Abstract
Factors in physiological fluids that regulate the chemotactic activity of complement activation peptides C5a and C5a des Arg are not well understood. The vitamin D binding protein (DBP) has been shown to significantly enhance chemotaxis to C5a/C5a des Arg. More recently, platelet-derived thrombospondin-1 (TSP-1) has been shown to facilitate the augmentation of C5a-induced chemotaxis by DBP. The objective of this study was to better characterize these chemotactic cofactors and investigate the role that cell surface TSP-1 receptors CD36 and CD47 may play in this process. The chemotactic activity in C-activated normal serum, citrated plasma, DBP-depleted serum or C5 depleted serum was determined for both normal human neutrophils and U937 cell line transfected with the C5a receptor (U937-C5aR). In addition, levels of C5a des Arg, DBP and TSP-1 in these fluids were measured by RIA or ELISA. Results show that there is a clear hierarchy with C5a being the essential primary signal (DBP or TSP-1 will not function in the absence of C5a), DBP the necessary cofactor and TSP-1 a dependent tertiary factor, since it cannot function to enhance chemotaxis to C5a without DBP. Measurement of the C5a-induced intracellular calcium flux confirmed the same hierarchy observed with chemotaxis. Moreover, analysis of bronchoalveolar lavage fluid (BALF) from patients with the adult respiratory distress syndrome (ARDS) demonstrated that C5a-dependent chemotactic activity is significantly decreased after anti-DBP treatment. Finally, results show that TSP-1 utilizes cell surface receptors CD36 and CD47 to augment chemotaxis, but DBP does not bind to TSP-1, CD36 or CD47. The results clearly demonstrate that C5a/C5a des Arg needs both DBP and TSP-1 for maximal chemotactic activity and suggest that the regulation of C5a chemotactic activity in physiological fluids is more complex than previously thought.
Keywords: Complement, C5a, Chemotaxis, Inflammation, Neutrophils
1. INTRODUCTION
Numerous leukocyte chemoattractants have been reported and extensively characterized. However, the vast majority of these studies utilized purified in vitro systems and their regulation in physiological fluids generally is not well understood. Complement (C) activation peptides C5a and C5a des Arg (the stable serum form) are among the most potent chemoattractants for a wide variety of cell types, particularly neutrophils, and the peptides can be generated very rapidly in almost all body fluids (Guo and Ward, 2005; Klos et al., 2009; Manthey et al., 2009). Accordingly, C activation with the consequent C5a-mediated recruitment and activation of neutrophils is associated with the pathogenesis of numerous inflammatory disorders, particularly diseases of the lung and kidney (Guo and Ward, 2005; Klos et al., 2009; Manthey et al., 2009). Besides anaphylatoxin inactivators (carboxypeptidases N and R) that convert C5a to the stable C5a des Arg form, factors that regulate the chemotactic activity of C5a in physiological fluids have not been well characterized nor widely appreciated. Previously, it has been demonstrated that the chemotactic activity of the C5-derived peptides can be enhanced significantly by the vitamin D binding protein (DBP), a plasma protein also known as Gc-globulin (Binder et al., 1999; Kew and Webster, 1988; Metcalf et al., 1991; Perez et al., 1988; Petrini et al., 1991; Piquette et al., 1994; Senior et al., 1988; Zwahlen and Roth, 1990). Several reports have shown in vitro that this positive chemotactic cofactor function of DBP (i.e., co-chemotactic activity) appears to be specific for C5a and cannot augment other C5a-mediated leukocyte functions (oxidant generation and degranulation) (Binder et al., 1999; Kew and Webster, 1988; Metcalf et al., 1991; Perez et al., 1988; Petrini et al., 1991; Piquette et al., 1994; Senior et al., 1988; Zwahlen and Roth, 1990).
DBP is an abundant multifunctional 56 kDa plasma protein that is part of the albumin gene family (White and Cooke, 2000). Although the protein by itself lacks chemotactic activity, it associates with the plasma membrane of many cell types and appears to bind with low avidity to multiple cell surface ligands such as chondroitin sulfate proteoglycans (DiMartino and Kew, 1999), CD44 (McVoy and Kew, 2005) megalin (Nykjaer et al., 1999), and cubulin (Nykjaer et al., 2001). A cell surface DBP binding site complex has been inferred by functional, structural and kinetic cell binding studies and its interaction with DBP is essential for chemotaxis enhancement of C5a (DiMartino and Kew, 1999), (DiMartino et al., 2001), (Kew et al., 1995), (Trujillo and Kew, 2004), (McVoy and Kew, 2005), (DiMartino et al., 2007). Formation of a DBP binding site complex in leukocytes is a dynamic, multi-step and transient process requiring cell activation (DiMartino et al., 2007) and perhaps several distinct macromolecules. This complex also appears to function independent of C5a interacting with the C5a receptor (C5aR1/CD88) since DBP does not alter C5a receptor-ligand interactions (Perez, 1994), and DBP does not bind to C5a or the C5a receptor (DiMartino et al., 2001), (Zhang et al., 2010). Previously, we reported that C-activated serum has significantly greater leukocyte chemotactic activity than C-activated plasma; this difference was due to thrombospondin-1 (TSP-1) released into serum from activated platelets (Trujillo and Kew, 2004). Therefore, the objective of this study was to further characterize the cofactors that regulate chemotactic activity of C5a in physiological fluids (serum, plasma and BALF) and investigate the role that cell surface TSP-1 receptors may play in augmenting C5a-mediated leukocyte chemotaxis.
2. MATERIALS AND METHODS
2.1 Reagents
Purified recombinant human C5a was purchased from Sigma-Aldrich (St. Louis, MO). Vitamin D binding protein (DBP) was purified from human plasma and purchased from Athens Research and Technology (Athens, GA). BSA, goat IgG, and zymosan A (yeast cell walls from Saccharomyces cerevisiae) were obtained from Sigma-Aldrich. The affinity-purified antibodies to CD36 (N-15 and L-17) and CD47 (S-19 and C-18), and their corresponding peptide antigens (antigen blocking peptide), were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Polyclonal anti-DBP was purchased from DiaSorin (Stillwater, MN) and then affinity-purified using immobilized DBP. Goat anti-gC1qR was prepared as previously described (Ghebrehiwet and Peerschke, 2004). Fab preparation kit was purchased from Pierce-Thermo Scientific (Rockford, IL). The TSP-1 agonist peptide (4N1K) for CD47 and the CD36 TSP-1 binding peptides 93–110 and 139–155 were purchased from Bachem (Torrence, CA). Radioimmunoassy for C5a/C5a des Arg was purchased from Amersham-GE Healthcare (Piscataway, NJ), ELISA for TSP-1 was obtained from Chemicon-Millipore (Temecula, CA). DBP ELISA was performed as previously described (Trujillo and Kew, 2004). Sterile, pyrogen-free water, HBSS, PBS, RPMI and 1 M HEPES solution were purchased from Mediatech (Herndon, VA).
2.2 Isolation of Human Samples and Blood Products
Neutrophils, serum, and plasma were isolated from the venous blood of healthy, medication-free, paid volunteers who gave informed consent. The Institutional Review Board (IRB) of Stony Brook University approved this procedure. These protocols have been described in detail previously (Trujillo and Kew, 2004). DBP-depleted serum was prepared using an anti-DBP as previously described (Trujillo and Kew, 2004). C5-depleted serum was purchased from Sigma-Aldrich. Bronchoalveolar lavage fluid (BALF) was collected as part of an IRB-approved study from patients with the adult respiratory distress syndrome (ARDS) and generously provided by Dr. George Matuschak, Pulmonary and Critical Care Division, St. Louis University School of Medicine (St. Louis, MO). All ARDS patient samples were provided de-identified of any individual information and randomly numbered.
2.3 In Vitro Culture of U937 Cells
U937 cells were originally obtained from the ATCC (Rockville, MD) and transfected with either the human C5a receptor (C5aR1/CD88) or the empty plasmid vector as detailed previously (Kew et al., 1997). U937 cells were cultured at 37°C, 5% CO2 in RPMI-1640 containing 10% FBS (Hyclone, Logan, UT) and 400 µg/ml of active G418 (Invitrogen, Carlsbad, CA) and maintained at a density between 2 × 105 and 1.5 × 106/ml. The cell surface expression of the C5a receptor was routinely verified by flow cytometry using PE-labeled mouse anti-human CD88 (clone S5/1) obtained from Biolegend (San Diego, CA).
2.4 Preparation of Complement-Activated Serum and Plasma
Serum and citrated plasma (1 ml each) were incubated for 45 min at 37°C with 10 mg of washed zymosan. Particulate matter was removed by centrifugation (15,000 × g) for 5 min at 4°C using a microfuge. Samples were then aliquoted and frozen at −80°C.
2.5 Flow Cytometry
U937-C5aR cells or neutrophils were resuspended at 5 × 106 in 1 ml PBS + 1% BSA and blocked with 4 µg of rat IgG for 15 min at room temperature. Cells were washed once in PBS-BSA then 0.1 ml of cells (5 × 105) were stained with 32 ng of either PE-labeled mouse anti-human CD36 (clone 5–271), PE-labeled mouse IgG2a isotype control, FITC-labeled mouse anti-human CD47 (clone CC2C6), or FITC-labeled mouse IgG1 isotype control, all purchased from BioLegend (San Diego, CA). After incubating for 15 min at room temperature in the dark, cells were washed twice in PBS-BSA and resuspended in 2% paraformaldehyde in PBS and stored at 4°C until analyzed using a BD FACScan analyzer.
2.6 Chemotaxis Assay
Cell movement was quantitated using a 48 well microchemotaxis chamber (Neuroprobe, Cabin John, MD) and 5.0 µm pore size cellulose nitrate filters (purchased from Neuroprobe) as previously described (Kew et al., 1995). In each assay, the migration of 200,000 neutrophils (50 µl of 4×106/ml) or 300,000 U937-C5aR cells (50 µl of 6×106/ml) was evaluated. Cell movement was quantitated microscopically by measuring the distance in microns (µm) that the leading front of cells had migrated into the filter according to the method described by Zigmond and Hirsch (Zigmond and Hirsch, 1973). In each experiment, five fields per duplicate filter were measured at 400 × magnification. The value of the background controls for random cell movement (cells responding to buffer) has been subtracted in all cases so that the data are presented as net movement in µm.
2.7 Measurement of Changes in Intracellular Calcium Concentrations
Changes in intracellular calcium concentrations in U937-C5aR cells (107 cells/ml) were measured using Fluo-3 AM (Invitrogen-Molecular Probes, Carlsbad, CA). Cells were resuspended in HBSS-1% BSA containing 2 µM Fluo-3 AM and incubated at 37°C for 40 minutes. Cells incubated without the dye were used as a control to measure autofluorescence (Fmin). Following dye uptake, cells were washed twice then suspended at 5 × 106 cells/ml in HBSS-1% BSA. In selected experiments cells were pretreated with either 50 nM DBP or 0.5 nM TSP-1 for 20 min at 22°C before they were stimulated with either 0.1 nM C5a, C5a + 50 nM DBP or C5a + 0.5 nM TSP-1, and the increase in intracellular calcium monitored by fluorescence. The concentrations of DBP and TSP-1 represent the approximate amount of those proteins in 1–2% serum, a DBP/TSP-1 molar ratio of 100:1. Immediately after cells were stimulated with C5a fluorescence was measured at 505 nm excitation, 526 nm emission. Calcium concentrations were calculated using the following formula: [Ca2+] = Kd (F − Fmin)/(Fmax − F), where Kd = 325 nM for Fluo-3 according to manufacturer. The Fmax value was obtained by treating labeled cells with 60 µM digitonin. Controls included untreated cells not stimulated (negative control), stimulated with 1 nM C5a (positive control).
2.8 Surface Plasmon resonance (SPR) measurement of DBP-cell binding
The interactions between cells and DBP were evaluated using a BIAcore 2000 (BIAcore AB, Upsala, Sweden). Purified DBP was covalently coupled to a CM5 sensor chip using N-ethyl-N-(dimethylaminopropyl) carbodiimide / N-hydroxysuccinimide (EDC/NHS) according to the manufacturer’s instructions. The surface of the CM5 sensor chip was activated with EDC/NHS for 20 min before adding either DBP (5 µM) in 10 mM sodium carbonate buffer, pH 5.0. Excess NHS was deactivated for 20 min using 1 M ethanolamine, pH 8.5. The efficiency of DBP coupling was determined by injecting 5 µg/ml affinity-purified goat anti-human DBP into the flow cell at 10 µl/min at 22°C. Cell-DBP binding interactions were determined by injecting cell suspensions at a flow rate of 5 µl/min at 22°C in Hanks’ balanced salt solution, pH 7.4 (HBSS) containing 0.005% Tween 20. The sensor chip was stripped and regenerated using 0.8 M glycine (pH 2.0) containing 0.6 M NaCl. The regeneration conditions were adjusted to achieve a subsequent binding response that was within 10% of the initial (first injection) binding value. A blank sensor chip that was EDC/NHS-activated and ethanolamine blocked was used as a background reference in all experiments. Net resonance response units (RU) were determined by subtracting of the background values using BIAevaluation software version 4.1. Each figure in the Results section shows a representative sensorgram of response units versus time, however, all experiments were repeated (minimum n ≥ 3) to verify results.
2.9 Data Analysis and Statistics
A minimum of 3 experiments was performed for each assay. Results of several experiments were analyzed for significant differences among group means using analysis of variance (ANOVA) followed by a multiple comparisons posttest utilizing the statistical software program InStat (GraphPad Software, San Diego, CA).
3. RESULTS
3.1 Cofactor Regulation of C5a Chemotactic Activity in Physiological Fluids
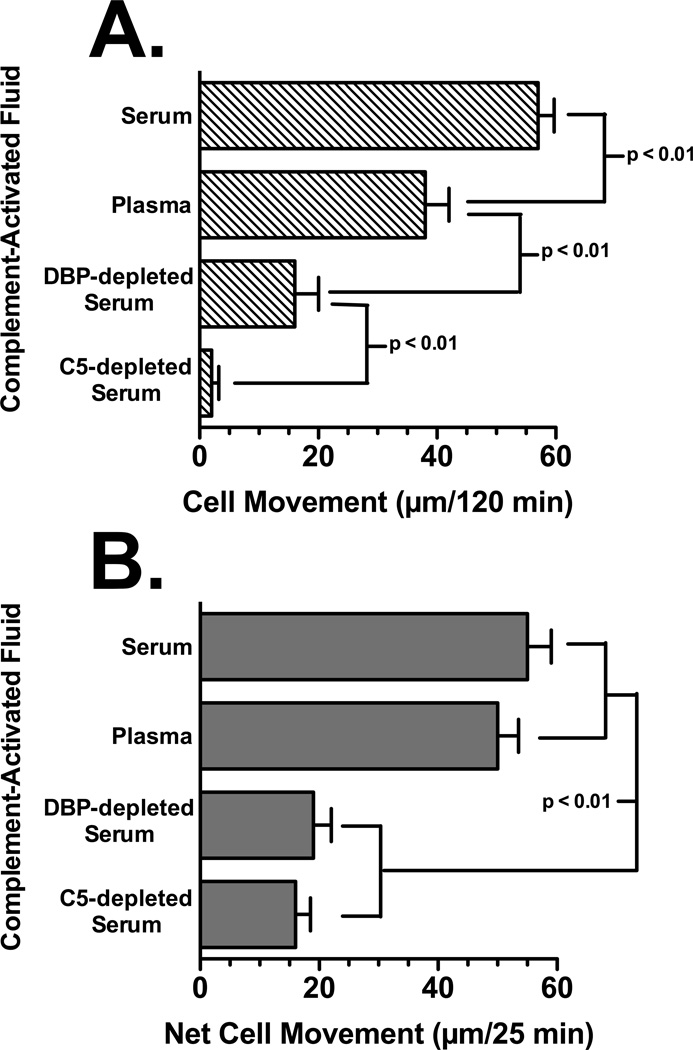
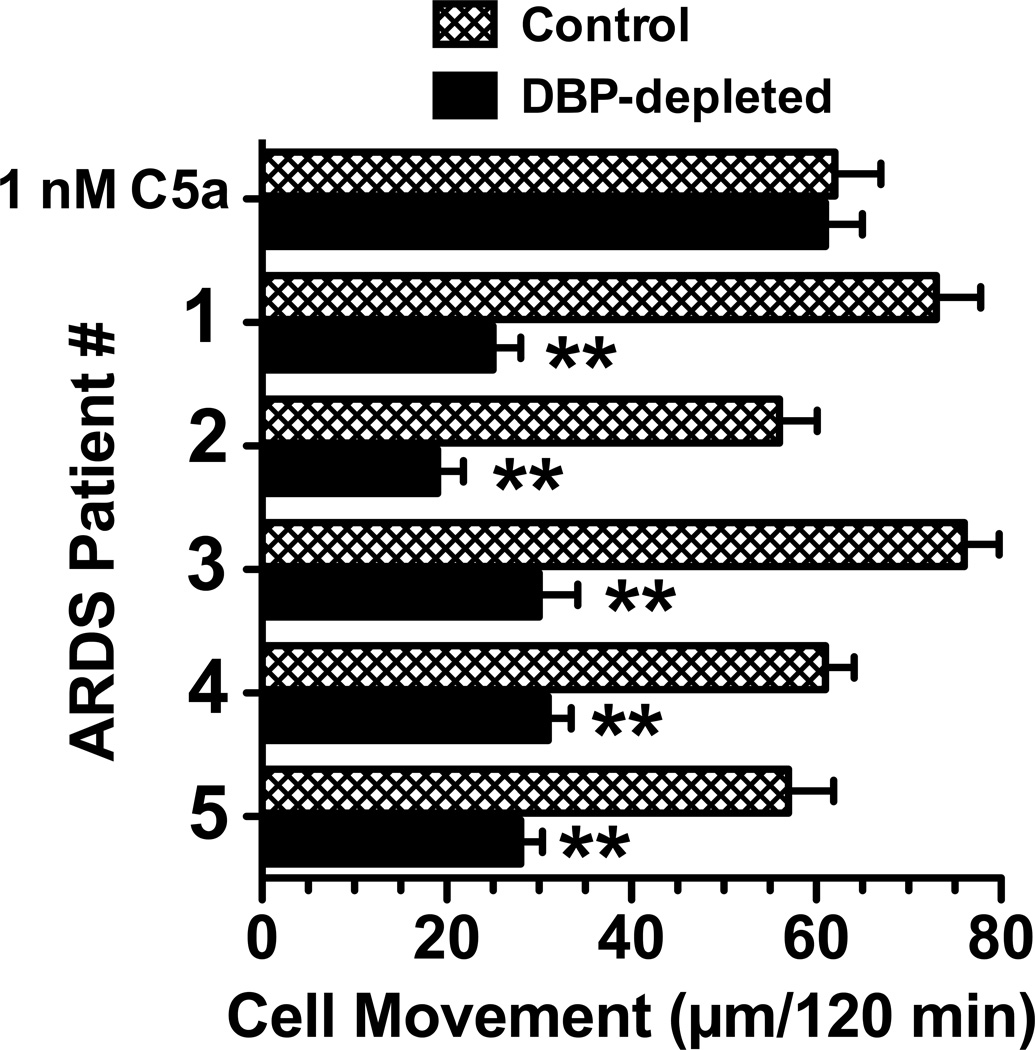
Figure 1 and Table I show that at least two cofactor proteins, DBP and TSP-1, are required for maximal C5a chemotactic activity in C-activated serum. The leukocyte chemotactic activity in serum, plasma, DBP-depleted serum and C5-depleted serum was determined using U937-C5aR cells and peripheral blood neutrophils from healthy donors. The U937-C5aR cell line was utilized because these cells migrate only to a C5a stimulus and will not respond to other chemotactic factors that may be present in serum and plasma (this contrasts with neutrophils that will respond to multiple factors in these samples). Figure 1 demonstrates the chemotactic activity in C-activated (using zymosan) normal serum, citrated plasma, DBP-depleted serum and C5-depleted serum. Table I shows the concentrations of C5a des Arg, DBP and TSP-1 (determined by RIA or ELISA) in these C-activated fluids. The C5 depleted serum, as expected, had no chemotactic activity for U937-C5aR cells (Fig. 1A) or significantly reduced activity for neutrophils (Fig. 1B), the residual activity is due to other non-C5a chemoattractants present in serum. There was no detectable C5a by RIA in C5-depleted serum but the sample contained the same amount of DBP and TSP-1 as control serum (Table I). The chemotactic activity of DBP-depleted serum is significantly reduced for U937-C5aR cells (70% reduction, Fig. 1A) and neutrophils (65% reduction, Fig. 1B) but the levels of C5a and TSP-1 are essentially the same as control C-activated serum (Table I). C-activated plasma had very little TSP-1 but had the same concentration of C5a and DBP as C-activated serum (Table I). The chemotactic activity for U937-C5aR cells in C-activated plasma is reduced by 30% for U937-C5aR cells (Fig. 1A) but less than 10% for neutrophils (Fig. 1B), most likely because neutrophils possess TSP-1 both on the cell surface and in rapidly mobilized intracellular granules (Kries et al., 1989; Suchard et al., 1991). To extend these observations further bronchoalveolar lavage fluid (BALF) from patients with the adult respiratory distress syndrome (ARDS) were analyzed for C5a-dependent chemotactic activity ex vivo using U937-C5aR cells. Figure 2 demonstrates that all five ARDS BALF samples had robust C5a-dependent chemotactic activity (mean migration of 65 ± 4.2 µm/120 min), equivalent to or exceeding the positive control 1 nM purified C5a. Moreover, pretreatment of BALF with polyclonal anti-DBP significantly reduced (p < 0.01) chemotactic activity in each specimen (mean migration of 27 ± 2.3 µm/120 min), a comparable percent reduction as the DBP-depleted serum in figure 1. These results show that C5a is the essential primary chemotactic signal in C-activated fluids but it also requires DBP to manifest full chemotactic activity.
Figure 1.
Chemotactic activity of C-activated fluids. Pooled human serum, citrated plasma, DBP-depleted serum, C5-depleted serum were treated with zymosan A for 45 min at 37°C to activate complement, and the activated fluid was centrifuged to remove zymosan particles. Panel A: U937-C5aR cells (3×105) were allowed to migrate toward 2.5% dilution of each fluid for 120 min at 37°C. Panel B: Neutrophils (2×105) were allowed to migrate toward 2.5% dilution of each fluid for 25 min at 37°C. Numbers represent mean migration distance ± SEM, n = 3–7. Statistical significance is indicated.
Table I.
| Protein Concentration (µg/ml) | |||
|---|---|---|---|
| C-activated Fluid | C5a des Arg | DBP | TSP-1 |
| Serum | 1.8 ± 0.2 | 385 ± 16 | 42 ± 7 |
| Citrated Plasma | 1.9 ± 0.3 | 390 ± 22 | < 1 |
| DBP-depleted Serum | 1.8 ± 0.4 | not detected | 39 ± 6 |
| C5-depleted Serum | not detected | 365 ± 24 | 36 ± 8 |
Figure 2.
Effect of DBP depletion on the C5a dependent chemotactic activity in ARDS BALF. The indicated ARDS BALF samples (normalized for total protein content), as well as 1 nM purified human C5a, were treated for 30 min at 22°C with 40 µg/ml of goat anti-DBP or an irrelevant goat IgG (sham control). U937-C5aR cells (3×105) were then allowed to migrate towards either C5a or a 20% dilution of BALF in chemotaxis buffer for 120 min at 37°C. Numbers represent mean migration distance ± SEM, n = 3. Asterisks denote that cell movement was significantly less (p < 0.01) than the sham control.
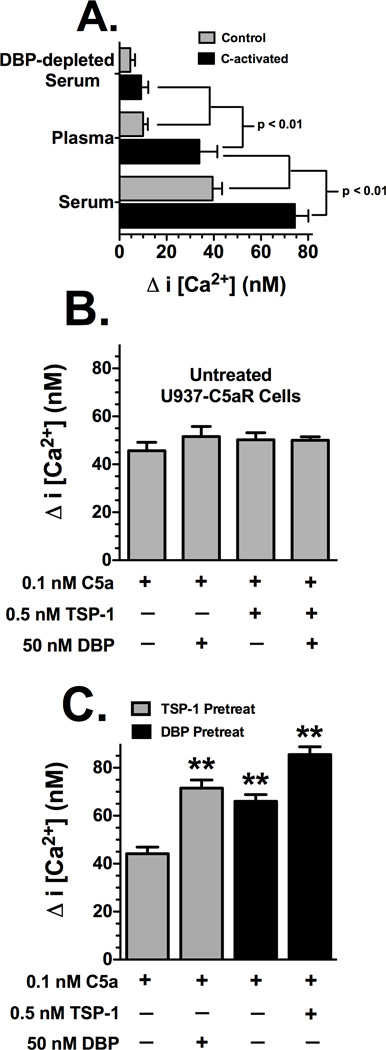
The effect of DBP and TSP-1 on rapid signaling activity was determined by measuring changes in intracellular calcium levels in U937-C5aR cells. Figure 3A shows that, as expected, C-activated serum and plasma (both at 1%) induce a greater C5a-dependent increase in intracellular calcium than their corresponding sham-activated controls. However, C-activated serum induces a significantly greater calcium influx than C-activated plasma, and both induce a significantly greater flux than 1% DBP-depleted serum (Fig. 3A). Treatment of U937-C5aR cells with C-activated C5-depleted serum generated almost no calcium response, essentially background levels (data not shown). These results support the chemotaxis data presented in figure 1A. To more precisely dissect the C5a cofactor effect of DBP and TSP-1 on the rapid calcium influx, purified proteins were utilized. Figure 3B demonstrates that DBP, TSP-1 or both together are not able to increase the C5a-mediated calcium signal when the proteins are added to cells simultaneously with C5a. The concentrations of DBP and TSP-1 represent the approximate amount of those proteins in 1–2% serum, a DBP/TSP-1 molar ratio of 100:1. However, a different picture emerges when cells are pretreated for 20 minutes with either purified DBP or TSP-1 and then stimulated with C5a (Fig. 3C). TSP-1 pretreatment does not alter the C5a-induced calcium signal, but in contrast, DBP pretreatment significantly increases calcium levels (Fig. 3C). Most interesting, TSP-1 pretreatment permits DBP to enhance the C5a signal immediately when cells were stimulated with C5a + DBP (Fig. 3C), possibly suggesting that TSP-1 facilitates the assembly of a DBP binding/signaling complex that allows DBP to augment the C5a-mediated calcium signal. Neither DBP nor TSP-1 can signal by itself in the absence of C5a as evidenced by the complete lack of a calcium signal in cells stimulated with DBP or TSP-1 alone (data not shown). These results demonstrate that pretreatment of cells with DBP alone or TSP-1 + DBP significantly augments a C5a-induced increase in intracellular calcium, further supporting the chemotaxis results in Figure 1 that DBP is the essential cofactor and TSP-1 a DBP-dependent tertiary cofactor.
Figure 3.
Effect of DBP and TSP-1 on C5a-induced intracellular calcium mobilization in U937-C5aR cells. Panel A: Cells treated with 1% (diluted in HBSS) C-activated or sham activated serum, citrated plasma or DBP-depleted serum. Panel B: Cells were treated with purified C5a, DBP and TSP-1 with no preincubation. Panel C: Cells were preincubated with either DBP or TSP-1 before stimulation with C5a. Cells (5×106 cells/ml) loaded Fluo-3 AM were washed and then suspended in HBSS containing 1% BSA. Cells were pretreated for 20 min at 22°C with either 0.5 nM TSP-1 or 50 nM DBP. For each measurement, 0.4 ml of cells was added to a cuvette and stimulated with either 1% serum or plasma (panel A) or 0.1 nM purified C5a, C5a + 0.5 nM TSP-1 or C5a + 50 nM DBP (panels B and C) at 22°C. Fluorescence was measured immediately at 505 nm excitation, 526 nm emmission for Fluo-3 AM. Fmax was measured by treating labeled cells with 60 µM digitonin. Data are presented as mean ± SEM (n = 4–6) of the peak increase in intracellular calcium concentration. Significance is indicated in panel A, double asterisks (panel C) indicates value is significantly higher (p < 0.01) than C5a alone.
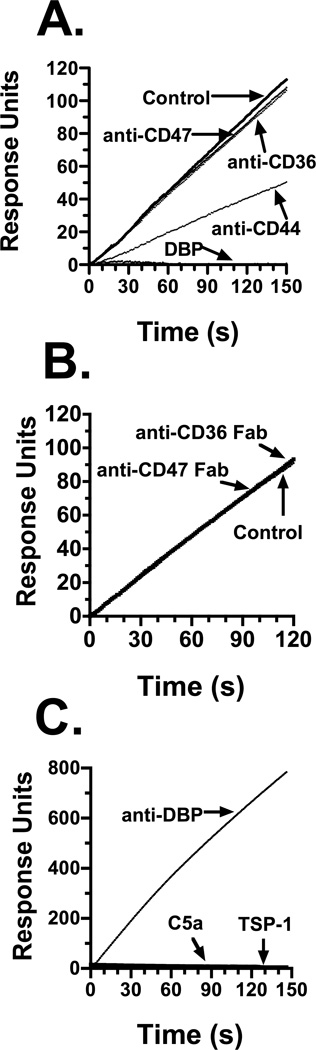
Previously, we demonstrated that the C5a chemotactic cofactor function of DBP is mediated in part by cell surface CD44 and annexin A2 (McVoy and Kew, 2005). Since TSP-1 is needed for maximal chemotactic activity in C-activated fluids, the role of TSP-1 receptors CD36 and CD47 were investigated to determine if they mediate the cofactor effect of this protein. Cell surface expression of CD36 and CD47 by flow cytometry (data not shown) confirmed previous reports that CD47 is constitutively expressed but the CD36 staining pattern is weakly positive on both cell types (Alessio et al., 1996); (Prieto et al., 1994); (Yamauchi et al., 2002). The potential interaction of DBP with CD36, CD47 and TSP-1 was next determined by binding assays using Biacore surface plasmon resonance (SPR). Figure 4 shows the results of either U937-C5aR cells (Figs. 4A and 4B) or purified proteins (Fig. 4C) interacting with DBP immobilized to an SPR chip. Figure 4, panel A demonstrates that U937-C5aR cells bind to immobilized DBP (Fig. 4A, Control) but binding is completely abolished if cells are first pretreated with soluble DBP (Fig. 4A, DBP), confirming our previous report (Zhang et al., 2010). Treatment of cells with anti-CD44 reduces cell binding to DBP by about 50%, this is almost the exact percent inhibition of binding we reported previously using a different binding assay (McVoy and Kew, 2005). However, pretreatment of cells with either anti-CD36 or anti-CD47 essentially had no effect on U937-C5aR cell binding to immobilized DBP. Treatment of cells with intact IgG molecules may cause receptor clustering and/or stearic inhibition and so produce a false negative result. Thus, Fab fragments of anti-CD36 and anti-CD47 were used to minimize these effects, and results also show that the Fab treatment did not diminish cell binding to DBP (Fig. 4B). Finally, purified human platelet derived TSP-1, as well as recombinant human C5a, did not bind to immobilized DBP (Fig. 4C). These results indicate that TSP-1, and its receptors CD36 and CD47, do not bind directly to DBP suggesting that DBP and TSP-1 may enhance C5a-mediated chemotaxis via two independent mechanisms.
Figure 4.
Ligand binding to DBP immobilized on a Biacore SPR sensor chip. Panel A: 106 cells/ml in HBSS were not treated (control), pretreated with 50 nM DBP, or with 25 µg/ml of the indicated goat polyclonal antibody. Panel B: Cells were treated with 50 µg/ml of either anti-CD36 or anti-CD47 Fabs. Panel C: binding to immobilized DBP by soluble goat anti-DBP (5 µg/ml), purified TSP-1 (50 nM) or purified C5a (0.5 µM). U937-C5aR cells at 106 cells/ml (panels A and B), or the indicated purified proteins (panel C), were allowed to interact with DBP immobilized on a Biacore sensor chip at a flow rate of 5 µl/min at 22°C in HBSS. Data is expressed as response units of the molecular interaction on the sensor chip.
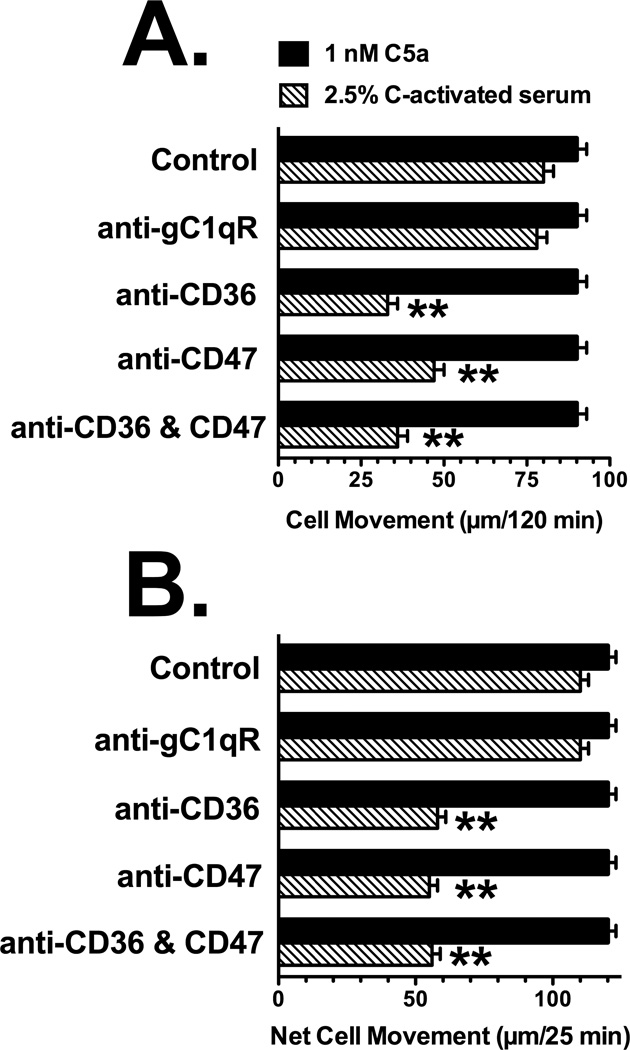
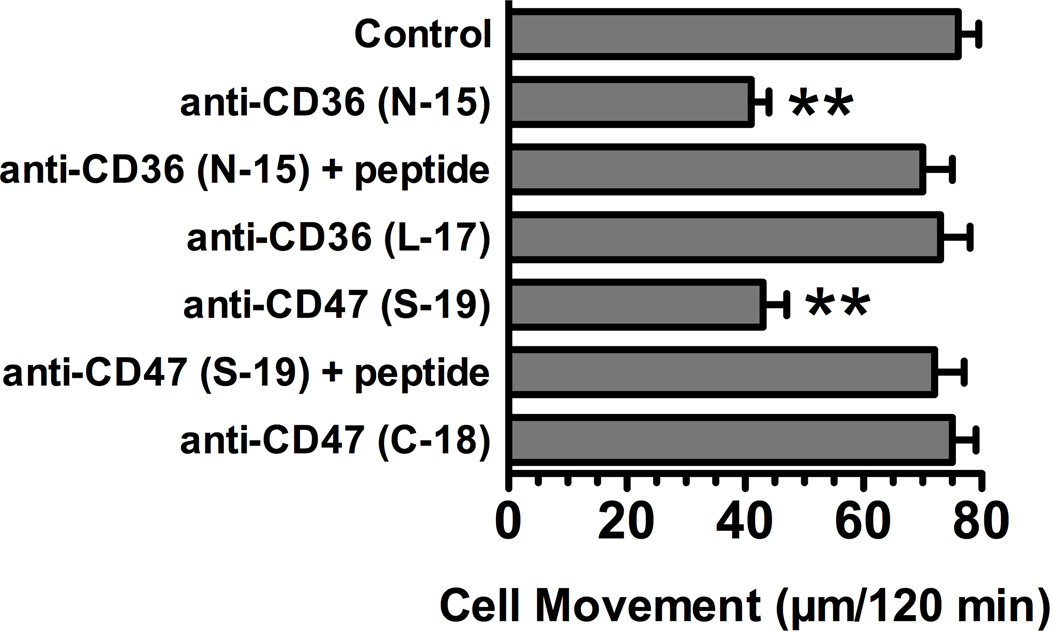
The effect of pretreating cells with anti-CD36 or anti-CD47 on chemotaxis to C-activated serum (DBP and TSP-1 dependent) versus purified C5a (DBP and TSP-1 independent) was investigated next. Figure 5 shows the effect of pretreating U937-C5aR cells (Fig. 5A) and neutrophils (Fig. 5B) with the indicated affinity-purified goat polyclonal antibodies on movement to either purified C5a or C-activated serum. None of the antibody treatments had any effect on either U937-C5aR (Fig. 5A) or neutrophil (Fig. 5B) chemotaxis to 1 nM purified C5a, a DBP and TSP-1 independent stimulus. Moreover, treating cells with a species matched (goat) antibody to the receptor for the globular head region of C1q (gC1qR), which is expressed on both neutrophils and U937 cells (Eggleton et al., 1995), also had no effect on chemotaxis to C-activated serum. In addition, an irrelevant purified goat IgG had no effect on chemotaxis to either C5a or C-activated serum (data not shown). However, either anti-CD36 or anti-CD47 alone significantly inhibited chemotaxis to C-activated serum to a similar extent for both U937-C5aR (Fig. 5A) and neutrophils (Fig. 5B). Combining the two antibodies did not result in a further inhibition of chemotaxis (Figs. 5A and 5B). As additional controls for the specificity of this effect, the anti CD36 (N-15) and anti-CD47 (S-19) utilized in Figure 5 were pretreated with their corresponding blocking antigenic peptides prior to cell treatment. Figure 6 demonstrates that pretreatment with the antigenic peptides completely reversed the inhibitory effect of each antibody. In contrast, treatment of cells with either the antigenic peptides alone had no effect on chemotaxis (data not shown). Both CD36 and CD47 are large cell surface receptors that bind multiple ligands and the two antibodies utilized (N-15 and S-19) were directed against extracellular epitopes near the N-terminus of each molecule. Different affinity-purified goat polyclonal antibodies to either an internal CD36 epitope in a heavily glycosylated region (L-17), or an antibody to the C-terminal cytoplasmic domain of CD47 (C-18), had no effect on chemotaxis of U937-C5aR cells to C-activated serum (Fig. 6). Finally, none of the treatments altered chemotaxis to 1 nM purified C5a (data not shown). These results demonstrate that antibody binding to N-terminal epitopes in the primary TSP-1 receptors CD36 or CD47 significantly reduces chemotaxis to C-activated serum but not to purified C5a.
Figure 5.
Effect of anti-CD36 and anti-CD47 on chemotaxis to C-activated serum. Panel A: U937-C5aR cells, Panel B: neutrophils. U937-C5aR cells (3×105) or neutrophils (2×105) in chemotaxis buffer were either not treated (control) or treated for 15 min at 22°C with 25 µg/ml of goat anti-CD36, anti-CD47 or anti-gC1qR. Cells were then allowed to respond to either 2.5% C-activated serum or 1 nM purified C5a for either 25 min (neutrophils) or 120 min (U937 cells) at 37°C. Numbers represent mean migration distance ± SEM, n = 4–6. Double asterisks denote that cell movement was significantly less (p < 0.01) than to the untreated control.
Figure 6.
Effect of different anti-CD36 and anti-CD47 on chemotaxis to C-activated serum. U937-C5aR cells (3×105) in chemotaxis buffer were either not treated (control) or treated for 15 min at 22°C with 25 µg/ml of the indicated goat anti-CD36 and anti-CD47 antibodies. Antibody + peptide samples, aliquots of anti-CD36 and CD47 (25 µg/ml) were treated with 40-fold molar excess of antigenic blocking peptide for 15 min at 22°C before they were added to cells. Cells were then allowed to respond to 2.5% C-activated serum for 120 min at 37°C. Numbers represent mean migration distance ± SEM, n = 3. Double asterisks denotes that cell movement was significantly less (p < 0.01) than the control.
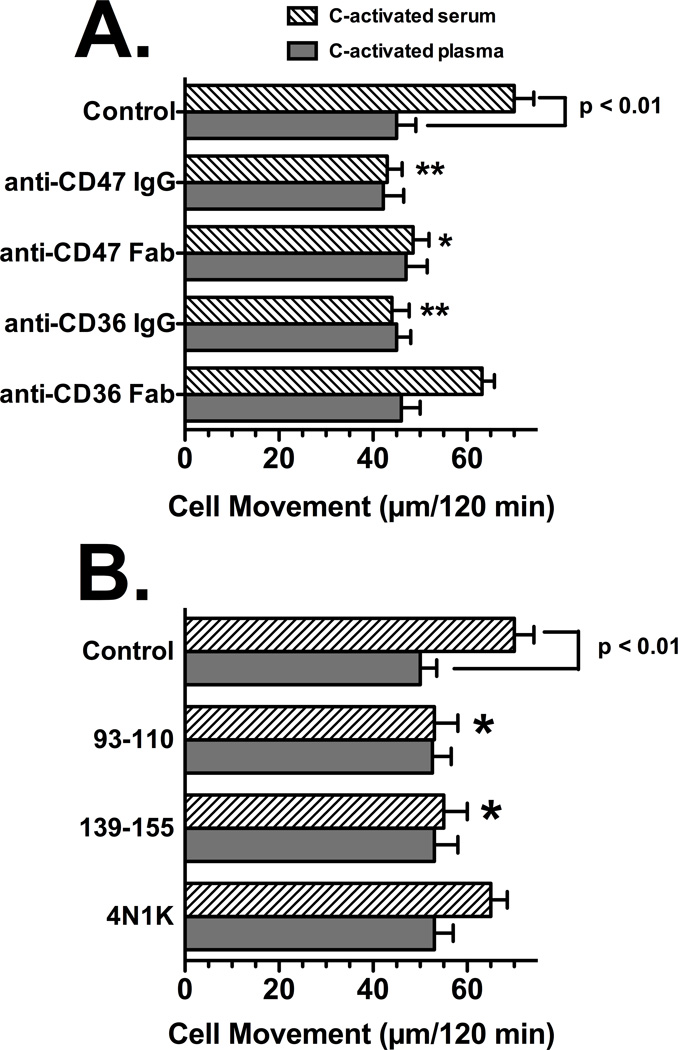
The results shown in Figures 5 and 6 suggest that blocking TSP-1 cell surface binding may explain the inhibitory effect of anti-CD36 and anti-CD47. Accordingly, to address this question directly, the differential chemotactic response of U937-C5aR cells to C-activated serum versus C-activated plasma (see Fig. 1) and (McVoy and Kew, 2005) was employed. In addition, Fab fragments of anti-CD36 and anti-CD47 were used to determine if univalent ligation of the cell surface antigens could mimic the inhibitory effect of intact bivalent antibodies. Figure 7A demonstrates that none of the antibody preparations altered U937-C5aR chemotaxis to C-activated plasma, which has almost undetectable levels of TSP-1 (Table I). Chemotaxis of U937-C5aR cells to C-activated serum was significantly reduced (p < 0.01) to the same level as C-activated plasma by either anti-CD36 or anti-CD47. However, only the Fab fragment of anti-CD47 significantly inhibited chemotaxis (p < 0.05) whereas the anti-CD36 Fab showed a slight but not significant diminution of cell movement to C-activated serum (Fig. 7A). These results show that the antibodies inhibit chemotaxis only when TSP-1 is present, presumably by preventing protein from binding to cell surface CD36 or CD47. In addition, univalent ligation of CD36 with Fab fragments is not as effective in inhibiting chemotaxis to C-activated serum as is the intact IgG antibody most likely because TSP-1 requires two distinct sites to bind CD36 but only one to bind CD47.
Figure 7.
Role of CD36 and CD47 in U937-C5aR chemotaxis to C-activated serum versus C-activated plasma. Panel A: Effect of univalent receptor ligation using Fab fragments, Panel B: Effect of peptides to the TSP-1 binding sites. U937-C5aR cells (3×105) in chemotaxis buffer were either not treated (control) or treated for 15 min at 22°C with 25 µg/ml of intact IgG to CD36 or CD47 or 50 µg/ml of the corresponding Fab fragments (panel A) or 100 µg/ml of the CD36 peptides (93–110, 139–155) or the TSP-1 peptide agonist for CD47 (4N1K). Cells were then allowed to respond to either 2.5% C-activated serum or 2.5% C-activated plasma for 120 min at 37°C. Numbers represent mean migration distance ± SEM, n = 4–5. Asterisks denote that cell movement was significantly less (**p < 0.01 or *p < 0.05) compared to the untreated control.
Inhibition of TSP-1 binding to either CD36 or CD47 using peptide mimics of the binding sites was investigated next. The TSP-1 binds to two sequences on CD36, amino acids 93–110 and 139–155. Synthetic peptide mimics of these regions can inhibit binding of TSP-1 to CD36 (Leung et al., 1992). The sequence in TSP-1 (amino acids 1016–1023) that binds to CD47 functions as an agonist peptide that can mimic binding of native TSP-1 and has been designated 4N1K (Kosfeld and Frazier, 1992). U937-C5aR cells were treated with these peptides and the chemotactic response to C-activated serum and C-activated plasma was evaluated. Figure 7B demonstrates that individual CD36 peptides 93–110 or 139–155 alone were effective at significantly reducing (p<0.05) the chemotactic response to C-activated serum but, as expected, had no effect on C-activated plasma. In contrast, treatment of cells with the CD47 agonist peptide 4N1K had no significant effect on U937-C5aR cell chemotaxis to either C-activated serum or plasma (Fig. 7B). These results indicate that that TSP-1 cell surface binding to CD36 and CD47 is essential for chemotaxis enhancement, and suggest that CD36 may be the more important of the TSP-1 receptors for enhancing chemotaxis to C5a.
4. DISCUSSION
This report demonstrates that the chemotactic activity of C5a in physiological fluids analyzed ex vivo requires both DBP and TSP-1 for maximal induction of cell migration. This is the first comprehensive analysis using depleted versus normal serum (and plasma) and correlating chemotactic activity (Fig. 1) with antigenic levels (Table I) of C5a des Arg, DBP and TSP-1 in these fluids. Results also show for the first time that DBP plays an essential role in the C-dependent chemotactic activity in BALF obtained from ARDS patients (Fig. 2), a severe inflammatory disorder where C5a generation has been linked to disease pathogenesis (Guo and Ward, 2005; Klos et al., 2009; Manthey et al., 2009). This study extends the findings of our previous paper that reported TSP-1 functions with DBP as a C5a chemotactic cofactor (Trujillo and Kew, 2004). Results herein (Figs. 1 to 3 and Table I) show that there is a clear hierarchy with C5a being the essential primary signal (DBP or TSP-1 will not function in the absence of C5a), DBP the necessary cofactor and TSP-1 a dependent tertiary factor, since it cannot function to enhance chemotaxis to C5a without DBP. Furthermore, this paper also reveals that DBP does not bind to TSP-1, CD36 or CD47 (Fig. 4) and that TSP-1 utilizes these cell surface receptors to augment chemotaxis to C5a (Figs. 5–7). Thus, C5a, DBP and TSP-1 all appear to bind to their cell surface receptors independently of one another. However, DBP and TSP-1 may have overlapping functions as cell surface proteins that facilitate assembly of multi-molecular complexes involved in cell movement to C5a. Alternatively, DBP and/or TSP-1 may neutralize an inhibitor of C5a or the C5a signal. However, either of these potential mechanisms requires further investigation.
This paper provides evidence that DBP enhances a C5a-induced parameter (calcium signal) other than chemotaxis (Fig. 3). The advantage of measuring changes in intracellular calcium levels is that it provides a very rapid assessment of the effect of cell treatment on a C5a signal. DBP, TSP-1 or both proteins are not able to increase the C5a-mediated calcium signal when the proteins are added to cells simultaneously with C5a. DBP, but not TSP-1, pretreatment significantly augments the C5a-induced calcium influx (Fig. 3C). Moreover, TSP-1 pretreatment permits DBP to enhance the C5a signal immediately when cells were stimulated with C5a + DBP. These experiments demonstrate the clear hierarchy of signals, reinforce the chemotaxis data (Fig. 1) and may indicate that TSP-1 facilitates the assembly of a DBP binding/signaling complex on the cell surface that allows DBP to augment the C5a-mediated calcium signal. Furthermore, since purified proteins were used (Fig. 3C) the results most likely indicate that DBP (and perhaps TSP-1) enhances a C5a signal directly rather than neutralizing an inhibitor as was speculated above.
Another novel aspect of this paper is the evidence that TSP-1 receptors CD36 and CD47 may mediate the C5a chemotactic cofactor function of this protein. Both neutrophils and U937 cells express several TSP-1 receptors including CD36, CD47, β1 and β3 integrins and cell surface proteoglycans (Yamauchi et al., 2002); (Febbraio et al., 2001); (Brown and Frazier, 2001). We chose to focus on CD36 and CD47 since previous work in our lab revealed that anti-CD29 (β1 integrin) had no effect on cell migration to C-activated serum (unpublished observations). The functions of TSP-1 are numerous and depend on the concentration of the protein, the interacting cell type and its expression profile of TSP-1 receptors (Adams and Lawler, 2004). TSP-1 is a 450 kDa homotrimer that is abundant in platelet α-granules and released during the clotting process, hence its concentration is very low in plasma but significantly elevated in serum (Table I). The differential chemotactic response to C-activated serum and plasma is due to the presence of platelet-derived TSP-1 in serum that facilitates the co-chemotactic activity of DBP (Trujillo and Kew, 2004). Neutrophils have been shown to express TSP-1 on their cell surface (Kries et al., 1989; Suchard et al., 1991) and thus migrate almost as well to C-activated plasma (Fig. 1). However, the neutrophil response to C-activated plasma is more variable (depending on the individual blood donor) while chemotaxis to C-activated serum produces very consistent results (unpublished observations).
Results presented in figures 5 to 7 demonstrate that treatment of cells with either anti-CD36 or anti-CD47 effectively eliminates the TSP-1 cofactor function, i.e., reduces migration of C-activated serum to the level of C-activated plasma. This raises several interesting questions concerning a potential mechanism of how TSP-1 assists DBP in enhancing chemotaxis to C5a in physiological fluids. Blocking TSP-1 cell surface binding by either anti-CD36 or anti-CD47 treatment of cells inhibits chemotaxis to C-activated serum essentially to the same extent, and dual antibody treatment has no additional effect (Fig. 5) perhaps indicating that both receptors are required. Indeed, TSP-1 can bind both to CD36 and CD47 simultaneously since the binding sequences for each receptor are distinct and in separate regions of TSP-1 (Adams and Lawler, 2004). Studies have also shown that ligation of CD36 and CD47 with TSP-1 often induces opposite effects (Yamauchi et al., 2002). However, it is not clear if this inhibition is due to blocking TSP-1 signaling through these receptors and/or preventing TSP-1 from assembling a multi-receptor complex needed for optimal chemotaxis. Although this latter concept would appear to be better supported by our data since, in the absence of DBP, purified TSP-1 does not enhance a C5a calcium signal (Fig. 3) or C5a-induced chemotaxis (Fig. 1). We believe that TSP-1 bridges cell surface receptors and facilitates (i.e., accelerates) formation of the DBP binding site complex, but there is no evidence that TSP-1 directly interacts with either CD44 or annexin A2, two molecules that we have previously shown are part of the DBP binding site (McVoy and Kew, 2005). However, there are indirect associations between CD44 and TSP-1 that may be relevant to understanding how TSP-1 assists DBP in enhancing chemotaxis to C5a. First, leukocytes from TSP-1 null mice have a significant reduction in cell surface CD44 compared to their wild-type counterparts (Kuznetsova et al., 2005). Second, CD44 is required for vascular smooth muscle cell migration to TSP-1 (Maier et al., 2009). These studies indicate a clear connection between the two molecules, and since both CD44 and TSP-1 bind multiple ligands there is a distinct possibility that one or more of these ligands could bridge TSP-1 with CD44, thus, facilitating the DBP binding site complex.
The results using Fab fragments and peptide mimics (Fig. 7) were very clear and consistent but on first impression are somewhat confusing. These results may be explained by the fact that TSP-1 requires two distinct sequences to bind CD36 (Leung et al., 1992) whereas there is one TSP-1 site in CD47 (Kosfeld and Frazier, 1992). In addition, TSP-1 induces dimerization of CD36 on the plasma membrane (Daviet et al., 1997). Consequently, a univalent ligation of CD36 (anti-CD36 Fab) may not completely block TSP-1 binding. The Fab fragment of anti-CD47 significantly inhibits chemotaxis to C-activated serum but the 41NK peptide, which is an effective agonist mimic of full-length TSP-1 for signaling via CD47 (Kosfeld and Frazier, 1992), has no effect on chemotaxis to C-activated serum suggesting that the role of CD47 is to localize TSP-1 on the membrane rather than induce signaling. Either of the CD36 peptide mimics (93–110 or 139–155) can inhibit chemotaxis to C-activated serum since both sequences are required for TSP-1 binding (Leung et al., 1992). This data would suggest that CD36 is the more important of the TSP-1 receptors since in the presence of 93–110 or 139–155 peptides, TSP-1 in C-activated serum will still bind to CD47 but yet chemotaxis is inhibited. Interestingly, a functional association between DBP and CD36 has been reported previously. A deglycosylated form of DBP, known as DBP-MAF that has macrophage activating function, reportedly also has anti-angiogenic properties that may be mediated via CD36 since treatment of endothelial cells with anti-CD36 blocks this activity (Kanda et al., 2002).
In conclusion, this paper clearly demonstrates that the chemotactic function of C5a in physiological fluids is more complex than previously thought and may involve an intricate balance between yet unrecognized inhibitory and enhancing cofactor signals. This information may have implications for current therapeutic approaches to block C5a using receptor antagonists or antibodies (Monk et al., 2007).
Highlights.
DBP and TSP-1 regulate chemotactic activity of C5a in physiological fluids
DBP is an essential chemotactic cofactor that can function without TSP-1
TSP-1 is a tertiary cofactor that is dependent on DBP
TSP-1 mediates it cofactor function via cell surface CD36 and CD47
Acknowledgments
This investigation was supported in part by grants from the U.S. National Institutes of Health (NIH) to R.R.K (GM063769) and B.G. (AI060866, AI084178). D.M.H. was supported by NIH training grant T32-GM008468. G.T. was supported by a W. Burghardt Turner Postdoctoral Fellowship and the NSF-funded AGEP Program (both at Stony Brook University).
Abbreviations used in this paper
- ARDS
adult respiratory distress syndrome
- BALF
bronchoalveolar lavage fluid
- DBP
vitamin D binding protein
- TSP-1
thrombospondin-1
- U937-C5aR cells
undifferentiated U937 cells transfected with the human C5a receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alessio M, De Monte L, Scirea A, Gruarin P, Tandon NN, Sitia R. Synthesis, processing, and intracellular transport of CD36 during monocytic differentiation. J Biol Chem. 1996;271:1770–1775. doi: 10.1074/jbc.271.3.1770. [DOI] [PubMed] [Google Scholar]
- Binder R, Kress A, Kan G, Herrmann K, Kirschfink M. Neutrophil priming by cytokines and vitamin D binding protein (Gc-globulin): impact on C5a-mediated chemotaxis, degranulation and respiratory burst. Mol Immunol. 1999;36:885–892. doi: 10.1016/s0161-5890(99)00110-8. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- Daviet L, Malvoisin E, Wild TF, McGregor JL. Thrombospondin induces dimerization of membrane-bound, but not soluble CD36. Thrombosis and haemostasis. 1997;78:897–901. [PubMed] [Google Scholar]
- DiMartino SJ, Kew RR. Initial characterization of the vitamin D binding protein (Gc-globulin) binding site on the neutrophil plasma membrane: evidence for a chondroitin sulfate proteoglycan. J Immunol. 1999;163:2135–2142. [PubMed] [Google Scholar]
- DiMartino SJ, Shah AB, Trujillo G, Kew RR. Elastase controls the binding of the vitamin D-binding protein (Gc-globulin) to neutrophils: a potential role in the regulation of C5a co-chemotactic activity. J Immunol. 2001;166:2688–2694. doi: 10.4049/jimmunol.166.4.2688. [DOI] [PubMed] [Google Scholar]
- DiMartino SJ, Trujillo G, McVoy LA, Zhang J, Kew RR. Upregulation of vitamin D binding protein (Gc-globulin) binding sites during neutrophil activation from a latent reservoir in azurophil granules. Mol Immunol. 2007;44:2370–2377. doi: 10.1016/j.molimm.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggleton P, Ghebrehiwet B, Sastry KN, Coburn JP, Zaner KS, Reid KBM, Tauber AI. Identification of a gC1q-binding protein (gC1q-R) on the surface of human neutrophils. Subcellular localization and binding properties in comparison with the cC1q-R. J.Clin.Invest. 1995;95:1569–1578. doi: 10.1172/JCI117830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest. 2001;108:785–791. doi: 10.1172/JCI14006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebrehiwet B, Peerschke EI. cC1q-R (calreticulin) and gC1q-R/p33: ubiquitously expressed multi-ligand binding cellular proteins involved in inflammation and infection. Mol Immunol. 2004;41:173–183. doi: 10.1016/j.molimm.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N. Effects of Vitamin D(3)-Binding Protein-Derived Macrophage Activating Factor (GcMAF) on Angiogenesis. J Natl Cancer Inst. 2002;94:1311–1319. doi: 10.1093/jnci/94.17.1311. [DOI] [PubMed] [Google Scholar]
- Kew RR, Fisher JA, Webster RO. Co-chemotactic effect of Gc-globulin (vitamin D binding protein) for C5a. Transient conversion into an active co-chemotaxin by neutrophils. J Immunol. 1995;155:5369–5374. [PubMed] [Google Scholar]
- Kew RR, Peng T, Dimartino SJ, Madhavan D, Weinman SJ, Cheng D, Prossnitz ER. Undifferentiated U937 Cells Transfected With Chemoattractant Receptors - a Model System to Investigate Chemotactic Mechanisms and Receptor Structure/Function Relationships. J. Leukoc. Biol. 1997;61:329–337. doi: 10.1002/jlb.61.3.329. [DOI] [PubMed] [Google Scholar]
- Kew RR, Webster RO. Gc-globulin (vitamin D binding protein) enhances the neutrophil chemotactic activity of C5a and C5a des Arg. J.Clin.Invest. 1988;82:364–369. doi: 10.1172/JCI113596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klos A, Tenner AJ, Johswich KO, Ager RR, Reis ES, Kohl J. The role of the anaphylatoxins in health and disease. Mol Immunol. 2009;46:2753–2766. doi: 10.1016/j.molimm.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld MD, Frazier WA. Identification of active peptide sequences in the carboxyl-terminal cell binding domain of human thrombospondin-1. J Biol Chem. 1992;267:16230–16236. [PubMed] [Google Scholar]
- Kries C, La Fleur M, Ménard C, Paquin R, Beaulieu AD. Thrombospondin and fibronectin are synthesized by neutrophils in human inflammatory joint disease and in a rabbit model of in vivo neutrophil activation. J Immunol. 1989;143:1961–1968. [PubMed] [Google Scholar]
- Kuznetsova SA, Day AJ, Mahoney DJ, Rugg MS, Mosher DF, Roberts DD. The N-terminal module of thrombospondin-1 interacts with the link domain of TSG-6 and enhances its covalent association with the heavy chains of inter-alpha-trypsin inhibitor. J Biol Chem. 2005;280:30899–30908. doi: 10.1074/jbc.M500701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LL, Li WX, McGregor JL, Albrecht G, Howard RJ. CD36 peptides enhance or inhibit CD36-thrombospondin binding. A two-step process of ligand-receptor interaction. J Biol Chem. 1992;267:18244–18250. [PubMed] [Google Scholar]
- Maier KG, Sadowitz B, Cullen S, Han X, Gahtan V. Thrombospondin-1-induced vascular smooth muscle cell migration is dependent on the hyaluronic acid receptor CD44. American journal of surgery. 2009;198:664–669. doi: 10.1016/j.amjsurg.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Manthey HD, Woodruff TM, Taylor SM, Monk PN. Complement component 5a (C5a) Int J Biochem Cell Biol. 2009;41:2114–2117. doi: 10.1016/j.biocel.2009.04.005. [DOI] [PubMed] [Google Scholar]
- McVoy LA, Kew RR. CD44 and annexin A2 mediate the C5a chemotactic cofactor function of the vitamin D binding protein. J Immunol. 2005;175:4754–4760. doi: 10.4049/jimmunol.175.7.4754. [DOI] [PubMed] [Google Scholar]
- Metcalf JP, Thompson AB, Gossman GL, Nelson KJ, Koyama S, Rennard SI, Robbins RA. Gc-globulin functions as a cochemotaxin in the lower respiratory tract. A potential mechanism for lung neutrophil recruitment in cigarette smokers. Am.Rev.Respir.Dis. 1991;143:844–849. doi: 10.1164/ajrccm/143.4_Pt_1.844. [DOI] [PubMed] [Google Scholar]
- Monk PN, Scola AM, Madala P, Fairlie DP. Function, structure and therapeutic potential of complement C5a receptors. Br J Pharmacol. 2007;152:429–448. doi: 10.1038/sj.bjp.0707332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen EI, Willnow TE. An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]
- Nykjaer A, Fyfe JC, Kozyraki R, Leheste JR, Jacobsen C, Nielsen MS, Verroust PJ, Aminoff M, de la Chapelle A, Moestrup SK, Ray R, Gliemann J, Willnow TE, Christensen EI. Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3) Proc Natl Acad Sci U S A. 2001;98:13895–13900. doi: 10.1073/pnas.241516998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez HD. Gc globulin (vitamin D-binding protein) increases binding of low concentrations of C5a des Arg to human polymorphonuclear leukocytes: An explanation for its cochemotaxin activity. Inflammation. 1994;18:215–220. doi: 10.1007/BF01534562. [DOI] [PubMed] [Google Scholar]
- Perez HD, Kelly E, Chenoweth D, Elfman F. Identification of the C5a des Arg cochemotaxin. Homology with vitamin D-binding protein (group-specific component globulin) J.Clin.Invest. 1988;82:360–363. doi: 10.1172/JCI113595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrini M, Azzara A, Carulli G, Ambrogi F, Galbraith RM. 1,25-dihydroxycholecalciferol inhibits the cochemotactic activity of Gc (vitamin D binding protein) J.Endocrinol.Invest. 1991;14:405–408. doi: 10.1007/BF03349090. [DOI] [PubMed] [Google Scholar]
- Piquette CA, Robinson-Hill R, Webster RO. Human monocyte chemotaxis to complement-derived chemotaxins is enhanced by Gc-globulin. Journal of Leukocyte Biology. 1994;55:349–354. doi: 10.1002/jlb.55.3.349. [DOI] [PubMed] [Google Scholar]
- Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- Senior RM, Griffin GL, Perez HD, Webster RO. Human C5a and C5a des Arg exhibit chemotactic activity for fibroblasts. Journal of Immunology. 1988;141:3570–3574. [PubMed] [Google Scholar]
- Suchard SJ, Boxer LA, Dixit VM. Activation of human neutrophils increases thrombospondin receptor expression. J Immunol. 1991;147:651–659. [PubMed] [Google Scholar]
- Trujillo G, Kew RR. Platelet-derived thrombospondin-1 is necessary for the vitamin D-binding protein (Gc-globulin) to function as a chemotactic cofactor for C5a. J Immunol. 2004;173:4130–4136. doi: 10.4049/jimmunol.173.6.4130. [DOI] [PubMed] [Google Scholar]
- White P, Cooke N. The multifunctional properties and characteristics of vitamin D-binding protein [Review] Trends in Endocrinology & Metabolism. 2000;11:320–327. doi: 10.1016/s1043-2760(00)00317-9. [DOI] [PubMed] [Google Scholar]
- Yamauchi Y, Kuroki M, Imakiire T, Uno K, Abe H, Beppu R, Yamashita Y, Shirakusa T. Opposite effects of thrombospondin-1 via CD36 and CD47 on homotypic aggregation of monocytic cells. Matrix Biol. 2002;21:441–448. doi: 10.1016/s0945-053x(02)00036-7. [DOI] [PubMed] [Google Scholar]
- Zhang J, Habiel DM, Ramadass M, Kew RR. Identification of two distinct cell binding sequences in the vitamin D binding protein. Biochim Biophys Acta. 2010;1803:623–629. doi: 10.1016/j.bbamcr.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S, Hirsch J. Leukocyte locomotion and chemotaxis. New methods for evaluation and demonstration of a cell-derived chemotactic factor. Journal of Experimental Medicine. 1973;137:387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwahlen RD, Roth DR. Chemotactic competence of neutrophils from neonatal calves. Functional comparison with neutrophils from adult cattle. Inflammation. 1990;14:109–123. doi: 10.1007/BF00914034. [DOI] [PubMed] [Google Scholar]