Abstract
Objectives
Antibody-mediated disruption of the annexin A5 (AnxA5) anticoagulant shield has been posited to be a thrombogenic mechanism in the antiphospholipid syndrome. We recently showed that the antimalarial drug, hydroxychloroquine, dissociates antiphospholipid immune complexes and restores AnxA5 binding to planar phospholipid bilayer. Using quantitative immunoassays, we demonstrated similar effects on BeWo trophoblasts. We therefore investigated the effects of the drug on localization of AnxA5 in primary cultures of human placental syncytiotrophoblasts (SCTs).
Study
Laser confocal microscopy with computer-based morphometric analysis was used to localize AnxA5 and antiphospholipid antibodies on SCTs exposed to polyclonal and monoclonal antiphospholipid and control IgGs.
Results
Hydroxychloroquine reversed the effects of the antiphospholipid antibodies on the SCTs by markedly reducing IgG binding and restoring AnxA5 expression.
Conclusions
These results provide the first morphologic evidence for this effect of hydroxychloroquine on human placental SCTs and support the possibility of novel treatments that target antiphospholipid antibody binding.
Keywords: syncytiotrophoblasts, antiphospholipid syndrome, annexin A5, hydroxychloroquine, confocal microscopy, pregnancy, miscarriage, thrombophilia
INTRODUCTION
The placental anticoagulant protein annexin A5 (AnxA5) is highly expressed by syncytiotrophoblasts (SCTs) in an apparently constitutive manner.1 The potent anticoagulant properties of AnxA5 result from its forming 2-dimensional crystals over anionic phospholipids2 that shield them from availability for serving as cofactors for coagulation enzyme reactions.3 AnxA5 localizes on apical membranes of placental SCTs,1 an optimal anatomic position for the protein to play a thrombomodulatory role in maintaining the fluidity of intervillous blood circulation. Evidence from animal studies supports this concept; pregnant mice infused with anti-AnxA5 antibodies developed placental necrosis and fibrosis along with fetal resorption.4 There is also evidence for such a role in humans, although it is less direct because of ethical concerns that limit such experimentation. Patients with preeclampsia and fetal growth restriction had reduced expression of placental AnxA5 compared to matched controls.5 Women with histories for unexplained recurrent spontaneous pregnancy losses have reduced AnxA5 levels and resistance to the anticoagulant activity of AnxA5.6 A common haplotype in the promoter region of the AnxA5 gene – designated M2 – was associated with reduced placental expression of AnxA57,8 and with increased risk for recurrent spontaneous pregnancy losses9,10
The antiphospholipid (aPL) syndrome (APS) is an acquired autoimmune thrombophilic condition that is a cause of pregnancy complications attributable to placental insufficiency including: recurrent pregnancy losses and other including IUGR, oligohydramnios, preeclampsia/toxemia and placental abruption.11 aPL antibodies reduced the levels of AnxA5 on placental villous SCTs,12 cultured BeWo trophoblasts,13–15 and primary cultures of SCTs,14 and reduce the anticoagulant activity of AnxA5 on the cells.14,15 The aPL-mediated reduction of AnxA5 has been confirmed to be due to competitive displacement of the protein by several different methods including atomic force microscopy,16 ellipsometry,17 microtiter plate assays,17,18 measurements of AnxA5 binding to phospholipid suspensions,17 flow cytometry,19,20, and fluorescence imaging.21
We were motivated to investigate whether hydroxychloroquine (HCQ) might directly affect the aPL-AnxA5 thrombogenic mechanism because of the drug’s interesting chemical structure and because it reduced thrombosis in an animal model of APS.22 Observational studies in humans have also suggested a beneficial effect for the drug in reducing the risk of thrombosis23–28 We showed, through ellipsometry and atomic force microscopic imaging of aPL immune complexes on planar phospholipid bilayers, that HCQ directly disrupts the formation of aPL immune complexes15,29 and that this restores AnxA5 binding and crystallization on the planar bilayers,15,29 Also, using quantitative immunoassays, we demonstrated that the drug also reduced aPL binding and restored AnxA5 expression on cultured BeWo trophoblasts.15 Since those results were obtained through immunoassay measurements on a choriocarcinoma-derived trophoblast model and did not provide information on the localization of the proteins, we thought it critical to image primary cultures of human syncytiotophoblasts (SCTs) to study the effects HCQ on the distribution of antibodies and AnxA5.
Materials and Methods
Reagents
The research protocol was approved by the institutional review board of Montefiore Medical Center, which granted permission for the use of excess plasmas from APS patients that had been obtained from clinical assays or plasmapheresate discards, and were anonymized. Human polyclonal antibody immunoglobulin G (IgG) fractions were isolated from citrated plasma of patient with severe APS and a normal control subject with a protein G column, as described by Sammaritano et al.30 The patient had severe primary APS, manifested by recurrent spontaneous pregnancy losses, deep vein thrombosis, pulmonary embolism, stroke and high titers of anticardiolipin (aCL) IgG (25.3–30.6 GPL) and antiphosphatidylserine IgG (78.0–92.5 GPS), and positive lupus anticoagulant tests by standard dilute Russell viper venom time assays performed with mixing and confirmatory steps. The preparation of aPL antibodies from the patient was compared to IgG isolated from control plasma.
The findings were validated with a previously characterized human aPL monoclonal antibody (mAb) IgG, designated IS4 that was generated from a cell line generously provided by Dr. Pojen P. Chen (Department of Medicine, Division of Rheumatology, University of California at Los Angeles, Los Angeles, CA) from the peripheral blood mononuclear cells of a patient with APS and was purified by affinity columns as previously described.31 The aPL mAb does not have lupus anticoagulant activity by dilute Russell viper venom time (dRVVT) or kaolin clotting time.31–33 A commercially available non-immune human IgG derived from patients with monoclonal gammopathies (Sigma, St. Louis, MO) was used as a control. A stock solution of HCQ (gift from Dr. Kirk Sperber of New York Medical College) was prepared with HEPES-buffered saline (HBS; 0.01 M HEPES, 0.14 M NaCl, pH 7.5) at 200 mg/mL and stored at 4°C.
Isolation and Syncytialization of Placental Cytotrophoblasts
To obtain human SCTs, cytotrophoblasts were isolated from placentas from women undergoing elective cesarean sections at term, using the method described by Kliman et al34 with modification that robustly yields syncytialized trophoblast.35 In this well-established model of trophoblast differentiation, syncytialization was confirmed based on morphological assessment of cell fusion as well as biochemical criteria including the synthesis of progesterone, estradiol, hCG and hPL.35 Briefly, placental villous tissue was dissected free of membranes, minced and rinsed with calcium and magnesium free Dulbecco’s phosphate buffered salt solution (Mediatech, Inc, VA), which were subjected to sequential enzymatic digestion in a solution containing 0.25% trypsin (Invitrogen, CA), 0.2% DNase I (Roche, IN), 25mM HEPES, 2mM CaCl2 and 0.8mM MgSO4 in Hanks’ Balanced Salt Solution (Invitrogen). The first digestion was carried out for 15min in 100ml of digestion solution, and the following two sequential digestions were carried out for 30min in 150ml of digestion solution. Cells were pelleted from the second and third digestion by centrifugation at 1,500 × G for 10 min. The cells were resuspended in Dulbecco’s Modified Eagle’s Medium/Ham’s Nutrient Mixture F12 (DMEM/F12; Sigma, MO) containing 10% fetal bovine serum (FBS; Gemini Bioproducts, CA) and purified on a discontinuous gradient of Percoll (50%, 45%, 35%, and 30%) (GE Healthcare, CT) by centrifugation for 20 min without brake at 1000 × G. The cells that migrated to the 45% Percoll layer were recovered and immunopurified using mouse anti-human CD9 (R&D Systems) and mouse anti-human CD45 (GeneTex) antibody, and goat anti-mouse IgG conjugated DynaBeads (Invitrogen). For immunopurification, the cells were incubated with anti-CD9 and anti-CD45 antibody at ratio of 1 μg antibody per 107 cells for 15min at 4°C. The cells were then incubated with 50 μl anti-mouse IgG conjugated DynaBeads per 107 cells for 30 min at 4°C, recentrifuged and washed using DMEM/F12 containing 10% FBS. Dynabeads and the attached cells were removed by placing the cells under a magnetic force for 5 min. The supernatant containing immunopurified cytotrophoblasts were then plated in 4-well culture slides (BD Falcon, NJ) at a concentration of 106 cells per well in the DMEM/F12/FBS medium and maintained at 37°C in humidified atmosphere containing 5 percent carbon dioxide and 95 percent air. After 72 hours of culture, SCTs were obtained following spontaneous differentiation of cytotrophoblasts and were used for the studies described below.
Incubation with Antiphospholipid Antibodies and Hydroxychloroquine
To determine the effects of aPL antibodies on AnxA5 and whether HCQ might alter the effect as previously described in other systems,15 aPL or control antibodies (polyclonal antibody at 0.2 mg/ml and mAb at 0.1 mg/ml), together with either HCQ (1 μg/ml in HBS) or buffer control (HBS) in the DMEM/F12/FBS medium were added to the SCTSs and incubated in humidified atmosphere for 24 hours. HCQ was used at a concentration of 1 μg/ml because that is in the therapeutic range of serum concentrations in patients who are administered the drug for SLE,36 and was shown in previous studies not to be toxic to cultured cells.14,15 Syncytialized trophoblasts exposed to the same concentration of HCQ for 24 hours were assessed for function by measuring hCG levels in the culture media Levels of hCG were measured using an Immulite 1000 analyzer (Siemens, Munich, Germany); this assay measures hCG using solid phase, two-site chemiluminescent immunometric technology and has a reportable range from 1.1 to 5,000 mIU/ml. The culture media of cells incubated for 24 hours in culture medium containing HCQ (1 μg/ml) had 1.2 mIU/μg cell protein, which was exactly the same concentration as cells incubated in control culture medium.”
The cells were then washed with HBS containing 1.25 mM CaCl2, fixed with 5 % formalin containing 1.25 mM CaCl2 for 4 min at room temperature, and washed 3× with the calcium-containing HBS. To visualize the cell-bound AnxA5, the SCTs were incubated with rabbit anti-human AnxA5 (2 μg/ml) for 1 hour at room temperature, washed 3× with the calcium-containing buffer, followed by incubation for 1 hr with FITC-conjugated goat anti-rabbit IgG (1:100 dilution in HBS-CaCl2 buffer) (Sigma, St. Louis, MO). The cell-bound IgG was visualized by incubating the SCTs for 1 hour with rhodamine-conjugated goat anti-human IgG (1:100 dilution in HBS-CaCl2 buffer) (Sigma, St. Louis, MO). For the experiments with polyclonal aPL and control IgG antibodies done with and without HCQ, 3 experiments were done for each condition, with consistent results. For the confirmatory experiments with monoclonal IgGs, one experiment performed in duplicate for each condition and these showed consistent results. The immunostained SCTs were then mounted with medium containing DAPI (Vector Laboratories, Inc, LA). The slides were viewed in Analytical Imaging Facility, Albert Einstein College of Medicine. To confirm findings, the experiment described above was carried out 3 times using the SCTs that were isolated from 2 term placentas.
Laser Confocal Microscopy
The SCT cells, treated as described above, were observed and images of random areas were taken using a Leica TCS SP2 AOBS confocal microscope (Mannheim, Germany) equipped with Argon lase (set at 488 nm for excitation of FITC), diode lasers (set at 561 nm and 405 nm for excitations of rhodamine and DAPI, respectively), and objective lens HCX PL APO CS 40.0 × 1.25 OIL UV. To display three-dimensional (3D) images, a series of images in the Z-axis were taken at every 1.5 μm voxel. Line-by-line sequential scanning was used to eliminate crosstalk between channels. Conditions for imaging were set with the cells that produced the strongest fluorescent signals and all of the settings were kept constant during the imaging sessions.
Quantitative analysis of the immunofluorescent distributions of anti-AnxA5 and anti-human IgGs was performed using the ImageJ software (available at http://rsb.info.nih.gov/ij/). Z-stack image was prepared from the Z-axis slices, and Z-projection images with average intensity were then created from the original Z-stacks. The area covered by SCTs were contoured and the total cellular areas were determined along with the areas that were positive for fluorescence in each image; positivity was defined objectively as having fluorescence intensity band of 155–255. The results were expressed as percentage of total cellular area with positive fluorescence. The data obtained from six culture wells for polyclonal antibody treatment and two wells for monoclonal antibody treatment, without and with HCQ added, were then statistical analyzed using unpaired t test using GraphPAd InStat software (GraphPad Software, San Diego California USA, www.graphpad.com).
Results
Confocal microscopic imaging of SCTs exposed to the control polyclonal and monoclonal IgGs (Figs. 1A & 2A) showed very little binding of antibodies and strong expression of AnxA5 on the cell membranes (Fig 1B & Fig 2B). In striking contrast, exposure of the cells to polyclonal and monoclonal aPL IgGs resulted in significant binding of the antibodies to the cells (Figs 1D & 2D) and in marked reduction of AnxA5 on the cells (Figs 1E & 2E). Remarkably, HCQ (1 μg/ml) completely reversed the effects of aPL antibodies by markedly decreasing the binding of the IgG antibodies (Figs 1G & 2G) and restoring AnxA5 expression (Fig 1H & 2H). HCQ had no effect on cells exposed to the control IgGs (images not shown).
Figure 1. Effects of HCQ on polyclonal aPL IgG binding and AnxA5 expression on cultured SCTs.
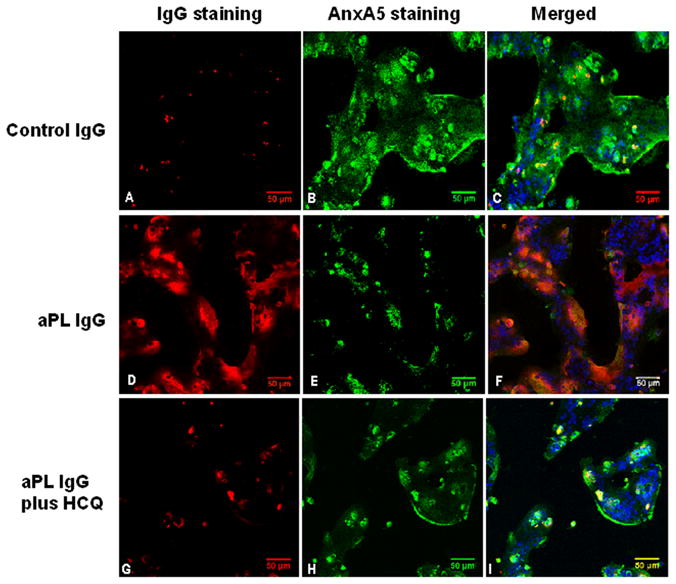
Representative images of three-channel laser confocal microscopy show that in the absence of HCQ, cells treated with polyclonal control IgG showed A) very little bound-IgG (red fluorescence) and B) normal expression of AnxA5 (green fluorescence). Cells treated with polyclonal aPL IgG showed D) a large amount of bound-IgG (red fluorescence) and E) markedly reduced expression of AnxA5 (green fluorescence).
Treatment with 1μg/ml of HCQ G) reduced the amount of bound-aPL IgG and H) increased the expression of AnxA5 on the cells. Addition of HCQ to the polyclonal control IgG-treated cells had no discernible effect (data not shown). C, F and I show merged images of green (FITC), red (rhodamine) and blue (DAPI) fluorescence marking AnxA5, IgG and nuclei, respectively. [bars = 50 μm. Confocal microscopy 3-dimensional projections of Z-axis image stacks, voxel size (μm): width 0.73, height 0.73, and depth 1.50]
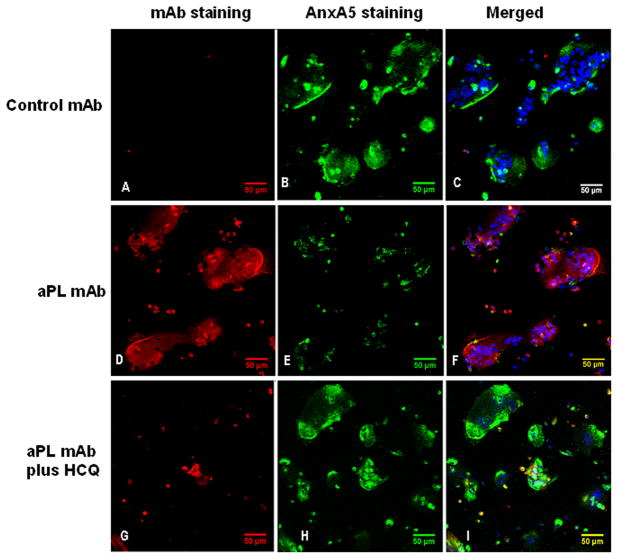
Figure 2. Effects of HCQ on monoclonal aPL IgG binding and AnxA5 expression on cultured SCTs.
Representative images of three-channel laser confocal microscopy are similar to the results with polyclonal IgGs shown in Figure 1. In the absence of HCQ, cells treated with control mAb showed A) very little binding of IgG (red fluorescence) and B) normal expression of AnxA5 (green fluorescence). Cells treated with aPL mAb showed D) extensive binding of IgG (red fluorescence) and E) markedly reduced expression of AnxA5 (green fluorescence).
Treatment with 1 μg/mL HCQ G) reduced the amount of bound aPL-mAb on the cells and H) increased the expression of AnxA5. Addition of HCQ to the control mAb-treated cells had no discernible effect (data not shown). C, F and I show merged of green (FITC), red (rhodamine) and blue (DAPI) fluorescence marking AnxA5, IgG and nuclei, respectively. [bars = 50 μm. Confocal microscopy 3-dimensional projections of Z-axis image stacks, voxel size (μm): width 0.73, height 0.73, and depth 1.50]
These confocal microscopic observations were confirmed with computer-based quantitative measurements of percentage of areas with positive immunofluorescence on the cells (Fig 3). In the absence of HCQ, polyclonal aPL IgG significantly reduced the area of AnxA5 as compared to polyclonal control IgG (mean±SEM: 4.5±0.9 % for aPL IgGs versus 20.7±1.5 % for control IgGs; n=6, p<0.0001;Fig 3A). HCQ reversed the aPL Ig G-mediated reduction of AnxA5 and restored AnxA5 expression on the SCTs (20.8±0.4 % for aPL IgG plus HCQ versus 20.0±1.3 % for control IgG plus HCQ, n=6, p=N.S.; Fig 3A). Similar results for measurements of the percentage of the areas with positive immunofluorescence for AnxA5 were observed on the SCTs treated with monoclonal aPL and control IgGs (data not shown).
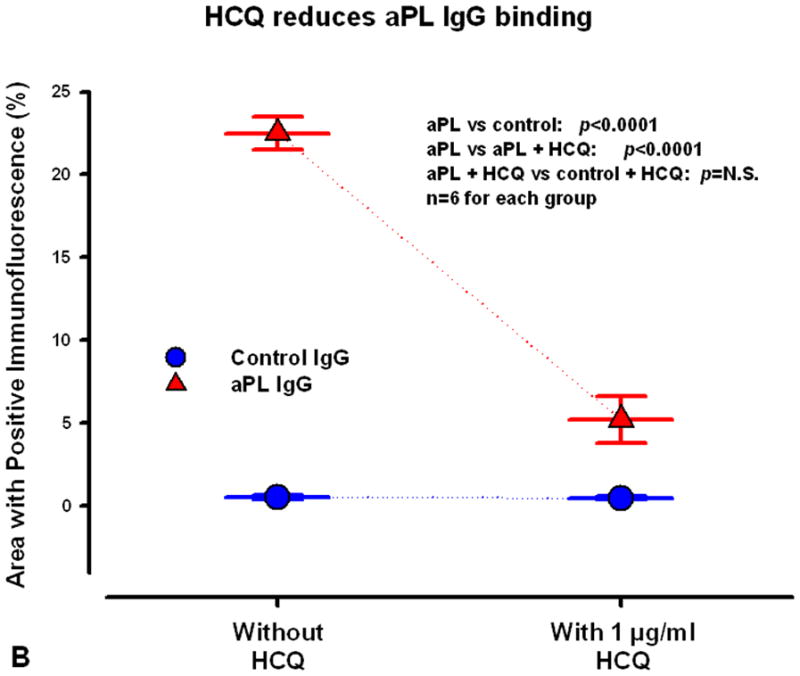
Figure 3. Computer-based quantitative measurements of areas with positive immunofluorescence for AnxA5 and IgG antibodies on the SCTs.
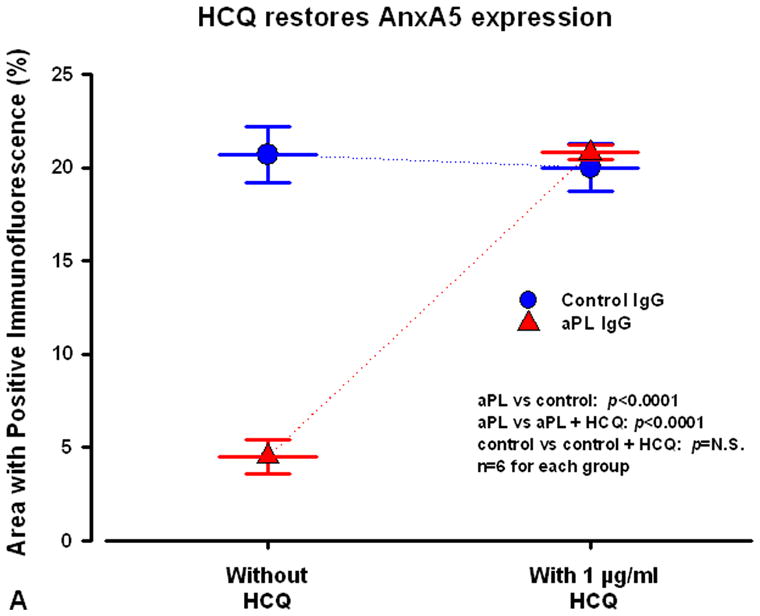
A) In the absence of HCQ, polyclonal aPL IgG significantly reduced the area of AnxA5 with positive immunofluorescence as compared to polyclonal control IgG. In the presence of HCQ, the drug reversed the aPL IgG-mediated reduction of AnxA5 and restored AnxA5 expression on the SCTs.
B) HCQ reversed the binding of aPL IgG to the SCTs. In the absence of HCQ, the cells incubated with polyclonal aPL IgG had a significantly larger area of intense fluorescence for rhodamine-labeled IgG than the cells incubated with polyclonal control IgG. Incubation of the SCTs with aPL IgG together with HCQ markedly reduced the area containing aPL IgG to a level that were much close to the control IgG.
Computer-based quantitative measurements of IgG localization showed that HCQ markedly reduced the binding of aPL IgG to the SCTs. In the absence of HCQ, the cells incubated with polyclonal aPL IgG had a significantly larger area of positive fluorescence for rhodamine-labeled IgG than the cells incubated with polyclonal control IgGs (22.5±1.0 % of area for aPL IgG versus 0.53±0.1 % for control IgG, n=6; p<0.0001, Fig 3B). However, incubation with HCQ markedly reduced the cellular area displaying aPL IgGs (5.2±1.4 % for aPL IgG plus HCQ, compared to 22.5±1.0 % for aPL IgG alone, n=6, p<0.0001; Fig 3B). There were no significant differences between bindings of control IgGs in the absence or presence of HCQ (p=N.S.; Fig 3B). Similar quantitative results were obtained on the SCTs treated with monoclonal aPL and control IgGs (data not shown).
COMMENTS
Obstetrical APS is currently treated with anticoagulant medications,37,38 a treatment that is associated with the risk of bleeding complications and that does not specifically target an APS disease process. It would therefore be beneficial to identify molecules that might target specific early steps in the disease mechanism as candidates for clinical trials. For this reason, we investigated the possibility that a synthetic antimalarial drug, HCQ, might be effective in reversing the adverse effects of aPL antibodies on earlier steps in the APS disease process in vitro. HCQ is an “old” drug that had, through astute clinical observations, been found to be beneficial for treating SLE and for which clinical studies and animal models had indicated a protective effect against thrombosis, An additional factor that motivated our investigation of HCQ was the relatively long experience that rheumatologists and obstetricians have had in treating pregnant women with this drug. Treatment of pregnant women with SLE was first described over 30 years ago,39,40 and its safety during pregnancy and during lactation have been extensively documented36,41,42 and systematically reviewed.43 These results offer a novel therapeutic mechanism for HCQ. Previously, the beneficial effect of HCQ in autoimmune diseases has mainly been attributed to its increasing pH within intracellular vacuoles that reduces proteolysis by acidic hydrolases and the subsequent antigen presentation that is required for generating the immune responses.44 Also, HCQ may disrupt T-cell receptor crosslinking-dependent calcium signaling.45
The current data add to our prior work on HCQ that demonstrated through biophysical studies on planar phospholipids and by atomic force microscopic imaging that the drug dissociates aPL immune complexes,29 and also restores the formation of AnxA5 crystals over sites that had been disrupted by the antibodies.15 Furthermore, using quantitative immunoassays for IgG and AnxA5 in a cultured BeWo trophoblast model, we showed that HCQ reduced the amount of IgG on the surfaces of cells incubated with aPL antibodies and restored AnxA5 expression.13–15 We also demonstrated that HCQ restored AnxA5 expression as well as its functional anticoagulant activity on these cells.13–15 The current results provide the first confocal microscopic imaging evidence that HCQ reverses the binding of aPL antibodies to human placental SCTs and that the drug restores AnxA5 expression. We plan, in future studies, to investigate this effect in placental villous explants.12
As mentioned above, HCQ is a synthetic antimalarial compound has become widely used for the immunosuppressive treatment of systemic SLE.46–49 It was first suggested more than two decades ago that HCQ may reduce the frequency of thrombosis among SLE patients.23 Several observational studies have supported this concept for patients with SLE24–26 and with APS,26,27 although there have not yet been prospective controlled randomized studies. In an animal model of APS, the drug significantly reduced the extent of experimentally provoked thrombosis22 and also reversed aPL-mediated platelet activation.50
Taking together the above reports with the current confocal microscopic imaging studies, the prior quantitative studies with HCQ in cultured cells, and the extensive clinical experience with the drug during pregnancy and lactation,39–43,51 there is the intriguing possibility that this drug might be an effective candidate for targeting specific steps in the obstetric APS. The important questions of whether or not this treatment would be more effective than the current anticoagulant treatment approaches, or whether it might be useful in supplementing current approaches, would need to be established with prospective randomized clinical trials.
Acknowledgments
These studies were supported by grant HL-61331 from the National Institutes of Health /the National Heart Lung and Blood. We thank Mr. Zhonghua Tang for his assistance in preparing the cells for culture.
Abbreviations
- AnxA5
annexin A5
- APS
antiphospholipid syndrome
- HBS
HEPES buffer saline
- IgG
immunoglobulin G
- aPL
antiphospholipid
- mAb
monoclonal antibody
- SCTs
human syncytiotrophoblasts
- SLE
systemic lupus erythematosus
Footnotes
Reprints will not be available
DISCLOSURE: None of the authors have conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Krikun G, Lockwood CJ, Wu XX, Zhou XD, Guller S, Calandri C, et al. The expression of the placental anticoagulant protein, annexin V, by villous trophoblasts: immunolocalization and in vitro regulation. Placenta. 1994;15:601–12. doi: 10.1016/s0143-4004(05)80407-2. [DOI] [PubMed] [Google Scholar]
- 2.Reviakine I, Bergsma-Schutter W, Mazeres-Dubut C, Govorukhina N, Brisson A. Surface topography of the p3 and p6 annexin V crystal forms determined by atomic force microscopy. J Struct Biol. 2000;131:234–39. doi: 10.1006/jsbi.2000.4286. [DOI] [PubMed] [Google Scholar]
- 3.Tait JF, Gibson D, Fujikawa K. Phospholipid binding properties of human placental anticoagulant protein-I, a member of the lipocortin family. J Biol Chem. 1989;264:7944–49. [PubMed] [Google Scholar]
- 4.Wang X, Campos B, Kaetzel MA, Dedman JR. Annexin V is critical in the maintenance of murine placental integrity. Am J Obstet Gynecol. 1999;180:1008–16. doi: 10.1016/s0002-9378(99)70674-5. [DOI] [PubMed] [Google Scholar]
- 5.Ornaghi S, Vergani P, Urban G, Giardini V, Moltrasio F, Leone BE. Immunohistochemical expression of Annexin A5 in preeclamptic placentas. Placenta. 2011;32:264–68. doi: 10.1016/j.placenta.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 6.Rand JH, Arslan AA, Wu XX, Wein R, Mulholland J, Shah M, et al. Reduction of circulating Annexin A5 levels and resistance to Annexin A5 anticoagulant activity in women with recurrent spontaneous pregnancy losses. Am J Obstet Gynecol. 2006;194:182–88. doi: 10.1016/j.ajog.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 7.Chinni E, Tiscia GL, Colaizzo D, Vergura P, Margaglione M, Grandone E. Annexin V expression in human placenta is influenced by the carriership of the common haplotype M2. Fertil Steril. 2009;91:940–42. doi: 10.1016/j.fertnstert.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 8.Markoff A, Gerdes S, Feldner S, Bogdanova N, Gerke V, Grandone E. Reduced allele specific annexin A5 mRNA levels in placentas carrying the M2/ANXA5 allele. Placenta. 2010;31:937–40. doi: 10.1016/j.placenta.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Bogdanova N, Horst J, Chlystun M, Croucher PJ, Nebel A, Bohring A, et al. A common haplotype of the annexin A5 (ANXA5) gene promoter is associated with recurrent pregnancy loss. Hum Mol Genet. 2007;16:573–78. doi: 10.1093/hmg/ddm017. [DOI] [PubMed] [Google Scholar]
- 10.Tiscia G, Colaizzo D, Chinni E, Pisanelli D, Scianname N, Favuzzi G, et al. Haplotype M2 in the annexin A5 (ANXA5) gene and the occurrence of obstetric complications. Thromb Haemost. 2009;102:309–13. doi: 10.1160/TH09-02-0123. [DOI] [PubMed] [Google Scholar]
- 11.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS) J Thromb Haemost. 2006;4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 12.Rand JH, Wu XX, Guller S, Gil J, Guha A, Scher J, et al. Reduction of annexin-V (placental anticoagulant protein-I) on placental villi of women with antiphospholipid antibodies and recurrent spontaneous abortion. Am J Obstet Gynecol. 1994;171:1566–72. doi: 10.1016/0002-9378(94)90403-0. [DOI] [PubMed] [Google Scholar]
- 13.Vogt E, Ng AK, Rote NS. Antiphosphatidylserine antibody removes annexin-V and facilitates the binding of prothrombin at the surface of a choriocarcinoma model of trophoblast differentiation. Am J Obstet Gynecol. 1997;177:964–72. doi: 10.1016/s0002-9378(97)70302-8. [DOI] [PubMed] [Google Scholar]
- 14.Rand JH, Wu XX, Andree HA, Lockwood CJ, Guller S, Scher J, et al. Pregnancy loss in the antiphospholipid-antibody syndrome--a possible thrombogenic mechanism. N Engl J Med. 1997;337:154–60. doi: 10.1056/NEJM199707173370303. [DOI] [PubMed] [Google Scholar]
- 15.Rand JH, Wu XX, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood. 2010;115:2292–99. doi: 10.1182/blood-2009-04-213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rand JH, Wu XX, Quinn AS, Chen PP, McCrae KR, Bovill EG, et al. Human monoclonal antiphospholipid antibodies disrupt the annexin A5 anticoagulant crystal shield on phospholipid bilayers: evidence from atomic force microscopy and functional assay. Am J Pathol. 2003;163:1193–200. doi: 10.1016/S0002-9440(10)63479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rand JH, Wu XX, Andree HAM, Alexander Ross JB, Rosinova E, Gascon-Lema MG, et al. Antiphospholipid antibodies accelerate plasma coagulation by inhibiting annexin-V binding to phospholipids: a “lupus procoagulant” phenomenon. Blood. 1998;92:1652–60. [PubMed] [Google Scholar]
- 18.Hanly JG, Smith SA. Anti-beta2-glycoprotein I (GPI) autoantibodies, annexin V binding and the anti-phospholipid syndrome. Clin Exp Immunol. 2000;120:537–43. doi: 10.1046/j.1365-2249.2000.01248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomer A. Antiphospholipid antibody syndrome: Rapid, sensitive, and specific flow cytometric assay for determination of anti-platelet phospholipid autoantibodies. J Lab Clin Med. 2002;139:147–54. doi: 10.1067/mlc.2002.121551. [DOI] [PubMed] [Google Scholar]
- 20.Tomer A, Bar-Lev S, Fleisher S, Shenkman B, Friger M, Abu-Shakra M. Antiphospholipid antibody syndrome: the flow cytometric annexin A5 competition assay as a diagnostic tool. Br J Haematol. 2007;139:113–20. doi: 10.1111/j.1365-2141.2007.06751.x. [DOI] [PubMed] [Google Scholar]
- 21.Gaspersic N, Ambrozic A, Bozic B, Majhenc J, Svetina S, Rozman B. Annexin A5 binding to giant phospholipid vesicles is differentially affected by anti-beta2-glycoprotein I and anti-annexin A5 antibodies. Rheumatology. 2007;46:81–86. doi: 10.1093/rheumatology/kel200. [DOI] [PubMed] [Google Scholar]
- 22.Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation. 1997;96:4380–84. doi: 10.1161/01.cir.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 23.Wallace DJ. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus? Arthritis Rheum. 1987;30:1435–36. doi: 10.1002/art.1780301219. [DOI] [PubMed] [Google Scholar]
- 24.Petri M. Thrombosis and systemic lupus erythematosus: the Hopkins Lupus Cohort perspective. Scand J Rheumatol. 1996;25:191–93. doi: 10.3109/03009749609069986. [DOI] [PubMed] [Google Scholar]
- 25.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009;68:238–41. doi: 10.1136/ard.2008.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum. 2009;61:29–36. doi: 10.1002/art.24232. [DOI] [PubMed] [Google Scholar]
- 27.Erkan D, Yazici Y, Peterson MG, Sammaritano L, Lockshin MD. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology (Oxford) 2002;41:924–29. doi: 10.1093/rheumatology/41.8.924. [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2009 doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 29.Rand JH, Wu XX, Quinn AS, Chen PP, Hathcock JJ, Taatjes DJ. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood. 2008;112:1687–95. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sammaritano LR, Gharavi AE, Soberano C, Levy RA, Lockshin MD. Phospholipid binding of antiphospholipid antibodies and placental anticoagulant protein. J Clin Immunol. 1992;12:27–35. doi: 10.1007/BF00918270. [DOI] [PubMed] [Google Scholar]
- 31.Zhu M, Olee T, Le DT, Roubey RA, Hahn BH, Woods VLJ, et al. Characterization of IgG monoclonal anti-cardiolipin/anti-beta2GP1 antibodies from two patients with antiphospholipid syndrome reveals three species of antibodies. Br J Haematol. 1999;105:102–09. [PubMed] [Google Scholar]
- 32.Lin WS, Chen PC, Yang CD, Cho E, Hahn BH, Grossman J, et al. Some antiphospholipid antibodies recognize conformational epitopes shared by beta2-glycoprotein I and the homologous catalytic domains of several serine proteases. Arthritis Rheum. 2007;56:1638–47. doi: 10.1002/art.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horkko S, Olee T, Mo L, Branch DW, Woods VL, Jr, Palinski W, et al. Anticardiolipin antibodies from patients with the antiphospholipid antibody syndrome recognize epitopes in both beta(2)-glycoprotein 1 and oxidized low-density lipoprotein. Circulation. 2001;103:941–46. doi: 10.1161/01.cir.103.7.941. [DOI] [PubMed] [Google Scholar]
- 34.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF., III Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–82. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- 35.Guller S, LaCroix NC, Kirkun G, Wozniak R, Markiewicz L, Wang EY, et al. Steroid regulation of oncofetal fibronectin expression in human cytotrophoblasts. J Steroid Biochem Mol Biol. 1993;46:1–10. doi: 10.1016/0960-0760(93)90202-8. [DOI] [PubMed] [Google Scholar]
- 36.Costedoat-Chalumeau N, Amoura Z, Hulot JS, Aymard G, Leroux G, Marra D, et al. Very low blood hydroxychloroquine concentration as an objective marker of poor adherence to treatment of systemic lupus erythematosus. Ann Rheum Dis. 2007;66:821–24. doi: 10.1136/ard.2006.067835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Empson M, Lassere M, Craig J, Scott J. Prevention of recurrent miscarriage for women with antiphospholipid antibody or lupus anticoagulant. Cochrane Database Syst Rev. 2005;2:CD002859. doi: 10.1002/14651858.CD002859.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoppe B, Burmester GR, Dorner T. Heparin or aspirin or both in the treatment of recurrent abortions in women with antiphospholipid antibody (syndrome) Curr Opin Rheumatol. 2011 doi: 10.1097/BOR.0b013e328344c3f7. [DOI] [PubMed] [Google Scholar]
- 39.Parke AL. Antimalarial drugs, systemic lupus erythematosus and pregnancy. J Rheumatol. 1988;15:607–10. [PubMed] [Google Scholar]
- 40.Parke A. Antimalarial drugs and pregnancy. Am J Med. 1988;85:30–33. doi: 10.1016/0002-9343(88)90359-2. [DOI] [PubMed] [Google Scholar]
- 41.Levy RA, Vilela VS, Cataldo MJ, Ramos RC, Duarte JL, Tura BR, et al. Hydroxychloroquine (HCQ) in lupus pregnancy: double-blind and placebo-controlled study. Lupus. 2001;10:401–04. doi: 10.1191/096120301678646137. [DOI] [PubMed] [Google Scholar]
- 42.Costedoat-Chalumeau N, Amoura Z, Huong DL, Lechat P, Piette JC. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Review of the literature. Autoimmun Rev. 2005;4:111–15. doi: 10.1016/j.autrev.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 43.Khamashta MA. Systemic lupus erythematosus and pregnancy. Best Pract Res Clin Rheumatol. 2006;20:685–94. doi: 10.1016/j.berh.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Fox RI. Mechanism of action of hydroxychloroquine as an antirheumatic drug. Semin Arthritis Rheum. 1993;23:82–91. doi: 10.1016/s0049-0172(10)80012-5. [DOI] [PubMed] [Google Scholar]
- 45.Goldman FD, Gilman AL, Hollenback C, Kato RM, Premack BA, Rawlings DJ. Hydroxychloroquine inhibits calcium signals in T cells: a new mechanism to explain its immunomodulatory properties. Blood. 2000;95:3460–66. [PubMed] [Google Scholar]
- 46.Rothfield N. Efficacy of antimalarials in systemic lupus erythematosus. Am J Med. 1988;85:53–56. doi: 10.1016/0002-9343(88)90363-4. [DOI] [PubMed] [Google Scholar]
- 47.Tsakonas E, Joseph L, Esdaile JM, Choquette D, Senecal JL, Cividino A, et al. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus. 1998;7:80–85. doi: 10.1191/096120398678919778. [DOI] [PubMed] [Google Scholar]
- 48.Molad Y, Gorshtein A, Wysenbeek AJ, Guedj D, Majadla R, Weinberger A, et al. Protective effect of hydroxychloroquine in systemic lupus erythematosus. Prospective long-term study of an Israeli cohort. Lupus. 2002;11:356–61. doi: 10.1191/0961203302lu203ra. [DOI] [PubMed] [Google Scholar]
- 49.Fessler BJ, Alarcon GS, McGwin G, Jr, Roseman J, Bastian HM, Friedman AW, et al. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005;52:1473–80. doi: 10.1002/art.21039. [DOI] [PubMed] [Google Scholar]
- 50.Espinola RG, Pierangeli SS, Ghara AE, Harris EN. Hydroxychloroquine reverses platelet activation induced by human IgG antiphospholipid antibodies. Thromb Haemost. 2002;87:518–22. [PubMed] [Google Scholar]
- 51.Abarientos C, Sperber K, Shapiro DL, Aronow WS, Chao CP, Ash JY. Hydroxychloroquine in systemic lupus erythematosus and rheumatoid arthritis and its safety in pregnancy. Expert Opin Drug Saf. 2011 Mar 22;2011 doi: 10.1517/14740338.2011.566555. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]