Abstract
Objectives
We evaluated the expression of human-trophoblast-cell-surface-marker (Trop-2) and the potential of hRS7, a humanized-monoclonal-anti-Trop-2-antibody, against treatment-refractory cervical cancer.
Study Design
Trop-2 expression was evaluated by immunohistochemistry (IHC), real-time polymerase-chain-reaction (RT-PCR) and flow-cytometry. Sensitivity to hRS7 antibody-dependent-cell-mediated-cytotoxicity (ADCC) and complement-dependent-cytotoxicity was tested in 5-hour-chromium-release-assays. The effect of interleukin-2 on hRS7 ADCC was also investigated.
Results
Membrane Trop-2 expression was observed in 8 out of 8 (100%) of the cancer samples tested by IHC, but not in normal cervix. High messenger RNA expression by RT-PCR and high Trop-2 surface expression by flow-cytometry were detected in 80% of cervical cancers (4 of 5 cell lines). Although these tumors were resistant to natural-killer-cell-dependent-cytotoxicity in vitro (mean killing 6.0%), Trop-2-positive cell lines showed high sensitivity to hRS7 ADCC (range of killing: 30.6–73.2%). Incubation with interleukin-2 further increased the level of cytotoxicity against Trop-2-positive tumors.
Conclusions
hRS7 may represent a novel treatment option for patients with cervical cancer refractory to conventional treatment modalities.
Keywords: cervical cancer, hRS7, immunotherapy, natural killer cell, Trop-2
Introduction
Cancer of the uterine cervix is a significant public health issue as it is the fourth leading cause of cancer death in women worldwide, with about 529,800 new cases and 275,100 deaths each year.1 In the United States, 12,200 new cases of cervical cancer and 4,210 deaths were estimated for 2010.2 Cervical cancers caught in earlier stages can be treated by radical surgery or radiotherapy with equal effectiveness.3 However, despite screening programs, many patients still present with advanced stage disease. For patients with locally advanced disease, pelvic radiation represents the standard of therapy. However, up to 35% of patients will develop recurrent disease for which treatment results are poor. Thus, there is a large need to develop targeted therapies for cervical cancer patients who have failed chemotherapy, radiation, and/or surgery.
Trop-2 (also termed human trophoblast cell-surface marker, TACSTD2, GA733-1, M1S1, and EGP-1) is a surface glycoprotein originally identified in human placental trophoblastic tissue and subsequently reported to be highly expressed by various types of human carcinomas, but rarely in normal adult tissues.4–9 The biological function of Trop-2 is unclear, although recently it has been speculated that it exerts its action via activation of the ERK/MAPK pathway.10 However, its overexpression has been found to correlate with invasive behavior and poor prognosis in various human carcinomas.4, 11–15 Due to the overexpression of Trop-2 by tumor cells and its location on the cell surface, it is an attractive target for cancer immunotherapy, as recently demonstrated by our group against a rare but highly aggressive variant of endometrial cancer (i.e., uterine serous carcinoma).16
hRS7 is a humanized IgG1 monoclonal antibody (MAb) developed against Trop-2 using complementary-determining-region and transfection techniques of the murine RS7-3G11 antibody (Immunomedics, Inc., Morris Plains, NJ).17–19 RS7-3G11 was shown to be rapidly internalized by target cells,17–19 so hRS7 was initially tested labeled with 131I-IMP-R4 to evaluate its effectiveness in preclinical radioimmunotherapy studies on breast cancer xenograft models.19 These results suggested that hRS7 may be promising as a carrier for radiometabolic therapy after labeling with suitable radionuclides. Nevertheless, our group has recently shown that hRS7 also induces antibody-dependent cellular cytotoxicity (ADCC) against biologically aggressive (i.e., Type II) endometrial carcinoma cell lines overexpressing Trop-2.16 To our knowledge, however the potential ability of hRS7 in inducing ADCC against primary cervical carcinoma cell lines has not been previously studied.
We, therefore, studied the expression of Trop-2 in multiple cervical carcinoma cell lines and investigated the in vitro potential of hRS7 as an innovative immunotherapeutic agent against cervical cancer cell lines overexpressing Trop-2.
Materials and Methods
Trop-2 immunostaining of Formalin-fixed Cervical cancer Tissues
Formalin-fixed, paraffin-embedded tissue blocks from 8 patients harboring stage Ib (6 patients), stage II (1 patient) and stage IIIb (1 patient) cervical carcinomas (i.e., 5 squamous and 3 adenocarcinomas) and 5 normal cervical control tissues obtained from similar age women were evaluated by standard immunohistochemical staining (IHC) for Trop-2 surface expression. Specimens were reviewed by a surgical pathologist (NB). Briefly, IHC stains were performed on 4-µm-thick sections of formalin-fixed, paraffin-embedded tissue as previously described (16). The purified goat polyclonal antibody against the recombinant human Trop-2 extracellular domain (R&D Systems, Inc., Minneapolis, MN; diluted 1:100) was applied for 1 hour. A secondary biotinylated anti-goat antibody (Vector Laboratories, Burlingame, CA; diluted 1:250) and the streptavidin-biotin complex (StreptABComplex/HRP, Dako, CA, USA) were applied, then 3’3-diaminobenzidine (Dako, CA, USA) was used as chromogen and the sections were counterstained by hematoxylin (Dako). Cases with less than 10% membranous staining in tumor cells were considered negative for Trop-2 expression. The intensity of membranous immunoreactivity for Trop-2 in tumor cells was subjectively scored as follow: (a) 0, negative; (b) 1+, weak membrane staining; (c) 2+, medium staining; and (d) 3+, strong membrane staining. Appropriate negative and positive controls were performed with each case.
Establishment of Primary Cervical Cancer Cell Lines
Primary cervical tumor cell lines from five patients were established after sterile processing of fresh tumor biopsies collected at the time of primary surgery under approval of the Institutional Review Board. Tumors were staged according to the International Federation of Gynecology and Obstetrics staging system. Source-patient characteristics of these five cell lines are described in Table 1.
Table 1.
Patient characteristics and mRNA expression in cervical cancer cell lines
| Cell Line | Age at diagnosis | FIGO Stage | Histology | HPV Status | mRNA copy # |
|---|---|---|---|---|---|
| CVX-SCC-1 | 42 | recurrence | Squamous Cell | 18 | 120.35 |
| CVX-SCC-2 | 40 | IB | Squamous Cell | 16 | 3035.66 |
| ADX-1 | 25 | IB | Adenocarcinoma | 18 | 351.28 |
| ADX-2 | 33 | IB | Adenosquamous | 18 | 9.4 |
| ADX-3 | 33 | IB | Adenosquamous | 18 | 117.56 |
Quantitative Real-time Polymerase Chain Reaction (qRT-PCR) in Primary Cell Lines
RNA isolation from the five primary cervical cancer cell lines (Table 1) was performed using TRIzol Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. Since Trop-2 is an intronless gene, all RNA samples were treated with TURBO DNase enzyme (TURBO DNA-free kit; Ambion, Inc., Applied Biosystems, Foster City, CA) to remove any contaminating DNA that was present. qRT-PCR was performed in duplicate by using a primer set and probe specific for the Trop-2 (i.e., Trop2-EX56) with a 7500 Real Time PCR System using the manufacturer's recommended protocol (Applied Biosystems). The comparative threshold cycle (CT) method (Applied Biosystems) was used to determine gene expression in each sample relative to the value observed in the lowest nonmalignant cervical keratinocyte cell sample, using glyceraldehyde-3-phosphate dehydrogenase (Assay ID Hs99999905_m1) RNA as internal control.
Flow Cytometry
The humanized anti-Trop-2 monoclonal antibody (MAb) hRS7 (Immunomedics, Inc., Morris Plains, NJ) was used for flow cytometry studies. Briefly, five primary cervical cancer cell lines established from the above described patients were treated with 2 µg/mL of hRS7. The chimeric anti-CD20 MAb rituximab (Rituxan; Genentech, San Francisco, CA) at the dose of 2.5 µg/mL was used as negative control. A goat anti-human F(ab’)2 immunoglobulin (BioSource International, Camarillo, CA) was used as a secondary reagent. Analysis was conducted with a FACScan, using Cell Quest software (Beckton Dickinson, Franklin Lakes, NJ).
Tests for ADCC
A standard five-hours chromium (51Cr) release assay was performed to measure the cytotoxic reactivity of Ficoll-Paque™ PLUS (GE Healthcare) separated peripheral blood lymphocytes (PBL) obtained from several healthy donors against all five cervical cancer cell lines. The release of 51Cr from the target cells was measured as evidence of tumor cell lysis after exposure of tumor cells to 2 µg/mL of hRS7. This dose was used because previous hRS7 dose-titration experiments demonstrated that killing of cervical cancer cells plateaued at a hRS7 concentration of 2µg/mL (data not shown). Controls included the incubation of target cells alone or with PBL or MAb separately. The chimeric anti-CD20 MAb rituximab was used as a negative control for hRS7 in all bioassays. ADCC was calculated as the percentage of killing of target cells observed with hRS7 plus effector cells compared with 51Cr release from target cells incubated alone.
Interleukin-2 enhancement of ADCC
To investigate the effect of interleukin-2 (IL-2) on hRS7-mediated ADCC, effector PBL were incubated for 5 hours at 37°C at a final concentration of IL-2 (Aldesleukin; Chiron Therapeutics, Emeryville, CA) ranging from 50–100 IU/mL in 96-well microtiter plates. Target cells were primary cervical cancer cell lines exposed to 2 µg/mL of hRS7, whereas controls included the incubation of target cells alone or with PBL in the presence or absence of IL-2 or MAb, respectively. Rituximab was used as a control MAb. ADCC was calculated as the percentage of killing of target cells observed with MAb plus effector PBL, as compared with target cells incubated alone.
Tests for complement-mediated target cell lysis and γ-globulin inhibition
A standard 5-hours 51Cr-release assay identical to those performed for ADCC assays was used. However, human serum was added as a source of complement to test for complement-mediated target cell lysis. To test for the possible inhibition of ADCC against cervical cancer cell lines by physiological human serum concentrations of γ-globulin, human serum diluted 1:2 was added in the presence or absence of effector PBL. The effect of heat-inactivated human serum (56°C for 60 minutes) was also tested in the presence of effector PBL. Controls included the incubation of target cells alone or with either lymphocytes or MAb separately. Rituximab was used as a control MAb.
Statistical analysis
qRT-PCR data were evaluated using unequal-variance t-test for cervical carcinoma-versus-normal difference. Differences in Trop-2 expression by flow cytometry were analyzed by the unpaired t-test, and a p-value of <0.05 among samples was considered to be significant. Kruskal-Wallis test and chi-square analysis were used to evaluate differences in hRS7-induced ADCC levels in primary tumor cell lines. Statistical analysis was performed using PASW Statistics version 18 (SPSS, Chicago, IL).
Results
Trop-2 expression by IHC in Cervical Cancer samples
We performed immunohistochemical analysis of Trop-2 protein expression on formalin fixed tumor tissue from eight [5 squamous cell carcinoma (SCC) and 3 adenocarcinoma (AC)] paraffin-embedded cervical cancer specimens. As representatively shown in Figure 1, with no exception, all cervical cancer sample tested showed moderate to strong membrane immunoreactivity for Trop-2 (i.e., 8 out of 8 samples = 100%), while the non-neoplastic cervical controls were either negative or demonstrated low to negligible Trop-2 expression. Four of five SCC showed strong, intense immunopositivity (3+), one case showed moderate (2+) immunostaining. All three cases of AC showed strong (3+) immunopositivity
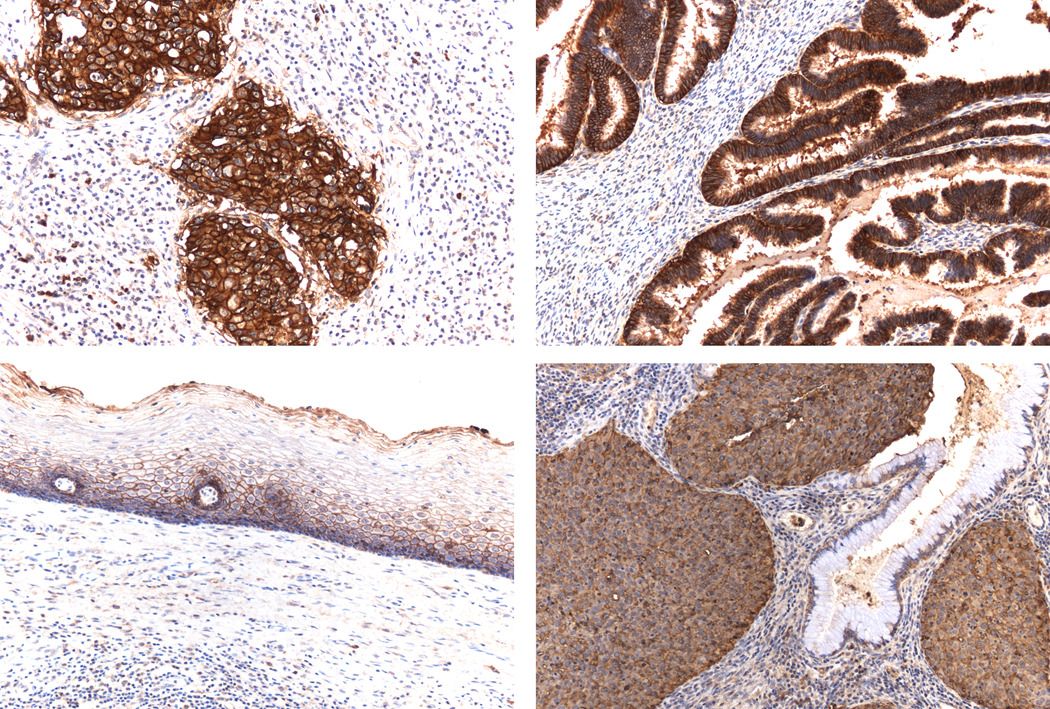
Figure 1.
Trop2 immunostaining in cervical carcinoma and normal cervical tissues. Upper left panel: Intense positive immunoreaction in invasive squamous cell carcinoma. Upper right panel: Intense positive immunoreaction in invasive adenocarcinoma. Lower left panel: Low level of Trop2 expression in normal ectocervical squamous epithelium. Lower right panel: Moderately Trop2 positive immunoreaction in invasive squamous cell carcinoma with no expression in normal endocervical glandular epithelium. (All images at 200× original magnification).
Establishment of primary cell lines
A total of five primary cervical cancer cell lines including 1 squamous cell carcinoma, 1 recurrent squamous cell carcinoma, 1 adenocarcinoma, and 2 adenosquamous tumors were available for this study. All primary cervical cancer cell lines were found to harbor human papillomavirus (HPV) 16 or 18 genotypes (Table 1 and data not shown). CVX-SCC-1 cell line was derived from a recurrent squamous cell carcinoma from a patient in progression after chemo-radiation therapy and multiple additional chemotherapy regimens including topotecan and gemzar. The remaining 4 cell lines were established from biopsies collected at the time of surgery from the primary site of disease (i.e. cervix uteri).
Trop-2 expression by qRT-PCR in primary cell lines
Of the five primary cervical cancer cell lines tested, 4 carcinomas showed a high mRNA copy number, ranging from 117.56 to 3035.66 (Table 1). Trop-2 expression between these tumor cells versus normal cells was significant (p=0.01). Among the cell lines tested, there was no significant difference between expression levels of Trop-2 between squamous cell cancers and adenocarcinomas (p=0.5).
Trop-2 surface expression by flow cytometry in cervical carcinomas
Given the qRT-PCR results, we investigated whether these results correlated with Trop-2 protein expression on the surface of tumor cells using flow cytometry. The 4 cell lines demonstrating high Trop-2 mRNA expression also demonstrated high protein expression (Figure 2). Mean fluorescence intensity (MFI) for Trop-2 in high-expressing cervical cell lines ranged from 180.9 to 355.2 (mean±SD = 237.7±38.3), while the 1 low-expressing cervical cell line had an MFI±SD of 10.9±2.5. The p-value between MFI seen in high Trop-2 expressing cell lines versus the low expressing cell line was <0.001.
Figure 2.
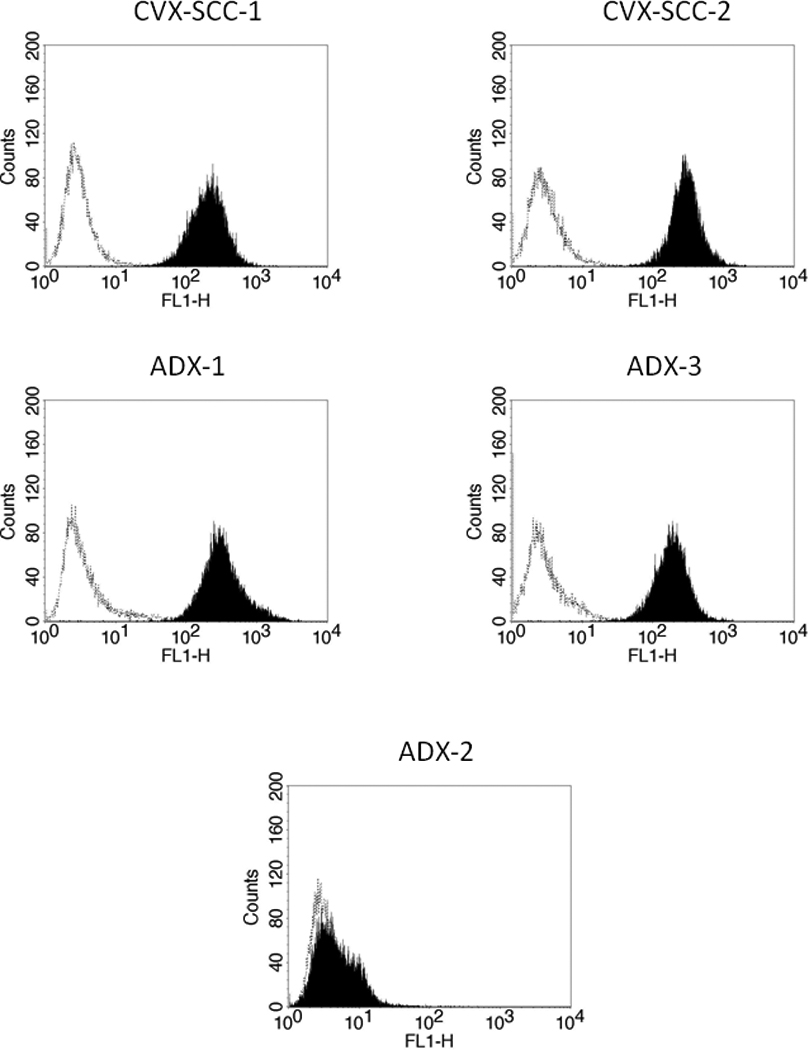
Flow cytometry histograms of primary cervical carcinoma cell lines showing high (CVX-SCC-1, CVX-SCC-2, ADX-1, & ADX-3) and low (ADX-2) expression of Trop-2. Rituximab (dashed line); hRS7 (solid black).
Cervical tumors overexpressing Trop-2 are resistant to natural killer (NK) cell activity but sensitive to hRS7-mediated ADCC
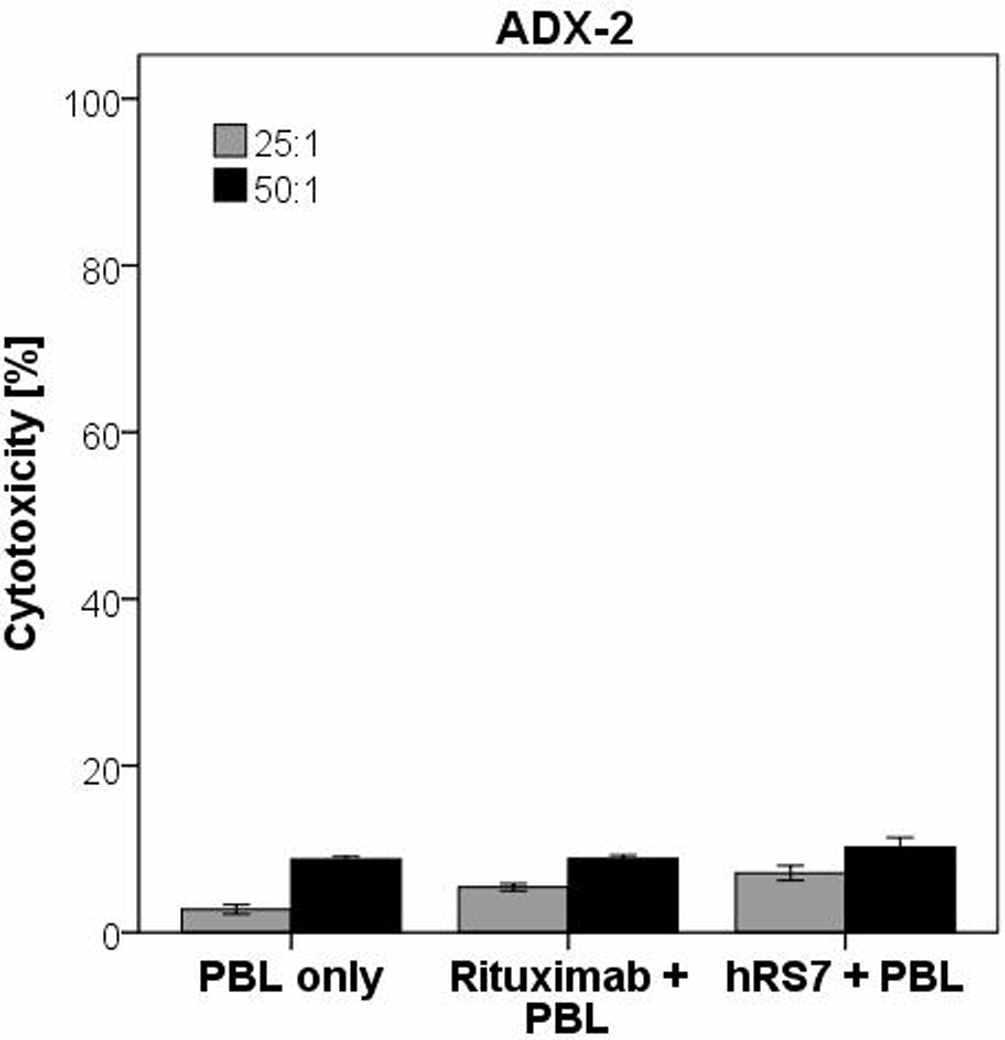
All five cervical cancer cell lines were tested for their sensitivity to NK cell activity, by exposure to peripheral blood lymphocytes (PBL) collected from several healthy donors. Cytotoxicity was measured using a standard 5-hrs 51Cr release assay. Cervical cancer cell lines were found to be highly resistant to NK-mediated lysis with exposure to PBL at two effector to target ratios (E/T) (25:1 and 50:1) (mean killing 6.01% ± 1.9 SD; Table 2). However, after incubation with hRS7, high Trop-2 expressing cell lines demonstrated significant killing with a mean killing of 54.7% (range: 30.6–73.1%; p<0.001; Figure 3a). ADX-2 demonstrated no significant difference in killing after incubation with PBL versus hRS7 (p=0.5), consistent with its limited Trop-2 expression seen by qRT-PCR and flow cytometry. All cells were resistant to ADCC mediated by rituximab antibody in the presence of PBL (Figure 3b & Table 2).
Table 2.
hRS7-dependent mean cytotoxicity results in cervical cancer cell lines
| Cell Line | PBLa ± SD | Rituximab ± SD | hRS7 ± SD |
|---|---|---|---|
| CVX-SCC-1 | 4.89 ± 0.29 | 7.27 ± 3.59 | 43.88 ± 12.21 |
| CVX-SCC-2 | 4.46 ± 2.09 | 2.15 ± 1.01 | 71.57 ± 2.28 |
| ADX-1 | 7.01 ± 1.20 | 7.81 ± 1.50 | 59.08 ± 6.84 |
| ADX-2 | 8.61 ± 0.69 | 8.63 ± 0.87 | 10.19 ± 1.68 |
| ADX-3 | 4.58 ± 0.70 | 5.40 ± 0.94 | 47.50 ± 0.19 |
| Averageb | 6.01 ± 1.9 | 6.25 ± 2.81 | 47.28 ± 20.86 |
Peripheral Blood Lymphocytes
Average of 36 cytotoxicity experiments
Figure 3.
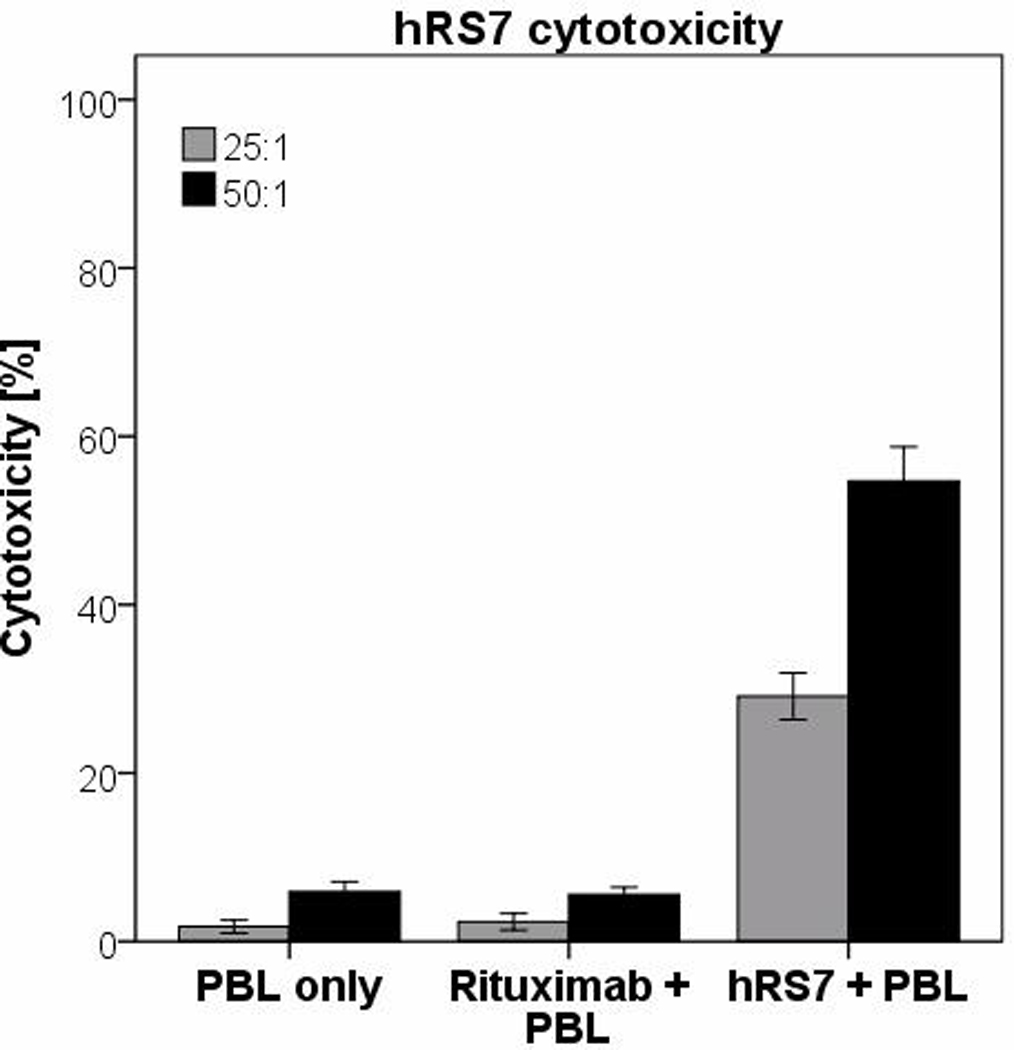
A. Pooled cytotoxicity data from experiments using hRS7 against high Trop-2 expressing cervical cancer cell lines at effector cell to target cell ratios of 50:1 and 25:1.
B. Cytotoxicity data from low Trop-2 expressing cervical cancer cell line (ADX-2) at effector cell to target cell ratios of 50:1 and 25:1. In all cell lines tested, no significant cytotoxicity was detected in the absence of hRS7 or in the presence of rituximab control monoclonal antibody.
IL-2 enhancement of ADCC against cervical carcinomas
As IL-2 has previously been shown to activate NK cell cytotoxicity and expansion of the percentage of NK cells within a PBL population20, we investigated the effect of IL-2 in combination with hRS7 (2µg/mL) on ADCC against cervical cancer cell lines overexpressing Trop-2. As representatively shown in Figure 4, hRS7-mediated ADCC was significantly increased in the presence of IL-2 in all primary cell lines tested (p=0.02). However, the presence of IL-2 did not significantly increase cytotoxicity in the absence of hRS7 or in the presence of rituximab (Figure 4).
Figure 4.
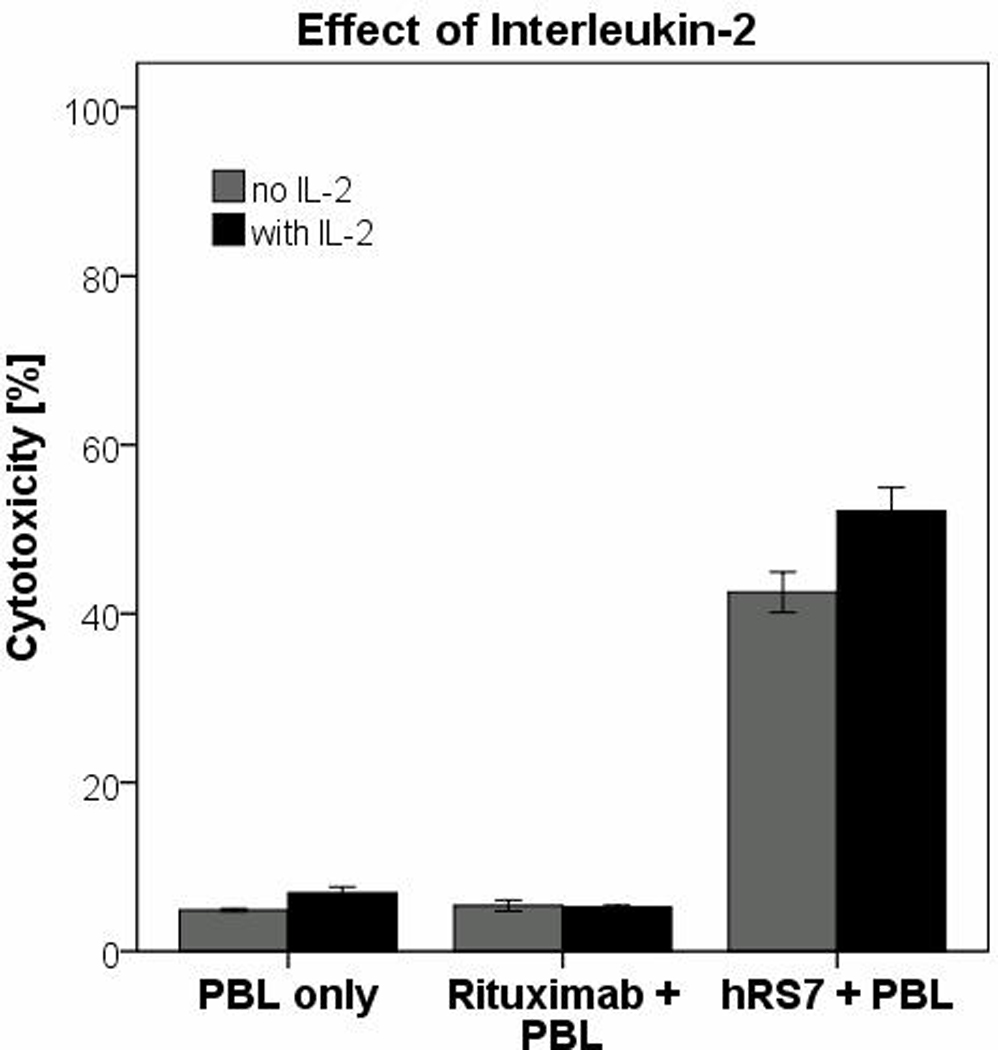
Representative effect of low doses of interleukin-2 (IL-2) in combination with hRS7 on antibody-dependent cellular cytotoxicity against cervical cancer cell lines.
Effect of serum complement and physiologic concentrations of IgG on hRS7-mediated ADCC
To assess the feasibility of hRS7-mediated ADCC functioning in vivo, we evaluated the effects of physiological serum IgG concentrations and serum complement on hRS7-mediated ADCC. After addition of diluted human serum, no significant decrease in killing was noted compared to incubation without serum, suggesting that irrelevant IgG does not alter the ability of hRS7 to mediate ADCC in Trop-2 expressing cells. In one squamous cervical cancer line (CVX-SCC-1), the addition of serum lead to a significant increase in killing (p=0.03), while the addition of heat-inactivated serum with hRS7 and PBL to the same cell line resulted in a decrease in killing (Figure 5). These results suggest a peculiar high sensitivity of this cell line to complement in vitro.
Figure 5.
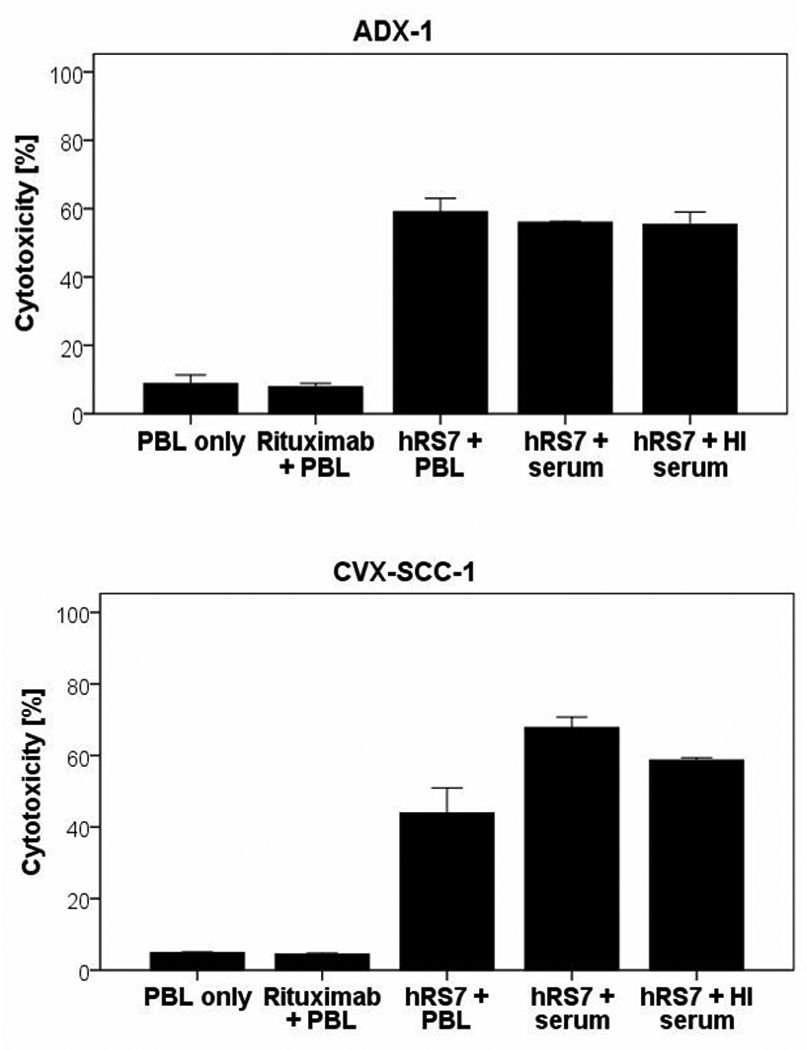
Representative cytotoxicity experiments adding human serum to hRS7 against cervical carcinoma cell lines to assess the feasibility of hRS7 ADCC in the presence of irrelevant human immunoglobulins. No significant decrease in killing was seen in the cell lines. In CVX-SCC-1, a significant increase in killing was seen in the presence of human serum which declined to baseline killing in the presence of heat-inactivated serum and hRS7 suggesting that this cell line is highly sensitive to complement in vitro.
Comment
This study is the first of its kind to evaluate Trop-2 expression in multiple cervical carcinoma cell lines established from patients refractory to standard treatments. In addition, we report on the sensitivity of Trop-2 expressing cervical cancer cell lines to hRS7, a humanized anti-Trop-2 monoclonal antibody. Finally, we have investigated Trop-2 expression and localization by immunohistochemistry in normal cervical keratinocytes and glands, and compared such expression to cervical squamous and adenocarcinomas. Our findings demonstrate that Trop-2 mRNA and protein are significantly upregulated in cervical tumors and cancer cell lines with both adenocarcinoma and squamous cell histology. In addition, we have shown that 4 out of 5 freshly established cervical tumor cell lines express high levels of Trop-2 on their cell surfaces and are highly susceptible to ADCC mediated by hRS7, a humanized anti-Trop-2 monoclonal antibody. Our results may therefore have important implications for patients with cervical cancer, particularly those who have previously failed multiple treatment modalities, including chemotherapy, radiation, and/or surgery.
Previous studies have demonstrated that Trop-2 expression is correlated with more aggressive tumors,4, 11–15 although its exact function is not well understood. In trophoblastic cells, where Trop-2 was initially found to be highly expressed, it allows for penetration of uterine stroma and the establishment of fetal-maternal vascular communications.10 This feature may contribute to the increased aggressiveness of Trop-2 expressing cancers.
The results from our study suggest that multiple cervical tumors including chemotherapy and radiation-resistant cervical cancers such as CVX-SCC-1, overexpress Trop-2 and therefore, Trop-2 may be a target to potentially treat patients with cervical cancer refractory to currently available therapies. All primary cervical cancer cell lines tested that demonstrated high Trop-2 expression were susceptible to hRS7-mediated cytotoxicity by NK cells. While in vivo experiments are needed to confirm our experimental results, we have shown that hRS7-mediated cytotoxicity may be feasible in cervical cancer patients in the in vivo setting by performing ADCC experiments in the presence of high concentrations of human IgG that could potentially block NK cells from interacting with hRS7 at the Trop-2 receptor. hRS7-mediated ADCC was not significantly decreased in the presence of human serum in our experiments. In fact, in some cell lines (i.e. CVX-SCC-1), an increase in cytotoxicity was noted in the presence of effector cells and non-heat-inactivated human serum. These results suggest that the binding of hRS7 to the Fc receptor on NK cells would likely succeed in the in vivo situation.
To show further relevance to clinical practice, we tested hRS7-mediated cytotoxicity in the presence of IL-2. Previous studies have demonstrated that treatment of cancer patients with monoclonal antibodies combined with cytokines can have a synergistic effect and can increase the number and function of circulating NK cells.20, 21 This is an important interaction because decreased ADCC responses have been reported in oncology patients, but cytotoxicity can be increased in these patients by exposing effector cells to IL-2.20, 21 As our experiments demonstrate a significant increase in ADCC after pre-treatment of PBLs with low doses of IL-2, this combined cytokine/antibody therapy may be useful to increase the efficacy of hRS7 cytotoxicity in treatment-refractory cervical cancer patients.
In summary, this is the first study to report on the potential therapeutic use of hRS7, a humanized anti-Trop-2 antibody, in cervical cancer. Our results show that Trop-2 is highly expressed at both mRNA and protein levels in 80% of the primary cell lines established from patients with cervical cancer resistant to currently available treatment modalities. The cell surface localization of high levels of Trop-2 in cervical tumors combined with the low to negligible expression of Trop-2 in normal human tissues 4–9 suggests that this protein could be used as a therapeutic target antigen for antibody-based therapies, specifically hRS7.
Acknowledgements
The authors thank Immunomedics, Inc. (Morris Plains, NJ) for supplying hRS7 free of charge for this study.
Financial Support: Supported by NIH R01 CA122728-01A2 and grants 501/A3/3 and 00227557 from the Italian Institute of Health (ISS) to ADS. This investigation was also supported by NIH Research Grant CA-16359 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors have a conflict of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011 Feb 4; doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage ib-IIa cervical cancer. Lancet. 1997;350:535–540. doi: 10.1016/S0140-6736(97)02250-2. [DOI] [PubMed] [Google Scholar]
- 4.Cubas R, Li M, Chen C, Yao Q. Trop2: A possible therapeutic target for late stage epithelial carcinomas. Biochim Biophys Acta. 2009;1796:309–314. doi: 10.1016/j.bbcan.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Lipinski M, Parks DR, Rouse RV, Herzenberg LA. Human trophoblast cell-surface antigens defined by monoclonal antibodies. Proc Natl Acad Sci U S A. 1981;78:5147–5150. doi: 10.1073/pnas.78.8.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miotti S, Canevari S, Menard S, Mezzanzanica D, Porro G, Pupa SM, et al. Characterization of human ovarian carcinoma-associated antigens defined by novel monoclonal antibodies with tumor-restricted specificity. Int J Cancer. 1987;39:297–303. doi: 10.1002/ijc.2910390306. [DOI] [PubMed] [Google Scholar]
- 7.Alberti S, Miotti S, Stella M, Klein CE, Fornaro M, Menard S, et al. Biochemical characterization of trop-2, a cell surface molecule expressed by human carcinomas: Formal proof that the monoclonal antibodies T16 and MOv-16 recognize trop-2. Hybridoma. 1992;11:539–545. doi: 10.1089/hyb.1992.11.539. [DOI] [PubMed] [Google Scholar]
- 8.Nakashima K, Shimada H, Ochiai T, Kuboshima M, Kuroiwa N, Okazumi S, et al. Serological identification of TROP2 by recombinant cDNA expression cloning using sera of patients with esophageal squamous cell carcinoma. Int J Cancer. 2004;112:1029–1035. doi: 10.1002/ijc.20517. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Zhou W, Velculescu VE, Kern SE, Hruban RH, Hamilton SR, et al. Gene expression profiles in normal and cancer cells. Science. 1997;276:1268–1272. doi: 10.1126/science.276.5316.1268. [DOI] [PubMed] [Google Scholar]
- 10.Cubas R, Zhang S, Li M, Chen C, Yao Q. Trop2 expression contributes to tumor pathogenesis by activating the ERK MAPK pathway. Mol Cancer. 2010;9:253. doi: 10.1186/1476-4598-9-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muhlmann G, Spizzo G, Gostner J, Zitt M, Maier H, Moser P, et al. TROP2 expression as prognostic marker for gastric carcinoma. J Clin Pathol. 2009;62:152–158. doi: 10.1136/jcp.2008.060590. [DOI] [PubMed] [Google Scholar]
- 12.Fong D, Moser P, Krammel C, Gostner JM, Margreiter R, Mitterer M, et al. High expression of TROP2 correlates with poor prognosis in pancreatic cancer. Br J Cancer. 2008;99:1290–1295. doi: 10.1038/sj.bjc.6604677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fong D, Spizzo G, Gostner JM, Gastl G, Moser P, Krammel C, et al. TROP2: A novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod Pathol. 2008;21:186–191. doi: 10.1038/modpathol.3801001. [DOI] [PubMed] [Google Scholar]
- 14.Fang YJ, Lu ZH, Wang GQ, Pan ZZ, Zhou ZW, Yun JP, et al. Elevated expressions of MMP7, TROP2, and survivin are associated with survival, disease recurrence, and liver metastasis of colon cancer. Int J Colorectal Dis. 2009;24:875–884. doi: 10.1007/s00384-009-0725-z. [DOI] [PubMed] [Google Scholar]
- 15.Bignotti E, Todeschini P, Calza S, Falchetti M, Ravanini M, Tassi RA, et al. Trop-2 overexpression as an independent marker for poor overall survival in ovarian carcinoma patients. Eur J Cancer. 2010;46:944–953. doi: 10.1016/j.ejca.2009.12.019. [DOI] [PubMed] [Google Scholar]
- 16.Varughese J, Cocco E, Bellone S, de Leon M, Bellone M, Todeschini P, et al. Uterine serous papillary carcinomas overexpress human trophoblast-cell-surface marker (Trop-2) and are highly sensitive to immunotherapy with hRS7, a humanized anti-trop-2 monoclonal antibody. Cancer. 2011 doi: 10.1002/cncr.25891. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein R, Basu A, Chen S, Shih LB, Goldenberg DM. Specificity and properties of MAb RS7-3G11 and the antigen defined by this pancarcinoma monoclonal antibody. Int J Cancer. 1993;55:938–946. doi: 10.1002/ijc.2910550611. [DOI] [PubMed] [Google Scholar]
- 18.Stein R, Govindan SV, Mattes MJ, Shih LB, Griffiths GL, Hansen HJ, et al. Targeting human cancer xenografts with monoclonal antibodies labeled using radioiodinated, diethylenetriaminepentaacetic acid-appended peptides. Clin Cancer Res. 1999;5:3079s–3087s. [PubMed] [Google Scholar]
- 19.Govindan SV, Stein R, Qu Z, Chen S, Andrews P, Ma H, et al. Preclinical therapy of breast cancer with a radioiodinated humanized anti-EGP-1 monoclonal antibody: Advantage of a residualizing iodine radiolabel. Breast Cancer Res Treat. 2004;84:173–182. doi: 10.1023/B:BREA.0000018417.02580.ef. [DOI] [PubMed] [Google Scholar]
- 20.Caligiuri MA, Murray C, Robertson MJ, Wang E, Cochran K, Cameron C, et al. Selective modulation of human natural killer cells in vivo after prolonged infusion of low dose recombinant interleukin 2. J Clin Invest. 1993;91:123–132. doi: 10.1172/JCI116161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caron PC, Lai LT, Scheinberg DA. Interleukin-2 enhancement of cytotoxicity by humanized monoclonal antibody M195 (anti-CD33) in myelogenous leukemia. Clin Cancer Res. 1995;1:63–70. [PubMed] [Google Scholar]