined the turnover of Borrelia-reactive human γδ T cells compared with αβ T cells during in vitro culture using γδ T cell clones of the Vδ1 subset that were previously derived from synovial fluid of Lyme arthritis patients [10]. Here, we show that Borrelia-reactive human γδ T cell clones have a high rate of both proliferation and cell death, consistent with studies of murine γδ T cell turnover [14]. This paralleled greater overall caspase activity in Borrelia-reactive human γδ T cell clones as well as primary synovial γδ T cells than Borrelia-reactive αβ T cells. Thus, high ambient levels of caspase activity may prime γδ T cells for death signals, and contribute to their high turnover.
2. Materials and Methods
2.1. Culture of αβ and γδ T cell clones
All studies were conducted with approval from the Institutional Human Studies Committee and synovial fluid samples were obtained with written informed consent by patients prior to inclusion in the study. Cultures of previously derived Borrelia-reactive γδ T cell clones [10] and αβ T cell clones [25] were expanded as previously described [3, 10, 25, 26] at approximately 21-day intervals using 10 μg/ml of a sonicate of B. burgdorferi, strain N40, grown in Barbour-Stoenner-Kelly II (BSKII) medium (Sigma, St. Louis, MO) or 1 μg/ml phytohemagglutinin (PHA) (Murex Biotech, Dartford, Kent, England). Clones were cultured in AIM-V (Invitrogen, Carlsbad, CA) containing 10% Hyclone characterized FBS (ThermoScientific, Rockford, IL) and 100 U/ml human recombinant IL-2 (Cetus, Emeryville, CA).
2.2. Proliferation assay by 3H-thymidine incorporation
Triplicates of 3×105 cells/well in 96 well plates were pulsed with 1 μCi/well 3H-thymidine (GE Lifesciences, Piscataway, NJ) and incubated for 18 h at 37° C. Cells were then lysed and DNA retained on nitrocellulose filters using Tomtec Harvester 96 (Tomtec, Hamden, CT). 3H-thymidine was subsequently counted using a Microbeta counter (PerkinElmer, Shelton, CT).
2.3. Antibodies, TUNEL, and flow cytometry
Antibodies used were to the following determinants: TCR-αβ (BMA 031; Invitrogen/BioLegend, San Diego, CA), TCR-γδ (5A6.E9; Invitrogen/BioLegend), CD25 (CD25-3G10; Invitrogen), Fas (DX2; BD Pharmingen, San Jose, CA/Invitrogen/BioLegend), and CD3 (HIT3a; BioLegend). Anti-CD3 antibody (TR66, gift of Dr. Antonio Lanzavecchia) at a final concentration of 10 μg/ml was used for short-term stimulation experiments. Cells were treated with DMSO (Sigma, St. Louis, MO) or benzyloxycarbonyl-valine-alanine-aspartic acid-fluoromethylketone (z-VAD-fmk; zVAD) (50μM) (MP Biomedicals, Solon, OH) for 30 min prior to anti-CD3 stimulation for caspase inhibition studies. To assess the extent of apoptosis, DNA strand breaks were measured by TUNEL staining. Briefly, single-cell suspensions were treated with or without plate-bound anti-CD3 for 8 h, and then washed twice with cold PBS. Cells were then stained with surface antibodies and fixed with fresh 1% formaldehyde (Ted Pella, Redding, CA), and after two washes, permeabilized with ice-cold 70% ethanol for 15 min. DNA strand break labeling was achieved by incubation at 37°C in labeling mix (1× TdT buffer, 2.5 mM CoCl2, 1 U/5μl terminal deoxynucleotidyl transferase (TdT), and 0.1 nM FITC-dUTP (Roche Diagnostics, Mannheim, Germany)). Cells were washed twice in cold PBS containing 1% BSA and stored in PBS/BSA/1% formaldehyde. Samples were analyzed on an LSR II flow cytometer (BD Biosciences, San Jose, CA).
2.4. Immunoprecipitation of TCR complex
T cells were lysed in 20 mM Tris, pH 7.5, 150 mM NaCl, 10 mM Hepes, 1 mM EDTA, 1 mM Na3VO4, protease inhibitors (Roche Diagnostics, Indianapolis, IN) and 1% Triton X-100 (Calbiochem, La Jolla, CA). Cleared lysates were then subjected to one round of immunoprecipitation with pan-TCRαβ and pan-TCRγδ monoclonal antibodies (ThermoScientific). Immunoprecipitated proteins were resolved by SDS-PAGE, transferred to PVDF membranes and blotted with antibodies against CD3ε (Dako, Carpinteria, CA), TCRζ (BD Pharmingen), and FcRγ (Upstate Biotechnology, Lake Placid, NY).
2.5. Caspase activity assay
Overall caspase activities were determined using the Apo-ONE Assay (Promega, Madison, WI), which measures the cleavage of DEVD-rhodamine, according to the manufacturer's recommendations. Spectrophotometric readings were taken using a Fluorescence reader (Biotek Instruments, Winooski, VT).
2.6. Biotin-VAD-fmk active caspase precipitation assay
Cells were lysed in lysis buffer (20 mM Tris, pH 7.4, 150 mM NaCl, 2mM sodium orthovanadate, 10% glycerol, protease inhibitors, and 0.2% NP-40) containing 20 μM biotin-VAD-fmk (bVAD) (MP Biomedicals). 450 μg of protein lysate in 300 μl lysis buffer were precleared by rocking with 40 μl Sepharose 6B agarose beads (Sigma) at 4°C for 2 h. Supernatants were then rocked with 60 μl streptavidin-sepharose beads (Invitrogen) at 4°C overnight. Beads were washed 5 times in lysis buffer, then boiled in loading buffer. Beads were removed by centrifugation and immunoblot analysis was performed on supernatants.
2.7. Immunoblot analysis
Protein lysates from T cell clones were separated by SDS-PAGE using 10% or 12.5% gels, transferred onto PVDF membranes (BioRad Laboratories, Hercules, CA), and blocked using 4% milk in Tris-buffered saline plus 0.1% Tween-20 (American Bioanalytical, Natick, MA) at room temperature for 1 h as previously described [26]. Membranes were incubated at 4°C overnight in milk/TBS-Tween containing mouse anti-human caspase-8 monoclonal antibody (3-1-9) (BD Pharmingen), anti-caspase-3 585 rabbit polyclonal antibody (the kind gift of Dr. Yuri Lazebnik), caspase-9 monoclonal antibody (5B4) (Stressgen Assay Designs, Ann Arbor, MI), affinity-purified goat anti-human DFF45/ICAD (R&D Systems, Minneapolis, MN), or affinity-purified goat anti-human/mouse BID (R&D Systems). Immunoreactive proteins were visualized using species-specific secondary antibodies conjugated to horse radish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA; Southern Biotech, Birmingham, AL; Biomeda, Foster City, CA; Jackson Immunoresearch, West Grove, PA) and developed using LumiGLO (KPL, Gaithersburg, MD). Quantitative assessment of band densities on immunoblots were performed using the Quantity-One software (Bio-Rad) and are displayed as the sum of the intensities of the pixels inside the volume boundary × area of a single pixel (in mm2) after correction for background signal.
2.8. Statistical Analyses
A random coefficients growth model was used to examine 3H-thymidine incorporation, cell death by TUNEL, CD25, Fas, and CD3 changes over time in the two groups of cells, with individual cell types within group (αβ versus γδ). Data from up to six experimental replications were used, and a random effect was included in the model to control for differences across experiments. Planned post-hoc tests were used to examine differences between cell groups on individual days. Group differences in caspase activity on day 14 were examined using a general linear mixed model, with cell groups defined as above and a random effect included to control for differences across experimental replications. An analysis of variance was used to examine the effect of restimulation on caspase activity. Data for TUNEL assays did not meet distributional assumptions; thus, all analyses were based on log-transformed data. A repeated-measures analysis of variance was used to examine differences in αβ versus γδ cells in human subjects. Because of lack of conformity to distributional assumptions as well as the small number of subjects involved, these analyses were based on ranked data, which allows a non-parametric test. All analyses were performed using SAS version 9.2. Student t-test was conducted to assess densitometry readings for γδ versus αβ T cells.
3. Results
3.1. Increased cell growth and death in vitro by human γδ T cells
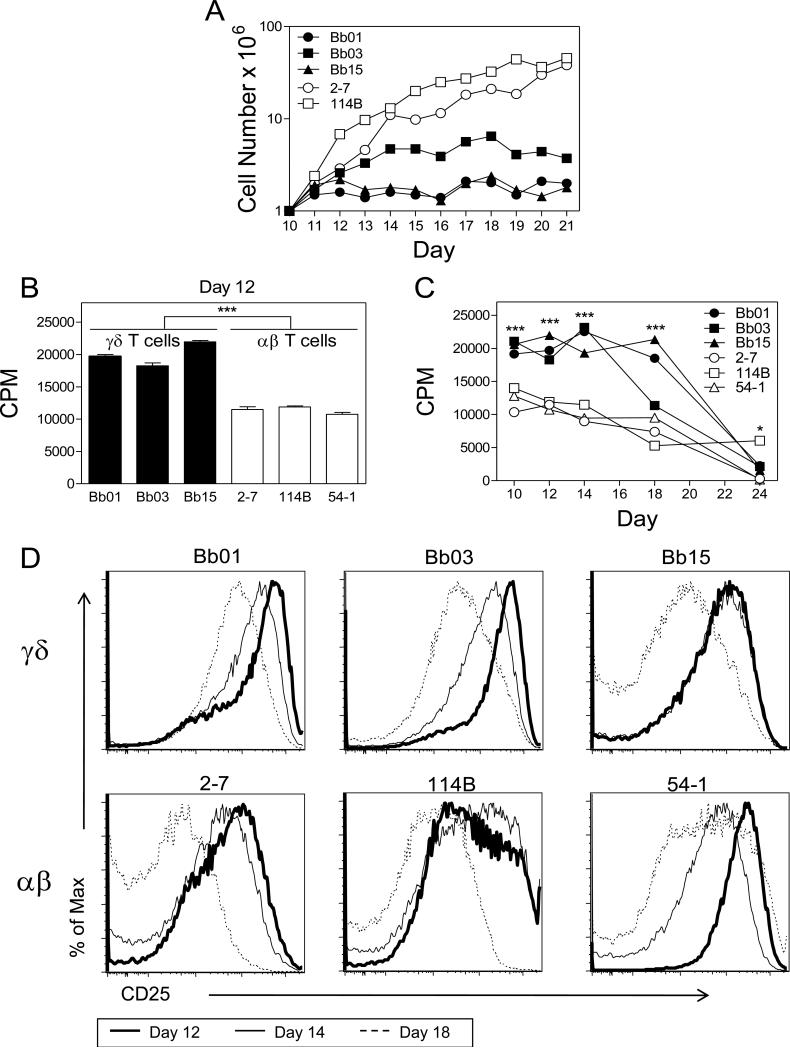
We used a previously described panel of human αβ T cell clones and synovial γδ (Vδ1) T cell clones from Lyme arthritis patients, all of which are reactive to Borrelia burgdorferi [10, 25] to compare proliferation, death, and related caspase activity in the two T cell subsets. The γδ and αβ T cell clones were monitored for expansion in cell number following stimulation and culture in IL-2-containing medium over 21 days. Under these conditions, γδ T cell clones, Bb01, Bb03, and Bb15, showed considerably less expansion compared to αβ T cell clones, 2-7 and 114B (Fig. 1A). This distinct difference in expansion raised the question whether these two T cell subsets actually proliferated at different rates or whether this reflected differences in cell death.
Figure 1. Reduced expansion of γδ T cells in vitro compared to αβ T cells is not explained by reduced levels of proliferation by γδ T cells.
(A) γδ T cell clones Bb01, Bb03, Bb15 (closed symbols) and αβ T cell clones 2-7, 114B (open symbols) were stimulated on day 0 and plated in fresh medium containing IL-2. Equal numbers (1 × 106 cells) of T cell clones were seeded at day 10 and cell number was then monitored until day 21. Data shown are representative of 2 independent experiments. (B, C) 3H-thymidine incorporation. T cell clones were pulsed with 3H-thymidine (1μCi/well) for 18h between days 10 to 24 following stimulation (C) and one time point is shown at day 12 (B). Data shown are representative of 2 independent experiments of triplicate samples. Values are mean ± sem for each group. Statistical analyses are of the combined data (*** p<0.0001; * p<0.04). The overall significance over time was p<0.0001. (D) CD25 profiles for γδ and αβ T cell clones at day 12 (thick line), day 14 (intermediate line), and day 18 (dashed line) post stimulation. Data shown are representative of 6 independent experiments and analysis is of the combined data measured as mean fluorescent intensity. There was a decline in CD25 levels over time for both γδ T cells (Bb01, Bb03, Bb15) and αβ T cells (2-7, 114B, 54-1) (p<0.01).
Proliferation, as assessed by DNA replication, was determined by incorporation of 3H-thymidine. Somewhat surprisingly, γδ T cell clones (Bb01, Bb03, and Bb15) actually manifested considerably higher levels of DNA replication than αβ T cell clones (2-7, 114B, and 54-1) over several time points, with the differences decreasing over time after stimulation (Fig. 1B, 1C). Time points were assessed beginning at day 10 to 12, when the clones had outgrown the feeder cell (irradiated PBL) population, and IL-2 receptor-α (CD25) expression levels were high, and end between day 18 to 24, when expression levels of CD25 were downregulated and cell cycling abating (Fig. 1C, D). This is consistent with earlier studies showing T cell proliferation parallels the level of IL-2 receptor expression, which increases and then decreases over several days following TCR stimulation [27].
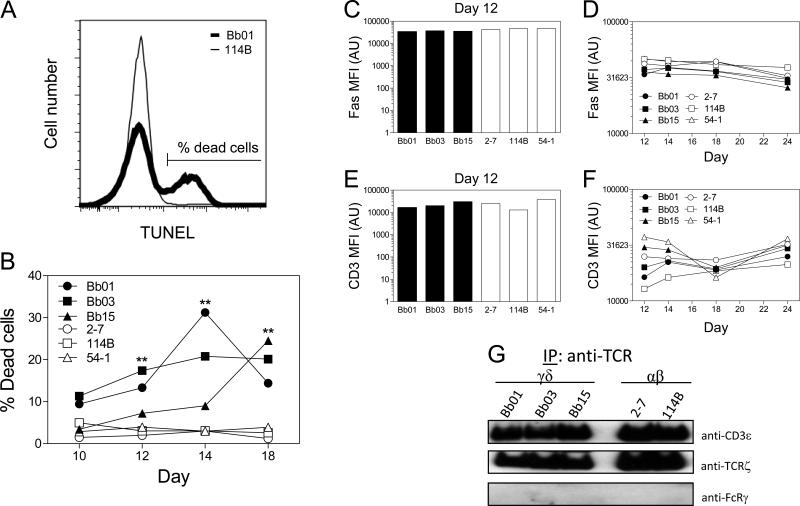
Because the greater expansion of αβ T cell numbers could not be explained by their greater proliferative capacity, spontaneous cell death was quantitated by TUNEL assay. The findings revealed a considerably higher percentage of spontaneous cell death in the γδ T cell clones compared to the αβ T cells over an extended period of time (Fig. 2A, B). This suggested that the reduced expansion in cell number by γδ T cells during this growth period was not explained by reduced proliferation by γδ T cells but rather by their augmented cell death.
Figure 2. γδ T cells manifest augmented levels of death relative to αβ T cells.
(A) Representative profile of TUNEL staining at day 14 post stimulation for γδ T cell clone, Bb01 (thick line), versus αβ T cell clone, 114B (thin line). (B) Cell death measured by TUNEL between days 10 to 18 following stimulation of γδ T cell clones (closed symbols) compared to αβ T cell clones (open symbols). Data shown are representative of 2 independent experiments. Differences between γδ T cells and αβ T cells trended towards significance on day 10 (p=0.063) and were significant on days 12, 14, and 18 (**, p≤0.01). The overall significance over time between γδ T cells (Bb01, Bb03, Bb15) and αβ T cells (2-7, 114B, 54-1) was p<0.01. (C-F) T cell clones between days 12 to 24 following stimulation were analyzed for expression of surface Fas and CD3. Data shown in C-F are consistent over at least 3 independent experiments and statistical analysis of the combined experiments show no significant differences (p=0.88, p=0.99, p=0.99, and p=0.45, respectively). (G) Clones were lysed and lysates immunoprecipitated with anti-TCR (γδ or αβ), resolved by SDS-PAGE, and immunoblotted for anti-CD3ε, anti-TCRζ, and anti-FcRγ. Data are representative of 2 independent experiments.
To assess why the γδ T cell clones manifested a higher rate of spontaneous cell death, we initially examined the expression of cell surface molecules known to influence susceptibility to cell death. These included the death receptor, Fas (CD95), and surface CD3, given that recurrent TCR stimulation promotes activation-induced cell death (AICD) [28]. However, mean fluorescent intensities of Fas and CD3, as measured by flow cytometry, revealed no differences in the levels of these molecules between the γδ and αβ T cell clones (Fig. 2C-F). We next examined the TCR complex composition as a possible explanation for the differences in cell death. Our previous studies have shown that the composition of the TCR-γδ complex might differ from TCR-αβ, with the TCR-γδ containing a greater proportion of FcεR1γ (FcRγ) and less TCRζ following activation [29]. However, the composition of the TCR complex did not differ between these particular γδ and αβ T cell clones, with the TCRs on all clones containing TCRζ and no FcRγ (Fig. 2G). This suggested that another mechanism was likely responsible for the augmented cell death of γδ T cells.
3.2. Increased caspase activity in γδ T cells
Because caspases are now recognized to promote both cell proliferation and cell death [15, 19-21, 30], we considered that differences in the levels of caspase activity might exist between γδ and αβ T cells that could influence both their cell growth and death. Overall caspase activity was first examined using a DEVD-rhodamine assay in which cleavage of the terminal aspartic acid of the substrate releases fluorescent rhodamine. The γδ T cell clones all manifested higher caspase activity than the αβ T cell clones at days 12, 14 and 18, as exemplified for day 14 in Figure 3A. These ambient levels of caspase activity increased considerably in absolute value following 8 h stimulation by anti-CD3, particularly among the γδ T cell clones (Fig. 3B). This was paralleled by augmented spontaneous cell death of the γδ T cells compared to the αβ T cells, as well as a tendency toward increased cell death of γδ T cells following anti-CD3 stimulation (Fig. 3C). Furthermore, the augmented CD3-induced cell death of the γδ T cell clones was blocked by the pan-caspase inhibitor zVAD (Fig. 3D), demonstrating that the cell death was caspase dependent.
Figure 3. Higher overall caspase activity in γδ T cells compared to αβ T cells.
(A-C) DEVD-rhodamine release assay was used to determine levels of caspase activity in T cell clones. (A) Data shown are representative of 4 independent experiments of triplicate samples with equal number of cells per sample using clones on day 14 following stimulation. Analysis by general linear mixed model across 4 independent experiments indicate that these two cell types have significantly different levels of caspase activity (*, p=0.02). This same trend occurred at day 12 (p=0.08) and 18 (p=0.04) following stimulation based on a random coefficients growth model (data not shown). (B) T cells on day 15 post stimulation were cultured with or without plate-bound anti-CD3 and assessed for caspase activity after 8 h. Data are representative of 3 independent experiments and statistical analysis by ANOVA of the combined data show that caspase activity is higher in anti-CD3 restimulated γδ T cell clones than αβ T cell clones (p<0.01), and that anti-CD3 treatment leads to greater differences in levels of caspase activity in γδ T cells compared to αβ T cells (p=0.03). (C) Proportion of dead cells assessed by TUNEL staining of T cell clones at day 18 post stimulation following CD3 restimulation for 8 h. Data are representative of 6 independent experiments. Analysis performed on the combined experiments was based on log-transformed data. Cell death was significantly increased upon anti-CD3 restimulation in γδ T cell clones (p=0.0003) and αβ T cell clones (p<0.0001), but no significance was found when comparing γδ versus αβ T cell clones (p=0.17). (D) TUNEL assay of cell death of T cell clones post restimulation and treated with DMSO control or z-VAD-fmk (zVAD) for 30 min prior to anti-CD3 restimulation for 8 h. Data are representative of 5 independent experiments. (E) Synovial fluid from three Lyme arthritis patients was activated with B. burgdorferi and expanded in vitro. At day 7 γδ and αβ T cells were sorted from each culture and equal numbers of cells were used to assess caspase activity (***, p<0.0001 using student t-test for samples performed in triplicates from patient 2 and patient 3. Culture from patient 1 did not provide sufficient number of cells to perform the assay in triplicate. p=0.04 for γδ versus αβ T cells for all three patients combined by repeated measures of ANOVA, with cell type as the within-subjects factor. Because the original data did not meet the distributional assumptions, all values were transformed to ranks prior to analysis). Data shown here are representative of 2 independent experiments.
It is possible that the differences in caspase activity observed in the γδ and αβ T cell clones might reflect artifacts resulting from long-term culture. We thus examined caspase activity in primary synovial fluid γδ and αβ T cells from three patients with Lyme arthritis. Synovial fluid lymphocytes were stimulated with B. burgdorferi and after seven days, at a time when the γδ T cells had expanded [10], the γδ and αβ T cells were sorted and examined directly for their level of caspase activity. As shown in Figure 3E, in all three cases the synovial fluid γδ T cells manifested considerably greater caspase activity than αβ T cells from the same cultures. Thus, the augmented caspase activity is intrinsic to γδ T cells and unlikely the result of selection during the cloning process.
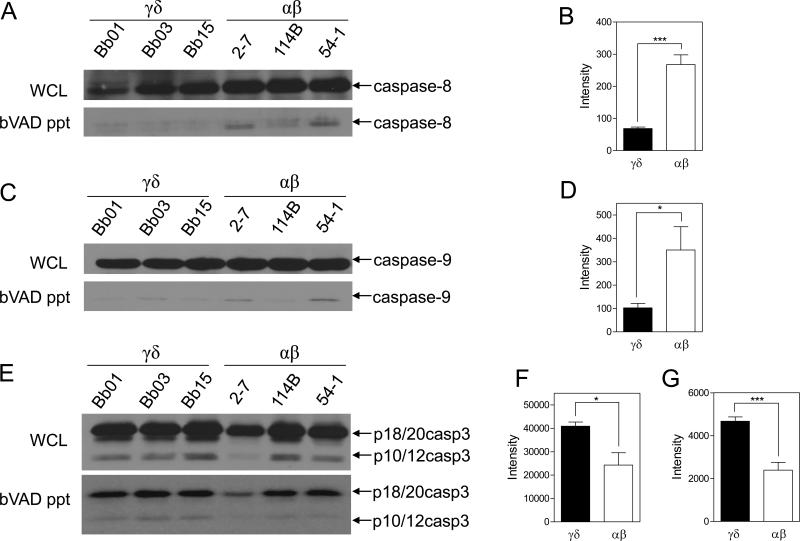
To more completely define which caspases were activated in the γδ and αβ T cell clones, we used biotin-VAD-fmk (bVAD) to selectively label all active caspases in cell lysates. The active caspase fraction was precipitated using streptavidin-sepharose beads and then examined by immunoblot for specific caspases. Consistent with the DEVD-rhodamine assay findings, immunoblot of bVAD precipitates revealed that the γδ T cells had increased amounts of cleaved active caspase-3, p18/20 and p10/12 compared to αβ T cells (Fig. 4E-G). However, the levels of total caspase-3 in whole cell lysates showed no detectable difference between the γδ and αβ T cells (Fig. 4E), and hence the increased amount of active caspase-3 in γδ T cells (Fig. 4F, G) was not explained by greater total caspase-3. Of further interest was that the levels of active upstream initiator caspases, which can cleave effector caspases into active forms, did not parallel the trend of caspase-3. Total and active caspase-8 levels were significantly lower in γδ compared to αβ T cells (Fig. 4A, B). Furthermore, although the total levels of caspase-9 in whole cell lysates were comparable between the γδ and αβ T cells, the proportion of caspase-9 that was active was substantially reduced in the γδ T cells (Fig. 4C, D). This suggested that the greater active caspase-3 in γδ T cells could not be explained by greater activity of upstream caspase-8 or caspase-9.
Figure 4. γδ T cells have higher levels of active caspase-3, but lower levels of active caspase-8 and caspase-9 than αβ T cells.
γδ (Bb01, Bb03, Bb15) and αβ (2-7, 114B, 54-1) T cell clones were lysed in buffer containing biotin-VAD-fmk (bVAD) to selectively label active caspases, which were then precipitated with streptavidin-sepharose beads. Precipitates were separated by SDS-PAGE and immunoblotted for caspase-8, caspase-9, and caspase-3 (A, C, and E, respectively) and compared to whole cell lysates from the same cells. Immunoblots shown are representative of 2 independent experiments. (B, D, F, and G) Densitometry of immunoblot results of bVAD precipitates. Values are the readings of γδ versus αβ T cell clones combined from 2 independent experiments. Statistics based on student t-test for γδ versus αβ T cells. (B) Active caspase-8 (***, p=0.0002). (D) Active caspase-9 (*, p=0.0406). (F, G) Active caspase-3, p18/20 (*, p=0.0172) and p10/12 (***, p=0.0006).
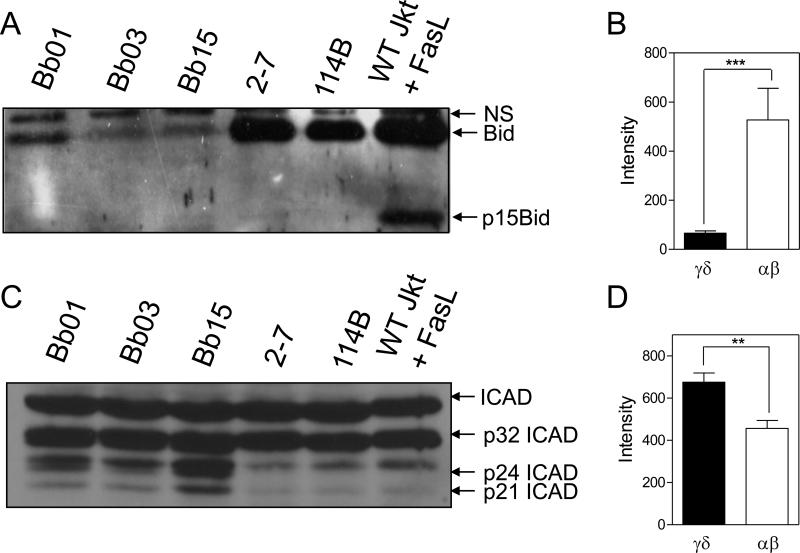
These findings raised the question of how γδ T cells survive in the presence of such elevated levels of active caspase-3. We have previously determined that active caspases are detectable within 30 min following TCR ligation of murine αβ T cells, and that the active caspases appear in lipid rafts in the cell membrane during T cell cycling [22, 23]. Consistent with this notion, no evidence of BID cleavage was observed in either γδ or αβ T cell clones. Of interest, however, was that the inactive full-length form of BID (p24) was found at considerably lower levels in the γδ T cell clones compared to αβ T cell clones (Fig. 5A, B). The reasons for this are currently under examination. In contrast to BID, cleavage of the caspase-3-specific death substrate, ICAD, was observed to a partial extent (p32) in all the T cell clones, but the γδ T cells manifested a clearly greater level of completely cleaved ICAD (p24/21) (Fig. 5C, D), which mirrored the increased levels of active caspase-3 and spontaneous cell death found in γδ T cells.
Figure 5. γδ T cells have lower levels of BID yet higher levels of the cleaved caspase-3 substrate, ICAD, than αβ T cells.
20 μg of whole cell lysate from effector γδ T cell clones (Bb01, Bb03, Bb15) and αβ T cell clones (2-7, 114B) were separated by SDS-PAGE and immunoblotted for the caspase-8 substrate, BID (A), and the caspase-3 substrate, ICAD (C). NS= non-specific band. (B, D) Densitometry of immunoblot results. Values are the readings of γδ versus αβ T cell clones combined from 4 and 3 separate experiments, respectively. Statistics based on student t-test for γδ versus αβ T cells (***, p=0.0003; **, p=0.0038).
4 Discussion
This study demonstrates that actively cycling human γδ T cells of the Vδ1 subset manifest greater overall caspase activity, particularly of effector caspase-3, than αβ T cells. As a consequence, human γδ T cells are more prone to both spontaneous cell death as well as cell death following TCR restimulation, and this occurs in a caspase-dependent manner. This was reflected in the reduced expansion of γδ T cells compared to αβ T cells despite higher levels of DNA replication in the former subset. These findings are consistent with previous observations that murine γδ T cells demonstrate greater rates of proliferation in vivo, as measured by greater CFSE dilution [29] and BrdU incorporation [14], despite their greater rate of turnover and loss. The augmented caspase activity of γδ T cells provides a potentially unifying model that may explain both of these phenomena. In addition, the increased caspase activity was also observed in primary synovial fluid γδ T cells from patients with Lyme arthritis, suggesting our findings were not the result of the cloning process or long periods in culture. Furthermore, we have also observed similar differences in caspase activity between murine γδ and αβ T cells (A. Koenig, unpublished observations).
Analysis of active caspases in human γδ T cells revealed that elevated caspase activity was due more to effector caspase-3 than initiator caspase-8 or caspase-9. This suggested that upstream initiator caspases were not primarily responsible for the augmented active caspase-3 in γδ T cells. These data are in agreement with an earlier study showing the presence of active caspase-3 in Jurkat cells, which also could not be explained by active caspase-8 [31]. The unanticipated finding of an active effector caspase in cycling T cells raises the obvious question of how these cells avoid cell death. Our previous findings that active caspases appear in lipid rafts in the cell membrane during T cell cycling [22, 23] suggest a sequestration model in which basal levels of active caspases are confined to the plasma membrane of non-apoptotic cycling effector T cells. In this state active caspase-8 and caspase-3 are denied access to cytoplasmic substrates involved with cell death, such as BID or ICAD, but may interact with other as yet unknown substrates important for effector T cell function. This is consistent with our observations that there was only minimally detectable complete cleavage of the caspase-3 substrate, ICAD, in αβ T cells. However, more extensive ICAD cleavage was observed in effector γδ T cells, indicating some active caspase-3 is likely present in the cytoplasm of effector γδ T cells. This observation is consistent with the increased spontaneous death observed in the γδ T cells.
The greater caspase activity in γδ T cells might result from more intense TCR signaling in γδ T cells compared to αβ T cells, as reflected by our earlier observations of increased levels of intracellular calcium and phosphorylated-ERK following TCR stimulation of γδ T cells [29]. This might not only promote effector function, but also would prime effector T cells for subsequent death signals. Conceivably, the greater intensity of TCR signals could drive higher levels of caspase activity to promote a rapid effector response followed by equally rapid cell death to ensure their elimination at the end of an immune response. This is consistent with our earlier study in the murine system showing that enhanced signaling intensity of TCR-γδ leads to a stronger proliferative response upon anti-CD3 restimulation compared to TCR-αβ [29]. Our findings are also consistent with previous work showing that murine γδ T cells turn over at a surprisingly high rate. Using BrdU labeling in vivo, Tough, et al. [14] demonstrated that during a 21-day BrdU labeling period, 70% of γδ T cells had cycled, whereas only 20% of CD4 T cells cycled during the same period. However, fully 70% of the labeled γδ T cells disappeared during the subsequent 28-day washout period without BrdU, reflecting a rapid turnover. In a separate yet relevant system, we previously demonstrated that the level of caspase activity in cycling T cells was considerably increased by overexpression of the caspase-8 regulator, cellular Fas-associated death domain-like interleukin-1-β-converting enzyme-inhibitory protein long form (c-FLIPL) [24]. This also resulted in both augmented proliferation, as well as increased subsequent cell death. The collective results imply that the intrinsically higher caspase activity in γδ T cells might promote both their increased proliferation and death in vivo. The current findings thus support a model that γδ T cells have evolved to function quickly and transiently, as in an innate response.
Acknowledgments
We thank Ms. Colette Charland for technical assistance with flow cytometry, Janice Bunn for statistical analysis, Dr. Antonio Lanzavecchia for the kind gift of anti-CD3 antibody (TR66), and Yuri Lazebnik for anti-caspase-3 585 rabbit polyclonal antibody. This work was supported by grants from the National Institutes of Health (AI36333, AR43520, AI45666, and AI 055402).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burk MR, Mori L, De Libero G. Human V gamma 9-V delta 2 cells are stimulated in a cross-reactive fashion by a variety of phosphorylated metabolites. Eur J Immunol. 1995;25:2052. doi: 10.1002/eji.1830250737. [DOI] [PubMed] [Google Scholar]
- 2.Tanaka Y, Morita CT, Nieves E, Brenner MB, Bloom BR. Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature. 1995;375:155. doi: 10.1038/375155a0. [DOI] [PubMed] [Google Scholar]
- 3.Collins C, Wolfe J, Roessner K, Shi C, Sigal LH, Budd RC. Lyme arthritis synovial gammadelta T cells instruct dendritic cells via fas ligand. J Immunol. 2005;175:5656. doi: 10.4049/jimmunol.175.9.5656. [DOI] [PubMed] [Google Scholar]
- 4.Poccia F, Boullier S, Lecoeur H, Cochet M, Poquet Y, Colizzi V, et al. Peripheral V gamma 9/V delta 2 T cell deletion and anergy to nonpeptidic mycobacterial antigens in asymptomatic HIV-1-infected persons. J Immunol. 1996;157:449. [PubMed] [Google Scholar]
- 5.Boullier S, Cochet M, Poccia F, Gougeon ML. CDR3-independent gamma delta V delta 1+ T cell expansion in the peripheral blood of HIV-infected persons. J Immunol. 1995;154:1418. [PubMed] [Google Scholar]
- 6.Brennan FM, Londei M, Jackson AM, Hercend T, Brenner MB, Maini RN, et al. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J Autoimmun. 1988;1:319. doi: 10.1016/0896-8411(88)90002-9. [DOI] [PubMed] [Google Scholar]
- 7.Bucht A, Soderstrom K, Hultman T, Uhlen M, Nilsson E, Kiessling R, et al. T cell receptor diversity and activation markers in the V delta 1 subset of rheumatoid synovial fluid and peripheral blood T lymphocytes. Eur J Immunol. 1992;22:567. doi: 10.1002/eji.1830220240. [DOI] [PubMed] [Google Scholar]
- 8.Jacobs MR, Haynes BF. Increase in TCR gamma delta T lymphocytes in synovia from rheumatoid arthritis patients with active synovitis. J Clin Immunol. 1992;12:130. doi: 10.1007/BF00918143. [DOI] [PubMed] [Google Scholar]
- 9.Shen Y, Li S, Quayle AJ, Mellbye OJ, Natvig JB, Forre O. TCR gamma/delta+ cell subsets in the synovial membranes of patients with rheumatoid arthritis and juvenile rheumatoid arthritis. Scand J Immunol. 1992;36:533. doi: 10.1111/j.1365-3083.1992.tb03221.x. [DOI] [PubMed] [Google Scholar]
- 10.Vincent MS, Roessner K, Lynch D, Wilson D, Cooper SM, Tschopp J, et al. Apoptosis of Fashigh CD4+ synovial T cells by borrelia-reactive Fas-ligand(high) gamma delta T cells in Lyme arthritis. J Exp Med. 1996;184:2109. doi: 10.1084/jem.184.6.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rust C, Kooy Y, Pena S, Mearin ML, Kluin P, Koning F. Phenotypical and functional characterization of small intestinal TcR gamma delta + T cells in coeliac disease. Scand J Immunol. 1992;35:459. doi: 10.1111/j.1365-3083.1992.tb02881.x. [DOI] [PubMed] [Google Scholar]
- 12.Balbi B, Moller DR, Kirby M, Holroyd KJ, Crystal RG. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J Clin Invest. 1990;85:1353. doi: 10.1172/JCI114579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salerno A, Dieli F. Role of gamma delta T lymphocytes in immune response in humans and mice. Crit Rev Immunol. 1998;18:327. doi: 10.1615/critrevimmunol.v18.i4.30. [DOI] [PubMed] [Google Scholar]
- 14.Tough DF, Sprent J. Lifespan of gamma/delta T cells. J Exp Med. 1998;187:357. doi: 10.1084/jem.187.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543. doi: 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Zhu H, Xu CJ, Yuan J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- 17.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 18.Sakahira H, Enari M, Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy NJ, Kataoka T, Tschopp J, Budd RC. Caspase activation is required for T cell proliferation. J Exp Med. 1999;190:1891. doi: 10.1084/jem.190.12.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alam A, Cohen LY, Aouad S, Sekaly RP. Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med. 1999;190:1879. doi: 10.1084/jem.190.12.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra RS, Jelley-Gibbs DM, Russell JQ, Huston G, Swain SL, Budd RC. Effector CD4+ T cells generate intermediate caspase activity and cleavage of caspase-8 substrates. J Immunol. 2005;174:3999. doi: 10.4049/jimmunol.174.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra RS, Russell JQ, Koenig A, Hinshaw-Makepeace JA, Wen R, Wang D, et al. Caspase-8 and c-FLIPL associate in lipid rafts with NF-kappaB adaptors during T cell activation. J Biol Chem. 2007;282:19365. doi: 10.1074/jbc.M610610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koenig A, Russell JQ, Rodgers WA, Budd RC. Spatial differences in active caspase-8 defines its role in T-cell activation versus cell death. Cell Death Differ. 2008;15:1701. doi: 10.1038/cdd.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dohrman A, Russell JQ, Cuenin S, Fortner K, Tschopp J, Budd RC. Cellular FLIP long form augments caspase activity and death of T cells through heterodimerization with and activation of caspase-8. J Immunol. 2005;175:311. doi: 10.4049/jimmunol.175.1.311. [DOI] [PubMed] [Google Scholar]
- 25.Roessner K, Fikrig E, Russell JQ, Cooper SM, Flavell RA, Budd RC. Prominent T lymphocyte response to Borrelia burgdorferi from peripheral blood of unexposed donors. Eur J Immunol. 1994;24:320. doi: 10.1002/eji.1830240207. [DOI] [PubMed] [Google Scholar]
- 26.Collins C, Shi C, Russell JQ, Fortner KA, Budd RC. Activation of gamma delta T cells by Borrelia burgdorferi is indirect via a TLR- and caspase-dependent pathway. J Immunol. 2008;181:2392. doi: 10.4049/jimmunol.181.4.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantrell DA, Smith KA. Transient expression of interleukin 2 receptors. Consequences for T cell growth. J Exp Med. 1983;158:1895. doi: 10.1084/jem.158.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150:4338. [PubMed] [Google Scholar]
- 29.Hayes SM, Love PE. Distinct structure and signaling potential of the gamma delta TCR complex. Immunity. 2002;16:827. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 30.Budd RC. Activation-induced cell death. Curr Opin Immunol. 2001;13:356. doi: 10.1016/s0952-7915(00)00227-2. [DOI] [PubMed] [Google Scholar]
- 31.Aouad SM, Cohen LY, Sharif-Askari E, Haddad EK, Alam A, Sekaly RP. Caspase-3 is a component of Fas death-inducing signaling complex in lipid rafts and its activity is required for complete caspase-8 activation during Fas-mediated cell death. J Immunol. 2004;172:2316. doi: 10.4049/jimmunol.172.4.2316. [DOI] [PubMed] [Google Scholar]