Abstract
Sperm is not a simple carrier of paternal genetic information but its role extends clearly beyond fertilization. Integrity of sperm genome is an essential pre-requisite for birth of healthy offspring and evaluation of sperm should entail DNA integrity analysis. DNA integrity analysis is a better diagnostic and prognostic marker of sperm reproductive potential. Conventional semen analysis emphasizes on sperm concentration, viability, motility and morphology and has been proven to be a poor indicator of reproductive potential and pregnancy outcome. To overcome the drawbacks associated with conventional semen analysis more useful fertility tests and molecular biomarkers have been explored. Among the different tests which have evolved for assessing the sperm reproductive potential, tests for sperm DNA quality are most promising. Sperm DNA damage has been closely associated with numerous indicators of reproductive health including fertilization, embryo quality, implantation, spontaneous abortion, congenital malformations and childhood diseases. It therefore has great potential as a prognostic test for both in vitro and in vivo conception. This review presents an updated account of tests that have better diagnostic and prognostic implications in the evaluation of sperm DNA damage. The basic principles, outline of methodology, advantage, disadvantage, clinical significance of each technique and implications of these tests have been discussed. The logistics of each test with respect to available resources and equipment in an andrology laboratory, the feasibility of performing these tests in routine diagnostic workup of infertile men and the opportunities and challenges provided by DNA testing in male fertility determination are also presented.
Keywords: Sperm DNA integrity; Semen analysis; DNA integrity tests; Comet, TUNEL, SCSA; Acridine orange test; Comet assay; Toluidine blue staining; Chromomycin A-3; Sperm chromatin dispersion; Infertility, recurrent spontaneous abortion, assisted reproduction technique
Introduction
Sperm DNA integrity is an important parameter of sperm quality in the prognosis of infertility and in the outcome of assisted reproductive procedures. In a basic andrology laboratory the assessment of the sperm quality relies on the World Health Organization [1, 2] (WHO) guidelines, which are poor predictors of reproductive outcome. In semen analysis, according to WHO guidelines sperm parameters as concentration, motility and morphology are emphasized. The conventional analysis establishes qualitative as well as quantitative threshold values for above mentioned parameters. Although fertile men as a group have higher mean sperm parameters (concentration, motility, and morphology) than infertile men, there is significant overlap between fertile and infertile men [3]. Approximately 15% of infertile men have a normal spermiogram [4]. Hence routine semen analysis may describe some aspect of the function of testis and sperm but they do not address the functional competence of the sperm attributed DNA integrity.
Supplementing the parameters referred in the WHO guidelines by additional tests of sperm function as sperm-zona pellucida binding and sperm- penetration [5] has not improved the IVF success rate significantly and helped nominally in clinical decision on treatment of patients with infertility. In addition, sperm function test do not have clinical value in ICSI since sperm bypass the zona pellucida and oolemma by injecting a single sperm directly into cytoplasm of oocyte. The use of sperm selection method by motile sperm organellar morphology examination (MSOME; done under 6600X) as an alternative to sperm function tests for ICSI patients [6] is unreliable due to the structural variability of human sperm and the observer subjectivity involved in such assessment. Due to this, the clinician cannot be sure that the sperm cells selected by MSOME for insemination represent those with the best reproductive potential. Moreover small sample size and limited number of studies weaken the credential of this technique as a tool for assessment of reproductive capacity of the sperm and therefore there exists lacunae in the diagnostic evaluation of male infertility for IVF/ICSI and infertility in general.
Recent studies have highlighted the significance of sperm DNA integrity as an important factor which affects functional competence of the sperm [7]. Different assays have been developed and applied in research laboratories to assess sperm DNA damage, which is more clinically informative and relevant. But so far very few andrology laboratories have implemented DNA integrity assessment as a part of routine semen analysis. Assessment of sperm DNA damage in patients opting for ART is crucial since these advanced assisted conception techniques bypass the natural selection barriers of conception and therefore sperm with high DNA damage may increase the possibility of transmitting the genetic aberrations to the conceptus and may affect the fetal and post natal development [8]. Such transfer of aberrant sperm genome may result in early pregnancy loss or birth of offspring with major or minor congenital malformation or even cancer.
The percentage of sperm with fragmented DNA is comparable in idiopathic infertile men having normal sperm parameters and in idiopathic infertile men with abnormal sperm parameters, which is significantly higher in both groups as compared to fertile controls [9–11].Thus standard sperm parameters (SSP) are poor predictors of functional competence of sperm. Recent study by Venkatesh et al., 2009 and Lewis et al., 2010 [12, 13] showed that there is no correlation between oxidative stress (OS) or DNA damage with SSP. So there is a need to have specific diagnostic tests for the assessment of OS and DNA damage
Studies both, in vitro and in vivo have shown that there is a negative correlation between sperm DNA integrity and fertility [14–20]. DNA damage is associated with poor embryo development, decreased implantation, and poor pregnancy outcome [21]. Birth of offspring with use of sperm with DNA damage results in increased chances of morbidity and childhood cancer [22, 23].
A study by [24] Bungum et al., 2004 has shown that 30% of men opting for ART have high percentage of sperm with DNA breaks. In our earlier studies [25] it was observed that in male partners of couples experiencing abortions 47.7% of sperm had high DNA damage, where as in infertile men with severe sperm pathologies 40.06% sperm had damaged DNA. Also these men had high levels of free radicals (reactive oxygen species; ROS) as detected by chemiluminescence, which is one of the leading cause of DNA damage [12] and such men may benefit immensely by early diagnosis and prompt antioxidant treatment [26].
The chromatin restructuring during spermiogenesis makes the sperm chromatin transcriptionally and translationally inert as a result of which the DNA repair capacity of the sperm is limited. The breaks in the DNA that may have escaped repair prior to compaction or damage occurring after chromatin remodelling has been completed are delivered to the oocyte. Accumulated products of oxidative DNA damage as ethenonucleosides impair nucleotide excision repair (NER) in oocyte [27, 28]. Consequently, the biological effect of abnormal sperm chromatin structure depends on both, the magnitude of sperm chromatin damage and the capacity of the oocyte to repair it after fertilization.
The etiology of sperm DNA damage is multifactorial. Sperm DNA fragmentation may result from aberrant chromatin packaging during spermatogenesis [22, 29, 30], defective apoptosis [31] excessive reactive oxygen species (ROS) production [12, 22, 29, 30, 32–35] decreased seminal antioxidants [26]. External factors such as drugs, pollution, cigarette smoking, fever, xenobiotics, high testicular temperature, varicocele, and advanced age have also been associated with increased sperm DNA damage [36–40].
A variety of assays have been developed to measure sperm DNA damage. These assays can be broadly categorized into two types. The first category include assays where DNA fragmentation is measured directly by incorporating probes at the site of damage, which detect actual DNA strand breaks. The signals from these probes quantify the DNA fragmentation in the sperm. Terminal deoxynucleotidyl transferase mediated dUTP nick end labeling assay (TUNEL) and in situ nick translation (ISNT) belong to this category. Additionally, the differential binding of the dyes with single stranded and double stranded DNA is utilized in assays belonging to this category. The second category includes assays which utilize the property of fragmented DNA to easy denaturation under certain conditions. Since nicked DNA denatures more easily than double-stranded DNA this approach measure the susceptibility of DNA to denaturation—that is, the formation of single-strand DNA from native double-strand DNA. Sperm chromatin dispersion test (SCD), Single cell gel electrophoresis (SCGE) or comet assay, sperm chromatin structure assay (SCSA), acridine orange staining are few examples, where the chromatin is subjected to denaturation treatment and then the DNA damage is quantified.
It is worthwhile to mention that in the available literature little information can be found regarding the differential assessment of mitochondrial DNA fragmentation and the nuclear DNA fragmentation in the sperm. As predicted and also reported by Kopper AJ et al., 2010 [41] mitochondrial genome being in proximity to the site of ROS generation is comparatively more susceptible to OS induced DNA damage, thus sperm mitochondria and mitochondrial DNA (mt DNA) are reduced to disposable elements at time of fertilization. Also during evolution majority of genes on mt DNA have translocated to the nucleus and only those genes that regulate electron transport and oxidative phosphorylation are retained in the mt genome. As mt DNA accumulate sequence variations it leads to mt dysfunctions, producing higher levels of free radicals and lower levels of ATP [42, 43].
Since the treatment of idiopathic infertility is mostly empirical therefore clinical validation and routine application of sperm DNA fragmentation assay in the workup of infertile men may help to decide the suitable therapeutics in these men and also minimize the risk of iatrogenic transmission of abnormalities to the offspring conceived through advanced assisted conception techniques.
Considering the significance of sperm DNA fragmentation assay and the lack of consensus for the most clinically relevant techniques, this review discusses basic principles, mechanisms, efficiency, logistics and research and clinical perspective of these tests.
Aetiology of sperm DNA damage
DNA integrity is defined as the absence of both single strand or double strand and breaks absence of nucleotide modifications in the DNA [44]. The loss of integrity in sperm DNA may occur at any level from the transformation of the spermatogonial germ cells to the ejaculated sperm, thereby the DNA damage may be present in the testicular sperm, epididymal sperm or the ejaculated sperm.
In transformation of mitotic spermatogonia to spermatocytes in meiosis, the DNA double strand breaks (DSB’s) are introduced and normally ligated after the crossing over. An unligated nick may transfer the DSB to the next phase of cell cycle i.e. round spermatids which if unrepaired in successive steps may be present in the ejaculated sperm. During spermiogenesis which is marked by conversion of round spermatid to elongated spermatid, the replacement of histones by protamines and the compaction of the genome take place. Improper chromatin packaging makes the sperm DNA more prone to damage. To relieve the torsional stress during protamination, both single strand breaks (SSB’s) and DSB are introduced in elongating spermatids [29, 30, 45, 46]. These breaks are temporary and are repaired in a healthy sperm but if unrepaired, they may lead to increased DNA fragmentation in the mature ejaculated sperm. During epididymal maturation, the protamine disulfide cross linking is completed, confering a highly compact structure to sperm chromatin. If the disulphide cross linking is incomplete it may lead to suboptimal compaction and therefore high degree of DNA fragmentation [47]. DNA damage incurred during sperm transit and storage in epididymis or post ejaculation cannot be repaired by sperm because post spermiogenesis there is negligible transcription and translation [28, 48].
Studies by Greco et al. [49], have shown that sperm DNA damage is higher in ejaculated sperm as compared to testicular sperm which supports the premise that sperm DNA damage load increases as the sperm transits from testis to epididymis and then to ejaculate.
As the sperm pass through epididymis it is exposed to ROS (free radicals), which are released by leukocytes, immature sperm or by dysfunctional mitochondrial metabolism. The electron loss from mitochondrial electron transport chain (ETC) in sperm leads to mt DNA mutations which further enhances ROS production [12] and leads to mitochondrial and nuclear DNA damage [25].
During the sperm maturation most of the antioxidants which are localized in the cytoplasm are lost so, antioxidant defense against the free radicals is mainly conferred by the seminal plasma. And if the seminal antioxidants are also compromised the sperm has a higher susceptibility to oxidative stress induced DNA damage [26].
The abortive apoptosis of fas expressed cells also contributes to sperm with DNA damage in the ejaculate. This dysfunctional apoptosis is responsible for presence of defective sperm in fertile men [50–52].
The damage in sperm DNA may not always be in the form of single or double strand breaks. The oxidizing capacity of ROS, produces nucleotide modifications or base loss. These cause aberrations in the chromatin packing and expose the genome to further oxidative insult [22, 44].
The most common type of nucleotide modification is 7,8-dihydro 8-oxo 2 deoxyguanosine (8-OH dG) an oxidative adduct of guanosine [53]. If sperm with DNA adducts are successful in achieving a pregnancy, paternally originating errors in DNA replication, transcription and translation can occur, potentially predisposing the offspring to a number of cancers and other degenerative disorders [54, 55]. Moreover, with time such base modifications may also lead to discrete DNA strand breaks [56].
Reduced expression of molecular factors HspA2 and p53 have also been shown to be associated with increased DNA damage. In an unpublished study from our laboratory increased number of non synonymous sequence variations were detected in p53 gene in infertile men. Expression levels of HspA2 are related to sperm maturity, DNA integrity, chromatin maturity, chromosomal aneuploidy frequency and sperm function, including fertilizing potential [57]. Reduced expression of HspA2 chaperone in the male germ cells leads to various defects during the meiotic phase and during spermiogenic maturation (delivery of DNA repair enzymes, cytoplasmic extrusion, plasma membrane remodelling), all of which may be interrupted in the absence of the HspA2 [58]. Optimal expression of p53 is essential for the elimination of sperm by apoptotic mechanism thereby reducing the sperm with DNA damage from the ejaculate. It also participates in many DNA repair mechanisms, the under expression of p53 may therefore lead to increased DNA damage in the sperm [59, 60].
These mechanisms of DNA damage are in vivo but the clinically induced DNA damage in ART procedures are an additional concern for the in vitro conceptions. Assisted conception procedures as ICSI has made the fertilization of oocyte possible by an immature testicular sperm, which are more vulnerable to damage than that of ejaculated sperm [49, 56], and have an incomplete methylation imprint. Since these sperm do not have the complete disulphide cross linking and even the epigenetic programming is incomplete this increases the risk of genetic and epigenetic defects in children conceived through these techniques [61]. Also these techniques require sperm preparation and processing in which the seminal plasma is removed by centrifugation and washing which makes sperm more vulnerable to oxidative damage [32].
Recent trends of delaying the age of parenthood have added to the use of DNA damaged sperm and oocyte with lower repair capacity. In a study by Singh NP et al. [62], it was found that percentage of DNA damaged sperm increased with paternal age. Use of centrifugation and freeze-thawing of cryopreserved sperm induces DNA damage. Study by [63] Holt W.V. 2000., showed that success rates with frozen samples are lower than with fresh sperm.
Basics of DNA integrity assays
Assays used to quantify DNA fragmentation are based on different principles. These assays in general utilize the difference in the properties of fragmented and non fragmented DNA.
In assays utilizing chromatin probes, DNA without nicks which is in supercoiled state has greater affinity for intercalating dyes as acridine orange because this reduces the free energy of torsion stress. On the contrary, the affinity for intercalation is low in relaxed DNA and is lost in fragmented DNA. The binding of dyes to the phosphate residues of fragmented DNA is observed when intercalation of dyes does not take place [64, 65].
The accessibility of DNA specific dyes is decreased by the protamination of sperm DNA since the positive charge on protamines neutralizes the negative charge of DNA. Therefore the intensity of fluorescence is low in sperm as compared to fluorescence intensity of round spermatid [66–68].
In assays, where the removal of nuclear proteins (eg by acid extraction/denaturation) is done the staining potential of DNA is dependent on the steric structure of probe and the interaction of probe with the substrate DNA [69]. The generation of ss DNA at DNA breaks by acid denaturation also forms the basis of DNA break quantification.
Chromatin proteins interact differentially to supercoiled DNA, relaxed DNA or fragmented DNA. This determines the affinity of probes to the substrate DNA and forms the mechanism for DNA damage assessment in various assays. The negative supercoils of intact sperm DNA, are supported by covalent bonds between nuclear matrix proteins, and tight ionic interactions between DNA and chromatin proteins [70]. Thus the binding of a probe is possible only through intercalation in the supercoiled DNA whereas in a fragmented DNA the loose interionic interactions between DNA and chromatin proteins allows the external binding of the dye to the DNA phosphate groups which produces a metachromatic shift (change of colour). Both mechanisms of dye binding, external and intercalating, compete within each constraint loop-domain (toroid) depending on its conformational state.
In assays where incorporation of probes is catalyzed by enzymes, the choice of enzyme determines the nature of DNA damage assessed for example, in in situ nick translation (ISNT), the template-dependent enzyme, DNA polymerase I incorporates probes at single stranded breaks where as terminal deoxynucleotidyl transferase incorporates probes at double stranded breaks in TUNEL assay. The pH conditions also affect the sensitivity of an assay eg. COMET assay quantifies single and/or double-stranded DNA breaks depending on the pH conditions. The difference in the pattern of forming a loop around lysed nuclear membrane carcass is used to differentiate the degree of intactness in chromatin deprived DNA in tests as sperm chromatin dispersion (SCD).
Tests for sperm DNA damage quantification
Acridine orange (AO) test
Acridine orange is a nucleic acid specific, fluorescent, cationic dye. It interacts with DNA by intercalation and by electrostatic interaction with RNA or single stranded DNA. When bound to ds DNA it has an excitation maximum at 502 nm and an emission maximum at 525 nm (green). When it associates with RNA or single stranded DNA produced by single stranded DNA breaks, the excitation maximum shifts to 460 nm (blue) and the emission maximum shifts to 650 nm (red). This metachromatic shift is utilised in DNA integrity assays for the quantification of DNA damage by AO test.
During the assay, mild acid treatment denatures DNA with single stranded or double stranded breaks. Acridine orange binds to ds DNA (non denatured) to produce green fluorescence while binding of acridine orange to single stranded DNA regions/ss DNA breaks produces red fluorescence. The measured parameter is number of cells with red fluorescence which is an approximation of DNA damaged sperm in the sample.
The technique utilizes flourescence microscope and is rapid, simple and inexpensive. On the other hand heterogeneous staining of slide and color fading shortly after staining are drawbacks of this technique. Another disadvantage of this staining technique is the indistinct colours i.e. a series of intermediate colors (between red and green) that relate with a differential sensibility to sperm denaturation. Inter-laboratory variations and lack of reproducibilty is also associated with this assay.
Sperm chromatin structure assay (SCSA)
The sperm chromatin structure assay (SCSA) is the flow cytometric version of acridine orange staining. Both SCSA and Acridine Orange Test measure the susceptibility of sperm nuclear DNA to acid-induced conformational transition in situ by quantifying the metachromatic shift of AO fluorescence from green (native DNA) to red (denatured or relaxed DNA). Compared to visual counting of red and green cells in AO test, in SCSA the red-green fluorescence is detected using a flow cytometer. Though the technique has high statistical robustness and inter- and intra-laboratory reproducibility, the assay requires a flow cytometer and a dedicated software. The ratio of red fluorescence to the total (sum of red and green fluorescence) gives the DNA fragmentation index of the sperm sample being analyzed unlike in AO test which gives the approximation of DNA in terms of number of damaged cells.
Aniline blue staining
Aniline blue is an acidic dye which has a greater permeability/affinity for proteins in the loose chromatin of sperm nucleus. This is due to the presence of the residual histones and increased accessibility of the basic groups of the nucleoprotein. Increased aniline blue staining of sperm indicates loose chromatin packing.
The technique is simple, inexpensive and requires a simple bright field microscope for the analysis. Though the results of aniline blue staining correlates well with AO test [71, 72], hetrogenous slide staining is a prominent drawback of this technique.
Chromomycin A3 (CMA3) staining
CMA3 is a fluorochrome specific for GC-rich sequences and interacts with DNA at the same site at which protamine binds with DNA. The extent of staining is related to the degree of protamination of mature spermatozoa [73, 74]. Therefore greater intensity of CMA3 staining indicates protamine deficiency or aberrant chromatin packing. Chromomycin A3 staining requires a fluorescence microscope and is inexpensive and simple. Inter-observer subjectivity in establishing classification groups is an important limitation of this assay.
Toluidine blue
Toluidine blue is a basic stain that stains phosphate residues of the sperm DNA with loosely packed chromatin and fragmented ends. When the stain attaches with lysine rich regions of histone it produces violet-bluish intense coloration whereas a pale-blue colour is produced with interactions with protamines in the chromatin. The sample can be analyzed using a ordinary microscope but intermediate coloration increases inter-user variability. Flow cytometer can also be used for evaluation. The results correlate well with SCSA and TUNEL.
In situ nick translation (ISNT)
In situ nick translation incorporates biotinylated dUTP at ss DNA breaks with template-dependent DNA polymerase I. The measured parameter is number of fluorescent sperm which represent incorporated dUTP. The ISNT is simple, inexpensive and requires only a fluorescence microscope for analysis. The limitation of this technique is that since it uses a template dependent polymerase it can only quantify single stranded breaks and also has low sensitivity as compared to other techniques.
Terminal deoxy nucleotidyl transferase mediated dUTP nick end labeling assay (TUNEL)
The TUNEL assay quantifies the incorporation of biotinylated dUTP at double strand breaks in DNA using a reaction catalyzed by template independent terminal deoxynucleotidyl transferase. The assay scores florescent cells with labeled DNA. TUNEL can be applied in both bright field and fluorescence microscopy, and also using flow cytometry. Unlike ISNT, which quantifies only single stranded breaks TUNEL is sensitive for both single and double stranded breaks. TUNEL correlates well with SCSA, comet and toluidine blue.
Sperm chromatin dispersion (SCD)
In SCD, the agarose embedded sperm are treated with a denaturing acid solution or alkaline solution to remove the nuclear proteins and to generate ss DNA from the nicks. The sperm are then subjected to lysis. The sperm with intact DNA produce a characteristic halo whereas in sperm with fragmented DNA the halo is either not observed or is minimal.
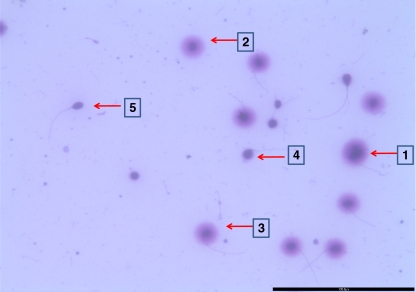
Sperm chromatin dispersion test is based on the ability of intact DNA deprived of chromatin proteins to loop around the sperm nucleus carcass [75–77]. It has been previously established that DNA breaks produces ssDNA from the ends of the DNA breaks after treatment with denaturing agents (eg, heat, acid, or alkali) [78]. With the increase in DNA breaks, more ssDNA is generated and the denaturing solution transforms the regions with extensive DNA breaks into ss DNA motifs [79, 80]. The generation of increased amounts of ssDNA is a necessary condition for suppressing the generation of DNA dispersion halos in sperm with fragmented DNA. The ss DNA interact within the sperm head in such a way that the removal of most nuclear proteins by lysing solutions does not result in dispersion of the DNA fragments. As evident in Fig. 1, sperm 1 to 3 have intact DNA (non fragmented DNA), on treating them with denaturing agents the large non fragmented DNA fragments, coiled with the nuclear protein deprived remains of the nucleus leading to the formation of halo. In sperm 4 and 5 small DNA fragments in absence of nuclear proteins could not disperse and therefore have small or no halo.
Fig. 1.
DNA integrity by sperm chromatin dispersion test. Sperm 1 to 3: Large halo- unfragmented DNA. Sperm 4 and 5: Small halo- fragmented DNA
Halos can be observed by bright field microscope if the staining is done by eosin and Azure B solution. If DNA directed fluorochromes are used, the analysis requires a fluorescence microscope. The technique is simple and does not require complex instrumentation. Inter-observer subjectivity to categorize the halos is an important setback to this technique which otherwise is a competent assay for DNA damage quantification.
Single cell gel electrophoresis (SCGE) or comet assay
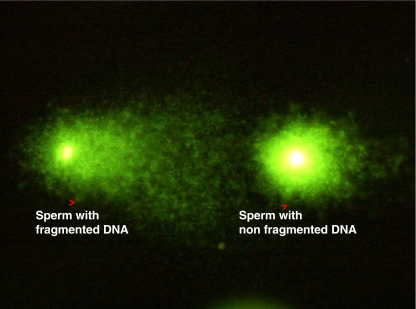
In the comet assay, sperm are sandwiched between agarose layers and then lysed and electrophoresed. The movement of fragmented DNA from a damaged sperm chromatin becomes visible as a comet with a tail. The assay is a microscopic variant of the normal electrophoresis in which the smaller DNA fragments migrate farther than the larger fragments. During the lysis step of comet assay the sulfhydryl groups in protamines are reduced which eases the movement of fragmented DNA on electrophoresis. The staining intensity and length of the comet tail represents the amount of migrated DNA, indicating different degrees of DNA fragmentation [81] (Fig. 2). Dyes such as propium iodide, SYBR-Green and YOYO-1 Iodide are used for staining. The major limitation of this assay is that it is labor intensive, has observer subjectivity and requires experience to evaluate the comets. Expensive softwares are commercially available to analyze the comets.
Fig. 2.
Comet image of sperm with fragmented and non fragmented DNA
Clinical aspects and relevance of DNA integrity tests
Intact sperm genomic integrity is an essential prerequisite for the successful reproductive outcome in vivo and in vitro. DNA integrity is a relatively independent parameter of sperm quality that gives diagnostic and prognostic information complementary to, but distinct from, that obtained from conventional sperm analysis. Numerous studies have indicated that evaluation of the sperm DNA fragmentation is more predictive of rate of conception and successful pregnancy outcome.
In a study by Evenson DP and Spano M [14, 82], probability of fertilization in natural conception and in IUI was found to be close to zero if the proportion of sperm cells with DNA damage exceeds 30% as detected by SCSA. Venkatesh S et al., 2011 proposed the threshold for DFI >30% in infertile men and also reported poor ART outcome when DFI was raised (>30%) [83]. Several other studies have reported a predictive threshold of 27% for successful pregnancy by IVF and ICSI as assessed by SCSA [16, 84]. If the DNA fragmentation is higher than 12% as detected by TUNEL assay low rates of pregnancy by IUI have been observed [85]. Sperm DNA damage is negatively correlated with embryo quality in IVF procedures [86]. In an ongoing study in our laboratory we found that men opting for ART had 39%% sperm with DNA damage when quantified by SCD (preliminary unpublished results), which is higher than the clinical accepted threshold (27–30%) of DNA damage for successful pregnancy.
In male partner of couples experiencing recurrent pregnancy loss it has been shown that sperm DNA damage is approximately 38% as compared to about 22% in fertile controls as detected by TUNEL [87]. Similar results for pregnancy loss were also reported by Virro et al., 2004 [87] using SCSA. Shamsi MB et al., 2011 reported DFI >24% in couples experiencing idiopathic RSA [88]. Since the paternal genome gets activated between four to eight cell stage in human embryos [89], therefore high DNA damage load presumably has no affect on the fertilization and manifests itself in the later stages of embryonic development [90].
A negative correlation between sperm DNA damage and quality of embryo, development of blastocyst, implantation rate has been found in men opting for ICSI when the DNA damage was assessed by ISNT and TUNEL [91–94].
The clinical significance of comet assay in correlating the DNA damage with sperm concentration, morphology, mitochondrial function and oocyte peneteration has been documented in previous studies [95–97]. In a study by O’Connell [98] it was reported that sperm isolated from semen by density centrifugation for ART have less DNA fragmentation. Furthermore, the predictive values of DNA damage in embryo quality [99] and pregnancy with ejaculated and testicular sperm [100] have also been reported.
In our previously reported work using alkaline comet assay, it was observed that oligozoospermic (O) men had 20%, asthenozoospermic (A) men had 24%, teratozoospermic (T) men had 28% and men with a combination of all these sperm pathologies (OAT) had 43% sperm with high DNA damage [44]. In a different subset of infertile men evaluated for DNA damage by SCD, we observed that infertile men with abnormal sperm parameters had 39% sperm with high DNA fragmentation as compared to 30% in infertile men with normal sperm parameters (unpublished results). In a similar study by Fernandez JL et al., 2005 [101] 27% sperm DNA damage was found in normozoospermic patients as compared to 16.3% in fertile controls. Study by Piasecka et al., 2006 [102] found that approximately 9% sperm of normozoospermic men had DNA damage as quantified by TUNEL assay. The DNA fragmentation Index, evaluated by SCSA assay, provides a weak or a moderate correlation with conventional sperm parameters (Giwercman A et al., 2003 and Peris SI et al. 2004 [103, 104]).
Though there is technique specific variation in the threshold value of DNA damage to predict the reproductive outcome, it has been suggested that sperm chromatin integrity is an independent index of sperm quality and has better diagnostic and prognostic potential in association with routine semen analysis for both in vivo and in vitro reproductive procedures.
Research laboratory aspects and relevance of DNA integrity tests
Several assays used to evaluate DNA structure and integrity in human sperm have different setbacks and advantages when performed practically in a research laboratory. Their correlation with other assays and accuracy in predicting the chances of conception or outcome of a pregnancy depends on precision with which a technique detects the actual pathological DNA damage. So far no single test is competent enough to detect the actual DNA damage load with cent percent accuracy to predict the reproductive profile of the sperm.
Among all the DNA integrity assays used, comet assay has the unique ability to measure DNA damage within an individual cell as opposed to an aggregate measure of damage versus undamaged as in tests like TUNEL, SCSA etc. This is an important feature with regard to study on infertility since semen is one of the most heterogeneous biological fluids in men. Selection of best subpopulation of sperm may help to achieve higher successful pregnancy rates and birth of healthy ART conceived offsprings. Comet assay has been used in vivo and in vitro in variety of cells to study the response to genotoxic stimuli as UV radiation, carcinogen, radiotherapy and chemotherapy. The other advantage of comet assay is that it requires fewer sperm (100 cells) for analysis so it is particularly useful for men with low sperm count and for DNA damage analysis on testicular sperm [100]. However for technical and biological reasons, the comet assay underestimates the true frequency of DNA breaks. This may be due to several possible causes: (i) masking, overlapping and entangling of migrating fragments (ii) incomplete chromatin decondensation may not allow all breaks to be revealed, (iii) due to loss of small pieces of DNA from agarose during various steps involved in the comet assay there may be fragments which are too small to be visualized. Thus the DNA damage observed is less than the actual DNA damage providing an approximate assessment for level of DNA damage [25].
The comet assay is exhaustively standardized by different research groups, each manipulating with the pH, temperature, salt concentrations, electrophoresis time etc. to produce a number of protocol variants with different sensitivities. The alkaline variant of the comet assay assesses both the single and double strand breaks and the alkali labile sites as compared to the neutral comet assay which measures only DSB’s. The alkaline comet assay overestimate true DNA strand breakage in spermatozoa because of artificial damage induced at alkali-labile sites within the DNA strand [105]. Regarding alkaline and neutral variant of comet assay different workers have varied opinion in deciding which is better. The alkaline comet assay cannot differentiate between single or double strand breaks, endogenous or induced breaks and moreover the alkali labile sites are not considered specific for infertility [106, 107]. The double strand breaks are difficult to repair by the oocyte DNA repair mechanism, making their evaluation more critical than single stranded breaks which can be repaired and hence have lower pathogenicity. On the contrary, few workers [108] suggest that since alkali labile sites are predisposed to single strand breaks under high pH, so their quantification along with native single and double strand breaks is more valuable for the overall assessment of sperm DNA damage.
Alkaline comet assay can detect damage equivalent to as few as 50 single-strand breaks (SSB) per cell. In the alkaline comet assay the sensitivity for the detection of single stranded breaks is provided by the use of alkaline lysis buffer which reverses DNA supercoiling and separates the DNA duplex into single strands. Additional sensitivity is provided by the use of proteinase k in the lysis buffer which removes protamine that otherwise impedes DNA migration through the agarose. During standardization of the sperm comet protocol in our laboratory we observed that this step is most crucial since protamination in sperm confer tight packing, and the lysis requires more stringent conditions compared to a somatic cell which has only histones as nuclear proteins.
The most commonly used parameters to express DNA damage in comet assay are length of tail (length of tail measured from periphery of comet head core),% tail DNA (percentage of DNA in the tail compared to the percentage of DNA in the head or unfragmented DNA), olive tail moment (OTM; integral function of DNA in tail and pixel fluorescence of tail and head). Tail length can be estimated by visual scoring through a microscope but OTM and% tail DNA requires specific commercially available softwares [44].
Unlike comet assay which only measures the DNA breaks, ISNT measures DNA breaks and the efficiency of protamination which decreases the specificity of the assay [109, 110]. The other disadvantage of ISNT is that it labels less than 10% of all spermatozoa even in the patient group [86].
The ISNT relies on the access of DNA polymerase I to the genome, which correlates with level of protamination [73]. Higher the degree of incomplete protamination of the sperm genome greater is the degree of nick end labeling. ISNT incorporates a measure of chromatin condensation that in turn reflects the quality of the processes controlling the differentiation and maturation of the spermatozoa thus this assay has an additional diagnostic significance over other assays which reveal only the degree of fragmentation in the sperm genome.
TUNEL assay labels the blunt 3′ OH ends of double strand breaks by the terminal deoxynucleotidyl transferase (TdT). The disadvantage of TUNEL assay is that since it does not include the lysis step, the accessibility of TdT to all the 3′OH ends in tightly packed sperm genome is limited [111]. As a result of which the suggested clinical thresholds is lower (18%) in different studies [85, 112–114] as compared with the established threshold of 27-30%. A recent study by Mitchell LA et al. 2010 [115], has suggested an improvised TUNEL assay which provides solution to increase the accessibility of TdT and also incorporates the assessment of the vitality in the same flow cytometry assay. In the modified TUNEL assay the use of dithiothreitol (DTT) was suggested which breaks the disulphide linkage between adjacent protamine molecules, relaxing the chromatin and thereby allowing the TdT to access the DNA strand breaks within sperm nucleus.
The SCSA has higher reproducibility and is clinically more validated than other techniques for DNA integrity assessment. The specificity of SCSA is lower than alkaline comet assay, ISNT or TUNEL since it detects DNA fragmentation, protamine content and disulphide cross linkage as well. SCSA can also quantify immature sperm since they have higher than normal stainibility (high density stainibility), though it can contaminate the fluorescence emitted from SSB and DSB thus generating an over estimation DNA fragmentation than actual.
SCD like comet assay requires the sperm to be embedded in the agarose but without electrophoresis, thus it is comparatively fast and easy. Neither does it requires colour or flouresence determination which makes its interpretation simple and without the use of any complex instrument. During the SCD, processing of agarose embedded sperm remove the protamine molecules. This removal leads to breakage of disulfide bonds in the otherwise tightly looped and compact sperm genome. As the disulfide bonds break, the loops of DNA relax, forming haloes around the residual nuclear central structure. Spermatozoa with fragmented DNA showed evidence of restricted DNA loop dispersions, showing very limited haloes or absence of them, unlike the sperm with non-fragmented DNA [77]. Since some sperm cells may have different nuclear sizes, it may lead to the variations in size of halo for sperm with equal fragmentation.
Though the application of the DNA integrity assays in andrology laboratory are low but with increased use of micromanipulative reproductive techniques and with reports which implicate DNA damage as an important causal factor in infertility and recurrent spontaneous abortions in large number of men, these test have gained significance. It is necessary to consider several issues when evaluating studies of sperm DNA integrity. Although many assays determine DNA fragmentation, but not all DNA nicks are detrimental. The methods used to assess sperm DNA status do not selectively differentiate between clinically important and clinically insignificant fragmentation. The present assays do not discriminate the physiological breaks with the pathological ones, since few nicks occur as a normal process during winding and unwinding of DNA. Moreover, the assays are incapable to evaluate the genes affected by fragmentation, which is significant since fragmentation in the inactive region of genome is not as detrimental as in the active regions of genome. This approach gives a higher level of percentage of sperm with DNA fragmentation and does not assess the actual level of pathological DNA fragmentation. Definitely, these drawbacks in these assays, have led to the ambiguity over establishing threshold values of sperm DNA fragmentation. However these assays have better diagnostic and prognostic value and far reaching clinical implications than traditional semen parameters.
Conclusion
Accumulated data shows that DNA damage correlates well with the reproductive potential of the sperm, its functional competence and therefore DNA damage assessment has a better predictive score than the conventional semen analysis. DNA integrity assays have clinical implications that are more informative and may be used to complement the classical parameters used in semen evaluation. Assisted conception techniques and in particular ICSI have a high probability of using a sperm, which otherwise would not achieve pregnancy spontaneously, so it becomes important to evaluate the quality of sperm genome. Moreover long term consequences of using sperm with compromised DNA integrity are unknown so it is imperative to improve the current assays or to develop a simplified novel DNA integrity tests which could be performed in a basic andrology laboratory and is robust enough to overcome the limitations of the presently used assays. Secondly, the modifications in the present assays or the development of a new assay should be oriented to non destructive determination of sperm reproductive potential so that the same germ cell could be used for fertilization; this would provide safe and effective diagnostics in cases opting for assisted reproduction technology.
Until further research develops a new assay for sperm DNA integrity which is more accurate and has higher predictive value for pathological DNA damage and can exactly calculate DNA damage per sperm, supplementing conventional semen analysis with a combination of assays could help to establish the etiology of infertility. This would further help to direct the case specific treatment which could be given to infertile men. There are varied reports [116–118] on the efficiency of various antioxidant therapy in infertile men. Recent meta analysis [119, 120] of these studies suggest that antioxidant therapy is beneficial and improves various indices of male fertility. However antioxidants should only be administered in cases with increased free radical levels as majority of enzymatic reactions in our body functions under redox conditions. Also minimal life style modifications like quitting smoking, reducing/stopping alcohol intake, exercise in moderation, prompt treatment of systemic and localized testicular inflammation/infection and varicocelectomy may benefit such cases.
To conclude sperm plays dynamic role which extends beyond fertilization and is critical for optimal embryonic development and birth of healthy offspring. Thus tests for analysis of sperm DNA have better diagnostic and prognostic value.
Footnotes
Capsule Sperm DNA integrity is an important parameter in assessment of sperm quality and has a predictive value in infertility & idiopathic recurrent spontaneous abortions following natural and assisted conception.
References
- 1.WHO laboratory manual for the examination of human semen and semen–cervical mucus interaction. 4. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 2.WHO Laboratory manual for the examination and processing of human semen. 5th ed. Cambridge: Cambridge University Press; 2010.
- 3.Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, et al. National Cooperative Reproductive Medicine Network. Sperm morphology, motility, and concentration in infertile and fertile men. New Engl J Med. 2001;345:1388–1393. doi: 10.1056/NEJMoa003005. [DOI] [PubMed] [Google Scholar]
- 4.Guzick DS, Sullivan MW, Adamson GD, Cedars MI, Falk RJ, Peterson EP, et al. Efficacy of treatment for unexplained infertility. Fertil Steril. 1998;70(2):207–213. doi: 10.1016/S0015-0282(98)00177-0. [DOI] [PubMed] [Google Scholar]
- 5.Liu DY, Baker HW. Evaluation and assessment of semen for IVF/ICSI. Asian J Androl. 2002;4(4):281–285. [PubMed] [Google Scholar]
- 6.Berkovitz A, Eltes F, Lederman H, Peer S, Ellenbogen A, Feldberg B, et al. How to improve IVF-ICSI outcome by sperm selection. Reprod Biomed Online. 2006;12(5):634–638. doi: 10.1016/S1472-6483(10)61191-1. [DOI] [PubMed] [Google Scholar]
- 7.Lewis SE, Agbaje I, Alvarez J. Sperm DNA tests as useful adjuncts to semen analysis. Syst Biol Reprod Med. 2008;54(3):111–125. doi: 10.1080/19396360801957739. [DOI] [PubMed] [Google Scholar]
- 8.Zini A. Are sperm chromatin and DNA defects relevant in the clinic? Syst Biol Reprod Med. 2011;57:78–85. doi: 10.3109/19396368.2010.515704. [DOI] [PubMed] [Google Scholar]
- 9.Host E, Lindenberg S, Smidt-Jensen S. DNA strand breaks in human spermatozoa: correlation with fertilization in vitro in oligozoospermic men and in men with unexplained infertility. Acta Obstet Gynecol Scand. 2000;79:189–193. doi: 10.1080/j.1600-0412.2000.079003189.x. [DOI] [PubMed] [Google Scholar]
- 10.Host E, Lindenberg S, Ernst E, Christensen F. DNA strand breaks in human spermatozoa: a possible factor, to be considered in couples suffering from unexplained infertility. Acta Obstet Gynecol Scand. 1999;78:622–625. doi: 10.1080/j.1600-0412.1999.780710.x. [DOI] [PubMed] [Google Scholar]
- 11.Saleh RA, Agarwal A, Nelson DE, Nada EA, El-Tonsy MH, Alvarez JG, et al. Increased sperm nuclear DNA damage in normozoospermic infertile men: a prospective study. Fertil Steril. 2002;78:313–318. doi: 10.1016/S0015-0282(02)03219-3. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesh S, Riyaz AM, Shamsi MB, Kumar R, Gupta NP, Mittal S, et al. Clinical significance of reactive oxygen species in semen of infertile Indian men. Andrologia. 2009;41(4):251–256. doi: 10.1111/j.1439-0272.2009.00943.x. [DOI] [PubMed] [Google Scholar]
- 13.Lewis SEM, Luke S. Clinical implications of sperm DNA damage. Hum Fertil. 2010;13(4):201–207. doi: 10.3109/14647273.2010.528823. [DOI] [PubMed] [Google Scholar]
- 14.Spano M, Bonde JP, Hjollund HI, Kolstad HA, Cordelli E, Leter G. Sperm chromatin damage impairs human fertility. The Danish First Pregnancy Planner Study Team. Fertil Steril. 2000;73:43–50. doi: 10.1016/S0015-0282(99)00462-8. [DOI] [PubMed] [Google Scholar]
- 15.Evenson DP, Jost LK. Sperm chromatin structure assay is useful for fertility assessment. Meth Cell Sci. 2000;22:169–189. doi: 10.1023/A:1009844109023. [DOI] [PubMed] [Google Scholar]
- 16.Larson-Cook KL, Brannian JD, Hansen KA, Kasperson KM, Aamold ET, Evenson DP. Relationship between the outcomes of assisted reproductive techniques and sperm DNA fragmentation as measured by the sperm chromatin structure assay. Fertil Steril. 2003;80:895–902. doi: 10.1016/S0015-0282(03)01116-6. [DOI] [PubMed] [Google Scholar]
- 17.Saleh RA, Agarwal A, Nada ES, El-Tonsy MH, Sharma RK, Meyer A. Negative effects of increased sperm DNA damge in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril. 2003;79(Suppl 3):1597–1605. doi: 10.1016/S0015-0282(03)00337-6. [DOI] [PubMed] [Google Scholar]
- 18.Gandini L, Lombardo F, Paoli D, Caruso F, Eleuteri P, Leter G, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19:1409–1417. doi: 10.1093/humrep/deh233. [DOI] [PubMed] [Google Scholar]
- 19.Check JH, Graziano V, Cohen R, Krotec J, Check ML. Effect of an abnormal sperm chromatin structural assay (SCSA) on pregnancy outcome following (IVF) with ICSI in previous IVF failures. Arch Androl. 2005;51:121–124. doi: 10.1080/014850190518125. [DOI] [PubMed] [Google Scholar]
- 20.Evenson D, Wixon R. Meta-analysis of sperm DNA fragmentation using the sperm chromatin structure assay. Reprod Biomed Online. 2006;12:466–472. doi: 10.1016/S1472-6483(10)62000-7. [DOI] [PubMed] [Google Scholar]
- 21.Fernández-Gonzalez R, Moreira PN, Pérez-Crespo M, Sánchez-Martín M, Ramirez MA, Pericuesta E, et al. Long term effects of mouse intracytoplasmic sperm injection with DNA fragmented sperm on health and behavior of adult offspring. Biol Reprod. 2008;78(4):761–772. doi: 10.1095/biolreprod.107.065623. [DOI] [PubMed] [Google Scholar]
- 22.Shamsi MB, Kumar R, Dada R. Evaluation of nuclear DNA damage in human spermatozoa in men opting for assisted reproduction. Indian J Med Res. 2008;127(2):115–123. [PubMed] [Google Scholar]
- 23.Agarwal A, Allameneni SSR. The effect of sperm DNA damage in assisted reproduction outcomes. Mineva Ginecol. 2004;56:235–245. [PubMed] [Google Scholar]
- 24.Bungum M, Humaidan P, Spano M, Jepson K, Bungum L, Giwercman A. The predictive value of sperm chromatin structure assay (SCSA) parameters for the outcome of intrauterine insemination, IVF and ICSI. Hum Reprod. 2004;19:1401–1408. doi: 10.1093/humrep/deh280. [DOI] [PubMed] [Google Scholar]
- 25.Shamsi MB, Venkatesh S, Tanwar M, Singh G, Mukherjee S, Malhotra N, et al. Comet assay: a prognostic tool for DNA integrity assessment in infertile men opting for assisted reproduction. Indian J Med Res. 2010;131:675–681. [PubMed] [Google Scholar]
- 26.Shamsi MB, Venkatesh S, Kumar R, Gupta NP, Malhotra N, Singh N, et al. Antioxidant levels in blood and seminal plasma and their impact on sperm parameters in infertile men. Indian J Biochem Biophys. 2010;47(1):38–43. [PubMed] [Google Scholar]
- 27.Ahmadi A, Ng SC. Fertilizing ability of DNA-damaged spermatozoa. J Exp Zool. 1999;284:696–704. doi: 10.1002/(SICI)1097-010X(19991101)284:6<696::AID-JEZ11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 28.Steger K, Cavalcanti MC, Schuppe HC. Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int J Androl. 2010, Dec 3. doi:10.1111/j.1365-2605.2010.01129.x. [Epub ahead of print]. [DOI] [PubMed]
- 29.Boissonneault G. Chromatin remodeling during spermiogenesis: a possible role for the transition proteins in DNA strand break repair. FEBS Lett. 2002;514:111–114. doi: 10.1016/S0014-5793(02)02380-3. [DOI] [PubMed] [Google Scholar]
- 30.McLay DW, Hugh J. Clarke remodelling the paternal chromatin at fertilization in mammals. Reproduction. 2003;125:625–633. doi: 10.1530/rep.0.1250625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sakkas D, Moffatt O, Manicardi GC, Mariethoz E, Tarozzi N, Bizzaro D. Nature of DNA damage in ejaculated human spermatozoa and the possible involvement of apoptosis. Biol Reprod. 2002;66:1061–1067. doi: 10.1095/biolreprod66.4.1061. [DOI] [PubMed] [Google Scholar]
- 32.Venkatesh S, Shamsi MB, Dudeja S, Kumar R, Dada R. Reactive oxygen species measurement in neat and washed semen: comparative analysis and its significance in male infertility assessment. Arch Gynecol Obstet. 2011 Jan;283(1):121–6. Epub 2010 Sep 3. PMID: 20814688. [DOI] [PubMed]
- 33.Saalu LC. The incriminating role of reactive oxygen species in idiopathic male infertility: an evidence based evaluation. Pak J Biol Sci. 2010;13(9):413–422. doi: 10.3923/pjbs.2010.413.422. [DOI] [PubMed] [Google Scholar]
- 34.Aitken RJ, Baker MA, Iuliis GN, Nixon B. New insights into sperm physiology and pathology. Handb Exp Pharmacol. 2010;198:99–115. doi: 10.1007/978-3-642-02062-9_7. [DOI] [PubMed] [Google Scholar]
- 35.Moustafa MH, Sharma RK, Thornton J, Mascha E, Abdel-Hafez MA, Thomas AJ, Jr, et al. Relationship between ROS production, apoptosis and DNA denaturation in spermatozoa from patients examined for infertility. Hum Reprod. 2004;19:129–138. doi: 10.1093/humrep/deh024. [DOI] [PubMed] [Google Scholar]
- 36.Weber RF, Dohle GR, Romijn JC. Clinical laboratory evaluation of male subfertility. Adv Clin Chem. 2005;40:317–364. doi: 10.1016/S0065-2423(05)40008-6. [DOI] [PubMed] [Google Scholar]
- 37.Rubes J, Selevan SG, Evenson DP, Zudova D, Vozdova M, Zudova Z, et al. Episodic air pollution is associated with increased DNA fragmentation in human sperm without other changes in semen quality. Hum Reprod. 2005;20(10):2776–2783. doi: 10.1093/humrep/dei122. [DOI] [PubMed] [Google Scholar]
- 38.Colagar AH, Jorsaraee GA, Marzony ET. Cigarette smoking and the risk of male infertility. Pak J Biol Sci. 2007;10(21):3870–3874. doi: 10.3923/pjbs.2007.3870.3874. [DOI] [PubMed] [Google Scholar]
- 39.Soares SR, Melo MA. Cigarette smoking and reproductive function. Curr Opin Obstet Gynecol. 2008;20(3):281–291. doi: 10.1097/GCO.0b013e3282fc9c1e. [DOI] [PubMed] [Google Scholar]
- 40.Enciso M, Muriel L, Fernandez JL, Goyanes V, Segrelles E, Marcos M, et al. Infertile men with varicocele show a high relative proportion of sperm cells with intense nuclear damage level, evidenced by the sperm chromatin dispersion test. J Androl. 2006;27(1):106–111. doi: 10.2164/jandrol.05115. [DOI] [PubMed] [Google Scholar]
- 41.Koppers AJ, Garg ML, Aitken RJ. Stimulation of mitochondrial reactive oxygen species production by unesterified, unsaturated fatty acids in defective human spermatozoa. Free Radical Biology & Medicine. 2010;48:112–119. doi: 10.1016/j.freeradbiomed.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 42.St. John JC, Sakkas D, Barrat CL. A role of mitochondrial DNA and sperm survival. J Androl. 2000;21:189–199. [PubMed] [Google Scholar]
- 43.Gottlieb R. Mitochondria and apoptosis. Biol Signal Recep. 2001;10:147–161. doi: 10.1159/000046884. [DOI] [PubMed] [Google Scholar]
- 44.Shamsi MB, Venkatesh S, Tanwar M, Talwar P, Sharma RK, Dhawan A, et al. DNA integrity and semen quality in men with low seminal antioxidant level. Mutat Res Fundam Mol Mech Mutagen. 2009;665(1–2):29–36. doi: 10.1016/j.mrfmmm.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 45.Aitken RJ, Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16(1):3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 46.Tarozzi N, Bizzaro D, Flamigni C, Borini A. Clinical relevance of sperm DNA damage in assisted reproduction. Reprod Biomed Online. 2007;14(6):746–757. doi: 10.1016/S1472-6483(10)60678-5. [DOI] [PubMed] [Google Scholar]
- 47.Ozmen B, Koutlaki N, Youssry M, Diedrich K, Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online. 2007;14:384–395. doi: 10.1016/S1472-6483(10)60883-8. [DOI] [PubMed] [Google Scholar]
- 48.Aitken RJ, Koopman P, Lewis SE. Seeds of concern. Nature. 2004;432:48–52. doi: 10.1038/432048a. [DOI] [PubMed] [Google Scholar]
- 49.Greco E, Romano S, Iacobelli M, Ferrero S, Baroni E, Minasi MG, et al. ICSI in cases of sperm DNA damage: beneficial effect of oral antioxidant treatment. Hum Reprod. 2005;20:2590–2594. doi: 10.1093/humrep/dei091. [DOI] [PubMed] [Google Scholar]
- 50.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Canc Res. 1993;53:1945–1951. [PubMed] [Google Scholar]
- 51.McVicar CM, McClure N, Williamson K, Dalzell LH, Lewis SE. Incidence of Fas positivity and deoxyribonucleic acid. Fertil Steril. 2004 Mar;81 Suppl 1:767–74. [DOI] [PubMed]
- 52.Soleimani M, Tavalaee M, Aboutorabi R, Adib M, Bahramian H, Janzamin E, et al. Evaluation of Fas positive sperm and complement mediated lysis in subfertile individuals. J Assist Reprod Genet. 2010;27(8):477–482. doi: 10.1007/s10815-010-9425-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iuliis GN, Thomson LK, Mitchell LA, Finnie JM, Koppers AJ, Hedges A, et al. DNA damage in human spermatozoa is highly correlated with the efficiency of chromatin remodeling and the formation of 8-hydroxy-20-deoxyguanosine a marker of oxidative stress. Biol Reprod. 2009;81:517–524. doi: 10.1095/biolreprod.109.076836. [DOI] [PubMed] [Google Scholar]
- 54.Baker MA, Aitken RJ. Reactive oxygen species in spermatozoa: methods for monitoring and significance for the origins of genetic disease and infertility. Reprod Biol Endocrinol. 2005;3:67. doi: 10.1186/1477-7827-3-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. FASEB J. 2003;17:1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 56.Alvarez JG. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular sperm. Hum Reprod. 2005;20:2031–2032. doi: 10.1093/humrep/deh814. [DOI] [PubMed] [Google Scholar]
- 57.Cayli S, Jakab A, Ovari L, Delpiano E, Celik-Ozenci C, Sakkas D, et al. Biochemical markers of sperm function: male fertility and sperm selection for ICSI. Reprod Biomed Online. 2003;7(4):462–468. doi: 10.1016/S1472-6483(10)61891-3. [DOI] [PubMed] [Google Scholar]
- 58.Kovanci E, Kovacs T, Moretti E, Vigue L, Ward PB, Ward DC, et al. FISH assessment of aneuploidy frequencies in mature and immature human spermatozoa classified by the presence or absence of cytoplasmic retention. Hum Reprod. 2001;16(6):1209–1217. doi: 10.1093/humrep/16.6.1209. [DOI] [PubMed] [Google Scholar]
- 59.Chang FW, Sun GH, Cheng YY, Chen IC, Chien HH, Wu GJ. Effects of varicocele upon the expression of apoptosis-related proteins. Andrologia. 2010;42(4):225–230. doi: 10.1111/j.1439-0272.2009.00981.x. [DOI] [PubMed] [Google Scholar]
- 60.Paul C, Povey JE, Lawrence NJ, Selfridge J, Melton DW, Saunders PT. Deletion of genes implicated in protecting the integrity of male germ cells has differential effects on the incidence of DNA breaks and germ cell loss. PLoS One. 2007;2(10):e989. doi: 10.1371/journal.pone.0000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajender S, Avery K, Agarwal A. Epigenetics, spermatogenesis and male infertility. Mutat Res. 2011;727(3):62–71. doi: 10.1016/j.mrrev.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 62.Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80(6):1420–1430. doi: 10.1016/j.fertnstert.2003.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Holt WV. Basic aspects of frozen storage of semen. Anim Reprod Sci. 2000;62:3–22. doi: 10.1016/S0378-4320(00)00152-4. [DOI] [PubMed] [Google Scholar]
- 64.Erenpreisa EA, Zirne RA, Zaleskaia ND, S’iakste TG. Effect of single-stranded breaks on the ultrastructural organization and cytochemistry of the chromatin in tumor cells. Biull Eksp Biol Med. 1988;106:591–593. doi: 10.1007/BF00840855. [DOI] [PubMed] [Google Scholar]
- 65.Erenpreisa EA, Sondore O, Zirne RA. Conformational changes in the chromatin of tumor cells and the phenomenon of nuclear achromasia. Eksp Onkol. 1988;10(2):54–57. [PubMed] [Google Scholar]
- 66.Brewer LR, Corzett M, Balhorn R. Protamine-induced condensation and decondensation of the same DNA molecule. Science. 1999;286:120–123. doi: 10.1126/science.286.5437.120. [DOI] [PubMed] [Google Scholar]
- 67.Brewer L, Corzett M, Balhorn R. Condensation of DNA by spermatid basic nuclear proteins. J Biol Chem. 2002;277:38895–38900. doi: 10.1074/jbc.M204755200. [DOI] [PubMed] [Google Scholar]
- 68.Brewer L, Corzett M, Lau EY, Balhorn R. Dynamics of protamine 1 binding to single DNA molecules. J Biol Chem. 2003;278:42403–42408. doi: 10.1074/jbc.M303610200. [DOI] [PubMed] [Google Scholar]
- 69.Evenson D, Darzynkiewicz Z, Jost L, Janca F, Ballachey B. Changes in accessibility of DNA to various fluorochromes during spermatogenesis. Cytometry. 1986;7:45–53. doi: 10.1002/cyto.990070107. [DOI] [PubMed] [Google Scholar]
- 70.Benyajati C, Worcel A. Isolation, characterization, and structure of the folded interphase genome of Drosophila melanogaster. Cell. 1976;9:393–407. doi: 10.1016/0092-8674(76)90084-2. [DOI] [PubMed] [Google Scholar]
- 71.Erenpreiss J, Bars J, Lipatnikova V, Erenpreisa J, Zalkalns J. Comparative study of cytochemical tests for sperm chromatin integrity. J Androl. 2001;22:45–53. [PubMed] [Google Scholar]
- 72.Liu DY, Baker HW. Sperm nuclear chromatin normality: relationship with sperm morphology, sperm-zona pellucid binding, and fertilization rates in vitro. Fertil Steril. 1992;58:1178–1184. doi: 10.1016/s0015-0282(16)55566-6. [DOI] [PubMed] [Google Scholar]
- 73.Manicardi GC, Bianchi PG, Pantano S, Azzoni P, Bizzaro D, Bianchi U, et al. Presence of endogenous nicks in DNA of ejaculated human spermatozoa and its relationship to chromomycin A3 accessibility. Biol Reprod. 1995;52:864–867. doi: 10.1095/biolreprod52.4.864. [DOI] [PubMed] [Google Scholar]
- 74.Bianchi PG, Manicardi GC, Bizzaro D, Bianchi U, Sakkas D. Effect of deoxyribonucleic acid protamination on fluorochrome staining and in situ nick-translation of murine and human mature spermatozoa. Biol Reprod. 1993;49:1083–1088. doi: 10.1095/biolreprod49.5.1083. [DOI] [PubMed] [Google Scholar]
- 75.Ankem MK, Mayer E, Ward WS, Cummings KB, Barone JG. Novel assay for determining DNA organization in human spermatozoa: implications for male factor infertility. Urology. 2002;59:575–578. doi: 10.1016/S0090-4295(01)01619-3. [DOI] [PubMed] [Google Scholar]
- 76.Ward WS, Kimura Y, Yanagimachi R. An intact sperm nuclear matrix may be necessary for the mouse paternal genome to participate in embryonic development. Biol Reprod. 1999;60:702–706. doi: 10.1095/biolreprod60.3.702. [DOI] [PubMed] [Google Scholar]
- 77.Fernandez JL, Muriel L, Rivero MT, Goyanes V, Vazquez R, Alvarez JG. The sperm chromatin dispersion test: a simple method for the determination of sperm DNA fragmentation. J Androl. 2003;24:59–66. [PubMed] [Google Scholar]
- 78.Ahnström G. Techniques to measure DNA strand breaks in cells: a review. Int J Radiat Biol. 1988;54:695–707. doi: 10.1080/09553008814552151. [DOI] [PubMed] [Google Scholar]
- 79.Gorczyca W, Gong J, Darzynkiewicz Z. Detection of DNA strand breaks in individual apoptotic cells by the in situ terminal deoxynucleotidyl transferase and nick translation assays. Canc Res. 1993;53:945–951. [PubMed] [Google Scholar]
- 80.Gorczyca W, Traganos F, Jesionowska H, Darzynkiewicz Z. Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp Cell Res. 1993;207:202–205. doi: 10.1006/excr.1993.1182. [DOI] [PubMed] [Google Scholar]
- 81.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 82.Evenson DP, Jost LK, Marshall D, Zinaman MJ, Clegg E, Purvis K, et al. Utility of the sperm chromatin structure assay as a diagnostic and prognostic tool in the human fertility clinic. Hum Reprod. 1999;1999(14):1039–1049. doi: 10.1093/humrep/14.4.1039. [DOI] [PubMed] [Google Scholar]
- 83.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011. [DOI] [PubMed]
- 84.Larson KL, DeJonge CJ, Barnes AM, Jost LK, Evenson DP. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum Reprod. 2000;15:1717–1722. doi: 10.1093/humrep/15.8.1717. [DOI] [PubMed] [Google Scholar]
- 85.Duran EH, Morshedi M, Taylor S, Oehninger S. Sperm DNA quality predicts intrauterine insemination outcome: a prospective cohort study. Hum Reprod. 2002;17:3122–3128. doi: 10.1093/humrep/17.12.3122. [DOI] [PubMed] [Google Scholar]
- 86.Tomlinson MJ, Moffatt O, Manicardi GC, Bizzaro D, Afnan M, et al. Interrelationships between seminal parameters and sperm nuclear DNA damage before and after density gradient centrifugation: implications for assisted conception. Hum Reprod. 2001;16:2160–2165. doi: 10.1093/humrep/16.10.2160. [DOI] [PubMed] [Google Scholar]
- 87.Carrell DT, Liu L, Peterson CM, Jones KP, Hatasaka HH, Erickson L, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch Androl. 2003;49:49–55. doi: 10.1080/01485010290099390. [DOI] [PubMed] [Google Scholar]
- 88.Shamsi MB, Venkatesh S, Pathak D, Deka D, Dada R. Sperm DNA damage & oxidative stress in recurrent spontaneous abortion (RSA) Indian J Med Res. 2011;133(5):550–551. [PMC free article] [PubMed] [Google Scholar]
- 89.Virro MR, Larson-Cook KL, Evenson DP. Sperm chromatin structure assay (SCSA) parameters are related to fertilization, blastocyst development, and ongoing pregnancy in in vitro fertilization and intracytoplasmic sperm injection cycles. Fertil Steril. 2004;81:1289–1295. doi: 10.1016/j.fertnstert.2003.09.063. [DOI] [PubMed] [Google Scholar]
- 90.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332:459–461. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 91.Tesarik J, Greco E, Mendoza C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum Reprod. 2004;19:611–615. doi: 10.1093/humrep/deh127. [DOI] [PubMed] [Google Scholar]
- 92.Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82(2):378–383. doi: 10.1016/j.fertnstert.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 93.Mauri AL, Oliveira JBA, Baruffi RLR, Petersen CG, Vagnini LD, Massaro FC, Silva LFI, Nicoletti APM, Franco JG. Significance of extruded nuclear chromatin (regional nuclear shape malformation) in human spermatozoa: implications for ICSI. International Journal of Andrology. 2011. doi:10.1111/j.1365-2605.2010.01119.x. [DOI] [PubMed]
- 94.Henkel R, Hoogendijk CF, Bouic PJ, Kruger TF. TUNEL assay and SCSA determine different aspects of sperm DNA damage. Andrologia. 2010;42(5):305–313. doi: 10.1111/j.1439-0272.2009.01002.x. [DOI] [PubMed] [Google Scholar]
- 95.Donnelly ET, Steele EK, McClure N, Lewis SE. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Hum Reprod. 2001;16:1191–1199. doi: 10.1093/humrep/16.6.1191. [DOI] [PubMed] [Google Scholar]
- 96.Donnelly ET, O’Connell M, McClure N, Lewis SE. Differences in nuclear DNA fragmentation and mitochondrial integrity of semen and prepared human spermatozoa. Hum Reprod. 2000;15:1552–1561. doi: 10.1093/humrep/15.7.1552. [DOI] [PubMed] [Google Scholar]
- 97.Chan PJ, Corselli JU, Patton WC, Jacobson JD, Chan SR, King A. A simple alkaline comet assay for archived sperm correlates DNA fragmentation to reduced hyperactivation and penetration of zona-free hamster oocytes. Fertil Steril. 2001;75:186–192. doi: 10.1016/S0015-0282(00)01655-1. [DOI] [PubMed] [Google Scholar]
- 98.O’Connell M, McClure N, Powell LA, Steele EK, Lewis SE. Differences in mitochondrial and nuclear DNA status of highdensity and low-density sperm fractions after density centrifugation preparation. Fertil Steril. 2003;79(Suppl. 1):754–762. doi: 10.1016/S0015-0282(02)04827-6. [DOI] [PubMed] [Google Scholar]
- 99.Morris ID, Ilott S, Dixon L, Brison DR. The spectrum of DNA damage in human sperm assessed by single cell gel electrophoresis (alkaline comet assay) and its relationship to fertilization and embryo development. Hum Reprod. 2002;17:990–998. doi: 10.1093/humrep/17.4.990. [DOI] [PubMed] [Google Scholar]
- 100.Lewis SE, O’Connell M, Stevenson M, Thompson-Cree L, McClure N. An algorithm to predict pregnancy in assisted reproduction. Hum Reprod. 2004;19:1385–1394. doi: 10.1093/humrep/deh227. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez JL, Muriel L, Goyanes V, Segrelles E, Gosalvez J, Enciso M, et al. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil Steril. 2005;84(4):833–842. doi: 10.1016/j.fertnstert.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 102.Piasecka M, Gaczarzewicz D, Laszczynska M. Evaluation of sperm genomic integrity of normozoospermic men: a prospective study. Folia Histochem Cytobiol. 2006;44(2):117–122. [PubMed] [Google Scholar]
- 103.Giwercman A, Richthoff J, Hjollund H, Bonde JP, Jepson K, Frohm B, et al. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil Steril. 2003;80(6):1404–1412. doi: 10.1016/S0015-0282(03)02212-X. [DOI] [PubMed] [Google Scholar]
- 104.Peris SI, Morrier A, Dufour M, Bailey JL. Cryopreservation of ram semen facilitates sperm DNA damage: relationship between sperm andrological parameters and the sperm chromatin structure assay. J Androl. 2004;25(2):224–233. doi: 10.1002/j.1939-4640.2004.tb02782.x. [DOI] [PubMed] [Google Scholar]
- 105.Singh NP, Danner DB, Tice RR, McCoy MT, Collins GD, Schneider EL. Abundant alkali sensitive sites in DNA of human and mouse sperm. Exp Cell Res. 1989;184:461–470. doi: 10.1016/0014-4827(89)90344-3. [DOI] [PubMed] [Google Scholar]
- 106.Perreault SD, Aitken RJ, Baker HW, et al. Integrating new tests of sperm genetic integrity into semen analysis: breakout group discussion. Adv Exp Med Biol. 2003;518:253–268. doi: 10.1007/978-1-4419-9190-4_23. [DOI] [PubMed] [Google Scholar]
- 107.Evenson DP, Larson KL, Jost LK. Sperm chromatin structure assay: its clinical use for detecting sperm DNA fragmentation in male infertility and comparisons with other techniques. J Androl. 2002;23:25–43. doi: 10.1002/j.1939-4640.2002.tb02599.x. [DOI] [PubMed] [Google Scholar]
- 108.Aitken RJ, Luliis GN. Value of DNA integrity assays for fertility evaluation. Soc Reprod Fertil Suppl. 2007;65:81–92. [PubMed] [Google Scholar]
- 109.Leroy T, Hummelen P, Anard D, Castelain P, Kirsch-Volders M, Lauwerys R, et al. Evaluation of three methods for the detection of DNA single-strand breaks in human lymphocytes: alkaline elution, nick translation and single-cell gel electrophoresis. J Toxicol Environ Health. 1996;47:409–422. doi: 10.1080/009841096161573. [DOI] [PubMed] [Google Scholar]
- 110.Irvine DS, Twigg JP, Gordon EL, Fulton N, Milne PA, Aitken RJ. DNA integrity in human spermatozoa: relationships with semen quality. J Androl. 2000;21:33–44. [PubMed] [Google Scholar]
- 111.Tesarik J, Mendoza-Tesarik R, Mendoza C. Sperm nuclear DNA damage: update on the mechanism, diagnosis and treatment. Reprod Biomed Online. 2006;12:715–721. doi: 10.1016/S1472-6483(10)61083-8. [DOI] [PubMed] [Google Scholar]
- 112.Benchaib M, Braun V, Lornage J, Hadj S, Salle B, Lejeune H, et al. Sperm DNA fragmentation decreases the pregnancy rate in an assisted reproductive technique. Hum Reprod. 2003;18:1023–1028. doi: 10.1093/humrep/deg228. [DOI] [PubMed] [Google Scholar]
- 113.Benchaib M, Lornage J, Mazoyer C, Lejeune H, Salle B, Guerin JF. Sperm deoxyribonucleic acid fragmentation as a prognostic indicator of assisted reproductive technology outcome. Fertil Steril. 2007;87:93–100. doi: 10.1016/j.fertnstert.2006.05.057. [DOI] [PubMed] [Google Scholar]
- 114.Greco E, Scarselli F, Iacobelli M, et al. Efficient treatment of infertility due to sperm DNA damage by ICSI with testicular spermatozoa. Hum Reprod. 2005;20:226–230. doi: 10.1093/humrep/deh590. [DOI] [PubMed] [Google Scholar]
- 115.Mitchell LA, Iuliis GN, John Aitken R. The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int J Androl. 2010;34:2–13. doi: 10.1111/j.1365-2605.2009.01042.x. [DOI] [PubMed] [Google Scholar]
- 116.Ménézo YJ, Hazout A, Panteix G, Robert F, Rollet J, Cohen-Bacrie P, et al. Antioxidants to reduce sperm DNA fragmentation: an unexpected adverse effect. Reprod Biomed Online. 2007;14(4):418–421. doi: 10.1016/S1472-6483(10)60887-5. [DOI] [PubMed] [Google Scholar]
- 117.Moslemi MK, Tavanbakhsh S. Selenium-vitamin E supplementation in infertile men: effects on semen parameters and pregnancy rate. Int J Gen Med. 2011;4:99–104. doi: 10.2147/IJGM.S16275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lanzafame FM, Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reprod Biomed Online. 2009;19(5):638–659. doi: 10.1016/j.rbmo.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 119.Gharagozloo P, Aitken RJ. The role of sperm oxidative stress in male infertility and the significance of oral antioxidant therapy. Hum Reprod. 2011;26(7):1628–1640. doi: 10.1093/humrep/der132. [DOI] [PubMed] [Google Scholar]
- 120.Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for male subfertility. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No.: CD007411. doi:10.1002/14651858.CD007411.pub2. [DOI] [PubMed]