Abstract
Squalene-based oil-in-water emulsions have been used for years in some seasonal and pandemic influenza vaccines. However, concerns have been expressed regarding squalene source and potential biological activities. Little information is available regarding the immunomodulatory activity of squalene in comparison with other metabolizable oils in the context of oil-in-water emulsions formulated with vaccines. The present work describes the manufacture and physical characterization of emulsions composed of different classes of oils, including squalene, long chain triglycerides, a medium chain triglyceride, and a perfluorocarbon, all emulsified with egg phosphatidylcholine. Some differences were apparent among the non-squalene oils in terms of emulsion stability, including higher size polydispersity in the perfluorocarbon emulsion, more rapid visual instability at 60 °C for the long-chain triglyceride and perfluorocarbon emulsions, and an increased creaming rate in the medium-chain triglyceride emulsion at 60 °C as detected by laser scattering optical profiling. The biological activity of each of these emulsions was compared when formulated with either a recombinant malaria antigen or a split-virus inactivated influenza vaccine. Overall, vaccines containing the squalene emulsion elicited higher antibody titers and more abundant long-lived plasma cells than vaccines containing emulsions based on other oils. Since squalene-based emulsions show higher adjuvant potency compared to the other oils tested, non-squalene oils may be more suitable as carriers of amphiphilic or hydrophobic immunostimulatory molecules (such as TLR agonists) rather than as stand-alone adjuvants.
Keywords: squalene, long chain triglyceride, medium chain triglyceride, perfluorocarbon, Miglyol, oil-in-water emulsion, vaccine adjuvant
Introduction
Oil-in-water (o/w) emulsions are currently employed as safe and efficacious vaccine adjuvants in various vaccine products already approved for human use or in clinical trials. The leading o/w emulsions developed for vaccine applications, such as MF59® and AS03®, are squalene-based (AS03® also contains α-tocopherol). Squalene is a metabolizable linear triterpene derived from shark liver or other natural sources. Injectable drug and nutritional supplementation formulations employ several different vegetable-derived oils, such as soybean oil, sesame oil, cottonseed oil, and castor oil, which have been used in approved products [1]. These vegetable-based oils are generally triglyceride-based, with chain length and saturation dependent on source.
Millions of doses of MF59® and AS03® have been administered to humans, including children, and a good safety profile has been established [2]. Nevertheless, some have alleged that a vaccine containing squalene was responsible for induction of squalene antibodies in human recipients [3, 4]. Although the findings of these studies have been largely refuted in the scientific community [5-10] and it has been demonstrated that the vaccine in question did not even contain squalene [11], concerns remain regarding squalene-associated autoimmune pathogenesis observed in murine and rat models [12]. The implications of these findings are unclear, especially because several other commonly used pharmaceutical oils have also produced pathologies in the same models [13]. Recently, an increased risk of narcolepsy has been noted in recipients of Pandemrix®, an H1N1 pandemic influenza vaccine containing AS03® [14][14]. To date, no squalene-containing vaccine or other parenteral product has been approved for routine use in the US by the FDA, although MF59® and AS03® have been approved in Europe and conditionally approved in the US as stockpiled adjuvants in the event of pandemic influenza.
Since its widespread use in the 1980s as the emulsified oil in Syntex Adjuvant Formulation, squalene or its hydrogenated form, squalane, have been the most commonly used oils for vaccine adjuvant applications [15]. Although the majority of work with the Syntex formulation employed squalane, modern adjuvants MF59® and AS03® contain squalene. Given the concerns regarding both the safety and source of squalene, we assessed various oil-in-water emulsions composed of different structural classes of oils, including a medium chain triglyceride (MCT) oil, various long chain triglyceride (LCT) oils, a tocopherol (α-tocopherol), and a perfluorocarbon oil to determine if any could potentially be used as replacements for squalene. We report here the physical characterization, stability, and vaccine adjuvant activity of emulsions made from these various oils in the context of influenza and malaria vaccine formulations.
Materials and Methods
Adjuvant formulations
Shark liver squalene (≥98% purity) and Fluorinert® FC-40 were purchased from Sigma–Aldrich (St. Louis, MO). Sesame oil and soybean oil, both of USP grade, were obtained courtesy of Welch, Holme, & Clark Co., Inc. (Newark, NJ). Grapeseed oil was purchased from a local food store and was manufactured by Napa Valley Naturals (San Francisco, CA). Miglyol® 810 was obtained courtesy of Sasol (Witten, Germany). DL-α-tocopherol was purchased from Spectrum Chemical (Gardena, CA). Egg phosphatidylcholine (egg PC) was obtained from Avanti Polar Lipids, Inc. (Alabaster, AL). Egg-derived phospholipids were selected because of their demonstrated effectiveness as emulsifiers and use in approved parenteral products such as Intralipid®. For each emulsion, the oil phase was prepared by dissolving the phospholipid into 10 mL of each oil. The oil-PC mixture was then sonicated in a VWR B2500A-DTH sonicating water bath (West Chester, PA) at ~50°C until the phospholipid was dissolved into the oil. The aqueous phase (consisting of ultra pure water) was added at 90% (v/v) to the oil phase, such that the final concentration of phospholipid was 1.9% (w/v). The composition was then mixed with a Silverson Heavy Duty Laboratory Mixer Emulsifier (3/4 in. tubular square hole high shear screen attachment; East Longmeadow, MA) at ~7,000 RPM for ten minutes to yield a crude emulsion. The crude emulsion was processed through a Microfluidics M110P (Newton, MA) high-pressure homogenizer for 12 passes at ~207 MPa (~30,000 psi). The recirculating product was cooled by a water bath near room temperature. In general, formulations were monitored for stability over 6 months at 5 °C, room temperature, 37 °C, and 60 °C.
Particle size
Emulsion particle size and polydispersity were determined via dynamic light scattering (DLS) with the Malvern Instruments Zetasizer Nano-S, Zetasizer Nano-ZS, or the Zetasizer APS (Worcestershire, UK). In general, DLS measurements were carried out at as described earlier [16]. Formulations were measured for particle size on the day of manufacture and at periodic intervals thereafter for 6 months. Standard values for the viscosity and refractive index of water as the bulk phase were employed in the software analysis. The absorption and refractive index values of polystyrene latex particles were used to approximate that of the emulsion droplets. All DLS measurements were performed in triplicate on each of three separate aliquots for a total of nine measurements for each timepoint.
Zeta potential
Zeta potential measurements were performed on the Zetasizer Nano ZS from Malvern Instruments. Standard values supplied by the instrument software were used for the viscosity and refractive index of water. Data analysis was performed assuming the Smoluchowski limit of the Henry function. Nine measurements were made at 25 °C using an automatic software determination of the measurement duration between 30 and 100 runs. The laser attenuation and the applied voltage settings were also automatically optimized by the software.
Viscosity
The dynamic viscosities of the emulsions were determined by a Brookfield DV-E digital viscometer (Middleboro, MA). A 20 mL sample of emulsion was removed from storage at 5 °C and allowed to equilibrate to room temperature. The sample was then loaded into the viscometer operating at 50 RPM spindle speed. Measurements were taken after 5 minutes of equilibration with the viscometer.
Laser scattering optical profiling
Optical profile measurements were performed using the LUMiReader Separation Analyzer (LUM Americas, Boulder, CO). Light transmission of a near-infrared laser line (870 nm) through undiluted emulsion was detected using a vertical CCD line of 45 mm length and 7 μm resolution. Profiles were collected every 10 minutes during 4 hours at 60 °C.
Hemolysis assay
The hemolysis assay was a modified version of the technique described previously [17]. Blood from a human volunteer was heparinized and used on the day of collection. One mL of blood was washed 3 times by centrifuging at 1500 rpm for 5 min in a microcentrifuge, removing the plasma and replacing with saline. The resulting red blood cells (RBC) were diluted into 6 mL saline and 200 μl of the RBC suspension was added to each sample, which consisted of 600 μl saline and 200 μl emulsion formulation. Samples were incubated at room temperature (RT) with occasional gentle shaking for ~20 min, after which they were centrifuged as above. Supernatant (50 μl) was added to 950 μl of ethanol:HCl (39:1 v:v) in a cuvette and absorbance was measured at 398 nm with a UV-Vis spectrophotometer. A positive control (100% lysis) was prepared by adding the RBCs to distilled water. Sample-induced hemolysis is reported as a percentage normalized to the positive control. In general, hemolysis activity was assayed on duplicate samples of blood from different donors for each sample with the average value reported.
Immunization and serum collection
Female, six-seven week old, BALB/c mice, (Charles River Laboratories, Wilmington, MA) were immunized by intramuscular injection in each hind quadricep. Formulations were mixed with antigen immediately prior to injection to provide a final formulation consisting of 2% v/v oil in a 100 μl total injection volume (evenly split by 50 μl injection per muscle). Two distinct antigens were tested: 1) 2007-2008 Fluzone inactivated split-virus vaccine, incorporating the representative influenza strains A/Solomon Islands/2/2006 (H1N1), A/Wisconsin/67/2005 (H3N2) and B/Malaysia/2506/2004, used at 0.2 μg total HA per dose; and 2) recombinant Plasmodium berghei circumsporozoite protein (PbCSP), used at 2 μg per dose. Mice were immunized twice with antigen, at an interval of 3 weeks for Fluzone or an interval of 2 weeks for PbCSP. Serum was collected by retro-orbital bleed into microtainer serum collection tubes (VWR International, West Chester, PA) before each injection and 2 weeks (PbCSP) or 4 weeks (Fluzone) after the final injection. All procedures were performed under specific pathogen-free conditions in accordance with the regulations and guidelines of the IDRI animal care and use committee.
Antibody responses
Sera were analyzed for antigen-specific IgG, IgG1, IgG2a antibodies by capture ELISA. Polysorp ELISA plates (Nunc, Rochester, NY) were coated with Fluzone or PbCSP at a concentration of 1.0 μg/mL in 0.1 M bicarbonate coating buffer. Plates were allowed to incubate overnight at 4 °C. Plates were blocked with a PBS-Tween 0.5%, 1% BSA (Sigma) solution for two hours at RT at 200 μl per well. Plates were washed five times in PBS-Tween 0.1% and once in PBS. Serial dilutions (1:5) of sera were added, and plates were incubated at RT for 2 hours. Plates were washed and antigen-specific antibodies detected with either anti-mouse IgG-HRP, IgG1-HRP or IgG2a-HRP (Southern Biotech, Birmingham, AL) at a 1:2000 dilution. Plates were incubated at RT for 1 hour, washed as above, and developed using SureBlue tetramethylbenzidine (TMB) substrate solution (Kirkegaard and Perry Laboratories, Gaithersburg, MD). The enzymatic reaction was stopped by adding 1N H2SO4 and plates were read at 450 nm wavelength (ELX808, Bio-Tek Instruments Inc, Winooski, VT). For PbCSP, arbitrary anti-PbCSP units were assigned by comparison against a standard curve derived from a pool of hyper-immune anti-PbCSP serum. Each test serum was diluted, then arbitrary units calculated based upon the first dilution factor that allowed placement within the linear phase of the standard curve. For Fluzone components, endpoint titer was determined as the last dilution to render a response of greater than 0.1 mean optical density using Prism software (GraphPad Software, La Jolla, CA).
Hemagglutination inhibition (HAI) antibody responses
HAI antibody activity was determined using sera collected 4 weeks after the second injection of Fluzone alone or adjuvanted with emulsion formulations. HAI antibodies specific to the A/Solomon Islands/3/2006 (H1N1) or A/Wisconsin/67/2005 (H3N2) component of the vaccine, or the heterologous strains A/Brisbane/59/07 (H1N1) and A/Uruguay/716/07 (H3N2), were determined as previously described using 0.5% turkey RBC [18]. Each serum sample was treated with receptor-destroying enzyme (RDE; Vibrio cholera Denka-Seiken, Tokyo, Japan) to remove non-specific inhibitors. The HAI titer is defined as the reciprocal of the highest dilution of sera which completely inhibits the agglutination of the turkey RBCs.
Enumeration of long-lived antibody-secreting plasma cells
A bone marrow ELISPOT was used to determine the induction of vaccine-specific long-lived antibody-secreting plasma cells following Fluzone immunization with and without adjuvant as previously described [19] with minor modifications. Mice were euthanized four weeks after the second immunization with 2007-2008 Fluzone formulation plus or minus the adjuvant.
Statistical analysis
Mouse experiments typically consisted of five individual animals per group per timepoint. ELISPOT counts and log10-transformed antibody titers were compared using ANOVA with Tukey’s multiple comparison test. HAI titers were compared by ANOVA with Tukey’s multiple comparison test using the log2-transformed HAI titers.
Results
The composition of the oils employed in this study is described in Table 1. Squalene is a linear triterpene available at high purity (≥98%). Grapeseed, sesame, and soybean oils are long-chain triglycerides (LCTs) containing a mixture of fatty acid components as well as phytosterols and tocopherols [20, 21]. USP monographs for sesame and soybean oils are available, and grapeseed oil provides a different triglyceride composition and source for comparison. Miglyol® 810 is a highly purified triglyceride mixture of mainly capric and caprylic fatty acids, and conforms with the USP monograph for medium chain triglycerides (MCT). LCT and MCT oils have been used extensively in parenteral nutritional supplementation formulations. Fluorinert® FC-40 is comprised of a mixture of perfluorocarbon compounds, namely perfluorotri-n-butylamine and perfluoro-n-dibutylmethylamine. Perfluorocarbons have shown promise as oxygen-carrying blood substitutes with a good safety record [22]. We note here that the emulsion containing α-tocopherol became visually unstable immediately after manufacture and was not analyzed further (data not shown). The squalene emulsion described here is the same lot reported in an earlier study [23, 24]; some data for this lot are repeated in Table 2 in this manuscript for ease of comparison.
Table 1.
Composition of emulsion oils
| Oil | Chemical Structure | C18:1 | C18:2 | C18:3 | C18:0 | C16:0 | C10:0 | C8:0 | Other Components |
|---|---|---|---|---|---|---|---|---|---|
| squalene |
|
≥98% linear triterpene |
|||||||
| grapeseed |
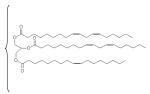
|
16 | 72 | ? | 4 | 7 | C16:1, steroids, tocopherols |
||
| sesame | 35-50 | 35-50 | <1.0 | 3.5-6 | 7-12 | C14:0, C16:1, C20:0, C20:1, C22:0, steroids, tocopherols |
|||
| soybean | 21.5 | 55.1 | 7.8 | 3.6 | 10.6 | C14:0, C16:1, C20:0, C20:1, steroids, tocopherols |
|||
| Miglyol 810 |
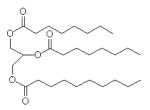
|
20-35 | 65-80 | C6:0, C12:0, C14:0 | |||||
| Fluorinert FC-40 |
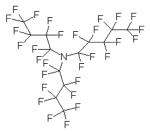
|
mixture of perfluorotri-n- butylamine (shown) with perfluoro-n- dibutylmethylamine |
Note: Numbers to the right of chemical structures refer to percentages of each compound present in the oil. The triglyceride notation refers to the length saturation of carbons in the triglyceride acyl chain (for example, C18:1 refers to 18 carbon chain length with 1 unsaturated bond). Composition information is obtained from the manufacturers or estimated based on the literature.
Table 2.
Emulsion physicochemical and biocompatibility characterization
| oil | Particle Size (nm, T=0) |
Polydispersity | Particle Size (nm, T=6mo) |
Polydispersity | Dynamic Viscosity (cP, T=0) |
Dynamic Viscosity (cP, T=6mo) |
Zeta Potential (mV, T=0) |
Zeta Potential (mV, T=6mo) |
Hemolysis % (T=0) |
|---|---|---|---|---|---|---|---|---|---|
| squalene | 119 | 0.18 | 134 | 0.21 | 1.6 | NM | −27.5 | −28.1 | 0.2% |
| grapeseed | 119 | 0.08 | 121 | 0.10 | 1.6 | 1.6 | −21.4 | −15.8 | 0.7% |
| sesame | 107 | 0.09 | 109 | 0.12 | 1.5 | 1.5 | −25.7 | −20.4 | 0.4% |
| soybean | 91.7* | 0.09* | 98.6 | 0.17 | 1.5 | 1.6 | −19.7 | −28.1 | 0.3% |
| Miglyol 810 | 119 | 0.15 | 129 | 0.21 | 1.6 | 1.6 | −20.2 | −24.4 | 0.3% |
| Fluorinert FC-40 | 119 | 0.30 | NM | NM | 1.6 | 1.6 | −5.8 | −19.6 | 0.2% |
Notes and abbreviations: T=0 refers to at or near the day of manufacture; T=6mo refers to ~6 months post-manufacture at 5°C storage temperature; NM, not measured due to insufficient sample volume or measurement quality failure. Size measurement is the z-avg, which represents the intensity-biased average size value from dynamic light scattering.
Measured using the Malvern Zetasizer Nano instead of the Malvern Zetasizer APS.
We compared the physicochemical and biocompatibility properties of the emulsions at time of manufacture and after 6 months of storage at 5 °C (Table 2). Initial particle size shows minor variation between the different emulsions, although the perfluorocarbon emulsion shows a higher polydispersity value than the other emulsions. The viscosity values are essentially the same for each formulation and are close to that of water (~1 cP), indicative of the low oil content in these emulsions (10% v/v when viscosity is measured, but diluted to 2% v/v for immunization). Zeta potential values are negative for each of the emulsions, although the magnitude varies somewhat between emulsions and within the same emulsion after 6 months of storage. Hemolytic activity of the emulsions when incubated with a suspension of red blood cells was equivalent to that of a negative saline control, indicating good hemocompatibility.
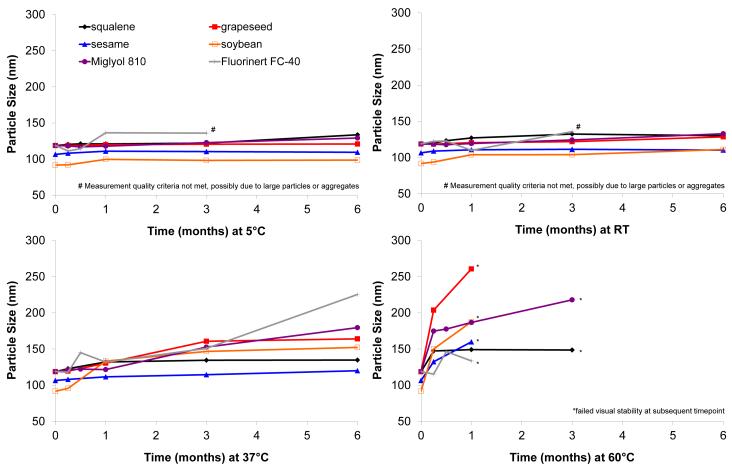
To assess the effect of varying temperatures on stability, particle size of the emulsions stored at different temperatures was monitored (Figure 1). At 5 °C and room temperature, the emulsions show little change in particle size over 6 months post manufacture. Notably, the perfluorocarbon emulsion particle size measurement at 6 months did not meet quality criteria of the instrument, indicating potential interference from large particles or aggregates. Unlike the other oils studied here, the perfluorocarbon is more dense than water, and the perfluorocarbon particles tend to settle instead of cream upon coalescence and/or aggregation. At 37 °C and 60 °C, more substantial particle size growth takes place, with all formulations showing visual inhomogeneities before the end of the 6 month period when stored at 60 °C, although the squalene and Miglyol-based emulsions appeared more visually stable at this temperature. With the exception of the perfluorocarbon emulsion, there do not appear to be major differences in particle size stability between the various emulsions.
Figure 1.
Emulsion particle size stability at various storage temperatures. Shown are results following storage at (a) 5 °C, (b) RT, (c) 37 °C and (d) 60 °C. Particle size was measured using Malvern Instruments Zetasizer Nano or Automated Particle Sizer. Size measurement is the z-avg, which represents the intensity-biased average size value from dynamic light scattering.
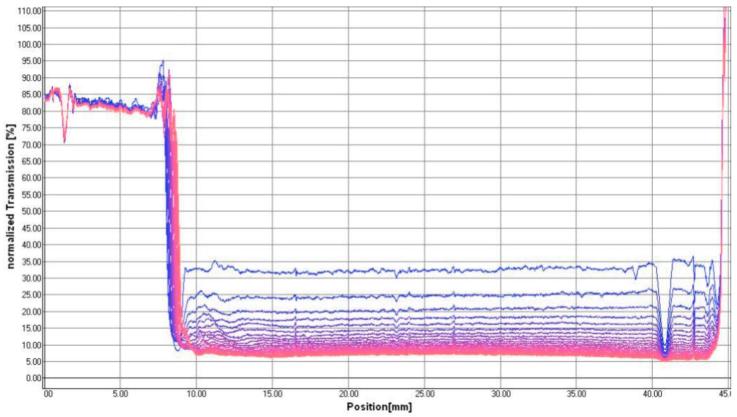
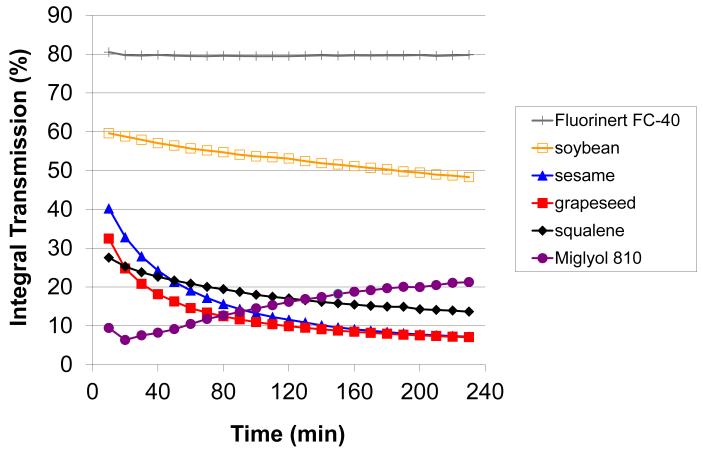
We have employed laser scattering optical profiling to provide information regarding emulsion stability parameters. A similar method was recently employed to investigate non-ionic surfactant vesicle stability [25]. In this technique, an 870-nm laser line simultaneously illuminates the vertical profile of the undiluted emulsion, and the light transmission is collected by a CCD detector with <40 μm vertical spatial resolution at a controlled temperature. Changes in the emulsion due to creaming, coalescence, etc. are detected as changes in light transmission through the vertical profile of the emulsion. For example, the laser scattering optical profile of the grapeseed emulsion, measured every 10 minutes over a period of 4 hours at 60 °C, is shown in Figure 2a. In the chronological color gradient, blue profiles represent earlier timepoints and pink profiles the later timepoints. The laser scattering profiles of the other emulsions (sesame, soybean, perfluorocarbon, Miglyol) are shown in Supplementary Figure 1. In order to compare data from different emulsions, the integral transmission between a specific region (27 to 32 mm) near the center of the vertical profile of each sample is plotted in Figure 2b. A decrease in integral transmission (squalene, grapeseed, sesame, and soybean emulsions) is indicative of coalescence or particle size growth since larger particles will scatter more light, allowing less light to be transmitted to the detector. The higher initial transmission value of the soybean emulsion is expected since this emulsion showed smaller initial particle size than the other formulations. An increase in integral transmission (Miglyol 810 emulsion) is generally indicative of creaming. In fact, the complete profile of the Miglyol 810 emulsion clearly shows a creamed layer appearing as a large high transmission peak at ~8 mm on the x-axis during the course of the experiment, presumably due to the creation of a transparent oil layer as droplets coalesce (Suppl Fig 1). Interestingly, the relatively translucent perfluorocarbon emulsion (high light transmission) shows little overall change in light transmission, indicating good stability at this temperature.
Figure 2.
Laser scattering optical profiling analysis of emulsion stability. (a) Laser transmission scans of undiluted grapeseed emulsion in polycarbonate cuvette measured every 10 minutes over 4 hours at 60°C, with blue color indicating initial scans and pink color indicating later scans. (b) Integral transmission profiles of the 25 – 30 mm region (see above) of cuvettes containing emulsion, measured over 4 hours at 60°C.
Overall, the physicochemical characterization data indicate good stability at lower temperatures for the emulsions studied with the exception of the failed measurement at the 6 month timepoint for the perfluorocarbon emulsion. At higher temperatures different kinetics of emulsion instability between the various formulations are apparent. None of the alternative oils offer improved stability compared to the squalene-based emulsion.
Squalene emulsion enhances total IgG antibody responses to a recombinant malaria protein
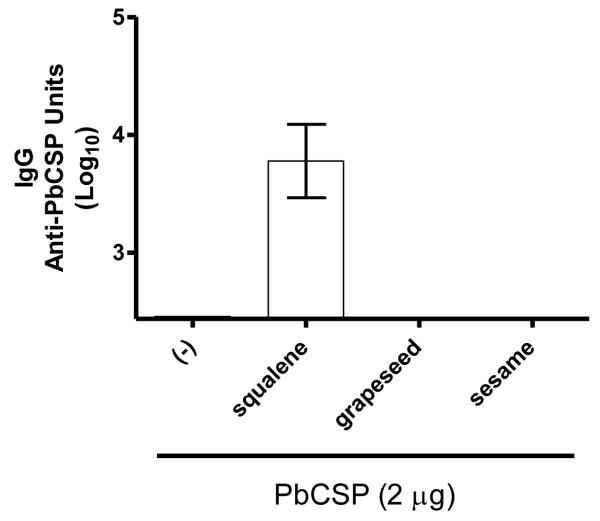
Antibody responses against PbCSP were measured in individual mouse sera two weeks after the boost immunization with protein alone or with protein combined with emulsion formulations. Antibody levels in four of the five mice receiving the antigen without adjuvant were below detection (Figure 3). The anti-PbCSP IgG antibody levels were also below detection in all mice receiving the vaccines containing the grapeseed or sesame emulsions, and only mice receiving the vaccine containing the squalene emulsion showed appreciable anti-PbCSP IgG antibody levels (Figure 3).
Figure 3.
Effect of emulsions on antibody response induced by immunization with recombinant malaria antigen. BALB/c mice were immunized twice with PbCSP antigen formulated with emulsions containing squalene, grapeseed, or sesame oil. Antigen-specific IgG were determined by ELISA. The antibody responses from the grapeseed and sesame oil groups were below the limit of detection. In the antigen alone group, only one mouse showed a response above the lower limit of detection. n = 5 per group, and data are shown as mean and s.e.
Antibody responses to influenza proteins is dependent on emulsion oil
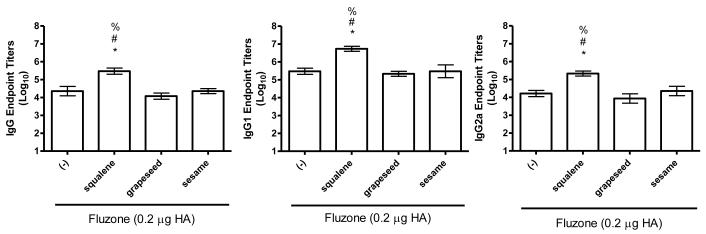
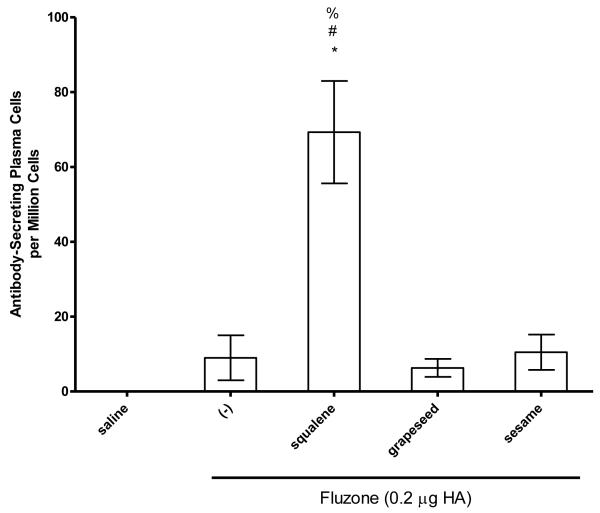
To determine if emulsions elevated the antibody response to native influenza proteins, we immunized mice with a low dose of Fluzone vaccine in the presence or absence of emulsion formulation incorporating squalene, grapeseed, or sesame oils. Compared to vaccination with the Fluzone vaccine alone, total IgG, IgG1 and IgG2 antibody titers were higher for all groups that received Fluzone with the squalene emulsion formulation (Figure 4, p-values < 0.05). Similar to the results generated with PbCSP, the antibody titers generated by the Fluzone vaccine containing the squalene emulsion were significantly greater than those generated by the vaccines containing grapeseed or sesame oil emulsion, which were not different from Fluzone alone. In agreement, the vaccine containing the squalene emulsion elicited higher numbers of antibody-secreting long-lived plasma cells than the Fluzone vaccine alone or the vaccine containing grapeseed or sesame oil emulsions (Figure 6a, p-values < 0.05).
Figure 4.
Effect of adjuvant formulation in humoral immune responses induced by immunization with inactivated split-virus influenza vaccine (Fluzone). BALB/c mice were immunized with Fluzone formulated with emulsions containing squalene, grapeseed, or sesame oil. Antigen-specific IgG (left), IgG1 (middle) and IgG2a (right) were determined by ELISA. Results are shown as the geometric mean endpoint titer (Log10) ± s.e., n = 5 per group.
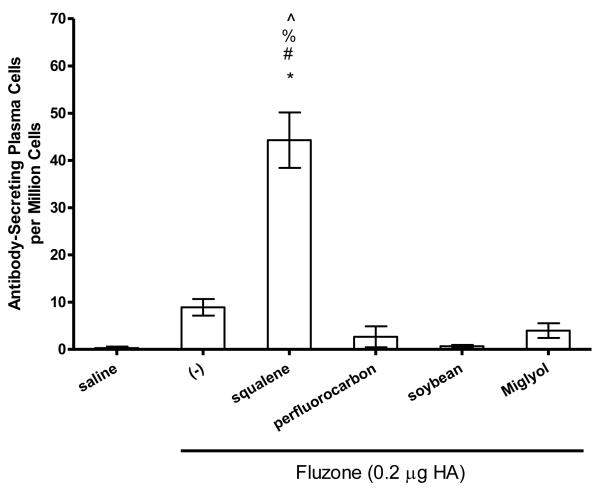
Figure 6.
IgG-secreting bone marrow plasma cells against Fluzone were determined by ELISPOT and data are represented by mean +/− SEM. (a) * = p-value < 0.05 versus immunization with vaccine alone; # = p-value < 0.05 versus immunization with the vaccine containing the grapeseed emulsion; % = p-value < 0.05 versus immunization with the vaccine containing the sesame emulsion. (b) * = p-value < 0.05 versus immunization with vaccine alone; # = p-value < 0.05 versus immunization with the vaccine containing the perfluorocarbon emulsion; % = p-value < 0.05 versus immunization with the vaccine containing the soybean emulsion; ^ = p-value < 0.05 versus immunization with the vaccine containing the Miglyol emulsion.
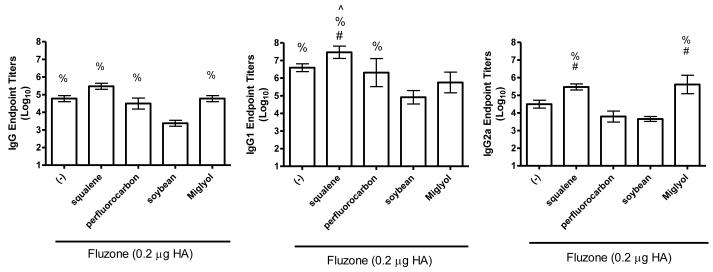
In order to evaluate the biological effects of different structural classes of emulsion oils, the squalene emulsion was compared to soybean, perfluorocarbon, and medium chain triglyceride (Miglyol® 810)–based emulsion adjuvants in the same influenza murine model. In contrast to the previous experiment, anti-influenza IgG, IgG1, and IgG2a antibody levels elicited by the vaccine containing the squalene emulsion did not achieve statistical significance compared to the Fluzone vaccine alone (Figure 5). However, total IgG responses in the soybean oil emulsion group were significantly lower than all of the other formulations, including the vaccine without adjuvant (Figure 5, p-values < 0.05). IgG1 responses induced by the vaccine containing the squalene emulsion were significantly higher than each of the other oil groups: perfluorocarbon, soybean, and Miglyol. In addition, IgG1 responses in the perfluorocarbon emulsion group were higher than the soybean oil emulsion group. Finally, IgG2a antibody responses were significantly higher in the squalene and Miglyol emulsion groups compared to the perfluorocarbon and soybean oil emulsion groups. The generation of antibody-secreting long-lived plasma cells was significantly enhanced by the vaccine containing the squalene emulsion compared to the vaccine alone or the vaccine containing any of the other emulsion oils (Figure 6b, p-values < 0.05). The vaccines containing non-squalene oils failed to induce more long-lived plasma cells compared to the vaccine alone.
Figure 5.
Effect of adjuvant formulation in humoral immune responses induced by immunization with inactivated split-virus influenza vaccine (Fluzone). BALB/c mice were immunized with Fluzone formulated with emulsions containing squalene, perfluorocarbon, soybean, or Miglyol 810 oil. Antigen-specific IgG (left), IgG1 (middle) and IgG2a (right) were determined by ELISA. Results are shown as the geometric mean endpoint titer (Log10) ± SEM, n = 5 per group except for the squalene group in (b) where n = 4.
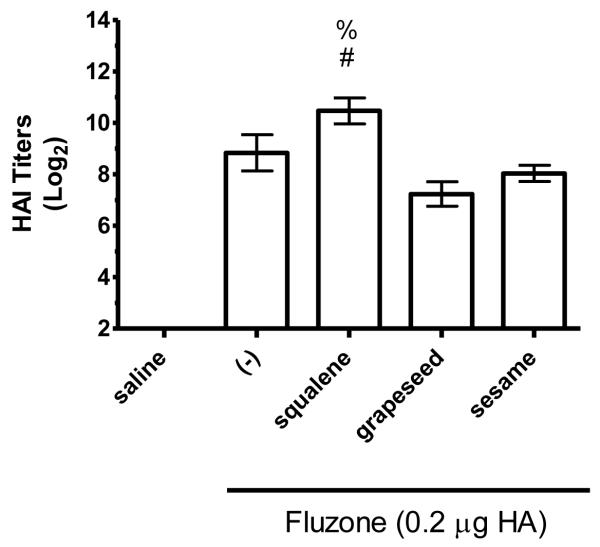
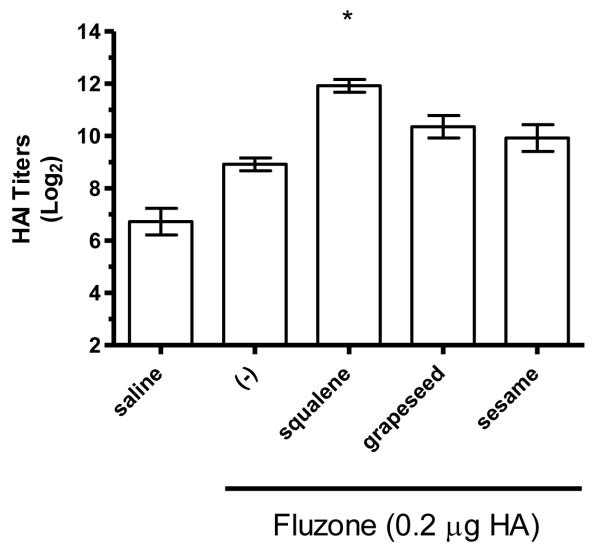
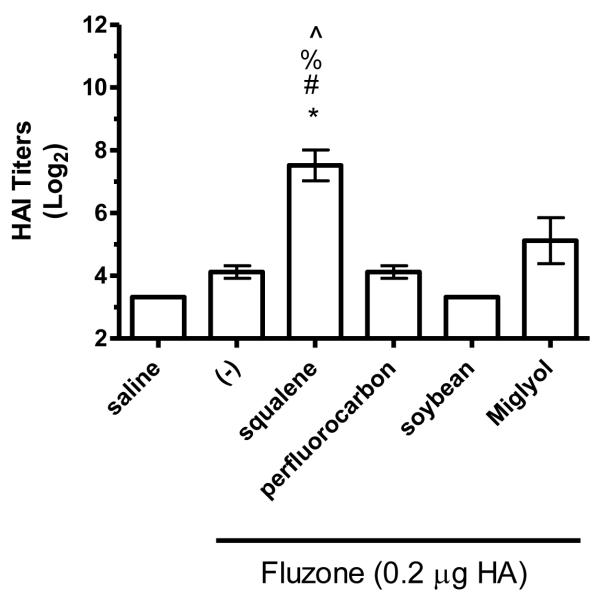
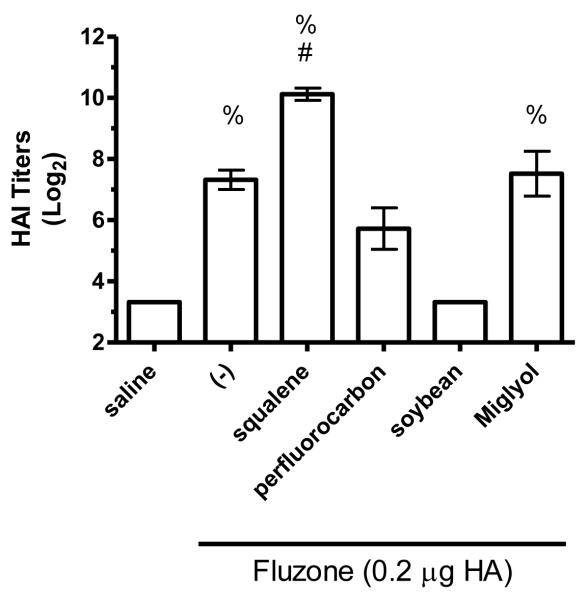
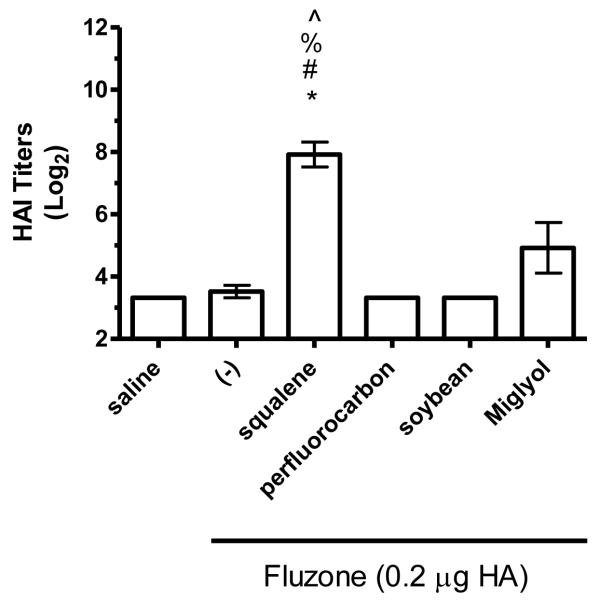
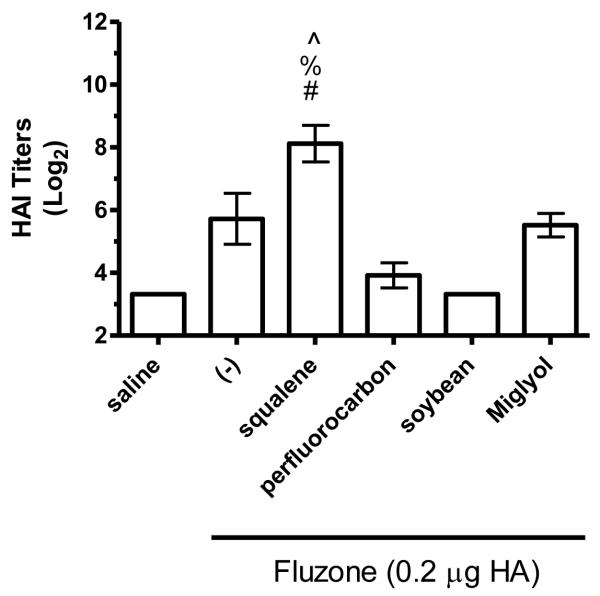
The hemagglutination inhibition (HAI) assay is a functional assay with a titer of ≥40 generally considered enough to provide protection against influenza [26]. To determine the impact of different emulsion oils on functional activity, we compared HAI titers of mice vaccinated with Fluzone alone or Fluzone with various emulsion formulations. Against the A/Solomon Islands/3/2006 (H1N1) vaccine component, the addition of squalene emulsion formulation induces higher HAI titers than the Fluzone vaccine containing any of the other oils studied (Figures 7a and 8a, p-values < 0.05). Against the A/Wisconsin/67/2005 (H3N2) vaccine component, results were more variable, with the vaccine containing the squalene emulsion the only formulation to induce higher HAI than the vaccine alone in one experiment (Figure 7b, p-values < 0.05); in another experiment, the vaccine containing the squalene emulsion elicited higher HAI titers than the vaccines containing either the soybean or perfluorocarbon emulsions, but not the Miglyol emulsion or the non-adjuvanted vaccine (Figure 8a; p-values < 0.05). Against the H1N1 and H3N2 heterologous strains (A/Brisbane/59/07 and A/Uruguay/716/07, respectively), the squalene-containing vaccine generated higher HAI titers than the vaccines containing soybean, perfluorocarbon, or Miglyol oils (Figure 8c-d, p-values < 0.05). Interestingly, inclusion of soybean oil emulsion appeared to have an inhibitory effect on HAI and antibody titers when determined at this low statistical power (Figure 8b; Figure 5; p-values < 0.05).
Figure 7.
HAI titers after immunization with Fluzone formulated with squalene, grapeseed, or sesame oil emulsion. BALB/c mice were immunized and then serum HAI titers determined four weeks after boosting. (a) HAI titers against the A/Solomon Islands/3/2006 (H1N1) component of Fluzone. (b) HAI titers against the A/Wisconsin/67/2005 (H3N2) component of Fluzone. Data are shown as the (Log2) titer for each individual animal, with the geometric mean and SEM represented. * = p-value < 0.05 versus immunization with vaccine alone; # = p-value < 0.05 versus immunization with the vaccine containing the grapeseed emulsion; % = p-value < 0.05 versus immunization with the vaccine containing the sesame emulsion.
Figure 8.
HAI titers after immunization with Fluzone formulated with squalene, perfluorocarbon, soybean, or Miglyol 810 oil emulsion. BALB/c mice were immunized and then serum HAI titers determined four weeks after boosting. (a) HAI titers against the A/Solomon Islands/3/2006 (H1N1) component of Fluzone. (b) HAI titers against the A/Wisconsin/67/2005 (H3N2) component of Fluzone. (c) HAI titers against the heterologous A/Brisbane/59/07 influenza strain. (d) HAI titers against the heterologous A/Uruguay/716/07 influenza strain. Data are shown as the (Log2) titer for each individual animal, with the geometric mean and SEM represented. * = p-value < 0.05 versus immunization with vaccine alone; # = p-value < 0.05 versus immunization with the vaccine containing the perfluorocarbon emulsion; % = p-value < 0.05 versus immunization with the vaccine containing the soybean emulsion; ^ = p-value < 0.05 versus immunization with the vaccine containing the Miglyol emulsion.
Some oils performed comparably to squalene in some assays. For instance, the vaccine containing the Miglyol emulsion elicited similar IgG and IgG2a antibody titers as the vaccine containing the squalene emulsion; however, HAI titers and long-lived plasma cells elicited by the vaccine containing the squalene formulation were generally superior to the vaccine containing Miglyol. In general, the immunological evaluation of the vaccines containing emulsions indicate that inclusion of squalene provides a greater immunostimulatory capacity,
Discussion
Although shark-derived squalene is widely and effectively used as a vaccine adjuvant, concerns regarding the source and safety of the material have prompted evaluation of substitutes. In an attempt to identify potential squalene replacements, we formulated oil-in-water emulsions using various oils with phosphatidylcholine emulsifier. We found in vitro physical stability of the emulsions to be comparable to the squalene-based emulsion. Immunological evaluations with a recombinant malaria antigen and an inactivated split-virus influenza vaccine indicate that none of the other oils tested generates biological activity equivalent to the squalene-based adjuvant formulation. Several differences were noted between the various oils in their biological activities when administered as vaccine formulations.
Given the significantly different structure, source, and purity of the oils studied here, it is perhaps surprising that their initial physicochemical characteristics are quite similar. This may be attributable to a highly efficient manufacturing method (microfluidization) and the use of a common emulsifier (egg PC). In contrast, oil-in-water emulsions of 18 different oils (from plant, animal, and mineral sources) have been produced by probe sonication with egg PC as emulsifier, showing that the squalene emulsion had smaller particle size and greater stability than most other emulsions from natural sources, such as sesame, soybean, and coconut oils [27].
Squalene is of higher molecular homogeneity than the other oils (which include multiple species), facilitating easier quantification and quality control. The question of molecular homogeneity as well as presence of undefined contaminants is especially important given historical and recent findings concerning both adjuvant activity and toxicity due to uncontrolled contaminants: first, the toxicity of IFA was considerably reduced due to purer materials and more refined emulsions [28]; second, regulatory requirements that more purified components be used in Adjuvant 65 resulted in loss of potency and caused an end of product development [29]; third, the w/o emulsion Montanide ISA 51 recently switched emulsifier source from animal to plant, which may be the cause of significantly reduced potency in clinical trials [30]. Moreover, plant-based oils contain various components besides triglycerides, such as phytosterols and α-tocopherol, [20]; the latter has shown adjuvant activity as an emulsion oil [31, 32]. Several drug-containing emulsions or other oil-based products are available commercially, including castor oil, cottonseed oil, MCTs, LCTs (e.g. soybean, sesame, safflower oils), fish oil, perfluorocarbon, and vitamins [1, 33]. Some of these products contain high percentages of oil or are injected continuously over long periods. The fact that there are many approved products with widespread use (at much higher doses than what is used in vaccines) indicates the favorable safety profile of these oils, despite their heterogeneous composition. One possible approach to alleviate concerns regarding animal source squalene is to switch to olive-derived squalene. Initial studies comparing biological activity show equivalence, although acceptable purity and stability still need to be demonstrated for olive squalene [16, 44].
In the context of oil-in-water emulsion adjuvants, soybean, hexadecane, peanut, mineral, squalene, and squalane oils have been employed, although results are highly dependent on differences in emulsion preparation, antigen, and emulsifier, making comparison between different studies difficult, although squalene and especially squalane appeared to be potent substitutes for mineral oil [34-38]. Recent research using both water-in-oil and oil-in-water emulsions has examined MCT-based emulsions vs. mineral oil emulsions; the latter were more reactogenic and induced higher antibody titers [39], although the MCT-based emulsion caused a higher influx of macrophages [40]. The Syntex Adjuvant Formulation was developed as a squalane- or squalene-based oil-in-water emulsion that was reported to have superior adjuvant activity compared to coconut, olive, peanut, and lanolin oils [41], although data was not reported in the literature. Current approved adjuvants MF59® and AS03® are squalene-based oil-in-water emulsions, while the latter also contains α-tocopherol.
In the present work, we have demonstrated that the squalene emulsion indeed has inherent adjuvant activity that is not shared by the other oils tested. Nevertheless, there were some differences in the biological activity of the other oils. For instance, the vaccine containing the Miglyol emulsion elicited higher IgG2a antibodies than either soybean- or perfluorocarbon-containing vaccines. In addition, the perfluorocarbon emulsion was associated with higher total IgG and IgG1 antibody titers than the soybean emulsion. Thus, our results show that the LCT-based emulsions elicited negligible adjuvant activity whereas the perfluorocarbon and MCT-based emulsions were associated with greater adjuvant activity compared to the LCT emulsions in some readouts. Only the squalene emulsion appeared effective as an adjuvant in all readouts. We note here that the low number of animals per group in these in vivo experiments causes the meaningfulness of some results to be difficult to interpret. A statistically higher-powered study would be necessary in order to definitively discriminate between the biological mechanisms of the different emulsions.
Finally, there may be another motivation for substitution of squalene with other metabolizable oils: ongoing development of well-defined, potent TLR agonists with highly specific adjuvant activity and an amphiphilic or hydrophobic structure (such as the lipid-based TLR2 or TLR4 agonists) may require emulsion formulations for solubility or delivery purposes only; that is, no additional adjuvant activity from the emulsion itself will be necessary. There are at least two reports describing the effective use of one of the most common nutritional supplementation emulsions, the soybean oil-based Intralipid®, as a vaccine formulation including antigen and additional immunostimulatory molecules (muramyl dipeptide or avridine) [34, 42]. Tsujimoto et al. found that the soybean emulsion with an influenza vaccine elicited similar HAI titers to the control vaccine (in PBS); however, when an additional immunostimulant (muramyl dipeptide analogue) was present, the vaccine containing the emulsion appeared to elicit higher HAI titers than the vaccine containing immunostimulant without the soybean oil emulsion [42], although the physical interaction between the immunostimulant and the emulsion was not characterized. Similarly, our results described in the present work showed negligible adjuvant activity associated with the soybean, grapeseed, and sesame oil emulsions. However, if combined with a suitable TLR agonist, the physical presentation of the agonist on the surface or in the core of the emulsion droplet may be all that is required for a potent immune response [43]. In this context, an emulsion could be largely inert on its own but potent as a formulation tool of a TLR agonist. Work along these lines is ongoing in our laboratory.
Supplementary Material
Highlights.
-
>
We evaluate stability and biological activity of various vaccine adjuvant emulsions employing different oils.
-
>
Stability is similar at lower temperatures.
-
>
Squalene-based emulsions showed higher adjuvant activity than other classes of oils.
Acknowledgments
The authors wish to thank Richard Roque from Tria Bioscience Corp. in Seattle, WA for conducting most of the HAI assays; Susan Lin, Sandra Sivananthan, Tim Dutill, Kristen Forseth, Tony Phan, Farah Mompoint, Tara Evers, Alison Bernard, and Marah Hay for skilled technical assistance; and Dr. Martin Friede for helpful discussions. This work was supported in part by grants AI-25038 and AI-044373 from the National Institutes of Health, contract HHSN272200800045C from the National Institutes of Health, and grant 42387 from the Bill and Melinda Gates Foundation.
The authors gratefully acknowledge Dr. Evelina Angov for kindly providing the codon harmonized PbCSP construct developed by Walter Reed Army Institute of Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Strickley RG. Solubilizing excipients in oral and injectable formulations. Pharm Res. 2004;21:201–30. doi: 10.1023/b:pham.0000016235.32639.23. [DOI] [PubMed] [Google Scholar]
- [2].Schultze V, D’Agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–22. doi: 10.1016/j.vaccine.2008.03.093. [DOI] [PubMed] [Google Scholar]
- [3].Asa PB, Cao Y, Garry RF. Antibodies to squalene in Gulf War Syndrome. Exp Mol Pathol. 2000;68:55–64. doi: 10.1006/exmp.1999.2295. [DOI] [PubMed] [Google Scholar]
- [4].Asa PB, Wilson RB, Garry RF. Antibodies to squalene in recipients of anthrax vaccine. Exp Mol Pathol. 2002;73:19–27. doi: 10.1006/exmp.2002.2429. [DOI] [PubMed] [Google Scholar]
- [5].Alving CR, Grabenstein JD. Letter to the Editor. Exp Mol Pathol. 2000;68:196–7. doi: 10.1006/exmp.2000.2314. [DOI] [PubMed] [Google Scholar]
- [6].Del Giudice G, Fragapane E, Bugarini R, Hora M, Henriksson T, Palla E, et al. Vaccines with the MF59 adjuvant do not stimulate antibody responses against squalene. Clin Vaccine Immunol. 2006 September 1;13:1010–3. doi: 10.1128/CVI.00191-06. 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fulco CE, Liverman CT, Sox HC. Gulf War and Health: Depleted Uranium, Pyridostigmine Bromide, Sarin, Vaccines. National Academy Press; Washington, DC: 2000. [PubMed] [Google Scholar]
- [8].Matyas GR, Rao M, Alving CR. Induction and detection of antibodies to squalene: II. Optimization of the assay for murine antibodies. J Immunol Methods. 2002;267:119–29. doi: 10.1016/s0022-1759(02)00180-1. [DOI] [PubMed] [Google Scholar]
- [9].Matyas GR, Rao M, Pittman PR, Burge R, Robbins IE, Wassef NM, et al. Detection of antibodies to squalene: III. Naturally occurring antibodies to squalene in humans and mice. J Immunol Methods. 2004;286:47–67. doi: 10.1016/j.jim.2003.11.002. [DOI] [PubMed] [Google Scholar]
- [10].Matyas GR, Wassef NM, Rao M, Alving CR. Induction and detection of antibodies to squalene. J Immunol Methods. 2000;245:1–14. doi: 10.1016/s0022-1759(00)00268-4. [DOI] [PubMed] [Google Scholar]
- [11].Spanggord RJ, Sun M, Lim P, Ellis WY. Enhancement of an analytical method for the determination of squalene in anthrax vaccine adsorbed formulations. J Pharm Biomed Anal. 2006;42:494–9. doi: 10.1016/j.jpba.2006.04.009. [DOI] [PubMed] [Google Scholar]
- [12].Beck F, Butters D, Matsumoto G, Whitehouse M. Queries about vaccines containing squalene. Immunol Cell Biol. 2010 doi: 10.1038/icb.2010.10. in press. [DOI] [PubMed] [Google Scholar]
- [13].Whitehouse MW, Orr KJ, Beck FWJ, Pearson CM. Freund’s adjuvants: relationship of arthritogenicity and adjuvanticity in rats to vehicle composition. Immunology. 1974;27:311–30. [PMC free article] [PubMed] [Google Scholar]
- [14].World Health Organization . Pandemrix vaccine and increased risk of narcolepsy. Feb 1, 2011. [Google Scholar]
- [15].Fox CB. Squalene emulsions for parenteral vaccine and drug delivery. Molecules. 2009;14:3286–312. doi: 10.3390/molecules14093286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fox CB, Anderson RC, Dutill TS, Goto Y, Reed SG, Vedvick T. Monitoring the effects of component structure and source and formulation stability and adjuvant activity of oil-in-water emulsions. Coll Surf B: Biointerfaces. 2008;65:98–105. doi: 10.1016/j.colsurfb.2008.03.003. [DOI] [PubMed] [Google Scholar]
- [17].Bock TK, Muller BW. A novel assay to determine the hemolytic activity of drugs incorporated in colloidal carrier systems. Pharm Res. 1994;11:589–91. doi: 10.1023/a:1018987120738. [DOI] [PubMed] [Google Scholar]
- [18].Kendal AP, Pereira MS, Skehel JJ. Hemagglutination inhibition. In: Kendal AP, Pereira MS, Skehel JJ, editors. Concepts and Procedures for Laboratory-Based Influenza Surveillance. Centers for Disease Control and Pan-American Health Organization; Atlanta, GA: 1982. pp. B17–35. [Google Scholar]
- [19].Baldwin SL, Shaverdian N, Goto Y, Duthie MS, Raman VS, Evers T, et al. Enhanced humoral and Type 1 cellular immune responses with Fluzone adjuvanted with a synthetic TLR4 agonist formulated in an emulsion. Vaccine. 2009;27:5956–63. doi: 10.1016/j.vaccine.2009.07.081. [DOI] [PubMed] [Google Scholar]
- [20].Hardy G, Puzovic M. Formulation, stability, and administration of parenteral nutrition with new lipid emulsions. Nutr Clin Pract. 2009;24:616–25. doi: 10.1177/0884533609342445. [DOI] [PubMed] [Google Scholar]
- [21].Forchielli ML, Bersani G, Tala S, Grossi G, Puggioli C, Masi M. The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. Lipids. 2010;45:63–71. doi: 10.1007/s11745-009-3371-x. [DOI] [PubMed] [Google Scholar]
- [22].Castro CI, Briceno JC. Perfluorocarbon-based oxygen carriers: review of products and trials. Artif Organs. 2010;34:622–34. doi: 10.1111/j.1525-1594.2009.00944.x. [DOI] [PubMed] [Google Scholar]
- [23].Fox CB, Baldwin SL, Duthie MS, Reed SG, Vedvick TS. Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions. 2011 doi: 10.1208/s12249-012-9771-x. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fox CB, Lin S, Sivananthan SJ, Dutill TS, Forseth KT, Reed SG, et al. Effects of emulsifier concentration, composition, and order of addition in squalene-phosphatidylcholine oil-in-water emulsions. Pharm Dev Technol. 2011 doi: 10.3109/10837450.2010.495397. in press. [DOI] [PubMed] [Google Scholar]
- [25].Marianecci C, Paolino D, Celia C, Fresta M, Carafa M, Alhaique F. Non-ionic surfactant vesicles in pulmonary glucocorticoid delivery: characterization and interaction with human lung fibroblasts. J Control Rel. 2010;147:127–35. doi: 10.1016/j.jconrel.2010.06.022. [DOI] [PubMed] [Google Scholar]
- [26].Falsey AR. Half-dose influenza vaccine. Arch Intern Med. 2008;168:2402–3. doi: 10.1001/archinte.168.22.2402. [DOI] [PubMed] [Google Scholar]
- [27].Chung H, Kim TW, Kwon M, Kwon IC, Jeong SY. Oil components modulate physical characteristics and function of the natural oil emulsions as drug or gene delivery system. J Control Rel. 2001;71:339–50. doi: 10.1016/s0168-3659(00)00363-1. [DOI] [PubMed] [Google Scholar]
- [28].Hilleman MR. Critical appraisal of emulsified oil adjuvants applied to viral vaccines. Progr Med Virol. 1966;8:131–82. [PubMed] [Google Scholar]
- [29].Hilleman MR. Personal historical chronicle of six decades of basic and applied research in virology, immunology, and vaccinology. Immunol Rev. 1999;170:7–27. doi: 10.1111/j.1600-065x.1999.tb01325.x. [DOI] [PubMed] [Google Scholar]
- [30].Rosenberg SA, Yang JC, Kammula US, Hughes MS, Restifo NP, Schwarz SL, et al. Different adjuvanticity of incomplete Freund’s adjuvant derived from beef or vegetable components in melanoma patients immunized with a peptide vaccine. J Immunother. 2010;33:626–9. doi: 10.1097/CJI.0b013e3181dac9de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Tengerdy RP, Lacetera NG. Vitamin E adjuvant formulations in mice. Vaccine. 1991;9:204–6. doi: 10.1016/0264-410x(91)90155-y. [DOI] [PubMed] [Google Scholar]
- [32].Morel S, Diderlaurent A, Bourguignon P, Delhaye S, Baras B, Jacob V, et al. Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29:2461–73. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- [33].Gupta PK, Cannon JB. Emulsions and microemulsions for drug solubilization and delivery. In: Liu R, editor. Water-Insoluble Drug Formulation. Interpharm Press; Denver, CO: 2000. pp. 169–211. [Google Scholar]
- [34].Woodard LF, Jasman RL. Stable oil-in-water emulsions: preparation and use as vaccine vehicles for lipophilic adjuvants. Vaccine. 1985;3:137–44. doi: 10.1016/0264-410x(85)90063-5. [DOI] [PubMed] [Google Scholar]
- [35].Ribi E, Anacker RL, Brehmer w, Goode G, Larson CL, List RH, et al. Factors influencing protection against experimental tuberculosis in mice by heat-stable cell wall vaccines. J Bacteriol. 1966;92:869–79. doi: 10.1128/jb.92.4.869-879.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Yarkoni E, Rapp HJ. Influence of type of oil and surfactant concentration on the efficacy of emulsified Mycobacterium bovis BCG cell walls to induce tumor regression in guinea pigs. Infect Immun. 1980;28:881–6. doi: 10.1128/iai.28.3.881-886.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Reynolds JA, Harrington DG, Crabbs CL, Peters CJ, Di Luzio NR. Adjuvant activity of a novel metabolizable lipid emulsion with inactivated viral vaccines. Infect Immun. 1980;28:937–43. doi: 10.1128/iai.28.3.937-943.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lasarow RM, Williams DL, Theis JH. Humoral responses following immunization with Leishmania infantum (ex. Oklahoma): a comparison of adjuvant efficacy in the antibody responses of Balb-C mice. Int J Immunopharmacol. 1992;14:767–72. doi: 10.1016/0192-0561(92)90074-u. [DOI] [PubMed] [Google Scholar]
- [39].Jansen T, Hofmans MPM, Theelen MJG, Manders F, Schijns VEJC. Structure- and oil type-based efficacy of emulsion adjuvants. Vaccine. 2006;24(26):5400–5. doi: 10.1016/j.vaccine.2006.03.074. [DOI] [PubMed] [Google Scholar]
- [40].Jansen T, Hofmans MPM, Theelen MJG, Schijns VEJC. Structure-activity relations of water-in-oil vaccine formulations and induced antigen-specific antibody responses. Vaccine. 2005;23:1053–60. doi: 10.1016/j.vaccine.2004.08.023. [DOI] [PubMed] [Google Scholar]
- [41].Allison AC. Squalene and squalane emulsions as adjuvants. Methods. 1999;19(1):87–93. doi: 10.1006/meth.1999.0832. [DOI] [PubMed] [Google Scholar]
- [42].Tsujimoto M, Kotani S, Okunaga T, Kubo T, Takada H, Kubo T, et al. Enhancement of humoral immune responses against viral vaccines by a non-pyrogenic 6-O-acylmuramyldipeptide and synthetic low toxicity analogues of lipid A. Vaccine. 1989;7:39–48. doi: 10.1016/0264-410x(89)90009-1. [DOI] [PubMed] [Google Scholar]
- [43].Retzinger GS, Meredith SC, Takayama K, Hunter RL, Kezdy FJ. The role of surface in the biological activities of trehalose 6,6′-dimycolate. J Biol Chem. 1981;256:8208–16. [PubMed] [Google Scholar]
- [44].Brito LA, Chan M, Baudner B, Gallorini S, Santos G, O’Hagan DT, Singh M. An alternative renewable source of squalene for use in emulsion adjuvants. Vaccine. 2011;29:6262–8. doi: 10.1016/j.vaccine.2011.06.067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.