Abstract
In this study, the functional role of INSM1 is examined with an AR42J acinar cell model for trans-differentiation into insulin-positive cells. Islet transcription factors (ITFs: INSM1, Pdx-1, and NeuroD1) are over-expressed in AR42J cells using adenoviral vectors. Addition of Ad-INSM1 alone or the combination of three ITFs to the AR42J cells triggers cellular trans-differentiation. Ectopic expression of INSM1 directly induces insulin, Pax6, and Nkx6.1 expression, whereas Pdx-1 and NeuroD1 were slightly suppressed by INSM1. Addition of Pdx-1 and NeuroD1 with INSM1 further enhances endocrine trans-differentiation by increasing both the numbers and intensity of the insulin positive cells with simultaneous activation of ITFs, Ngn3 and MafA. INSM1 expression alone partially inhibits dexamethasone-induced exocrine amylase expression. The combination of the three ITFs completely inhibits amylase expression and concomitantly induces greater acinar cell trans-differentiation into endocrine cells. Also, addition of the three ITFs promotes EGF and TGFβ receptors expression. Stimulation by the three ITFs along with the EGF/TGFβ growth factors strongly promotes insulin gene expression. The combination of the three ITFs and EGF/TGFβ growth factors with the primary cultured pancreatic acini also facilitates exocrine to endocrine cell differentiation. Taken together, both the AR42J cell line and the primary cultured mouse acinar cells support INSM1 induced acini trans-differentiation model.
Keywords: INSM1, AR42J, islet transcription factor, acinar cells, trans-differentiation, reprogramming
Introduction
In the present study, we employed a rat pancreatic acinar adenocarcinoma (AR42J) cell line as a model to investigate the functional role of a zinc-finger islet transcription factor († ITF), INSM1 (formerly named IA-1, insulinoma-associated antigen-1). INSM1 was originally isolated from a human insulinoma subtraction library (Goto et al, 1992). The temporal position of INSM1 during pancreatic endocrine cell differentiation is established to be located immediately after the neurogenin 3 (Ngn3) endocrine factor (Mellitzer et al, 2006;Breslin et al, 2007). Biochemical studies revealed that INSM1 exerts transcriptional repressor activities to the downstream target genes neurogenic differentiation 1 (NeuroD1), insulin, and its own gene expression (Breslin et al, 2002;Liu et al, 2006;Wang et al, 2008). Global deletion of Insm1 resulted in embryonic lethality and the impairment of β-cell development (Gierl et al, 2006). However, the normal functional effect of INSM1 on pancreatic acinar cells is not known.
AR42J is a pancreatic tumor cell line derived from azaserine-induced malignant nodules of the rat pancreas (Christophe, 1994). They resemble normal acinar cells by synthesizing and secreting digestive enzymes, but their exocrine function is partially abnormal. Also, they possess neuroendocrine-regulated pathways, which categorize them as acinar cells with an amphicrine phenotype. Numerous reports commonly use AR42J cells as an in vitro model to study peptide hormone receptor regulation and glucocorticoid induced amylase secretion (Rosewicz et al, 1992; Logsdon et al, 1995; Rosewicz and Logsdon, 1991; Ishiyama et al, 1998). In parallel, hormone factors such as betacellulin, activin A, transforming growth factor-β, glucagon-like peptide 1, and exendin-4 were shown to be able to convert AR42J cells into insulin-positive cells (Mashima et al, 1996; Yew et al, 2004; Zhou et al, 1999). During the endocrine trans-differentiation process, ITFs were induced and/or activated to promote endocrine cell conversion (Zhu et al, 2002; Ogihara et al, 2008). Therefore, AR42J cells represent an excellent model for studying ITFs involved in exocrine to endocrine cell trans-differentiation. In our previous report, AR42J cells were used as a model to study the ITF expression patterns after treatment with insulinoma-conditioned medium. This study revealed that AR42J cells produced insulin and induced INSM1, pancreatic duodenal homeobox-1 (Pdx-1), NeuroD1, and homeodomain transcription factor 6.1 (Nkx6.1) mRNA expression (Zhu et al, 2002). A recent study reported the use of the combination of Ngn3, Pdx-1, and musculoaponeurotic fibrosarcoma oncogene homolog A (MafA) adenoviral vectors could directly convert 20% of mouse acini into insulin-positive cells in vivo (Zhou et al, 2008). Thus, we investigated the functional role of INSM1 in pancreatic cells and evaluated whether INSM1 alone and/or in combination with other ITFs could induce AR42J and/or normal mouse pancreatic acinar cells to differentiate into insulin-positive cells.
In order to further investigate the molecular mechanisms underlying the ITFs functional effects, we also analyzed growth factor receptor expression and revealed that the epidermal growth factor receptor (EGFR) and transforming growth factor β receptor (TGFβR) were activated. With the combination of EGF and TGFβ growth factors with the three ITFs, we showed robust acini conversion into β-cells. Furthermore, the INSM1 effect was demonstrated by the direct conversion of normal mouse pancreatic acini into insulin-positive cells. The outcome of this study provides more insight into the functional role of INSM1 and how the different combinations of ITFs and growth factors can facilitate acinar cell trans-differentiation.
Materials and Methods
Cell lines and chemicals
A rat pancreatic acinar adenocarcinoma (AR42J) and a rat insulinoma (RINm5F) cell line were obtained from the American Type Culture Collection (ATCC) and maintained according to ATCC’s recommendation. Rabbit polyclonal anti-insulin antibody was purchased from Abbiotec (San Diego, CA) and mouse monoclonal anti-insulin antibody and anti-actin antibody were purchased from Sigma (St. Louis, MO). Anti-Ngn3 antibody was obtained from Developmental Studies Hybridoma Bank (F25A1B3). Anti-MafA antibody was purchased from Abcam (Cambridge, MA). Anti-TGFβR and EGFR antibody were purchased from Santa Cruz (Santa Cruz, CA). Horseradish peroxidase conjugated secondary antibodies were purchased from Pierce (Rockford, IL). Fluorescence conjugated (Alexa Fluor 594 or 488) secondary antibodies were purchased from Invitrogen (Carlsbad, CA). FITC-conjugated secondary antibody was purchased from Southern Biotechnology (Birmingham, AL). Recombinant TGFβ protein was purchased from R&D Systems (Minneapolis, MN) and EGF was purchased from Invitrogen. All the other chemicals were purchased from Sigma.
Adenovirus and infection
Recombinant adenovirus expressing human INSM1, mouse Pdx-1 and hamster NeuroD1 were generated using the AdEasy XL adenoviral vector system (Agilent, CA). AR42J cells were infected with either specific m.o.i. as indicated or 40:1 m.o.i. adenovirus for 3 days. In the ITFs and EGF/TGFβ induction, AR42J cells were first infected with adenoviral vectors for 24 hour and then EGF (50 ng/ml medium) and TGFβ (2 ng/ml medium) were added for an additional 48 hour before subjecting the cells to reverse transcription real-time polymerase chain reaction (RT-PCR), protein, or insulin content analysis.
RT-PCR and real-time PCR
RNA was isolated from the adenovirus-infected cells using the Trizol reagent (Invitrogen). Total RNA was treated with DNase I (Promega, Madison, WI), extracted with phenol/chloroform, and precipitated using isopropanol. A total of 2 μg of DNase I treated RNA was used for first strand reverse transcription using a cDNA synthesis kit (Invitrogen) according to the manufacturer’s instructions. PCR conditions were 95 °C for 5 minutes, followed by 40 cycles of 95 °C for 30 seconds, 30 seconds at 55 °C, and 72 °C for 30 seconds. For real-time PCR, one microliter of the reverse-transcribed cDNA obtained from the above procedure was used in a 25 μl reaction mixture including SYBR Green PCR Master Mix (BioRad, Hercules, CA) and 1 μl of 10 μM primers for the rat insulin gene, Ngn3, and MafA. Actin was used as an internal control. The cycling conditions were hold at 95 °C for 5 minute, followed by 25–40 cycles at 94 °C for 30 second, 60 °C for 30 second and 72 °C for 30 second. The fluorescent signals were analyzed using an ICycler IQ multicolor real-time detection system (BioRad). The PCR products were separated on 2% agarose gel. The PCR primer pairs and the product sizes are listed in the Table 1.
Table 1.
Primer Sequences for RT-PCR
| Gene Name | Forward Primer | Reverse Primer | Size (bps) |
|---|---|---|---|
| Actin | CGTAAAGACCTCTATGCCAA | AGCCAGGGCAGTAATCTCCT | 96 |
| INSM1 | AACTGTCCTTCGCTTGGA | ACGAGACAAACGCGTACAGCT | 80 |
| NeuroD1 | CTTGGCCAAGAACTACATCTGG | GGAGTAGGGATGCACCGGGAA | 228 |
| Pdx-1 | CTCGCTGGGAACGCTGGAACA | GCTTTGGTGGATTTCATCCACGG | 225 |
| Insulin2 | TGGATTCTTCTACACACCCATG | ACGATGCCGCGCTTCTGCC | 276 |
| Nkx6.1 | TACCCCCCATCAAGGATCCATT | GTGCTTCTTTCTCCACTTGGTCC | 223 |
| Nkx2.2 | CCGAGAAAGGTATGGAGGTGAC | CTGGGCGTTGTACTGCATGTGCTG | 187 |
| Pax4 | CTAATGGCTGTGTGAGCAAG | GGGAGAAGATAGTCCGATTC | 386 |
| Pax6 | GGAGTGAATCAGCTTGGTGGT | ATCTGTCTCGGATTTCCCAAGC | 297 |
| Ngn3 | AAGAGCGAGTTGGCACTGAGCAAG | GCTGTGGTCCGCTATGCGCAG | 222 |
| MafA | GCTTCAGCAAGGAGGAGGTCATC | GCCCGCCAACTTCTCGTATTTC | 221 |
| Glut2 | CCAGCACATACGACACCAGACG | CCTGATACGCTTCTTCCAGCAATG | 587 |
| GK | AAGGGAACAACATCGTAGGA | CATTGGCGGTCTTCATAGTA | 130 |
| GHR | CCAACTCCCCTCTACACCAA | GGGCTAACCCTGCCTTAATC | 134 |
| HGFR | CCAGAAGACCAGTTTCCCAA | TCCCATGATAGACACAGCC | 265 |
| IR | GCATCAAACGGACCTATGAT | ACCTGTGTGTTTTCAGGTCC | 281 |
| TGFβR | AACGTTCATGGTTCCGAGAG | ATGTGAAGATGGGCAAGACC | 238 |
| EGFR | AGATGACACATTCCTTCCCG | GTTGTCCAGGCTCATTTGGT | 262 |
Amylase activity assay
For amylase expression, AR42J cells were cultured in the presence or absence of 10 nmol/l dexamethasone and the different titers of ITFs treatment for 5 days. The cultured cells were washed with ice-cold PBS before suspension in phosphate buffer (50 mM pH 6.9 containing 50 mM sodium chloride). The cells were sonicated in phosphate buffer for 30 seconds at 4 °C. The cell lysate was collected for an amylase activity assay (Bernfeld, 1955). Briefly, the sonicated samples were diluted in reagent grade water before adding 1 ml of dinitrosalicyclic acid color reagent to each tube. The tubes were incubated in a boiling water bath for 5 minutes, cooled to room temperature, diluted with 10 ml reagent grade water, mixed, and finally read at absorbance (A540) versus blank on a spectrophotometer. Amylase activity was calculated against a maltose standard curve. The relative amylase activity was expressed as the fold increase of the total amylase activity with respect to the control cells.
Insulin content
AR42J cells treated or untreated with ITFs and RINm5F cells were suspended in acid-ethanol (1.5% HCl in 70% ethanol) solution overnight at −20 °C. The acid-ethanol extracted solution was centrifuged at 2000 rpm for 15 minute at 4 °C. The acid-ethanol extracts (100 μl) were neutralized with 100 μl 1 M Tris, pH 7.5. Samples were diluted for the insulin measurement using an ELISA kit (Alpco, Salem, NH) and protein measurement using BCA kit (Pierce).
Flow cytometric analysis
AR42J cells were subjected to various combinations of ITF treatment as described above. After 72 hour incubation, cells were permeabilized with PBS containing 0.1% Triton X-100 and subjected to primary antibody staining and fluorescence-activated flow cytometric analysis. Insulin-positive cells were immunostained with rabbit anti-insulin antibody overnight and a fluorescein isothiocyanate-labeled (FITC) secondary antibody was used for flow cytometric analysis. Ten thousand events were acquired and analyzed using FlowJo software (Tree Star, Inc., Ashland, OR).
Immunofluorescence staining of ITF treated AR42J cells
After ITF treatment, AR42J cells were washed and fixed onto glass slides with 4% paraformaldehyde and permeabilized with PBS containing 0.1% Triton X-100. The cells were blocked with 5% bovine serum albumin (BSA) and 1% goat antiserum in phosphate buffered saline (PBS). Insulin and transcription factor staining was performed using the appropriate primary antibody overnight and a fluorescence-conjugated secondary antibody for 1 hour at room temperature. The fluorescent signals were detected using a laser scanning confocal microscope (LSM 510, Carl Zeiss) and the nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI).
Glucose-stimulated insulin secretion of trans-differentiated AR42J cells
Insulin secretion was measured by placing the 3 ITFs/EGF/TGFβ treated AR42J cells in a transwell plate (New Transwell Clear, Costar Scientific, Cambridge, MA). The cells were pre-incubated in 1 ml of Krebs-Ringer bicarbonate buffer (KRBB) containing 143.5 mM Na+, 5.8 mM K+, 2.5 mM Ca2+, 25 mM HCO3, 0.3 % BSA (Fraction V, Sigma) and 3.3 mM glucose at 37 °C for 30 min. After pre-incubation, the cells were incubated in 1 ml of KRBB supplemented with 3.3 mM glucose for 1 h. Subsequently, the cells were transferred to medium containing 27.7 mM glucose and incubated for 1 h. Aliquots were removed and stored at −20 °C for insulin detection by ELISA.
Primary culture of pancreatic acinar cells
Normal C57BL/J6 mice were anesthetized and infused with 3 ml of collagenase solution (1.5 mg/ml in M199 medium, Sigma) through the pancreatic duct. The collagenase infused pancreas was isolated and incubated at 37 °C for 18 minutes. The digested pancreas was washed 3 times with M199 medium containing 10% fetal bovine serum and filtered through a 200 μm mesh before centrifugation. The pellet was subjected to Ficoll density gradient centrifugation and the acinar cell enriched fraction was recovered as a pellet. Prior to the induction, the residual islets were eliminated by overnight 10 mM streptozotocin (Sigma) treatment. The primary acinar culture was maintained in M199 medium containing 10% fetal bovine serum with the addition of Ad-LacZ, 3 Ad-ITFs, and/or EGF/TGFβ for 96 hours. After treatment, the cells were harvested for RNA isolation, real time PCR, and immunostaining for insulin expression. Animals used in this experiment are in compliance with the approved protocol by the Institutional Animal Care and Use Committee from Children’s Hospital, New Orleans.
Statistical analysis
All values were corrected and expressed relative to an untreated control group. All experiments were repeated at least three times. Results are presented as the mean ± SD. Statistical analysis was assessed either by the Student’s t-test when only two groups were included in the experiment or by an one-way ANOVA comparison of multiple groups using the Tukey-Kramer test with differences at p value of less than 0.05 being considered significant.
Results
INSM1 induces insulin expression in AR42J cells
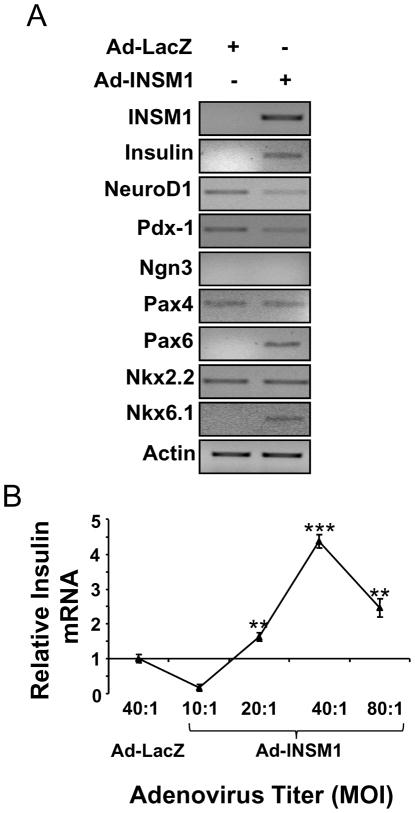
The AR42J cell line represents an excellent model for pancreatic exocrine to endocrine cell trans-differentiation. They exhibit the capacity of expressing both exocrine and neuroendocrine properties (Rosewicz et al, 1992; Christophe, 1994). In our previous study, we showed that INSM1 expression is associated with rat AR42J cell differentiation into insulin-positive cells (Zhu et al, 2002). However, it was unclear whether INSM1 alone was capable of inducing AR42J cell conversion into insulin-positive cells. In this study, we further investigate the functional role of INSM1, if any can contribute to the AR42J cell phenotype. Normally, AR42J cells do not express INSM1. First, we over-expressed INSM1 using an adenoviral expression system in AR42J cells or Ad-LacZ as a control. Then, we measured multiple ITF expression patterns during the Ad-INSM1 infection (Fig. 1A). Expression of INSM1 induced insulin, Pax6, and Nkx6.1 expression, whereas NeuroD1, Pdx-1, Pax4, and Nkx2.2 were either reduced or unchanged. Further we quantified the insulin mRNA levels induced at different Ad-INSM1 viral titers using real-time RT-PCR (Fig. 1B). A 40:1 multiplicity of infection (m.o.i.) induces the highest insulin message. Clearly, the results show that INSM1 alone is capable of inducing insulin gene expression in AR42J cells.
Fig. 1. INSM1 ectopic expression induces the insulin gene in AR42J cells.
(A) Adenoviral INSM1 infection (m.o.i.= 40:1) in AR42J cells for 3 days modulates multiple ITFs and insulin expression. (B) Various adenovirus m.o.i. show the most insulin message is induced at m.o.i.=40:1 titer. The p values represent **<0.01 and ***<0.001.
Combination of INSM1, Pdx-1, and NeuroD1 facilitates AR42J cell trans-differentiation
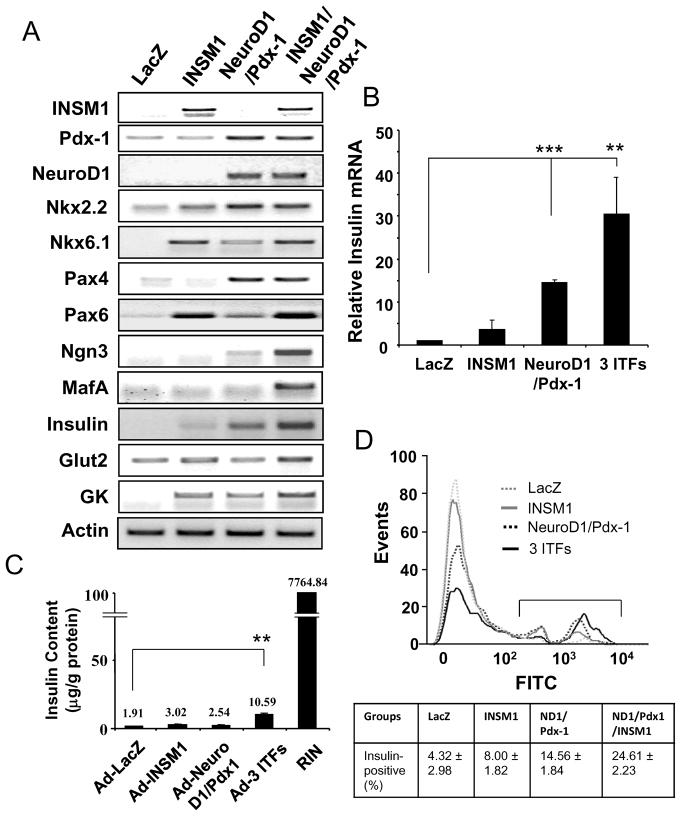
Both NeuroD1 and Pdx-1 have been previously shown to be important ITFs for β-cell differentiation and insulin gene expression (Kaneto et al, 2009). However, their expression levels in the Ad-INSM1 treated AR42J cells were relatively low or were slightly suppressed. Thus, we employed these two additional ITFs along with the Ad-INSM1 in AR42J cells to assess the ability of converting them into insulin-positive cells. As shown in Fig. 2, overexpression of the three Ad-ITFs reveals a concomitant increase of insulin message to 30 times the LacZ group with an increase in Nkx2.2, Pax4, and the activation of Ngn3 and MafA. Similarly, Pax6 and Nkx6.1 levels were either induced to the same level as the Ad-INSM1 treatment alone or slightly elevated in the three ITFs treatment group. Glucokinase (GK) was induced by Ad-INSM1 alone and elevated further by the three ITFs treatment. Glucose transporter 2 (Glut2) was only slightly increased by the three ITFs treatment. The relative insulin expression levels in the one, two, or three ITFs showed a gradual increase in insulin message from 4 times to 14 times to 30 times as compared with the Ad-LacZ control (Fig. 2B). Further, we performed insulin content analysis of the ITF treated AR42J cells using a rat insulinoma cell line (RINm5F) as a positive control (Fig. 2C). The three ITFs group show a 3.5-fold increase in the insulin content (10.59 μg/g protein) as compared with the INSM1 alone (3.02 μg/g protein). However, the total insulin content is very low in reference to the rat insulinoma cell line (0.14%) (Fig. 2C). Consistently, flow cytometric analysis of insulin positive cells revealed a higher percentage of insulin positive cells in the three ITFs treated AR42J cells (24.61%) than the Ad-INSM1 alone (8.00%) (Fig. 2D).
Fig. 2. ITF expression patterns with the addition of the three ITFs, Ad-INSM1, Ad-Pdx-1, and Ad-NeuroD1.
(A) Adenoviral vectors including control Ad-LacZ were added to the AR42J cells (m.o.i.=40:1) for 72 h. RT-PCR measurements of insulin, Nkx2.2, Nkx6.1, Pax4, Pax6, Ngn3, MafA, Glut2, and GK, mRNA expression levels from the four different treatment groups. Actin was used as an internal control. (B) Insulin RNA levels were quantified by real-time PCR. The p values represent **<0.01 and ***<0.001. (C) Insulin content. Four treatment groups and RINm5F cells were subjected to insulin content (μg/g protein) measurement using ELISA. (D) Flow cytometric analysis of insulin-positive cells. The insulin stained cells were labeled with FITC and subjected to flow cytometric analysis using FlowJo software. Percentage of insulin-positive cells is shown in the box below.
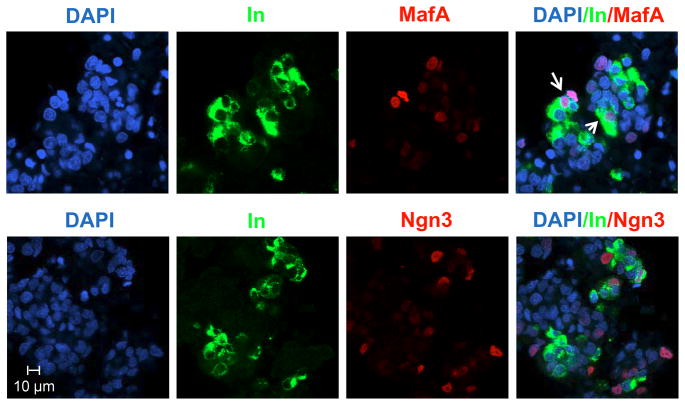
In the three ITFs group, two important ITFs, Ngn3 and MafA, are activated. Double immunofluorescence antibody staining clearly shows cytosolic insulin and nuclear Ngn3 and MafA staining. MafA and insulin are co-expressed in more of the cells suggesting that MafA is closely associated with mature β-cells (Fig. 3). In contrast, Ngn3 only co-localized with insulin in a limited number of cells supporting that Ngn3 is a pro-endocrine factor expressed in endocrine precursors, but not in the insulin-positive cells.
Fig. 3. Three ITFs adenovirus infection induces Ngn3 and MafA expression.
Co-localization of insulin (Alexa Fluor 488-green) with Ngn3 (Alexa Fluor 594-red) or MafA (Alexa Fluor 594-red) in the three ITFs and EGF/TGFβ treated AR42J cells. DAPI (blue) stains cell nuclear DNA. Arrows point to the double positive stained cells.
INSM1 presents a dual functional effect on AR42J cells
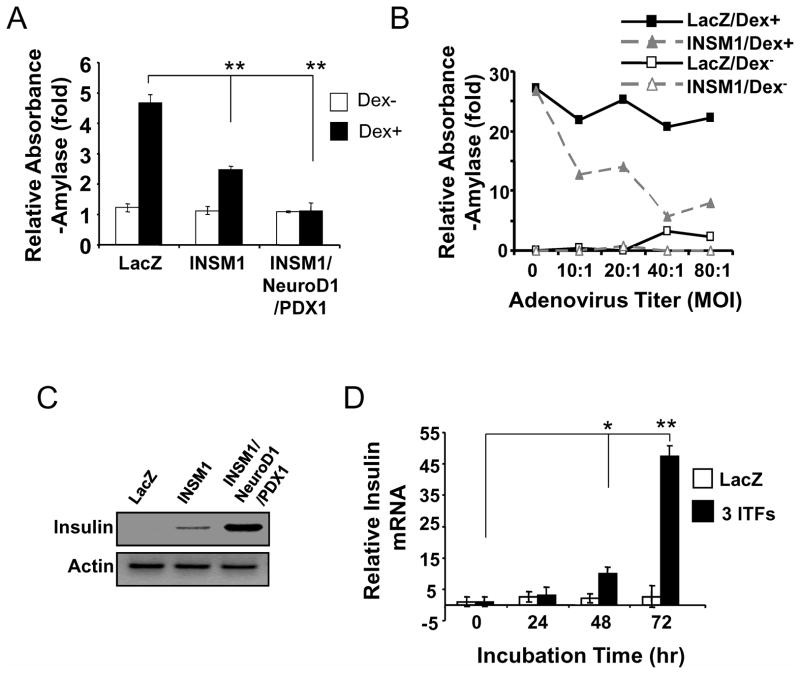
AR42J cells are capable of differentiation into an exocrine phenotype with dexamethasone treatment (Logsdon et al, 1995). We investigated whether over-expression of Ad-INSM1 could hinder amylase expression in dexamethasone treated AR42J cells. We compared the AR42J cells treated with Ad-LacZ, Ad-INSM1, or the three ITFs, in the presence or absence of dexamethasone (Fig. 4A). A 5-fold increase in amylase activity was observed in the dexamethasone treated control. In contrast, Ad-INSM1 suppressed amylase expression over 3-fold in response to the dexamethasone treatment. The INSM1 inhibitory effect on amylase expression corresponds to the INSM1 adenoviral titer (Fig. 4B). A m.o.i. of 40:1 suppresses most of the induced amylase activity. Notably, m.o.i. 40:1 was the same titer that demonstrated maximal induction of endocrine trans-differentiation. Therefore, INSM1 appears to exert a dual functional effect including a pro-endocrine and an anti-exocrine phenotype.
Fig. 4. Dual functional effects of INSM1 and the ITFs.
(A) INSM1 inhibits dexamethasone induced exocrine amylase expression. In the presence of dexamethasone (Dex+), Ad-INSM1 inhibits 50% of amylase expression as compared to the control Ad-LacZ group. The p value represents as **<0.01. The three ITFs inhibit 100% of the dexamethasone induced amylase expression. (B) Different titers of adenovirus showed variable inhibitory activities on amylase expression with the maximal inhibition at 40:1. (C) The three ITFs increase insulin expression dramatically as compared with Ad-INSM1 alone. (D) The three ITFs require 72 h to achieve maximal insulin expression. The p values represent as *<0.05 and **<0.01.
To further evaluate the dual effects of the ITFs on AR42J cells, we compared the insulin and amylase expression levels when AR42J cells were treated with the three ITFs. Figure 4A shows that the three ITFs completely suppressed amylase expression. In contrast, as we described above, the insulin message was greatly increased using three ITFs (Fig. 4C). Real-time quantitative RT-PCR revealed that a longer incubation time (72 h) with the three ITFs translated into higher insulin mRNA levels (Fig. 4D). These results support that INSM1 alone or in combination with Pdx-1 and NeuroD1 exert dual functional effects on the AR42J cells in favor of enhancing an exocrine to endocrine cell trans-differentiation.
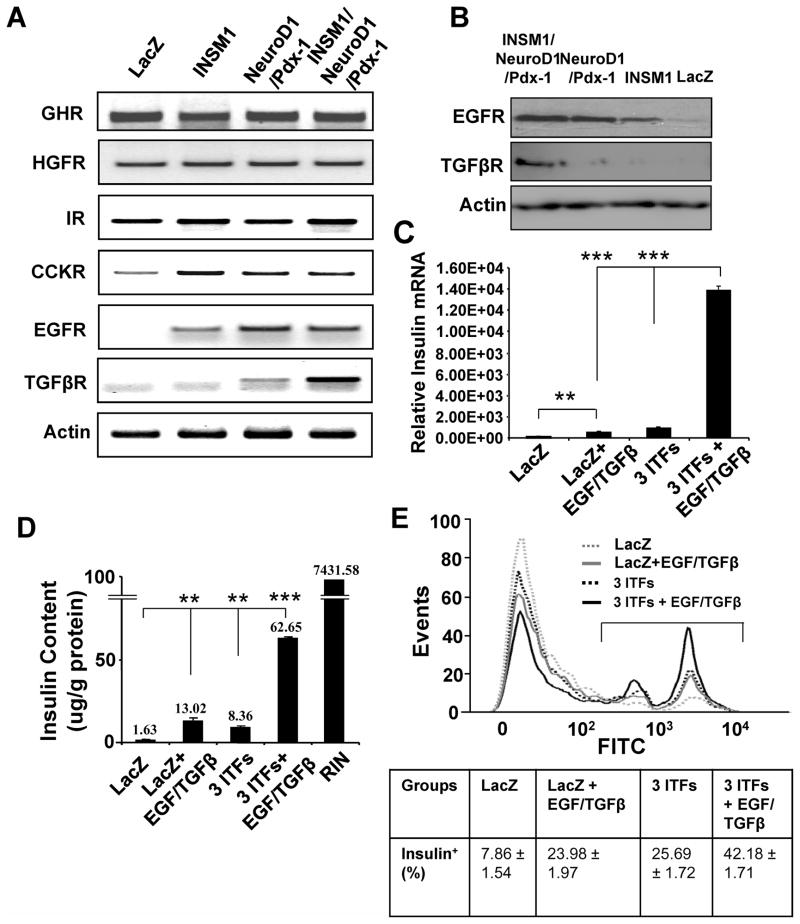
EGFR and TGFβR are activated after ITF addition and the inclusion of EGF and TGFβ greatly enhances insulin expression
AR42J cells will respond to growth hormone stimulation by differentiation into either exocrine or endocrine cell fates. Here, we examined the effect of the ITF expression on exocrine to endocrine cell trans-differentiation. We analyzed the expression of six growth factor receptors including growth hormone receptor (GHR), hepatocyte growth factor receptor (HGFR), insulin receptor (IR), cholecystokinin receptor (CCKR), epidermal growth factor receptor (EGFR), and transforming growth factor β receptor (TGFβR). When AR42J cells were treated with Ad-INSM1 alone, two, or three ITFs, the receptor levels were measured in the various treatment groups. In the three ITFs treatment, EGFR and TGFβR were highly induced over the control group both at the mRNA and protein levels (Fig. 5A and B). In order to evaluate whether the activation of the EGFR and the TGFβR could further potentiate the conversion of AR42J cells into insulin-positive cells, we added EGF/TGFβ growth factors and measured the insulin message using real-time PCR, the percentage of insulin-positive cells by flow cytometric analysis, and the insulin content of the various treatment groups. Real-time RT-PCR showed over a 14-fold increase in insulin message when comparing the three ITFs with three ITFs plus EGF/TGFβ groups. The EGF/TGFβ factors treatment alone with LacZ have very little effect on insulin expression. The three ITFs plus EGF/TGFβ treatment increases insulin content 7.49-fold over the three ITFs treatment alone, whereas the EGF/TGFβ treatment alone has minute insulin increase (Fig. 5D). Consistently, the percentage of insulin-positive cells were greatly increased with the three ITFs plus EGF/TGFβ combination (42.18% versus 25.69%) (Fig. 5E). These experiments suggest that modulation of the ITFs in AR42J cells could concomitantly activate the EGFR and the TGFβR, which respond to EGF/TGFβ stimulation and mediate endocrine cell trans-differentiation.
Fig. 5. ITFs combined with EGF/TGFβ enhance insulin expression.
(A) RT-PCR shows elevated levels of EGFR and TGFβR in the three ITFs induction group. (B) EGFR and TGFβR protein were detected by Western blot analysis. (C) Quantitative RT-PCR analysis of insulin message from LacZ, LacZ + EGF/TGFβ, 3 ITFs, and 3 ITFs + EGF/TGFβ. The p values represent as **<0.01 and ***<0.001. (D) Insulin content. The above four treatment groups were subjected to insulin content analysis using ELISA and expressed as μg/g protein using RINm5F cells as the control. (E) Flow cytometric analysis of insulin-positive cells. The insulin-positive cells were labeled with FITC and subjected to flow cytometric analysis using FlowJo software. The percentage of insulin-positive cells is shown in the box below.
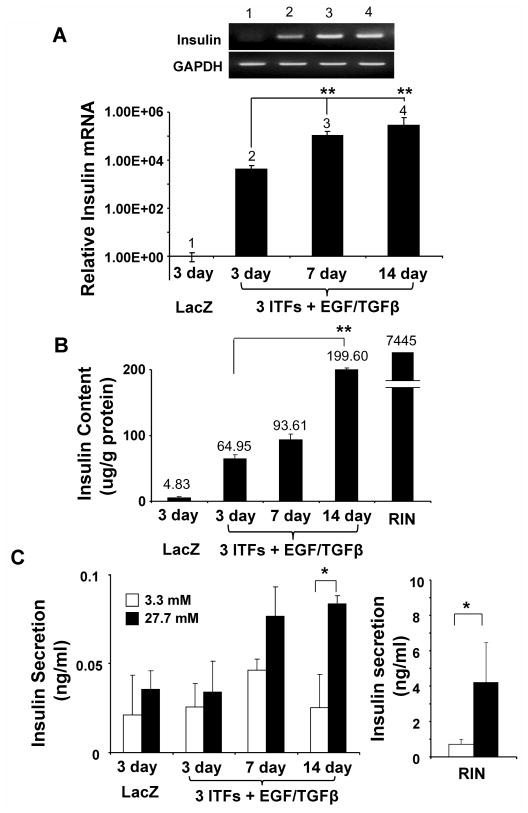
Time course induction of insulin expression from 3 ITFs/EGF/TGFβ treated AR42J cells
Later, we performed a time course to evaluate the 3 ITFs/EGF/TGFβ treated AR42J cells during long term culture. As shown in Figure 6, both 7 and 14 day treatment significantly increases insulin message as compared with the 3 day culture using real time RT-PCR analysis. Especially, the 14 day culture displays a 3-fold increase of insulin content over the 3 day culture. Although the 14 day culture responds to glucose stimulation, the amount of insulin secretion upon glucose stimulation is minimal as compared with the RIN cells (Fig. 6C).
Fig. 6. Time course induction of insulin expression from the 3 ITFs/EGF/TGFβ treated AR42J cells.
The AR42J cells were treated with Ad-LacZ or 3 ITFs with EGF/TGFβ for 3, 7, or 14 days. Cells were harvested from each time point and subjected to (A) RT-PCR, real time PCR analysis, (B) insulin content analysis, (C) and glucose-stimulated insulin secretion assays. RIN cells were used as positive control.
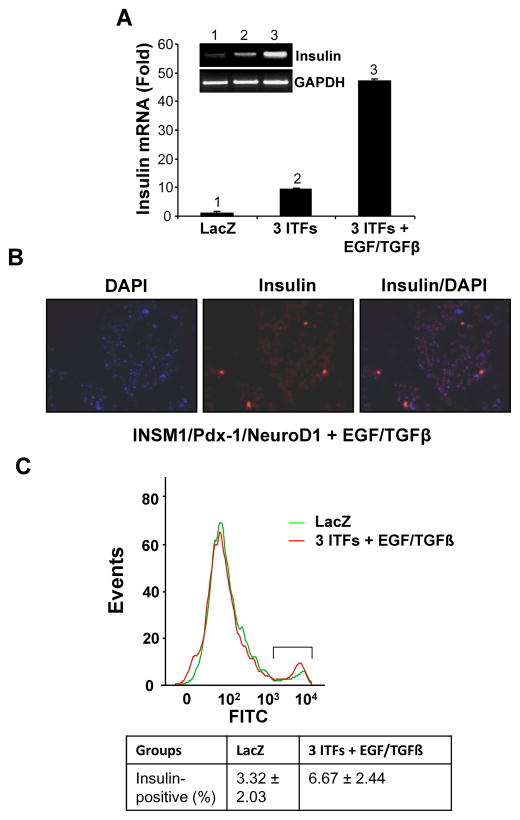
ITFs and EGF/TGFβ induce primary pancreatic acinar cell trans-differentiation
Primary mouse pancreatic acinar cells were isolated through collagenase infusion. We tested and confirmed that the combination of the three ITFs and EGF/TGFβ is capable of converting normal pancreatic acinar cells into insulin-positive cells. The addition of EGF/TGFβ growth factors to the three ITFs enhanced the insulin mRNA expression by five fold (Fig. 7A). Further, insulin antibody staining confirmed the presence of insulin-positive cells (Fig. 7B), but the percentage of insulin-positive cells only reached 6.67%. This is relatively low as compared to the AR42J cells (Fig. 7C). However, this study supports that the INSM1 containing ITFs along with EGF/TGFβ growth factors are capable of converting both AR42J and normal acinar cells into insulin-positive cells.
Fig. 7. Trans-differentiation of primary cultured acinar cells.
Mouse acinar cells were isolated by collagenase infusion. The primary cultured acinar cells were grown in M199 medium containing 10% FBS with the addition of Ad-LacZ, Ad-3 ITFs, with or without EFG/TGFβ for 96 hours. The treated cells were harvested for (A) RT-PCR and real time PCR analysis, (B) insulin antibody staining, and (C) flow cytometric analysis of insulin-positive cells. The percent of insulin-positive cells is shown in the box below.
Discussion
The functional role of INSM1 in pancreatic endocrine cell differentiation has been studied in several laboratories (Mellitzer et al, 2006; Gierl et al, 2006; Zhang et al, 2009). From the initial gene cloning, identification of both upstream and downstream target genes, to the deletion of Insm1 in an animal model revealed that Insm1 plays a key role in modulating the transcriptional cascade during pancreatic endocrine cell development (Gierl et al, 2006; Goto et al, 1992; Breslin et al, 2003; Breslin et al, 2007; Liu et al, 2006; Wang et al, 2008). INSM1 was shown to induce pancreatic duct cell trans-differentiation into insulin-positive cells (Zhang et al, 2010). Here, we provide evidence that both AR42J and primary cultured mouse pancreatic acinar cells are capable of conversion into insulin-positive cells when treated with ITFs and EGF/TGFβ. Similarly, a recent study reported by Zhou et al., employed a different combination of ITFs (Ngn3, Pdx-1, and MafA) to achieve the direct conversion of mouse pancreatic acini into insulin-positive cells in vivo (Zhou et al, 2008). Since Insm1 is the direct downstream target of Ngn3 and demonstrates effective induction of insulin gene expression in AR42J cells, we sought to explore the functional effects of expression of INSM1 on pancreatic acinar cells. Further, we investigated the combination of three ITFs including INSM1 to further enhance the conversion of pancreatic acinar cells into insulin-positive cells. Our results using the AR42J cell line as a model reveals that Pdx-1 and NeuroD1 ITFs were down regulated by sustained INSM1 over-expression. Thus, inclusion of Pdx-1 and NeuroD1 was our first choice. However, the measured insulin content with all three ITFs is far less than the RINm5F insulinoma cells (0.14%) suggesting additional factors are still needed.
Surprisingly, in our study both Ngn3 and MafA gene expression were induced when AR42J cells are treated with the combination of INSM1, Pdx-1, and NeuroD1. It is generally accepted that expression of multiple ITFs specifies pancreas development (Oliver-Krasinski and Stoffers, 2009). More than a dozen ITFs have been shown to be essential for the proper endocrine cell differentiation cascade. This information provides insight into how to reprogram a particular cell type into an insulin-producing endocrine cell. The Pdx-1 transcription factor was shown to be both the initial and final key factor that controls total pancreas formation and the formation of mature β-cells (Ohlsson et al, 1993). Deletion of Pdx-1 results in agenesis of pancreas both in human and mouse (Stoffers et al, 1997). Neurogenin3 (Ngn3) is a critical marker for pro-endocrine precursor cells in pancreas (Gradwohl et al, 2000; Schwitzgebel et al, 2000). Deletion of Ngn3 abolished development of the total pancreatic endocrine cells. INSM1 is a relatively newly characterized islet cell transcription factor that is situated immediately downstream of Ngn3 and plays an essential role in pancreatic endocrine cell development (Mellitzer et al, 2006; Gierl et al, 2006;Breslin et al, 2007). Therefore, in this study we used INSM1 to replace Ngn3 in an AR42J cell model with the additional factors Pdx-1 and NeuroD1. The unexpected induction of expression of Ngn3 in our model seems to reverse the order of the transcriptional cascade. Expression of Ngn3 could signify the initiation of a complete pancreatic endocrine cell differentiation or a reversion to a more “fetal-like” state. NeuroD1 represents another downstream transcription factor of Ngn3 that is essential for β-cell maturation and insulin gene expression (Naya et al, 1997). MafA functions as a transcription factor in mature β-cells and is critical to activate insulin gene expression (Zhang et al, 2005). Our combination of ITFs not only facilitates the conversion of AR42J cells into insulin-positive cells, but also induces Ngn3 and MafA expression indicating the involvement of Ngn3, Pdx-1, and MafA to induce endocrine cell trans-differentiation.
Although our previous study showed that INSM1 is a direct transcriptional repressor of the insulin gene, expression of INSM1 in pancreatic β-cells and/or precursor cells is transient during development (Mellitzer et al, 2006;Wang et al, 2008). INSM1 plays a role in suppressing NeuroD1, while NeuroD1 activates INSM1 gene expression (Breslin et al, 2002; Breslin et al, 2003). At the mature stage in β-cells, both INSM1 and Ngn3 genes are turned off in contrast to the NeuroD1 and insulin genes, suggesting that INSM1 is a transient differentiation factor that modulates multiple ITFs during early endocrine cell differentiation. In this study, we show that INSM1 can induce insulin, Pax6, and Nkx6.1 during the AR42J cell trans-differentiation process. However, our results do not imply that INSM1 activates those genes directly. It is most likely that the expression of INSM1 in AR42J cells triggers an endocrine differentiation cascade and the expression of multiple ITFs facilitates the differentiation process and results in insulin expression. We have reported that INSM1 can physically interact with cyclin D1 and induce cell cycle arrest or promote cell fate switching to a differentiation pathway (Liu et al, 2006; Zhang et al, 2009). Therefore, we tested the hypothesis whether INSM1 could change the cell fate of AR42J cells from exocrine to endocrine cells. As expected, INSM1 suppresses amylase expression during dexamethasone treatment. The suppression of the exocrine phenotype was further potentiated by the addition of Pdx-1 and NeuroD1 transcription factors suggesting that the AR42J cells were predominantly switching to the endocrine cell fate. Clearly, during the endocrine differentiation process insulin expression and insulin content were clearly increased and the direct INSM1 suppression on insulin gene expression may be overridden.
We demonstrate the involvement of INSM1 in directly converting AR42J cells into insulin-positive cells. The addition of Pdx-1 and NeuroD1 greatly facilitates the conversion efficiency (from 8% to ~25%). However, the insulin content in the converted AR42J cells was only 0.14% of the rat insulinoma cells. Therefore, further fine-tuning is needed. We were also curious to pursue the mechanisms underlying the effects these ITFs had on the AR42J cells. Next, we examined multiple hormone growth factor receptors associated with the ITF expression. Two hormone growth factor receptors, EGFR and TGFβR, were clearly induced with the combination of the three ITFs. During development, hormones and growth factors provide the proper inductive signals to guide normal pancreas organogenesis. Understanding the complex interaction between these inductive signals and transcription factors that orchestrate normal organ development will have a significant impact on the conversion of exocrine cells of the pancreas into islet cells for diabetes therapy. In this study, we demonstrate that the addition of three ITFs, all previously shown to be critical for the differentiation of the insulin-producing cells, stimulate the expression of the EGF and TGFβ receptors. The importance of the EGFR for pancreas development was shown in the EGFR knockout mouse. Embryonic pancreas cultures from EGFR −/− mice showed a ~50% reduction in the formation of insulin-positive cells while the formation of the other islet cells remained normal (Miettinen et al, 2000). Additionally, trans-differentiation of normal adult mouse or rat acinar cells into insulin-expressing cells has been achieved through stimulation of the EGF-receptor. Lineage tracing in mouse acinar cultures showed that the neogenic β-cells were derived from exocrine cells through stimulation of the EGFR (Minami et al, 2005). A similar approach was used in cultured rat acinar cells where the combination of EGF and LIF (leukemia inhibitory factor) induced new β-cell formation (Baeyens et al, 2005). TGF-β1 promotes the development of endocrine cells, in particular the insulin-containing β-cells. Cytokines can modulate the development of the pancreas and suggest a role for TGF-β1 in regulating the balance between the acinar and endocrine portions of the gland (Sanvito et al, 1994). Therefore, it seems likely that the addition of these growth factors to the induction protocol would have a positive benefit on the acinar cell trans-differentiation. Inclusion of EGF/TGFβ in the trans-differentiation process leads to a higher insulin-positive cell population (42.18% versus 25.69%), a dramatic increase in the insulin message (>14-fold), and an approximately 7.49-fold increase in insulin content as compared with the triple ITF-treated cells. A prolonged induction time (up to 14 days) promotes more insulin expression showing the effect is not transient (Fig. 6). The positive effects of the combination of the three ITFs and EGF/TGFβ also observed with the mouse primary cultured acinar cells. These findings are important since the combination of ITFs and growth factors could be a valuable formula for generating insulin-positive cells.
Acknowledgments
This work was supported by funds from the Research Institute for Children, Children’s Hospital at New Orleans and a grant from the NIDDK, National Institutes of Health, DK61436 (to MSL). We thank Dr. M.J. Tsai (Baylor College of Medicine, Houston, TX) for NeuroD1/beta 2 cDNA plasmid and Dr. C. V. E. Wright (Vanderbilt University Medical School, Nashville, TN) for Pdx-1 cDNA plasmid. The Ngn3 antibody (F25A1B3) developed by Dr. O. D. Madsen was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by the University of Iowa, Department of Biology, Iowa City, IA 52242.
Footnotes
Disclosure: The authors have nothing to disclose.
Duality of interest: The authors declare that there is no duality of interest associated with this manuscript.
The abbreviations used are: AR42J, a rat pancreatic acinar adenocarcinoma cell line; RINm5F, a rat insulinoma cell line; ITFs, islet transcription factors; INSM1/IA-1, insulinoma-associated antigen-1; Ngn3, neurogenin 3; Pdx-1, pancreatic duodenal homeobox-1; NeuroD1, neurogenic differentiation 1; Pax4/6, pair-box 4/6; Nkx2.2/6.1, homeodomain transcription factor 2.2/6.1; MafA, musculoaponeurotic fibrosarcoma oncogene homolog A; GK, glucokinase; Glut2, glucose transporter 2; DAPI, 4′,6-diamidino-2-phenylindole; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; FITC, fluorescein isothiocyanate-labeled; GHR, growth hormone receptor; HGFR, hepatocyte growth factor receptor; IR, insulin receptor; CCKR, cholecystokinin receptor; EGFR, epidermal growth factor receptor; TGFβR, transforming growth factor β receptor.
Literature Cited
- Baeyens L, DeBreuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing beta cells from adult exocrine pancreatic cells. Diabetologia. 2005;48:49–57. doi: 10.1007/s00125-004-1606-1. [DOI] [PubMed] [Google Scholar]
- Bernfeld DA. Amylases, a and b. In: Colowick SaKN., editor. Methods in Enzymology. NY: Academic Press; 1955. p. 149. [Google Scholar]
- Breslin MB, Wang HW, Pierce A, Aucoin R, Lan MS. Neurogenin 3 recruits CBP co-activator to facilitate histone H3/H4 acetylation in the target gene INSM1. FEBS Lett. 2007;581:949–954. doi: 10.1016/j.febslet.2007.01.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin MB, Zhu M, Lan MS. NeuroD1/E47 regulates the E-box element of a novel zinc-finger transcription factor, IA-1, in developing nervous system. J Biol Chem. 2003;278:38991–38997. doi: 10.1074/jbc.M306795200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin MB, Zhu M, Notkins AL, Lan MS. Neuroendocrine differentiation factor, IA-1, is a transcriptional repressor and contains a specific DNA-binding domain: identification of consensus IA-1 binding sequence. Nucleic Acids Res. 2002;30:1038–1045. doi: 10.1093/nar/30.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophe J. Pancreatic tumoral cell line AR42J: an amphicrine model. Am J Physiol. 1994;266:G963–G971. doi: 10.1152/ajpgi.1994.266.6.G963. [DOI] [PubMed] [Google Scholar]
- Gierl MS, Karoulias N, Wende H, Strehle M, Birchmeier C. The Zinc-finger factor Insm1 (IA-1) is essential for the development of pancreatic beta cells and intestinal endocrine cells. Genes Dev. 2006;20:2465–2478. doi: 10.1101/gad.381806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, DeSilva MG, Toscani A, Prabhakar BS, Notkins AL, Lan MS. A novel human insulinoma-associated cDNA, IA-1, encodes a protein with zinc-finger DNA-binding motifs. J Biol Chem. 1992;267:15252–15257. [PubMed] [Google Scholar]
- Gradwohl G, Dierich A, LeMeur M, Guillemot F. Neurogenin 3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiyama N, Kanzaki M, Seno M, Yamada H, Kobayashi I, Kojima I. Studies on the betacellulin receptor in pancreatic AR421J cells. Diabetologia. 1998;41:623–628. doi: 10.1007/s001250050959. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Matsuoka TA, Katakami N, Matsuhisa M. Combination of MafA, Pdx-1 and NeuroD is a useful tool to efficiently induce insulin-producing surrogate beta-cells. Curr Med Chem. 2009;16:3144–3151. doi: 10.2174/092986709788802980. [DOI] [PubMed] [Google Scholar]
- Liu WD, Wang HW, Muguira M, Breslin MB, Lan MS. INSM1 functions as a transcriptional repressor of the NeuroD.beta2 gene through the recruitment of cyclin D1 and histone deacetylase. Biochem J. 2006;397:169–177. doi: 10.1042/BJ20051669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logsdon CD, Moessner J, Williams JA, Goldfine ID. Glucocorticoids increase amylase mRNA levels, secretory organelles, and secretion in pnacreatic acinar AR42J cells. J Cell Biochem. 1995;100:1200–1208. doi: 10.1083/jcb.100.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mashima H, Ohnishi H, Wakabayashi K, Mine T, Miyagawa J, Hanafusa T, Seno M, Yamada H, Kojuma I. Betacellulin and activin A coordinately convert amylase-secreting pancreatic AR42J cells into insulin-secreting cells. J Clin Invest. 1996;97:1647–1654. doi: 10.1172/JCI118591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellitzer G, Bonne S, Luco RF, Van De Casteele M, Lenne N, Collombat P, Mansouri A, Lee J, Lan MS, Pipeleers D, Nielsen FC, Ferrer J, Gradwohl G, Heimberg H. IA-1 is Ngn3-dependent and essential for differentiation of the endocrine pancreas. EMBO J. 2006;25:1344–1352. doi: 10.1038/sj.emboj.7601011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen PJ, Huotari M, Koivisto T, Ustinov J, Paslgi J, Rasilainen S, Lehtonen E, Deski-Oja J, Otonkoski T. Impaired migration and delayed differentiation of pancreatic islet cells in mice lacking EGF-receptors. Development. 2000;127:2617–2627. doi: 10.1242/dev.127.12.2617. [DOI] [PubMed] [Google Scholar]
- Minami K, Okuno M, Miyawaki K, Okumachi A, Ishizaki K, Oyama K, Kawaguchi M, Ishizuka N, Iwanagam T, Seino S. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Aci USA. 2005;102:15116–15121. doi: 10.1073/pnas.0507567102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Huang HP, Qiu Y, Mutoh H, DeMayo FJ, Leiter AB, Tsai MJ. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/NeuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara T, Fujitani Y, Uchida T, Kanno R, Choi JB, Hirose T, Kawamori R, Watada H. Combined expression of transcription factors induces AR42J-B13 cells to differentiate into insulin-producing cells. Endocr J. 2008;55:691–698. doi: 10.1507/endocrj.k07e-169. [DOI] [PubMed] [Google Scholar]
- Ohlsson H, Karlsson K, Edlund T. IPF-1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Krasinski JM, Stoffers DA. On the origin of the beta cell. Genes and Dev. 2009;22:1998–2021. doi: 10.1101/gad.1670808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosewicz S, Logsdon CD. Glucocorticoids stimulate ornithine decarboxylase gene expression in pancreatic AR42J cells. Gastroenterology. 1991;101:1102–1108. doi: 10.1016/0016-5085(91)90740-c. [DOI] [PubMed] [Google Scholar]
- Rosewicz S, Vogt D, Harth N, Grund C, Franke WW, Ruppert S, Schweitzer E, Riecken EO, Wiedenmann B. An amphicrine pancreatic cell line: AR42J cells combine exocrine and neuroendocrine properties. Eur J Cell Bio. 1992;59:80–91. [PubMed] [Google Scholar]
- Sanvito F, Herrera PL, Huarte J, Nichols A, Montesano R, Orci L, Vassalli JD. TGF-b1 influences the relative development of the exocrine and endocrine pancreas in vitro. Development. 1994;120:3451–3462. doi: 10.1242/dev.120.12.3451. [DOI] [PubMed] [Google Scholar]
- Schwitzgebel VM, Scheel DW, Conners JR, Kalamaras J, Lee JE, Anderson DJ, Sussel L, Johnson JD, German MS. Expression of neurogenin3 reveals an islet cell precursor popolation in the pancreas. Development. 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nuceotide deletion in the human IPF1 gene coding sequence. Nature Genetics. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Wang HW, Muguira M, Liu WD, Zhang T, Chen C, Aucoin R, Breslin MB, Lan MS. Identification of an INSM1 binding site in the insulin promoter: Negative regulation of the insulin gene transcription. J Endocrinol. 2008;198:29–39. doi: 10.1677/JOE-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew KH, Prasadan KL, Preuett BL, Hembree MJ, McFall CR, Benjes CL, Crowley AR, Sharp SL, Li Z, Tulachan SS, Mehta SS, Gittes GK. Interplay of glucagon-like peptide-1 and transforming growth factor-beta signaling in insulin-positive differentiation of AR42J cells. Diabetes. 2004;53:2824–2835. doi: 10.2337/diabetes.53.11.2824. [DOI] [PubMed] [Google Scholar]
- Zhang C, Moriguchi T, Kajihara M, Esaki R, Harada A, Shimohata H, Oishi H, Hamada M, Morito N, Hasegawa K, Kudo T, Engel JD, Yamamoto M, Takahashi S. MafA is a key regulator of glucose-stimulated insulin secretion. Mol Cell Biol. 2005;25:4969–4976. doi: 10.1128/MCB.25.12.4969-4976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Liu WD, Saunee NA, Breslin MB, Lan MS. Zinc-finger transcription factor INSM1 interrupts cyclin D1 and CDK4 binding and induces cell cycle arrest. J Biol Chem. 2009;284:5574–5581. doi: 10.1074/jbc.M808843200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Wang HW, Saunee NA, Breslin MB, Lan MS. Insulinoma-associated antigen-1 zinc-finger transcription factor promoters pancreatic duct cell trans-differentiation. Endocrinology. 2010;151:2030–2039. doi: 10.1210/en.2009-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang X, pineyro MA, Egan JM. Glucagon-like peptide-1 and exendin-4 convert pancreatic AR42J cells into glucagon- and insulin-producing cells. Diabetes. 1999;48:2358–2366. doi: 10.2337/diabetes.48.12.2358. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu M, Breslin MB, Lan MS. Expression of a novel zinc-finger cDNA, IA-1, is associated with rat AR42J cells differentiation into insulin-positive cells. Pancreas. 2002;24:139–145. doi: 10.1097/00006676-200203000-00004. [DOI] [PubMed] [Google Scholar]