Abstract
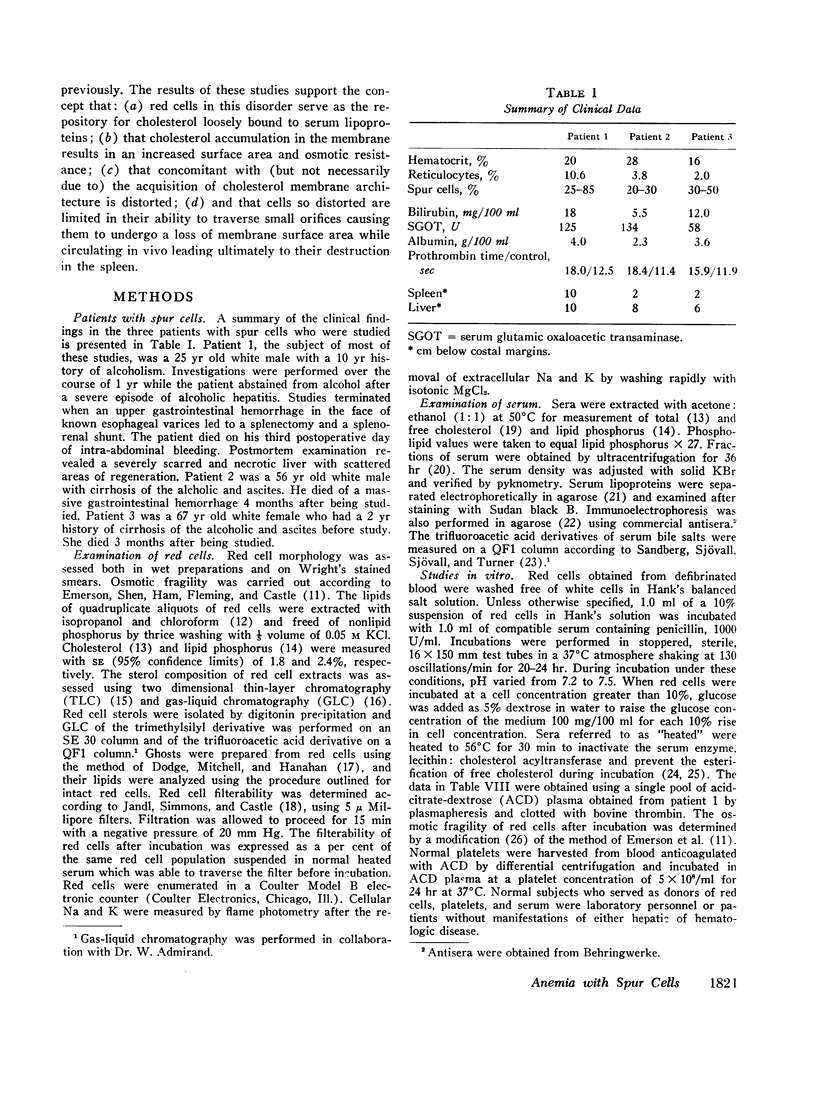
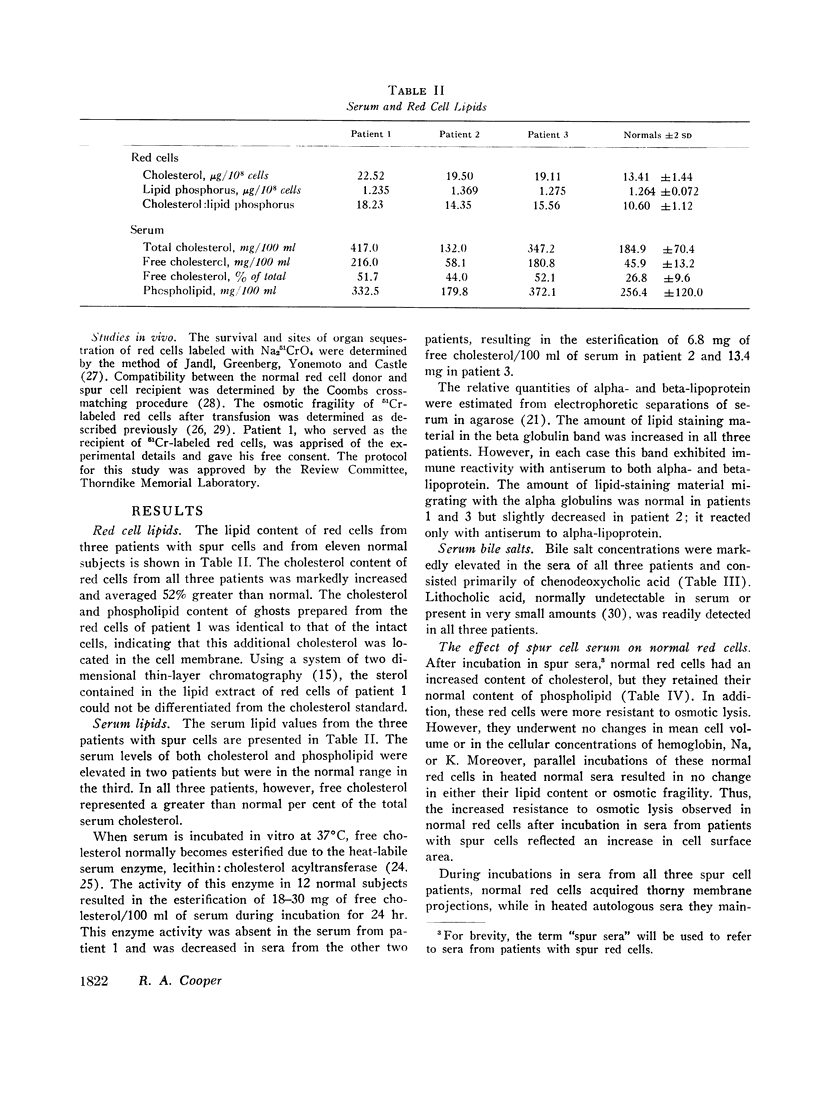
The sera and red cells of three patients with severe liver disease and “spur cells” were studied. In each case the per cent of serum cholesterol which was free (unesterified) was elevated, and the serum lecithin: cholesterol acyltransferase activity was depressed. Lipoproteins with beta mobility were increased, but exhibited immune reactivity with antisera to both alpha- and beta-lipoproteins. Serum bile salt concentrations were markedly elevated and consisted primarily of chenodeoxycholic acid, with small amounts of lithocholic acid present as well.
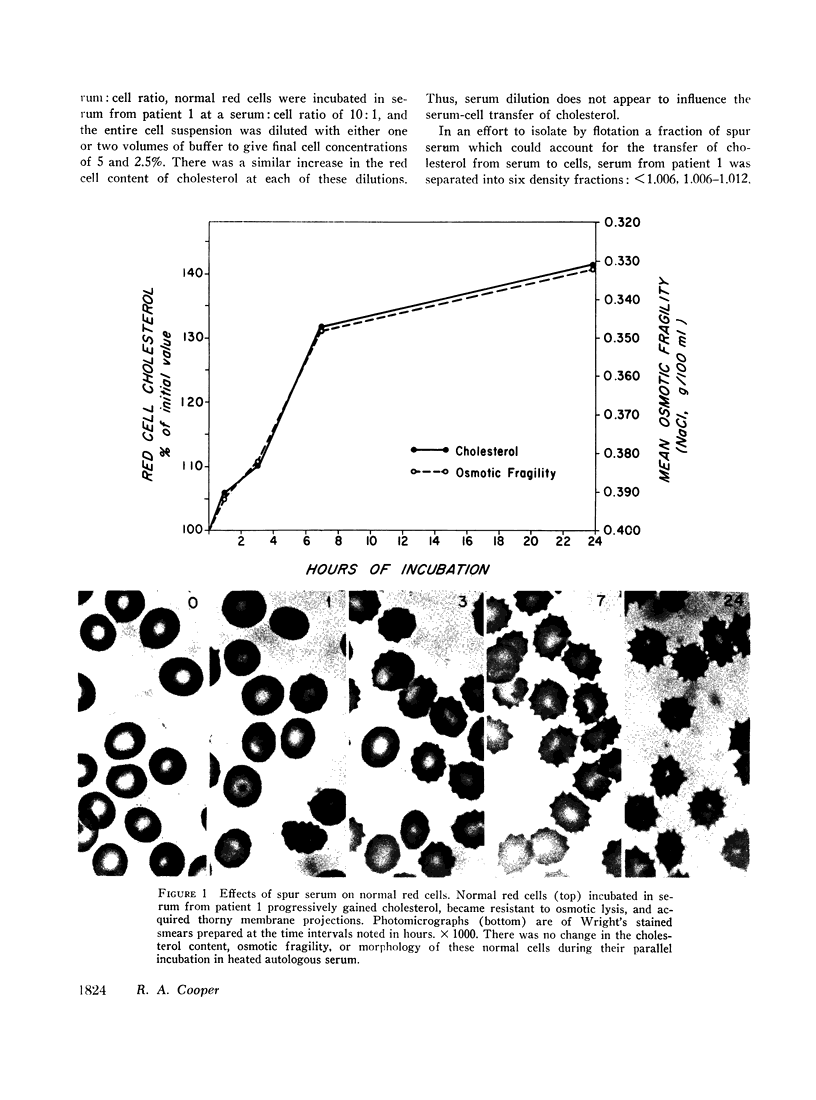
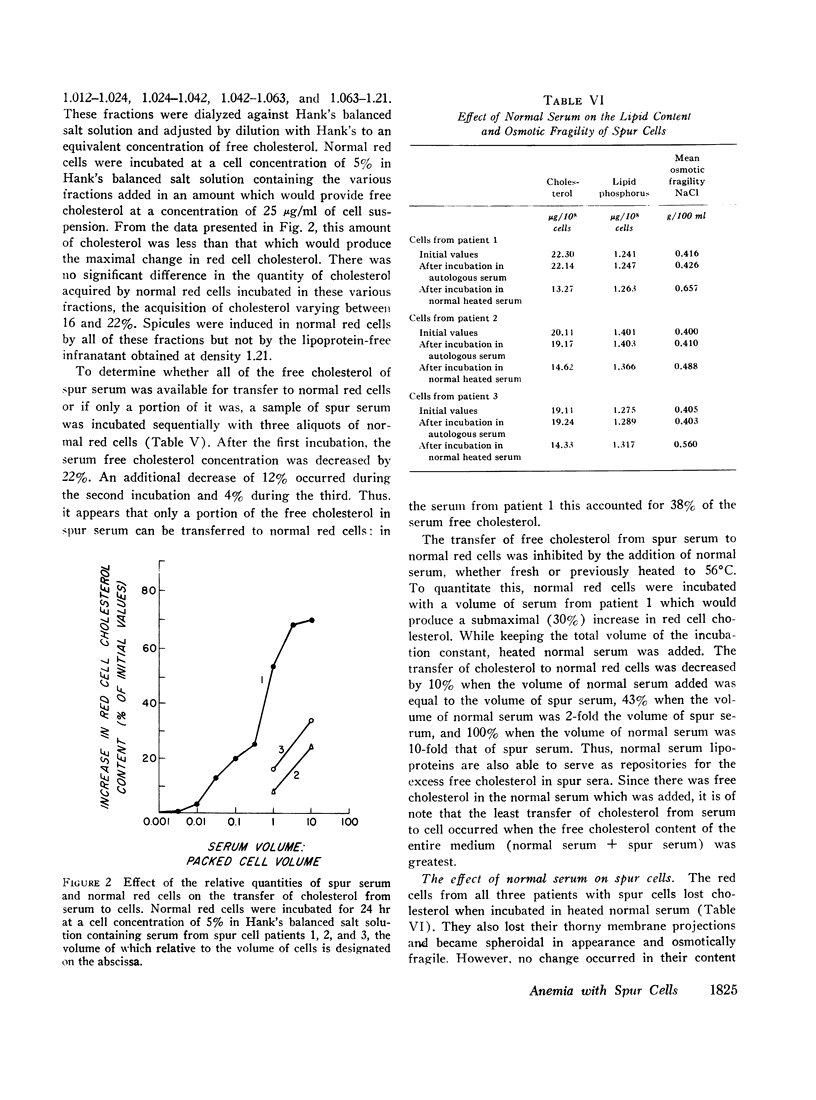
Spur cells manifested a striking increase in cholesterol content and in the cholesterol: phospholipid ratio, but a normal osmotic fragility. When incubated in heated normal serum, spur cells lost their excess cholesterol and became spherocytic and osmotically fragile. Conversely, sera from patients with spur cells readily transferred up to one-third of their free cholesterol to normal red cells, causing normal cells to become resistant to osmotic lysis. In addition, these sera caused normal red cells to acquire thorny membrane projections. Cholesterol transfer to normal cells also occurred from normal serum which had previously been incubated with spur cells. Changes in cell cholesterol were induced by all of the lipoprotein fractions of spur serum.
When transfused into a patient with spur cells, normal red cells became more resistant to osmotic lysis over the course of 24 hr. However, over the subsequent 7 days they underwent a progressive increase in osmotic fragility. These normal cells, as well as the patient's own cells, had a shortened survival. Correlating with the moderate decrease in the filterability of red cells spurred in vitro, red cell destruction occurred predominently in the spleen.
Red cells in this disorder appear to serve as repositories for free cholesterol loosely bound to serum lipoproteins. Cholesterol acquisition by the red cell membrane increases its surface area and causes the red cell to be resistant to osmotic lysis. The associated alteration in red cell shape leads to further changes in the cell membrane during circulation in vivo resulting in the loss of membrane surface area and culminating in the cell's premature destruction in the spleen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bruckdorfer K. R., Graham J. M., Green C. The incorporation of steroid molecules into lecithin sols, beta-lipoproteins and cellular membranes. Eur J Biochem. 1968 May;4(4):512–518. doi: 10.1111/j.1432-1033.1968.tb00242.x. [DOI] [PubMed] [Google Scholar]
- CAREY J. B., Jr The serum trihydroxy-dihydroxy bile acid ratio in liver and biliary tract disease. J Clin Invest. 1958 Nov;37(11):1494–1503. doi: 10.1172/JCI103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey J. B., Jr, Williams G. Lithocholic acid in human-blood serum. Science. 1965 Oct 29;150(3696):620–622. doi: 10.1126/science.150.3696.620. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. Bile salts and cholesterol in the pathogenesis of target cells in obstructive jaundice. J Clin Invest. 1968 Apr;47(4):809–822. doi: 10.1172/JCI105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Deuticke B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim Biophys Acta. 1968 Dec 10;163(4):494–500. doi: 10.1016/0005-2736(68)90078-3. [DOI] [PubMed] [Google Scholar]
- GOULD R. G., LEROY G. V., OKITA G. T., KABARA J. J., KEEGAN P., BERGENSTAL D. M. The use of C14-labeled acetate to study cholesterol metabolism in man. J Lab Clin Med. 1955 Sep;46(3):372–384. [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- HAGERMAN J. S., GOULD R. G. The in vitro interchange of cholesterol between plasma and red cells. Proc Soc Exp Biol Med. 1951 Oct;78(1):329–332. doi: 10.3181/00379727-78-19064. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., GREENBERG M. S., YONEMOTO R. H., CASTLE W. B. Clinical determination of the sites of red cell sequestration in hemolytic anemias. J Clin Invest. 1956 Aug;35(8):842–867. doi: 10.1172/JCI103338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L., CASTLE W. B. Red cell filtration and the pathogenesis of certain hemolytic anemias. Blood. 1961 Aug;18:133–148. [PubMed] [Google Scholar]
- Laurell C. B., Niléhn J. E. A new type of inherited serum albumin anomaly. J Clin Invest. 1966 Dec;45(12):1935–1945. doi: 10.1172/JCI105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Martinez-Maldonado M. Role of lipoproteins in the formation of spur cell anaemia. J Clin Pathol. 1968 Sep;21(5):620–625. doi: 10.1136/jcp.21.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neerhout R. C. Abnormalities of erythrocyte stromal lipids in hepatic disease. J Lab Clin Med. 1968 Mar;71(3):438–447. [PubMed] [Google Scholar]
- Neerhout R. C. Erythrocyte stromal lipids in hyperlipemic states. J Lab Clin Med. 1968 Mar;71(3):448–454. [PubMed] [Google Scholar]
- PHILLIPS G. B. Quantitative chromatographic analysis of plasma and red blood cell lipids in patients with acanthocytosis. J Lab Clin Med. 1962 Mar;59:357–363. [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- SANDBERG D. H., SJOEVALL J., SJOEVALL K., TURNER D. A. MEASUREMENT OF HUMAN SERUM BILE ACIDS BY GAS-LIQUID CHROMATOGRAPHY. J Lipid Res. 1965 Apr;6:182–192. [PubMed] [Google Scholar]
- SILVER M. M., MCMILLAN G. C., SILVER M. D. HAEMOLYTIC ANAEMIA IN CHOLESTEROL-FED RABBITS. Br J Haematol. 1964 Jul;10:271–280. doi: 10.1111/j.1365-2141.1964.tb00703.x. [DOI] [PubMed] [Google Scholar]
- SMITH J. A., LONERGAN E. T., STERLING K. SPUR-CELL ANEMIA: HEMOLYTIC ANEMIA WITH RED CELLS RESEMBLING ACANTHOCYTES IN ALCOHOLIC CIRRHOSIS. N Engl J Med. 1964 Aug 20;271:396–398. doi: 10.1056/NEJM196408202710804. [DOI] [PubMed] [Google Scholar]
- Silber R., Amorosi E., Lhowe J., Kayden H. J. Spur-shaped erythrocytes in Laennec's cirrhosis. N Engl J Med. 1966 Sep 22;275(12):639–643. doi: 10.1056/NEJM196609222751204. [DOI] [PubMed] [Google Scholar]
- Tchernia G., Navarro J., Becart R., Casasoprana A. Anémie hémolytique avec acanthocytose et dyslipidémie au cours de deux hépatitis néonatales. Arch Fr Pediatr. 1968 Aug-Sep;25(7):729–743. [PubMed] [Google Scholar]
- WAYS P., REED C. F., HANAHAN D. J. RED-CELL AND PLASMA LIPIDS IN ACANTHOCYTOSIS. J Clin Invest. 1963 Aug;42:1248–1260. doi: 10.1172/JCI104810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed R. I., Reed C. F. Membrane alterations leading to red cell destruction. Am J Med. 1966 Nov;41(5):681–698. doi: 10.1016/0002-9343(66)90030-1. [DOI] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]



