1. Introduction
Human lungs are constantly challenged with harmful environmental agents including infectious and non-infectious stimuli that initiate innate and adaptive immune responses in the airways. Airway epithelial cells provide an active mucosal barrier to protect lungs against various microbial and non-infectious agents in part through Toll-like receptors (TLRs) (Lafferty et al., 2010; Opitz et al., 2010; Randhawa and Hawn, 2008). Short Palate, Lung and Nasal epithelium Clone 1 (SPLUNC1), a newly described host defense protein, is abundant in airway lining fluid of healthy individuals, and is primarily expressed and secreted form large airway epithelial cells (Bingle and Bingle, 2000). SPLUNC1 is significantly reduced in allergic and cigarette smoke-exposed airways, which may render the hosts more susceptible to bacterial infections (Chu et al., 2007; Steiling et al., 2009). Our group as well as others have reported antimicrobial activity of SPLUNC1 against several respiratory pathogens including Mycoplasma pneumoniae and Pseudomonas aeruginosa (Chu et al., 2007; Gally et al., 2011; Lukinskiene et al., 2011; Zhou et al., 2008).
Although SPLUNC1 is an abundant protein in the airways, its gene regulation under basal as well as diseased conditions such as infection is poorly understood. We have shown that TLR2 activation by Mycoplasma pneumoniae and a TLR2 agonist Palmitoyl (3)-Cys-Ser-Lys (4)- OH (Pam3CSK4) enhanced SPLUNC1 expression in airway epithelial cells in part via activating NF-κB signaling pathway (Chu et al., 2010). This leads to the question of what other transcription factors also regulate SPLUNC1 at the transcriptional and/or post-transcriptional levels.
Mammalian cells express multiple mitogen-activated protein kinases (MAPK) to mediate the effects of extracellular signals on a wide variety of biological processes including cell growth, proliferation, differentiation and apoptosis (Garrington and Johnson, 1999). One of the downstream events following MAPK activation is activation and nuclear translocation of transcription factor c-Jun, a member of the activator protein-1 (AP-1) family. Activation of TLRs including TLR2 has been shown to activate MAPK/AP-1 signaling in airway epithelial cells, which may promote the production of inflammatory mediators and host defense proteins such as β-defensin-2 (Scharf et al., 2010; Schmeck et al., 2006). However, the role of TLR2-mediated MAPK/AP-1 activation in SPLUNC1 regulation remains to be determined. The aim of the present study is to investigate if TLR2-induced MAPK/AP-1 signaling regulates SPLUNC1 expression in lung epithelial cells. Understanding the functional role of key transcription factors/signaling pathways in SPLUNC1 gene regulation can provide new targets for add-on therapy aimed at improving mucosal immunity in diseased airways.
2. Material and methods
2.1 Lung epithelial cell culture
NCI-H292 cells, a human pulmonary mucoepidermoid carcinoma cell line (ATCC, Manassas VA) were cultured in RPMI-1640 medium supplemented with 10% FBS and penicillin-streptomycin at 37°C, 5% CO2. We selected NCI-H292 cells to study TLR2-mediated SPLUNC1 gene regulation because in our previous experiments they have been shown to express SPLUNC1 in response to TLR2 stimulation in a similar fashion to well-differentiated human primary airway epithelial cells (Chu et al., 2010). Since growth-arrested (100% confluence) NCI-H292 cells mimic most of the features of well-differentiated human primary airway epithelial cells including SPLUNC1 gene modulation, they were utilized in all the experiments except transient transfection study. NCI-H292 cells were cultured overnight in reduced serum (i.e., 1% FBS) containing RPMI-1640 medium. Next day, cells were stimulated with different doses (1–1000 ng/ml) of TLR2 agonist Palmitoyl(3)-Cys-Ser-Lys(4)-OH (Pam3CSK4) (InvivoGen, San Diego, CA) in reduced serum medium for indicated time points to measure various parameters.
2.2 Transient transfection of SPLUNC1 promoter construct and luciferase reporter assay
SPLUNC1 promoter reporter construct containing 5′ UTR region (−943bp/+47bp) of SPLUNC1 gene and a firefly luciferase reporter gene (pGL4-SPLUNC1) and renilla luciferase construct (pRL-TK) were purchased from SwitchGear Genomics (Menlo Park, CA). NCI-H292 cells were seeded into a 12-well plate (2×105 cells/well). Next day, cells reached 80–85% confluence, and were co-transfected with pGL4-SPLUNC1 and pRL-TK (5 to 1 ratio) by utilizing the DNApolyjet (SignaGen Laboratories, Rockville, MD) transfection reagent as per the manufacturers’ protocol. Next day cells were stimulated with or without Pam3CSK4 (100 ng/ml) for up to 48 h after overnight culture in reduced serum medium. Cells were then lysed in 1x passive lysis buffer. Firefly luciferase (F-luc) and renilla luciferase (R-luc) activity was determined using a dual luciferase reporter assay kit and a Glomax luminescent plate reader (Promega, Madison, WI). In order to normalize the transfection efficiency among different samples, ratio of F-luc and R-luc activity was utilized to determine the change in SPLUNC1 promoter activity.
2.3 mRNA stability
NCI-H292 cells were stimulated with Pam3CSK4 (100 ng/ml) for 16 h to induce SPLUNC1 mRNA expression. Cells were then divided into two groups where one group received 0.1% DMSO as a vehicle control, and other group received 5μg/ml actinomycin D (ACD) to inhibit de novo mRNA synthesis. Effect of Pam3CSK4 on SPLUNC1 mRNA stability was determined by comparing SPLUNC1 mRNA levels in the presence or absence of ACD for up to 12 h. Results were expressed as percent of change in SPLUNC1 mRNA levels with ACD over their respective controls without ACD. To determine if ACD was cytotoxic, lactate dehydrogenase (LDH) release was measured by using a cytotoxicity detection kit (Roche Applied Science, Indianapolis, IN).
2.4 c-Jun activation assay
NCI-H292 cells were stimulated with Pam3CSK4 (100 ng/ml) for up to 2 h. Cells were then harvested, and nuclear and cytosolic fractions were separated using a NXTRACT 1KT nuclear protein extraction kit (Sigma-Aldrich Corp, St. Louis, MO). Total protein was quantified using a BCA kit (Pierce, Rockford, IL) and 15μg nuclear proteins were utilized to determine c-Jun activation by using an ELISA-based TransAM c-Jun activation assay kit (Active Motif, Carlsbad, CA). This assay detects N-terminal Ser 73 phosphorylated c-Jun binding to oligonucleotide containing AP-1 consensus sequence [5′-TGA(C/G)TCA-3′]. Results of c-Jun activation assay were expressed as absorbance read at 450nm with a reference wavelength at 650nm.
2.5 Chromatin immunoprecipitation (ChIP) assay
NCI-H292 cells were stimulated with or without Pam3CSK4 in reduced serum medium for up to 2 h. Cells were harvested, and DNA and chromatin were cross-linked using 1.6% formaldehyde for 10min at room temperature. DNA-protein cross-linking was quenched by adding 0.125M glycine. Soluble chromatin (fragment size range, 0.3–1.5 kb) was prepared by mechanical shearing using a Bioruptor sonicator in ChIP lysis buffer containing 1% SDS, 10mM EDTA and 50mM Tris (pH8.0) with freshly added protease inhibitor cocktail and phosphate inhibitors. Chromatin was pre-cleared with protein A/G agarose beads for 1 h at 4 ºC. Equal amount of chromatin (50μg/IP) was utilized to perform immunoprecipitation with an anti-c-Jun antibody (Santa Cruz, sc-74543) and a corresponding mouse control IgG (CT IgG) using protein A/G agarose beads. Ten percent of immunoprecipitated chromatin (5μg) was collected as an input control for each sample. The immunoprecipitated chromatin was sequentially washed with low salt buffer, high salt buffer, LiCl2 buffer and then 50mM Tris-EDTA buffer. DNA-protein complex was eluted with 1% SDS elution buffer with 0.1 M NaHCO3 and reverse cross-linked, followed by protein digestion with proteinase K. DNA was extracted using the phenol-chloroform method and dissolved in molecular grade water. Immunoprecipitated DNA along with input DNA was subjected to PCR using specific primer sets amplifying DNA sequences flanking putative AP-1/c-Jun transcription factor binding motifs present on 5′ UTR region of SPLUNC1 gene. PCR products were visualized on the agarose gel, and the amplicon band densities were quantified using the NIH Image J software. Chromatin enrichment following Pam3CSK4 treatment was determined using the PCR amplicon band density ratio of c-Jun antibody over CT IgG.
2.6 Real-time quantitative PCR
Cells were harvested in Trizol (Invitrogen, Carlsbad, CA) to extract total RNA, followed by DNase treatment to remove any genomic DNA contamination. RNA (1μg/sample) was converted to cDNA by reverse transcription (RT) using Applied biosystems RT reagents. Primers and probe [forward primer, 5′ –GGGCCTGTTGGGCATTCT-3′; reverse primer, 5′-CCTCCTCCAGGCTTCAGGAT-3′; probe, 5′-AAACCTTCCGCTCCTGGA-3′] for human SPLUNC1 gene (gene bank accession # NM_016583) were designed using Primer Express Software (Applied biosystems, Carlsbad, CA). SPLUNC1 mRNA levels were determined by real-time quantitative PCR using 30ng of cDNA in a BioRad CFX96 real-time PCR machine. Housekeeping gene glyceraldehydes 3-phosphate dehydrogenase (GAPDH) mRNA levels were examined using 20x primers and probe mix from Applied biosystems. SPLUNC1 mRNA levels were normalized with GAPDH mRNA. Comparative threshold cycle (Ct) method was applied to determine the fold change of SPLUNC1 mRNA levels following Pam3CSK4 treatment relative to untreated samples.
2.7 Western blot analysis
Cells were lysed in RIPA lysis buffer containing protease and phosphate inhibitors. Total protein was quantified using the BCA kit (Pierce, Rockford, IL) and equal amount of proteins were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride membrane (BioRad Laboratories, Hercules, CA). Membranes were blocked in 2.5% dry fat milk prepared in PBS with 0.05% Tween 20 for 1 h, and then probed with primary antibodies against SPLUNC1 (R&D Systems), ERK (Millipore), JNK1/2 (Cell signaling), pERK1/2, pJNK1/2, p-p38MAPK, p38MAPK, GAPDH, β-actin (Santacruz), phospo c-Jun Ser63 (cell signaling) and total c-Jun (Abcam) at 1:1000 dilutions. Immuno-reactivity of primary antibody was detected using corresponding secondary HRP-conjugated antibody at 1:10,000 dilution and ECL substrate from Pierce.
2.8 Statistical analyses
Data presented as means ± SEM. Statistical significance (p ≤0.05) was determined among various treatment groups by applying unpaired Student’s t-test and ANOVA, followed by Tukey’s multiple comparison tests appropriately.
3. Results
3.1 Pam3CSK4 increases SPLUNC1 expression in lung epithelial cells
Growth-arrested NCI-H292 cells expressed SPLUNC1, thus serving as a valid cell model to study SPLUNC1 gene regulation (Fig 1). As we have reported previously (Chu et al., 2010), TLR2 stimulation by Pam3CSK4 increased SPLUNC1 mRNA expression in NCI-H292 cells in a dose- and time-dependent manner. Pam3CSK4 dose-dependently (up to 100 ng/ml) increased SPLUNC1 gene expression at 48 h (Fig 1A). A time course study with 100 ng/ml of Pam3CSK4 revealed a time-dependent effect of TLR2 stimulation on SPLUNC1 mRNA expression (Fig 1B). At 100 ng/ml, Pam3CSK4 maximally induced SPLUNC1 gene expression at 48 h (12 fold). Therefore, 100 ng/ml of Pam3CSK4 was chosen in subsequent experiments. Increased SPLUNC1 mRNA by Pam3CSK4 treatment was accompanied by enhanced SPLUNC1 protein expression (Fig 1C).
FIGURE 1. Pam3CSK4 increases SPLUNC1 expression in NCI-H292 cells.
NCI-H292 cells were stimulated with Pam3CSK4 after overnight growth in reduced serum medium. SPLUNC1 mRNA and protein expression was determined by real-time PCR and Western blot, respectively. Pam3CSK4 dose-dependently increased SPLUNC1 mRNA at 48 h (A). A time course study revealed a time-dependent increase in SPLUNC1 mRNA by Pam3CSK4 at 100 ng/ml (B). Pam3CSK4 also induced SPLUNC1 protein expression at 48 h as determined by Western blot (C) Data expressed as means ± SEM, n = 6–9.
3.2 Pam3CSK4 increases SPLUNC1 transcriptional activity, but not mRNA stability
NCI-H292 cells at 80–85% confluence were transiently transfected with SPLUNC1 promoter reporter construct and pRL-TK construct. Next day, cells were stimulated with Pam3CSK4 for up to 48 h. At 6 and 24 h, Pam3CSK4 treatment significantly increased SPLUNC1 promoter activity compared to their untreated controls (Fig 2A). However, we did not observe a significant increase in SPLUNC1 promoter activity at 48 h.
FIGURE 2. Pam3CSK4 increases SPLUNC1 transcriptional activity.
NCI-H292 cells were transiently co-transfected with SPLUNC1 promoter reporter construct (−943/+47bp) containing firefly luciferase (F-luc) as reporter gene and renilla luciferase (R-luc) gene for normalizing transfection efficiency. After Pam3CSK4 stimulation for up to 48 h, SPLUNC1 promoter activity was determined by normalizing F-luc to R-luc and expressed as % of Pam3CSK4–treated versus untreated cells (A). To determine the effect of Pam3CSK4 on SPLUNC1 mRNA stability, NCI-H292 cells were stimulated with 100 ng/ml Pam3CSK4 for up to 16 h, and then treated with either 0.1% DMSO or 5 μg/ml actinomycinD (ACD) to inhibit de novo mRNA synthesis. Degradation of SPLUNC1 mRNA was examined for up to 12 h after ACD addition and expressed as % of SPLUNC1 mRNA levels without ACD at indicated time points (B). Data expressed as means ± SEM, n = 3–9.
Inhibition of de novo mRNA synthesis by actinomycin D (ACD) attenuated Pam3CSK4-mediated SPLUNC1 mRNA induction within 2 h (16%) and 4 h (21%). Longer treatment (≥8 h) with ACD increased cell toxicity as determined by LDH release (data not shown), which is in accordance with the known toxic effect of blocking de novo mRNA synthesis. However, there was no significant difference observed in the SPLUNC1 mRNA degradation rate in ACD-treated Pam3CSK4 versus ACD-treated control cells at various time points (Fig 2B). Half-life of SPLUNC1 mRNA was estimated to be 6 h regardless of Pam3CSK4 treatment. This result suggests that Pam3CSK4-induced SPLUNC1 mRNA may result from SPLUNC1 gene transcription, but not from increased mRNA stability.
3.3 MAPK/AP-1 activation in Pam3CSK4-treated NCI-H292 cells
We determined MAPK/AP-1 activation by Pam3CSK4 in NCI-H292 cells. Among the three major MAPKs, ERK1/2 was strongly phosphorylated within 30 min and remained activated for up to 2 h (Fig 3A). JNK1/2 was activated at 1 h, but quickly deactivated after 1 h (Fig 3B). However, Pam3CSK4 treatment did not activate p38MAPK. AP-1 has been described as a down stream target of MAPK signaling especially ERK1/2 and JNK1/2 (Adiseshaiah et al., 2006; Karin, 1995; Pulverer et al., 1991). We determined the activation of c-Jun that belongs to the AP-1 transcription factor family. A maximal increase in Pam3CSK4-induced c-Jun protein and c-Jun phosphorylation was observed at 2 h (Fig 3C and 3D). Phosphorylation of c-Jun at Ser73 in N-terminal transactivation domain regulates its DNA binding activity. Therefore, we examined c-Jun Ser73 phosphorylation and its DNA binding activity to AP-1 consensus sequence containing oligo by using an ELISA-based TransAM c-Jun activation assay kit. At 2 h following Pam3CSK4 treatment, c-Jun Ser73 phosphorylation increased, indicating a potential increase of c-Jun binding to AP-1 consensus sequences (Fig 3E).
FIGURE 3. Pam3CSK4 activates MAPK/AP-1 in NCI-H292 cells.


After overnight growth in reduced serum medium, NCI-H292 cells were stimulated with 100 ng/ml Pam3CSK4 for up to 24 h. Activation of ERK (A) and JNK (B) and total c-Jun expression (C) were determined by Western blot. Upper panel in Figure A, B and C shows densitometry data from 3–6 independent replicates and lower panel is a representative picture of their Western blot. Activation of c-Jun transcription factor, a downstream target of ERK activation, was also determined by using Western blot (D) as well as an ELISA-based TransAM c-Jun activation assay (E). Data expressed as means ± SEM, n = 3–5.
3.4 Pam3CSK4 enhances c-Jun binding to the SPLUNC1 promoter
By searching TRANFAC database (http://www.cbrc.jp/research/db/TFSEARCH.html) and other publications (Chung et al., 1996; Kyo et al., 1997; Lee et al., 1987), several putative AP-1/c-Jun binding motifs (Fig 4A) were identified in the 5′ flanking region (−5kb) from the SPLUNC1 transcription start site. To further investigate the role of c-Jun in Pam3CSK4-mediated SPLUNC1 gene regulation, we determined c-Jun binding to the 5′ flanking DNA region (up to −2.5kb) of SPLUNC1 gene including the sequence of our SPLUNC1 promoter reporter construct (−943/+47 base). We designed 6 different primer sets to map the 9 putative AP-1/c-Jun binding motifs across −2.5kb downstream region of SPLUNC1 gene (Fig 4A). Pam3CSK4 stimulation significantly increased c-Jun binding (up to 2 fold) to SPLUNC1 promoter (−833/−1036 and −1773/−2004 region) at 2 h compared to untreated control (Fig. 4B). This result was in parallel with c-Jun activation assay where we observed a maximal increase in c-Jun activation at 2 h following Pam3CSK4 stimulation (Fig 3E).
FIGURE 4. Pam3CSK4 enhances c-Jun binding to the SPLUNC1 promoter.
Several putative AP-1/c-Jun binding motifs were identified in the −2.5kb flanking region of SPLUNC1 gene including SPLUNC1 promoter sequence. Six different primer sets were designed to map 9 putative AP-1/c-Jun binding motifs across −2.5kb flanking region of SPLUNC1 gene (A). After overnight growth in reduced serum medium, NCI-H292 cells were stimulated with Pam3CSK4 (100 ng/ml) for up to 2 h. Binding of c-Jun to SPLUNC1 promoter region (−833/−1036 and −1733/−2004 bases) containing putative Ap-1/c-Jun DNA binding motifs was determined by ChIP assay (B). Upper panel shows a representative picture of agarose gels; lower panel shows densitometric analysis of PCR products visualized on agarose gel electrophoresis. Data expressed as means ± SEM, n = 3.
3.5 Pam3CSK4-mediated ERK1/2 and c-Jun activation up-regulates SPLUNC1 mRNA
Having identified the ability of c-Jun to bind to SPLUNC1 promoter and shown increased ERK1/2 and c-Jun activation by Pam3CSK4, we then tested if Pam3CSK4-induced c-Jun activation was indeed responsible for SPLUNC1 up-regulation. NCI-H292 cells pretreated with a selective MEK1/2 inhibitor PD98059 for 1 h reduced Pam3CSK4-mediated ERK1/2 phosphorylation and c-Jun activation (Fig 5A and 5B). Importantly, PD98059 significantly attenuated Pam3CSK4-induced SPLUNC1 mRNA expression (Fig 5C). Together, our data suggest that ERK1/2-mediated c-Jun activation may be responsible for TLR2 agonist-induced SPLUNC1 gene expression.
FIGURE 5. Pam3CSK4-mediated ERK1/2 and c-Jun activation up-regulates SPLUNC1 expression.
NCI-H292 cells were pre-treated with DMSO (0.1%) or MEK1/2 inhibitor PD98059 (10 μM). An hour later, cells were stimulated with Pam3CSK4 for up to 24 h. ERK1/2 inhibition by PD98059 resulted in decreased ERK1/2 and c-Jun activation as determined by Western blot (A) and ELISA-based TransAM c-Jun activation assay (B). Effect of PD98059 on Pam3CSK4-induced SPLUNC1 mRNA was determined by real-time quantitative PCR (C). Data expressed as means ± SEM, n = 3–5.
4. Discussion
Although SPLUNC1 is an abundantly expressed host defense protein in the airways, little is known about its gene regulation. Our data have confirmed and extended our previous finding that TLR2 activation in lung epithelial cells increases SPLUNC1 expression in a dose- and time-dependent manner. Importantly, the present study demonstrates for the first time that TLR2-mediated ERK1/2 and c-Jun activation positively regulates SPLUNC1 expression in lung epithelial cells. Pam3CSK4, a TLR2 agonist, significantly increases SPLUNC1 gene expression at the transcription level without affecting its mRNA stability. Furthermore, we established that Pam3CSK4 enhances c-Jun activation and its binding to the SPLUNC1 promoter region. Inhibition of Pam3CSK4-induced ERK1/2 attenuates c-Jun activation and SPLUNC1 expression, suggesting a positive role of ERK1/2-c-Jun signaling in TLR2-mediated SPLUNC1 expression.
TLR activation in the airways has been known to initiate inflammatory response as well as to increase expression and secretion of many host defense proteins, which collectively provide protection against various respiratory pathogens (Randhawa and Hawn, 2008; Schmeck et al., 2006; Vora et al., 2004). Previously we have shown that TLR2 activation by Mycoplasma pneumoniae or by Pam3CSK4 increases SPLUNC1 expression in human airway epithelial cells (Chu et al., 2010; Chu et al., 2007). Our current results are in accordance with our previous finding that TLR2 activation by Pam3CSK4 increases SPLUNC1 expression in NCI-H292 cells as well as in well-differentiated human primary airway epithelial cells (Chu et al., 2010). We chose NCI-H292 cells to study SPLUNC1 gene regulation because they are much easier to be transiently transfected with a SPLUNC1 promoter reporter construct than well-differentiated primary human airway epithelial cells. We observed up to 50% increase in SPLUNC1 promoter activity following Pam3CSK4 treatment at 24 h, which is less than the magnitude of SPLUNC1 mRNA increase (>4 fold) observed at 24 h. This discrepancy could be due to the lower sensitivity of luciferase reporter assay than real-time PCR or the limited length of 5′ flanking region of SPLUNC1 gene in SPLUNC1 promoter reporter construct. Since SPLUNC1 promoter has not been characterized, it is difficult to predict the minimal length of SPLUNC1 promoter required to mediate a full SPLUNC1 transcriptional activity in response to Pam3CSK4 stimulation. Future experiments will address the impact of Pam3CSK4 on activity of various length of SPLUNC1 promoter. Nonetheless, Pam3CSK4-mediated increase in SPLUNC1 promoter reporter activity with −943/+47 base promoter construct suggests that Pam3CSK4-mediated SPLUNC1 mRNA expression may be attributed predominantly to increased SPLUNC1 transcription, as SPLUNC1 mRNA stability was not altered following TLR2 stimulation.
Mammalian gene transcription regulation is a tightly regulated event where multiple transcription factors can interact with basic transcription machinery to influence the final outcome of a gene expression. Earlier we have reported that TLR2-mediated SPLUNC1 expression was in part dependent on NF-κB activation. Inhibition of NF-κB partly suppressed Mycoplasma pneumoniae-induced SPLUNC1 expression whereas over-expression of IKKβ significantly increased SPLUNC1 expression in epithelial cells (Chu et al., 2010). TLR2 activation has been shown to induce MAPK/AP-1 signaling in lung epithelial cells (Scharf et al., 2010; Schmeck et al., 2006). Our data suggest that TLR2 activation following Pam3CSK4 stimulation increases SPLUNC1 in part through MAPK/AP-1 signaling. As agonists of other TLRs such as TLR4 can also activate MAPK/AP-1 signaling in lung epithelial cells (Guillot et al., 2004; Kanoh et al., 2011), it is conceivable that other TLR signaling may also increase SPLUNC1. Indeed, in a preliminary study, we found that TLR4 agonist LPS increases SPLUNC1 mRNA expression (up to 3 fold) in NCI-H292 cells in a dose-dependent manner. However, at the same dose (100 ng/ml), LPS versus Pam3CSK4 resulted in less (6-fold) SPLUNC1 mRNA induction. Why TLR2, as compared to TLR4 activation, more robustly up-regulates SPLUNC1 expression in epithelial cells deserves further studies.
In the present study we found that TLR2 activation by Pam3CSK4 induces a sustained ERK1/2 phosphorylation along with a transient increase in JNK1/2 phosphorylation in NCI-H292 cells. Traditionally ERK phosphorylation has been implicated in a wide variety of cellular responses including cell growth, proliferation and differentiation whereas JNK1/2 was more involved in inducing apoptosis or stress activated signaling (Ballif and Blenis, 2001; Gauthier et al., 2001; Li et al., 2004). Different MAPKs can regulate distinct cellular activities even though they share common substrate including transcription factor AP-1 (Adiseshaiah et al., 2006; Karin, 1995; Pulverer et al., 1991). AP-1 is a family of transcription factors that has been known to modulate various gene expressions via forming a homo- or hetero-dimer and also via interacting with other transcription factors (Bakiri et al., 2002; Hess et al., 2004). Transcription factor c-Jun is a component of AP-1 transcription family, which can be activated by ERK1/2 and JNK1/2 (Karin, 1995; Leppa et al., 1998). Although c-Jun has been suggested as a preferred substrate for JNK1/2, there are studies reporting N-terminal transactivation domain phosphorylation of c-Jun at Ser63 and Ser73 by ERK1/2 (Leppa et al., 1998; Pulverer et al., 1991). ERK activation has been shown to up-regulate de novo expression of c-Jun in rat phaeochromocytome PC12 cells (Leppa et al., 1998). Such dual input of increased c-Jun expression and phosphorylation of c-Jun by ERK1/2 and JNK1/2 have been shown to enhance cell differentiation in PC12 cells (Leppa et al., 1998). Our results also indicate a transient increase in c-Jun phosphorylation as well as increase in total c-Jun levels following Pam3CSK4 treatment at 2 h (Fig 3C and 3E). As our c-Jun Western blotting for total c-Jun revealed multiple bands, we speculate that Pam3CSK4 may induce c-Jun phosphorylation at other sites in addition to Ser63/Ser73. Based on the presence of multiple bands in c-Jun Western blot, similar speculations were reported by Satomi and colleagues in perillyl alcohol-treated human breast cancer cell line T47D-C4-2W (Satomi et al., 1999). Increased c-Jun phosphorylation by MAPK has been reported to increase c-Jun protein stability and de novo expression (Leppa et al., 1998; Musti et al., 1997). We observed in our previous studies that basal SPLUNC1 expression in airway epithelial cells depends on cell differentiation state. Pam3CSK4-induced ERK1/2 and c-Jun activation might enhance NCI-H292 cell growth and/or differentiation and therefore up-regulates SPLUNC1 expression in airway epithelial cells. Since we did not see a significant increase in the cell number after Pam3CSK4 treatment (1.09×106 cells/well vs. 1.01×106 cells/well, p=0.65), increased ERK1/2 and c-Jun activation may increase SPLUNC1 expression via increasing cell differentiation. But, this needs to be verified in future studies.
In the present study, we have focused on ERK1/2 and c-Jun signaling pathway as search for putative transcription factor binding sites revealed multiple AP-1/c-Jun binding motifs in the 5′ flanking region of SPLUNC1 gene. Our ChIP analyses indicated that Pam3CSK4 treatment enhanced binding of c-Jun to the SPLUNC1 promoter region (−833/−1036 base and −1733/−2004 base), which are consistent with the putative AP-1/c-Jun binding sites. This direct binding of c-Jun is likely responsible for TLR2-mediated SPLUNC1 expression as inhibition of ERK1/2 activation by PD98059 resulted in decreased c-Jun activation and Pam3CSK4-mediated SPLUNC1 mRNA expression in lung epithelial cells. As JNK1/2 inhibition alone did not attenuate Pam3CSK4-mediated SPLUNC1 mRNA expression in lung epithelial cells (data not shown), these results suggest a critical role of ERK1/2 activation in TLR2-mediated SPLUNC1 expression in lung epithelial cells.
There are several limitations to our current study. First, whether SPLUNC1 transcriptional mechanisms are identical between NCI-H292 cells and well-differentiated primary human airway epithelial cells awaits further studies. Nonetheless, unraveling SPLUNC1 transcriptional regulation in NCI-H292 cells should provide invaluable insights into the mechanistic studies in primary epithelial cells. Second, to more specifically study the role of c-Jun in SPLUNC1 transcription, direct inhibition of c-Jun activity may be needed in addition to the use of the chemical inhibitor PD98059. For example, we would consider c-Jun RNA interference approach in our future studies. Lastly, to increase the implications of our current work in human diseases, SPLUNC1 transcriptional regulation will be investigated in the context of bacterial infections and diseased conditions such as Th2 cytokines and cigarette smoke exposure.
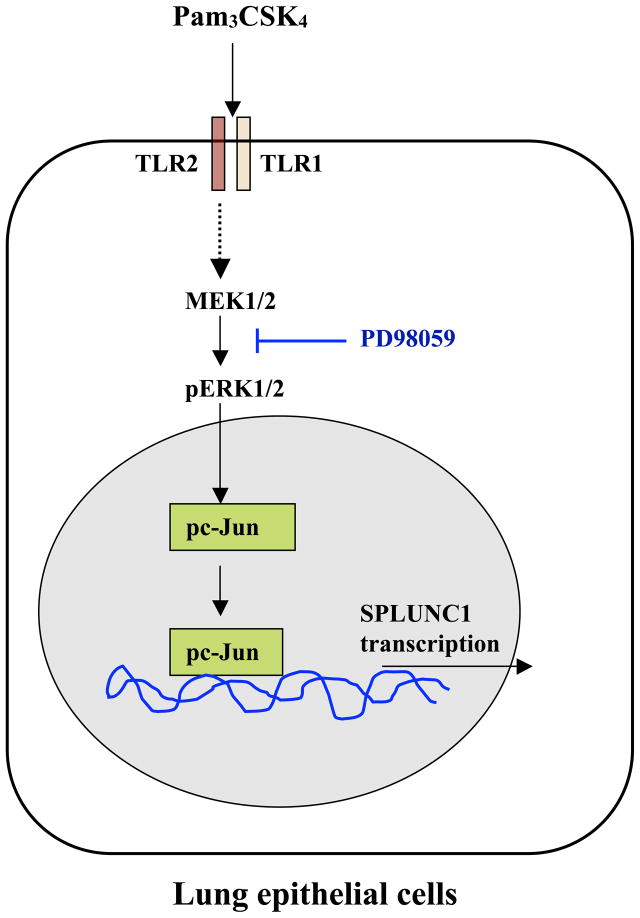
5. Conclusions (Figure 6)
FIGURE 6. Summary of our research findings.
Pam3CSK4-mediated TLR2 activation results in ERK1/2 and c-Jun activation, which enhances c-Jun binding to SPLUNC1 promoter region to up-regulate SPLUNC1 expression in lung epithelial cells.
In summary, we have demonstrated the TLR2 activation by Pam3CSK4 induces c-Jun phosphorylation via ERK1/2 activation in NCI-H292 cells. Pam3CSK4-induced ERK1/2 and c-Jun activation positively regulate SPLUNC1 expression at transcriptional levels. SPLUNC1, an important host defense protein, is significantly reduced in inflammatory lung diseases including asthma that may render the hosts more susceptible to bacterial infections. Our results will advance the study of SPLUNC1 regulation, and may provide new targets for add-on therapy to improve host innate immunity in diseased airways.
Acknowledgments
Financial support: NIH R01 HL088264
Authors kindly acknowledge technical support and guidance from Drs. James Hagman and Julita Ramirez for carrying out SPLUNC1 promoter reporter activity and ChIP assay.
Abbreviations
- ACD
Actinomycin D
- AP-1
Activator protein -1
- ChIP
Chromatin immunoprecipitation
- ERK1/2
Extracellular signal response kinase 1/2
- JNK1/2
c-Jun N-terminal kinase 1/2
- MAPK
Mitogen-activated protein kinase
- MEK1/2
Mitogen-activated protein kinase kinase 1/2
- Pam3CSK4
Palmitoyl (3)-Cys-Ser-Lys (4)- OH
- SPLUNC1
Short Palate, Lung and Nasal epithelium Clone 1
- TLR2
Toll-like receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adiseshaiah P, Kalvakolanu DV, Reddy SP. A JNK-independent signaling pathway regulates TNF alpha-stimulated, c-Jun-driven FRA-1 protooncogene transcription in pulmonary epithelial cells. J Immunol. 2006;177:7193–7202. doi: 10.4049/jimmunol.177.10.7193. [DOI] [PubMed] [Google Scholar]
- Bakiri L, Matsuo K, Wisniewska M, Wagner EF, Yaniv M. Promoter specificity and biological activity of tethered AP-1 dimers. Mol Cell Biol. 2002;22:4952–4964. doi: 10.1128/MCB.22.13.4952-4964.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- Bingle CD, Bingle L. Characterisation of the human plunc gene, a gene product with an upper airways and nasopharyngeal restricted expression pattern. Biochim Biophys Acta. 2000;1493:363–367. doi: 10.1016/s0167-4781(00)00196-2. [DOI] [PubMed] [Google Scholar]
- Chu HW, Gally F, Thaikoottathil J, Janssen-Heininger YM, Wu Q, Zhang G, Reisdorph N, Case S, Minor M, Smith S, Jiang D, Michels N, Simon G, Martin RJ. SPLUNC1 regulation in airway epithelial cells: role of Toll-like receptor 2 signaling. Respir Res. 2010;11:155. doi: 10.1186/1465-9921-11-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu HW, Thaikoottathil J, Rino JG, Zhang G, Wu Q, Moss T, Refaeli Y, Bowler R, Wenzel SE, Chen Z, Zdunek J, Breed R, Young R, Allaire E, Martin RJ. Function and regulation of SPLUNC1 protein in Mycoplasma infection and allergic inflammation. J Immunol. 2007;179:3995–4002. doi: 10.4049/jimmunol.179.6.3995. [DOI] [PubMed] [Google Scholar]
- Chung KY, Agarwal A, Uitto J, Mauviel A. An AP-1 binding sequence is essential for regulation of the human alpha2(I) collagen (COL1A2) promoter activity by transforming growth factor-beta. J Biol Chem. 1996;271:3272–3278. doi: 10.1074/jbc.271.6.3272. [DOI] [PubMed] [Google Scholar]
- Gally F, Di YP, Smith SK, Minor MN, Liu Y, Bratton DL, Frasch SC, Michels NM, Case SR, Chu HW. SPLUNC1 promotes lung innate defense against Mycoplasma pneumoniae infection in mice. Am J Pathol. 2011;178:2159–2167. doi: 10.1016/j.ajpath.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrington TP, Johnson GL. Organization and regulation of mitogen-activated protein kinase signaling pathways. Curr Opin Cell Biol. 1999;11:211–218. doi: 10.1016/s0955-0674(99)80028-3. [DOI] [PubMed] [Google Scholar]
- Gauthier R, Harnois C, Drolet JF, Reed JC, Vezina A, Vachon PH. Human intestinal epithelial cell survival: differentiation state-specific control mechanisms. Am J Physiol Cell Physiol. 2001;280:C1540–1554. doi: 10.1152/ajpcell.2001.280.6.C1540. [DOI] [PubMed] [Google Scholar]
- Guillot L, Medjane S, Le-Barillec K, Balloy V, Danel C, Chignard M, Si-Tahar M. Response of human pulmonary epithelial cells to lipopolysaccharide involves Toll-like receptor 4 (TLR4)-dependent signaling pathways: evidence for an intracellular compartmentalization of TLR4. J Biol Chem. 2004;279:2712–2718. doi: 10.1074/jbc.M305790200. [DOI] [PubMed] [Google Scholar]
- Hess J, Angel P, Schorpp-Kistner M. AP-1 subunits: quarrel and harmony among siblings. J Cell Sci. 2004;117:5965–5973. doi: 10.1242/jcs.01589. [DOI] [PubMed] [Google Scholar]
- Kanoh S, Tanabe T, Rubin BK. Dapsone inhibits IL-8 secretion from human bronchial epithelial cells stimulated with LPS and resolves airway inflammation in the ferret. Chest. 2011 doi: 10.1378/chest.10-2908. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Kyo S, Klumpp DJ, Inoue M, Kanaya T, Laimins LA. Expression of AP1 during cellular differentiation determines human papillomavirus E6/E7 expression in stratified epithelial cells. J Gen Virol. 1997;78 (Pt 2):401–411. doi: 10.1099/0022-1317-78-2-401. [DOI] [PubMed] [Google Scholar]
- Lafferty EI, Qureshi ST, Schnare M. The role of toll-like receptors in acute and chronic lung inflammation. J Inflamm (Lond) 2010;7:57. doi: 10.1186/1476-9255-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Mitchell P, Tjian R. Purified transcription factor AP-1 interacts with TPA-inducible enhancer elements. Cell. 1987;49:741–752. doi: 10.1016/0092-8674(87)90612-x. [DOI] [PubMed] [Google Scholar]
- Leppa S, Saffrich R, Ansorge W, Bohmann D. Differential regulation of c-Jun by ERK and JNK during PC12 cell differentiation. EMBO J. 1998;17:4404–4413. doi: 10.1093/emboj/17.15.4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gerrard ER, Jr, Balkovetz DF. Evidence for ERK1/2 phosphorylation controlling contact inhibition of proliferation in Madin-Darby canine kidney epithelial cells. Am J Physiol Cell Physiol. 2004;287:C432–439. doi: 10.1152/ajpcell.00020.2004. [DOI] [PubMed] [Google Scholar]
- Lukinskiene L, Liu Y, Reynolds SD, Steele C, Stripp BR, Leikauf GD, Kolls JK, Di YP. Antimicrobial Activity of PLUNC Protects against Pseudomonas aeruginosa Infection. J Immunol. 2011 doi: 10.4049/jimmunol.1001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musti AM, Treier M, Bohmann D. Reduced ubiquitin-dependent degradation of c-Jun after phosphorylation by MAP kinases. Science. 1997;275:400–402. doi: 10.1126/science.275.5298.400. [DOI] [PubMed] [Google Scholar]
- Opitz B, van Laak V, Eitel J, Suttorp N. Innate immune recognition in infectious and noninfectious diseases of the lung. Am J Respir Crit Care Med. 2010;181:1294–1309. doi: 10.1164/rccm.200909-1427SO. [DOI] [PubMed] [Google Scholar]
- Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353:670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Randhawa AK, Hawn TR. Toll-like receptors: their roles in bacterial recognition and respiratory infections. Expert Rev Anti Infect Ther. 2008;6:479–495. doi: 10.1586/14787210.6.4.479. [DOI] [PubMed] [Google Scholar]
- Satomi Y, Miyamoto S, Gould MN. Induction of AP-1 activity by perillyl alcohol in breast cancer cells. Carcinogenesis. 1999;20:1957–1961. doi: 10.1093/carcin/20.10.1957. [DOI] [PubMed] [Google Scholar]
- Scharf S, Hippenstiel S, Flieger A, Suttorp N, N’Guessan PD. Induction of human beta-defensin-2 in pulmonary epithelial cells by Legionella pneumophila: involvement of TLR2 and TLR5, p38 MAPK, JNK, NF-kappaB, and AP-1. Am J Physiol Lung Cell Mol Physiol. 2010;298:L687–695. doi: 10.1152/ajplung.00365.2009. [DOI] [PubMed] [Google Scholar]
- Schmeck B, Moog K, Zahlten J, van Laak V, N’Guessan PD, Opitz B, Rosseau S, Suttorp N, Hippenstiel S. Streptococcus pneumoniae induced c-Jun-N-terminal kinase-and AP-1 -dependent IL-8 release by lung epithelial BEAS-2B cells. Respir Res. 2006;7:98. doi: 10.1186/1465-9921-7-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiling K, Kadar AY, Bergerat A, Flanigon J, Sridhar S, Shah V, Ahmad QR, Brody JS, Lenburg ME, Steffen M, Spira A. Comparison of proteomic and transcriptomic profiles in the bronchial airway epithelium of current and never smokers. PLoS One. 2009;4:e5043. doi: 10.1371/journal.pone.0005043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora P, Youdim A, Thomas LS, Fukata M, Tesfay SY, Lukasek K, Michelsen KS, Wada A, Hirayama T, Arditi M, Abreu MT. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol. 2004;173:5398–5405. doi: 10.4049/jimmunol.173.9.5398. [DOI] [PubMed] [Google Scholar]
- Zhou HD, Li XL, Li GY, Zhou M, Liu HY, Yang YX, Deng T, Ma J, Sheng SR. Effect of SPLUNC1 protein on the Pseudomonas aeruginosa and Epstein-Barr virus. Mol Cell Biochem. 2008;309:191–197. doi: 10.1007/s11010-007-9659-3. [DOI] [PubMed] [Google Scholar]