Abstract
Specific activation of serotonin (5-HT) 5-HT2C G protein-coupled receptors may be therapeutic for obesity and neuropsychiatric disorders. Mutagenesis coupled with computational and molecular modeling experiments based on the human β2 adrenergic receptor structure were employed to delineate the interactions of different ligands at human 5-HT2C residues D3.32, S3.36 and Y7.43. No binding of the tertiary amine radioligand ([3H]-mesulergine) could be detected when the 5-HT2C D3.32 residue was mutated to alanine (D3.32A). The S3.36A point-mutation greatly reduced affinity of primary amine ligands, modestly reduced affinity of a secondary amine, and except for the 5-HT2C-specific agonist N(CH3)2-PAT, affinity of tertiary amines was unaffected. Molecular modeling results indicated that the primary amines form hydrogen bonds with the S3.36 residue, whereas, with the exception of N(CH3)2-PAT, tertiary amines do not interact considerably with this residue. The Y7.43A point-mutation greatly reduced affinity of 5-HT, yet reduced to a lesser extent the affinity of tryptamine that lacks the 5-hydroxy moiety present in 5-HT; modeling results indicated that the 5-HT 5-hydroxy moiety hydrogen bonds with Y7.43 at the 5-HT2C receptor. Additional modeling results showed that 5-HT induced a hydrogen bond between Y7.43 and D3.32. Finally, modeling results revealed two low-energy binding modes for 5-HT in the 5-HT2C binding pocket, supporting the concept that multiple agonist binding modes may stabilize different receptor active conformations to influence signaling. Ligand potencies for modulating WT and point-mutated 5-HT2C receptor-mediated phospholipase C activity were in accordance with the affinity data. Ligand efficacies, however, were altered considerably by the S3.36A mutation only.
Keywords: G protein-coupled receptor, molecular modeling, mutagenesis, serotonin 2C receptor
1. Introduction
Some of the diverse neurophysiological functions of serotonin (5-hydroxytryptamine, 5-HT) are mediated by the 5-HT2 G protein-coupled receptors (GPCRs), 5-HT2A, 5-HT2B, and 5-HT2C. The human 5-HT2C receptor (Saltzman et al., 1991), expressed with apparent exclusivity in the central nervous system, regulates ingestive behavior and is proposed to be involved in the pathophysiology of schizophrenia, psychostimulant abuse, and other neuropsychiatric disorders (Bubar and Cunningham, 2008; Jensen et al., 2010; Millan, 2005; Tecott et al., 1995).
Recently, (1R, 3S)-(−)-trans-1-phenyl-3-N,N-dimethylamino-1,2,3,4-tetrahydronaphthalene (N(CH3)2-PAT) was reported as the first 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist activity (Booth et al., 2009), and suggests a class of ligands suitable for pharmacotherapeutic development. In contrast, selective 5-HT2C agonists that also activate 5-HT2B and/or 5-HT2A receptors, may produce cardiopulmonary toxicity (Fitzgerald et al., 2000; Launay et al., 2002; Rothman et al., 2000) and/or hallucinations (Nichols, 2004). For example, lorcaserin is a selective 5-HT2C agonist that also activates 5-HT2A and 5-HT2B receptors (Thomsen et al., 2008) and produces “mood and perceptual adverse events” in humans at a dose four times higher than the recommended anorexient dose (Arena Pharmaceuticals, 2010).
The molecular determinants that govern the receptor-specific function of N(CH3)2-PAT at 5-HT2 subtypes are not yet clear. It is established that the basic amine moiety of most ligands interacts with the fully-conserved aspartate residue at position 3.32 (D3.32) of aminergic G protein-coupled receptors (Ballesteros et al., 2001a; Kristiansen et al., 2000). In 5-HT2 receptors, there is also a conserved serine residue, S3.36, approximately one helical turn from D3.32. When the 5-HT2A S3.36 residue is mutated to alanine (S3.36A), affinity of 5-HT, a primary amine, is reduced ~20-fold, whereas affinity of tertiary amines is barely affected. Together with molecular modeling based on homology to the bovine rhodopsin structure, these data suggest the 5-HT2A S3.36 residue is important for hydrogen bond interactions with primary amines, but not for tertiary amine ligands, likely due to steric hindrance of N-alkyl groups (Almaula et al., 1996; Ebersole et al., 2003).
Molecular interactions between the ligand amine moiety and the 5-HT2C receptor are less understood. Experiments here used a mutagenesis approach coupled with molecular modeling based on homology to the human β2 adrenergic receptor structure (Rasmussen et al., 2007) to characterize molecular determinants for ligand binding at 5-HT2C receptors. To probe ligand amine interactions, affinity of the tertiary amine N(CH3)2-PAT (Fig. 1), its secondary and primary amine analogs (NH(CH3)-PAT and NH2-PAT, respectively, Fig. 1), as well as, reference primary and tertiary amines were assessed at the human, nonedited 5-HT2C-INI (WT) receptor in comparison to the corresponding S3.36A point-mutated receptor. 5-HT2C receptor-mediated phospholipase C signaling data were also collected for class representative ligands. When building the 5-HT2C receptor model, it was observed that amino acid Y7.43 orients in the putative binding pocket such that it could hydrogen bond with a ligand amine moiety. Thus, we extended studies to include the Y7.43A point-mutated 5-HT2C receptor. The critical role of D3.32 in ligand amine binding was confirmed using the D3.32A point-mutated 5-HT2C receptor.
Fig. 1.
Structures of test compounds. Circled on each compound is the basic nitrogen atom that bonds with the D3.32 residue of the 5-HT2C receptor.
2. Materials and methods
2.1 DNA constructs and transfection of HEK cells
Amino acids were numbered according to the convention proposed for aminergic neurotransmitter G protein-coupled receptors (Ballesteros et al., 2001b). The cDNA encoding the human, 5-HT2C–INI (WT) receptor was obtained from UMR cDNA Resource Center (Rolla, MO). PCR-based methods were used to create the expression constructs of the S3.36A and Y7.43A point-mutated receptors based on the template WT 5-HT2C receptor. The PCR primers (sense and antisense) used were, 5'-gccccgtctggatttctttagctgttttattttcaacagcg-3' and 5’-gccccgtctggatttctttagatgttttattttcaacagcg-3’ and 5'-cgctgttgaaaataaaacagctaaagaaatccagacggggc-3' for the D3.32A mutation, 5'-ccgtctggatttctttagatgttttatttgcaacagcgtccatcat-3' and 5'-atgatggacgctgttgcaaataaaacatctaaagaaatccagacgg-3' for the S3.36A mutation, and, 5'-tgaatgtgtttgtttggattggcgctgtttgttcaggaatcaatcctc-3' and 5'-gaggattgattcctgaacaaacagcgccaatccaaacaaacacattca-3' for the Y7.43A mutation. The PCR primers (sense and antisense) used were, 5'-gccccgtctggatttctttagctgttttattttcaacagcg-3' and 5’-gccccgtctggatttctttagatgttttattttcaacagcg-3’ for the D3.32A mutation, 5'-ccgtctggatttctttagatgttttatttgcaacagcgtccatcat-3' and 5'-atgatggacgctgttgcaaataaaacatctaaagaaatccagacgg-3' for the S3.36A mutation, and, 5'-tgaatgtgtttgtttggattggcgctgtttgttcaggaatcaatcctc-3' and 5’-tgaatgtgtttgtttggattggctatgtttgttcaggaatcaatcctc-3’ for the Y7.43A mutation. The cDNA sequences were verified using the dideoxy chain termination method. PCR was carried out using the QuikChange Kit (Stratagene, Santa Clara, CA) with WT 5-HT2C in the pcDNA3.1+ vector as the template, primers with designated mutations, and a thermocycle of initial denaturing at 95°C for 30 sec, followed by 16 cycles of 95°C for 30 sec, 70°C for 1 min, and 68°C for 3 min. The WT parental DNA was digested by DpnI. PCR products were used to transform XL1-Blue Supercompetent Cells. A single colony was sequenced to confirm the designed mutation. HEK-293 cells, 50–80% confluent in a 10 cm plate, were transfected with the isolated DNA constructs by mixing 8–16 µg of construct DNA with 12–16 µl Lipofectamine 2000 (Invitrogen, Carlsbad, CA). Expression of receptors was confirmed by Western-blots (data not shown).
To generate receptors for radioligand binding studies, HEK-293 cells were grown to 90% confluency in DMEM (10-013-CV, Mediatech, Manassas, VA) supplemented with 5% dialyzed fetal bovine serum, 100 I.U./mL penicillin and 100 µg/mL streptomycin (30-0020-CI, Mediatech) in 10 cm plates. Cells were washed 1× with phosphate-buffered saline, and then transfected with 24 µg of human 5-HT2C WT, S3.36A or Y7.43A DNA mixed with 33 µl Lipofectamine 2000 reagent in Opti-MEM and placed in an incubator at 37°C, 5% CO2, 95% humidity for 24 to 48 hr. Membranes were then collected in 50 mM Tris, 10 mM MgCl2-6H2O, 0.1 mM EDTA (assay buffer) using previous methods (Booth et al., 2009) and stored at −80° Celsius until binding assays were performed.
2.2 Compounds
The radioligand [3H]-mesulergine (specific activity 92 Ci/mmol) was purchased from Perkin-Elmer. Serotonin hydrochloride was purchased from Alfa Aesar (Ward Hill, MA), tryptamine hydrochloride and mianserin hydrochloride was purchased from Sigma-Aldrich (St. Louis, MO), mesulergine hydrochloride was purchased from Tocris (Ellisville, MO), and lisuride hydrogen maleate was a gift from Dr. Elaine Sanders-Bush (Vanderbilt University, Nashville, TN). Synthesis of the primary, secondary, and tertiary amine analogues of (1R, 3S)-(±)-trans-1-phenyl-3-N,N-dimethylamino-1,2,3,4-tetrahydronaphthalene was performed according to previous laboratory methods (Booth et al., 2009; Bucholtz et al., 1999) to obtain the hydrochloride salt of the trans-stereoisomers (ligand docking studies considered only (1R, 3S)-(−) enantiomer). Ligand structures are given in Fig. 1.
2.3 Radioligand binding assay
Radioligand saturation isotherm and competitive displacement binding assays were performed in 96-well plates, using 3–5 µg of protein per well from membrane samples of HEK cells, similar to laboratory methods used previously (Booth et al., 2009). All binding experiments were performed using duplicates of samples, and each independent binding experiment was repeated a minimum of three times (with an average of six times). Briefly, 0.02 to 8 nM [3H]-mesulergine was used for saturation binding assays to obtain KD and Bmax values, and 1–2 nM [3H]-mesulergine (ca. KD) was used for competition binding assays to obtain Ki values for unlabeled ligands. Mianserin hydrochloride (10 µM) was used to define non-specific binding. After a 90 min equilibration period at room temperature, incubation mixtures were rapidly passed through GF/B filters using a Mach 2 cell harvester (Tomtec, Hamden, CT) and subsequently washed five times with 50 mM Tris-HCl at room temperature. Filters containing bound [3H]-mesulergine were dried, placed in vials containing 2 mL scintillation cocktail (ScintiVerse, Fisher), allowed to equilibrate overnight, and then were counted for 3H-induced scintillation using a Beckman-Coulter LS6500 counter.
2.4 Phosphoinositide hydrolysis assay
HEK cells grown to approximately 80% confluency in DMEM containing 10% fetal bovine serum and 1% antibiotic in 10 cm plates at 37°C, 5% CO2 (incubator) were washed one time with PBS, then transfected with 20 µg 5-HT2C pcDNA (5-HT2C-INI, 5-HT2C-INI S3.36A, or 5-HT2C-INI Y7.43A), and 30 µl lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA) in 5 mL DMEM containing 5% dialyzed fetal bovine serum and 5 mL Opti-MEM (transfection media). Cells were placed in an incubator, and approximately 16 hr. later, transfection media was removed and replaced with 16 mL inositol-free DMEM containing 2.5% dialyzed fetal bovine serum. Cells were then detached by vigorous pipetting, 0.1 µCi/mL [3H]-myo-inositol was added to the mixture (labeling media), and cells were seeded 300 µl per well into 48 well CellBind® plates (Corning, Lowell, MA), and placed in an incubator. 24 hr. later, plates were centrifuged at 2500 r.p.m. for 10 min. at room temperature, labeling media was discarded, and 450 µL inositol-free, serum-free DMEM was added to each well. Cells were placed in an incubator for one hour. Cells were then incubated for 30 min. with increasing concentrations of compounds (1 nM to 10 µM), 5-HT, N(CH3)2-PAT, or mesulergine, diluted in inositol-free, serum-free DMEM containing a final concentration of 50 mM LiCl and 10µM pargyline per well. Plates were again centrifuged at 2500 r.p.m. for 10 min. at room temperature, drug incubation media was discarded, and 400 µl of 50 mM formic acid was added to each well to lyse cells. one hour later, 200 µl of 150 mM NH4OH was added to each well to neutralize cells, and plates were stored at −20°C overnight. After thawing, 500 µl of solution from each well was added to individual anion-exchange columns to separate [3H]-inositol phosphates formed from [3H]-myo-inositol. Following a 10 mL wash with dH2O, bound [3H]-inositol phosphates were eluted with 4 mL 800 mM ammonium formate into vials. 1 mL of eluate was added to 10 mL scintillation fluid (ScintiVerse Cocktail, Fisher). After mixing, 3H-induced scintillations were counted with a Beckman-Coulter LS6500 counter. Experiments were performed with duplicates for 5-HT and triplicates for N(CH3)2-PAT and mesulergine, and each independent experiment was performed a minimum of three times.
2.5 Statistical analysis
Saturation and competition, radioligand binding data were analyzed using nonlinear regression curve-fitting algorithms in GraphPad Prism, 5.03 for Windows (San Diego, CA). Data points were limited (six to eight points per experiment), thus Hill slopes were not calculated (Motulsky and Christopoulos, 2003); data were fit using the “one site fit-Ki” model that constrains the Hill slope to 1.0. Two-site curve-fitting did not result in an improved fit (data not shown). Ligand affinity is expressed as an approximation of Ki values by conversion of the IC50 data using the equation Ki = IC50/1 + L/KD where L is the concentration of radioligand (Cheng and Prusoff, 1973). KD values for [3H]-mesulergine at 5-HT2C WT, S3.36A and Y7.43A receptors determined experimentally were used in calculating Ki values of cold ligands tested. Analysis of variance tests were performed for each ligand, comparing Ki or KD (affinity), EC50 or IC50 (potency), and EMAX or IMAX (efficacy) values obtained at WT 5-HT2C, S3.36A and Y7.43A receptors. Tukey’s multiple comparison post-hoc tests were performed when one-way analysis of variance tests resulted in P values less than 0.05, and tested for statistical differences in affinities, potencies, or efficacies at WT compared to S3.36A, WT compared to Y7.43A, and S3.36A compared to Y7.43A 5-HT2C receptors.
2.6 Homology modeling
The 3-D molecular models of the human WT and D3.32A, S3.36A, and Y7.43A point-mutated 5-HT2C receptor were constructed by homology modeling based on the crystal structure of the human β2 adrenergic receptor/T4-lysozyme chimera (Protein Data bank entry 2rh1) (Rasmussen et al., 2007). The 5-HT2C WT native sequence was aligned to the β2 adrenergic receptor sequence using Clustal W multiple sequence alignment (Larkin et al., 2007). The transmembrane domains (without T4L) were built using the Biopolymer module of Sybyl 8.1 (Tripos, St. Louis, MO), and point-mutations, additions and deletions were performed as needed according to the native amino acid sequence of the receptor after alignment. The inverse agonist carazolol, present in the crystal structure of the human β2 adrenergic receptor (2rh1) was deleted, and the resulting seven transmembrane domain bundle was optimized using Tripos force field and atomic charges. Then, the 5-HT2CN-terminus, C-terminus, extracellular and intracellular loop residues were added. The conserved bond formed between the cysteine amino acids C3.25 (at the end of transmembrane domain3) and C6.31 (in extracellular loop 3) present in the β2 adrenergic receptor structure was included in the 5-HT2C model. Water molecules present in the β2 adrenergic receptor structure were not incorporated in the 5-HT2C receptor model.
The crude models were minimized using the Powell method implemented in Sybyl with Tripos force field and atomic charges. The resulting model was inserted into a rectangular box containing a pre-equilibrated 1-palmitoyl-2-oleyl-sn-glycero phosphatidyl choline (POPC) bilayer (Heller, 1993), consisting of 200 lipid molecules, forming a rectangular patch, and 5,483 water molecules covering the head groups on each side of the bilayer. The system containing the 5-HT2C WT receptor model within the simulated membrane contained 37,775 atoms. The system was minimized initially using the Tripos force field to a convergence 0.05 Kcal/Å mol prior to molecular dynamics simulation in the PoPC membrane for 5000 ps, time step 1 fs, with snapshots collected every 5 fs. Run parameters used the NVT canonical ensemble, 300K temperature, Boltzmann initial velocities, with non-bonded cutoff set at 8 Å. Constraints for alpha carbons in the transmembrane domain were used. Finally the constraints were removed in 1000 ps runs, and the final structure was obtained from the average of the last 10 ps of the simulation, optimized using the Tripos force field to a convergence of 0.05 Kcal/Åmol.
2.7 Ligand docking and molecular dynamics simulations
Flexible ligand docking was performed with the FlexiDock utility in Sybyl 8.1 that uses an algorithm to probe the conformational space defining possible interactions between the ligand and its putative binding site. Ligand structures were built as monocations using HyperChem 8.0.8 and optimized using a PM3 model. The binding site was defined using residue D3.32 as definitive binding site interaction, and residues within a 7 Å radius (Kristiansen and Dahl, 1996; Kristiansen et al., 2000). Structures were prepared assigning amber atom types to both the receptor and the ligands. Amber charges were used for the protein and Gesteiger-Marsili charges for the ligand. The ligand was pre-positioned into the putative binding site, and the position of the ligand was refined prior to docking to ensure the interaction between the carboxylate oxygen at D3.32 and the protonated amine of the ligand, an interaction necessary for protonated amine ligands to bind to G protein-coupled receptors (Kristiansen and Dahl, 1996; Kristiansen et al., 2000). All rotatable bonds in the ligand and the side chains of the residues defining the active site were screened for optimal positioning of the ligand and the side chains in the conformational space. Default FlexiDock parameters were set at 80,000-generation, and the best docking solution, according to the highest FlexiDock score, was minimized using the Amber force field.
Using the selected high-score pose of the docked ligand, molecular dynamics was performed. Simulation times were 500 ps, with snapshots collected every 5 fs. Run parameters used the NVT cannonical ensemble, 300K temperature, Boltzmann initial and scaling velocities, with non-bonded cutoff set at 8 Å. The final structure of the ligand docked into the receptor was obtained from the last 10 ps of the dynamics simulation and optimized as described above.
2.8 Quantum mechanics model
Partial ligand–receptor models for the two poses of 5-HT in the 5-HT2C binding pocket were built for quantum mechanics calculations. These models utilized the 5-HT2C binding pocket–ligand structure extracted from the full receptor models docked with one or the other of the two 5-HT poses. Missing valences of amino acids resulting from breaking of peptide bonds during the extraction process were restored by adding the acetyl (COCH3) moiety to the N-terminus and the methyl amino (NHCH3) moiety to the C terminus. The structures were optimized using the semi-empirical PM3 Hamiltonian model to a gradient of 0.01 Kcal/mol to obtain the final energies, constraining the alpha-carbon atoms of the residues to preserve the configuration of the binding pocket.
3. Results
3.1 Affinity of ligands at S3.36A and Y7.43A point-mutated 5-HT2C receptors
Table 1 shows the mean and S.E.M. KD values derived from saturation binding isotherms of [3H]-mesulergine labeled WT, S3.36A and Y7.43A 5-HT2C receptors. No specific binding of [3H]-mesulergine to the D3.32A point-mutated 5-HT2C receptor was detected (not shown), consistent with results previously reported (Muntasir et al., 2006). Affinity of [3H]-mesulergine at the WT, S3.36A and Y7.43A point-mutated receptors was not significantly different (mean (S.E.M.) KD values = 1.8 (0.3) WT, 2.0 (0.4) S3.36A, and 3.0 (0.6) Y7.43A; F(2, 10)=2.14, P = 0.18). The Bmax values varied across experiments, and generally were 2–4 times higher for WT versus the point-mutated 5-HT2C receptors. The highest Bmax values for WT, S3.36A and Y7.43A receptors were 16.7, 7.6 and 2.6 pmol/mg of protein, respectively.
Table 1.
Affinity of [3H]-mesulergine for WT, S3.36A and Y7.43A 5-HT2C receptors. KDvalues were derived from saturation binding isotherms of [3H]-mesulergine at WT, S3.36A, and Y7.43A 5-HT2C point-mutated receptors expressed in HEK cells. Data shown are means and S.E.M. (in parentheses) of at least 3 independent experiments performed with triplicates.
| Receptor | [3H]-Mesulergine |
|---|---|
| Kd (nM) | |
| WT 5-HT2C | 1.8 (0.3) |
| S3.36A | 2.0 (0.4) |
| Y7.43A | 3.0 (0.6) |
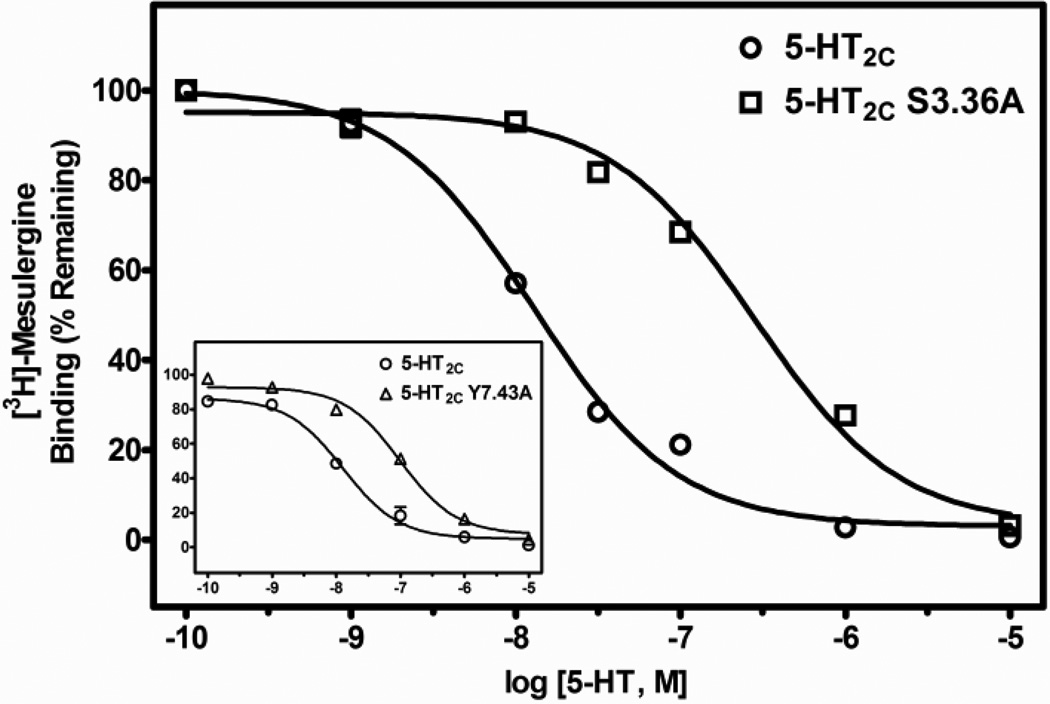
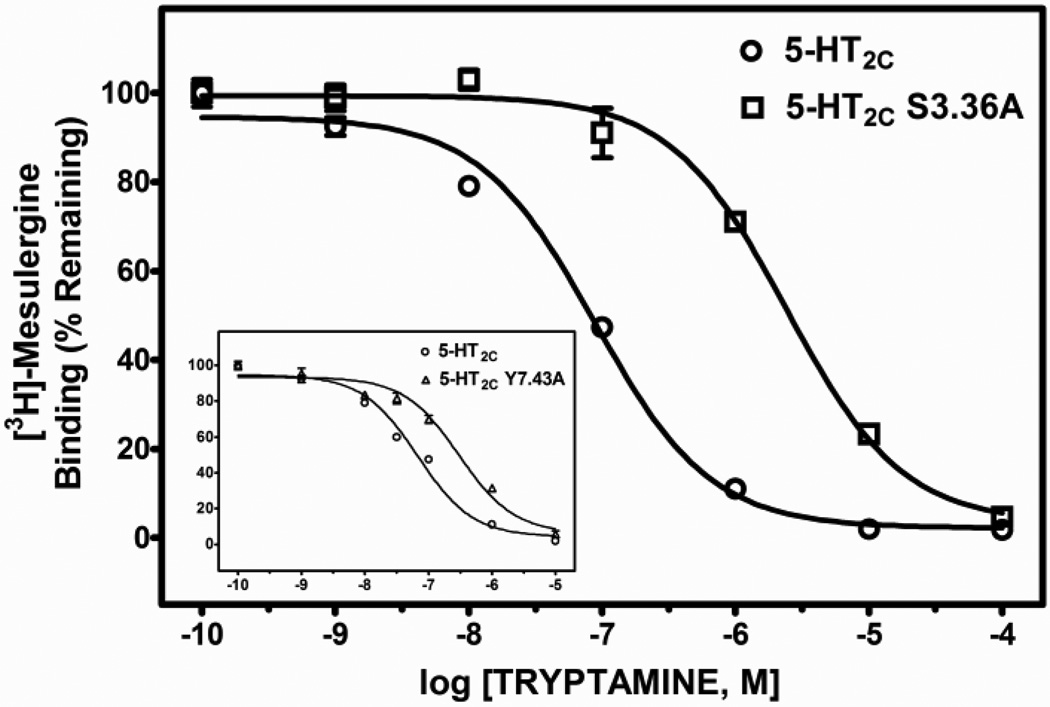
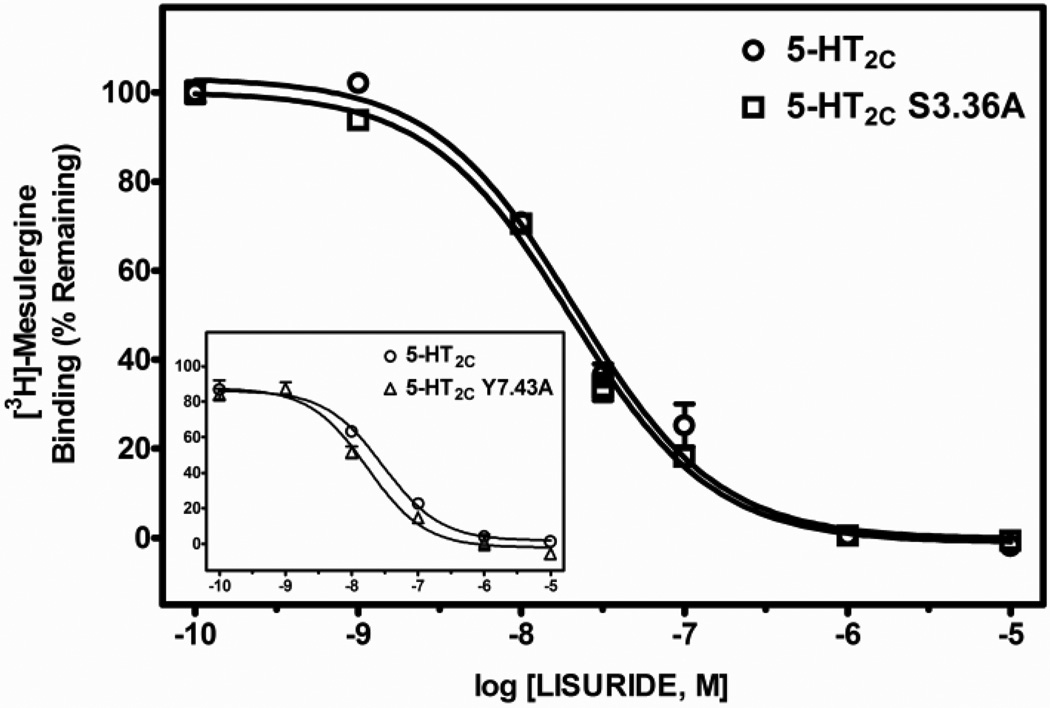
Table 2 shows the mean and S.E.M. of Ki values, as well as fold-differences in Ki values (mean Ki at the point-mutated receptor divided by the mean Ki at the WT receptor) derived from test ligand competitive inhibition of [3H]-mesulergine binding at WT, S3.36A and Y7.43A point-mutated 5-HT2C receptors expressed in HEK cells. The affinity of the primary amine 5-HT for the receptors was significantly different (F(2, 10) = 23.8, P < 0.001). The affinity of 5-HT was significantly less, approximately 13- and 17-times lower, at S3.36A and Y7.43A point-mutated 5-HT2C receptors, respectively, versus the WT receptor (post-hoc P values < 0.05). The affinity of tryptamine was also significantly different at WT and point-mutated receptors (F(2, 12) 133.6, P < 0.001). The affinity of tryptamine was significantly less, approximately 30- and 5-times lower, at S3.36A and Y7.43A receptors, respectively, versus the WT receptor (post-hoc P values < 0.05). In contrast, affinity of the tertiary amine lisuride at the S3.36A receptor was not significantly different from its affinity at the WT receptor (post-hoc P value > 0.05), but the affinity of lisuride was slightly, but significantly increased at the Y7.43A point-mutated receptor (post-hoc P values < 0.05) relative to the WT receptor (F(2, 12) = 11.2, P < 0.05). Representative competition binding curves for 5-HT, tryptamine and lisuride are shown in Figs. 2, 3 and 4.
Table 2.
Affinities of ligands for human WT, S3.36A, and Y7.43A 5-HT2C receptors. Ki values were derived from test ligand competitive inhibition of [3H]-mesulergine binding at the WT, S3.36A and Y7.43A point-mutated 5-HT2C receptors expressed in HEK cells. Data shown are means and S.E.M. (shown in parentheses) of at least 3 independent experiments performed with duplicates. Fold change represents the quantitative fold difference between Ki values of WT and S3.36A or WT and Y7.43A 5-HT2C receptors, respectively.
| LIGAND | WT 5-HT2C | S3.36A | Y7.43A | FOLD CHANGE |
|---|---|---|---|---|
| Ki(nM)Ki(nM) | Ki(nM) | |||
| Serotonin | 9.0 (1.6) | 118.2 (21.7)a | 152.0 (37.0)a | 13.1, 16.9 |
| Tryptamine | 57.1 (7.1) | 1513 (107.4)a | 298.5 (72.8)a | 29.5, 4.5 |
| Lisuride | 11.4 (0.6) | 11.3 (0.4) | 7.9 (0.5)a | 1.0, 0.7 |
| N(CH3)2-PAT | 78.4 (9.6) | 210 (21.9)a | 354 (46.8)a | 2.7, 4.5 |
| NHCH3-PAT | 145.1 (16.7) | 636.1 (69.6)a | 357.3 (45.6)a | 4.4, 2.5 |
| NH2-PAT | 1555 (146.6) | 9037 (820)a | 1353 (170.3) | 5.8, 0.9 |
significantly different from WT 5-HT2C.
Fig. 2.
Representative radioligand competition displacement curves for the primary amine 5-HT at WT vs. S3.36A and Y7.43A (inset) point-mutated 5-HT2C receptors. Data show means and S.E.M. from an individual experiment of percent specific binding of [3H]-mesulergine. The curves for the point-mutated receptors are significantly (P < 0.05) shifted to the right compared to the WT receptor.
Fig. 3.
Representative radioligand competition displacement curves for the primary amine tryptamine at WT vs. S3.36A and Y7.43A (inset) point-mutated 5-HT2C receptors. Data show means and S.E.M. from an individual experiment of percent specific binding of [3H]-mesulergine. The curves for the point-mutated receptors are significantly (P < 0.05) shifted to the right compared to the WT receptor.
Fig. 4.
Representative radioligand competition displacement curves for the tertiary amine lisuride at WT vs. S3.36A and Y7.43A (inset) point-mutated 5-HT2C receptors. Data show means and S.E.M. from an individual experiment of percent specific binding of [3H]-mesulergine. There is no difference (P = 0.12) between the curves for the WT and S3.36A receptor. Lisuride, however, showed a slight, yet significant (P < 0.05) enhancement in affinity for the Y7.43A relative to the WT receptor.
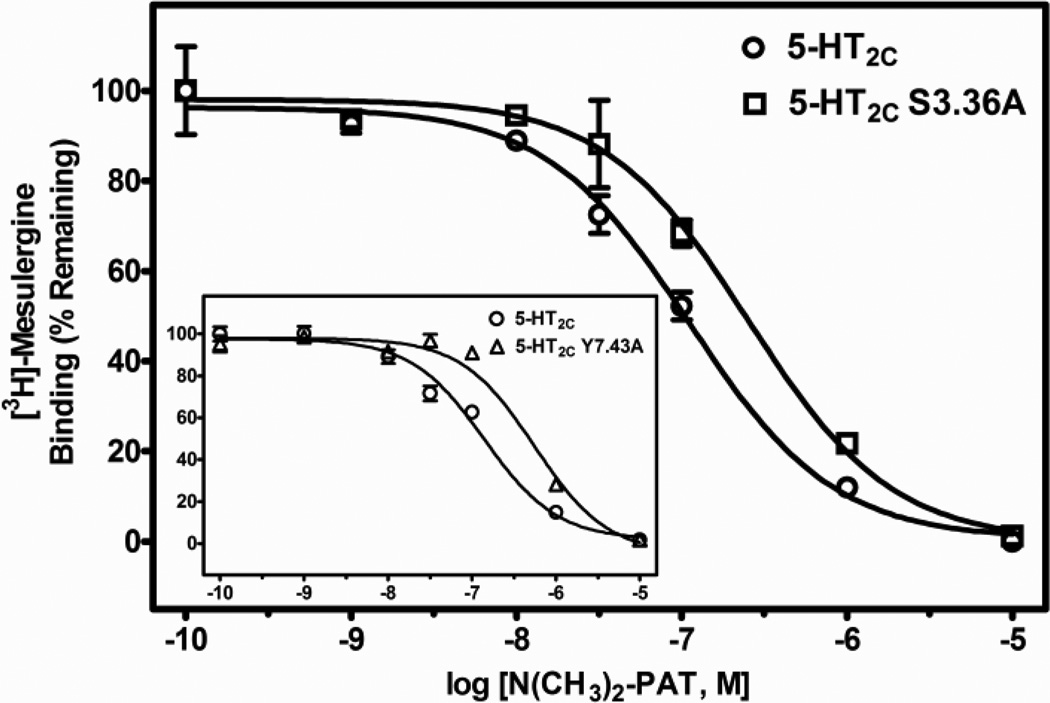
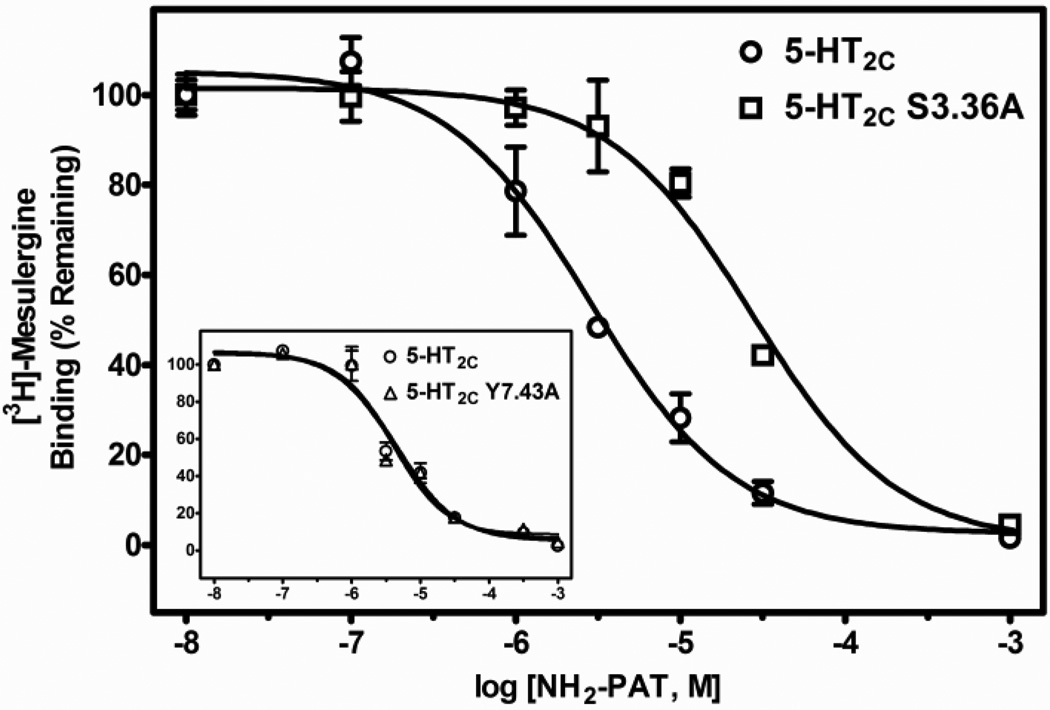
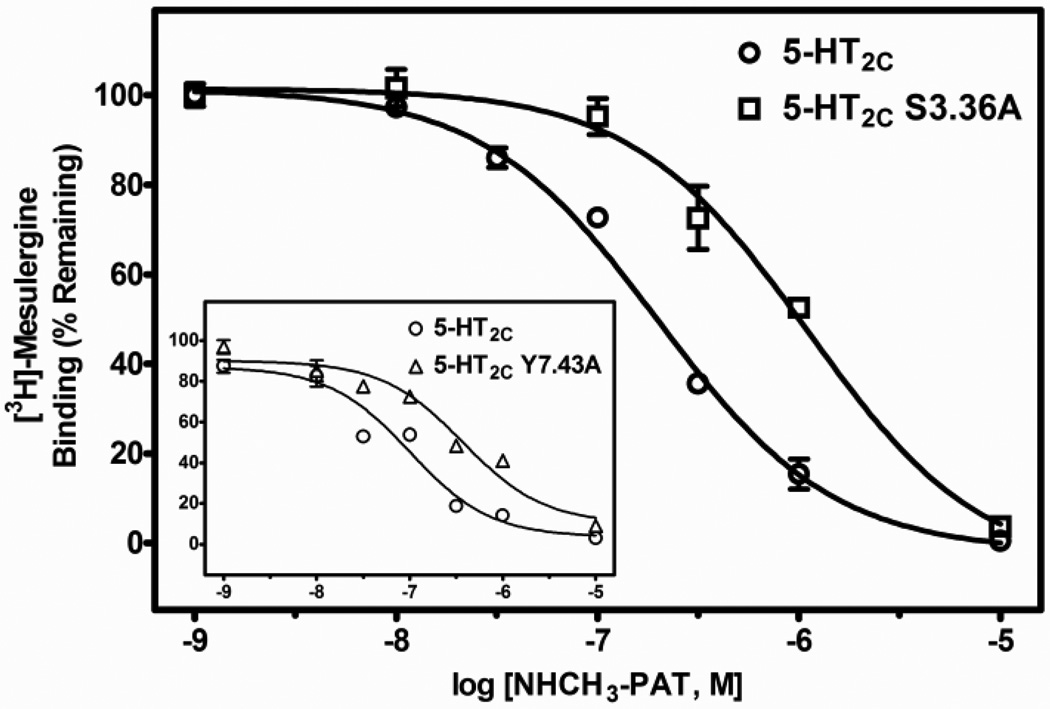
Regarding the PAT analogs, relative to WT 5-HT2C receptors, the affinity of the tertiary amine N(CH3)2-PAT was significantly lower at both the S3.36A (post-hoc P value < 0.05, about 3-times lower) and the Y7.43A (post-hoc P values < 0.05, about 5-times lower) point-mutated receptors (F (2, 21) = 35.3, P < 0.001). Affinity of the secondary amine NH(CH3)-PAT was also significantly different at WT and point-mutated receptors (F (2, 29) = 41.4, P < 0.001), having about 4- and 3-times lower affinity for the S3.36A and Y7.43A receptors, respectively (post-hoc P values < 0.05). Finally, the primary amine NH2-PAT showed significant differences in affinity at WT and point-mutated receptors (F(2, 24) = 108.9, P < 0.001), but the effects on affinity of the mutations were different relative to the tertiary and primary amine PAT compounds. NH2-PAT demonstrated a significant reduction in affinity for the S3.36A point-mutated receptor relative to the WT receptor (post-hoc P value < 0.05, about 6-times lower). Affinity of NH2-PAT, however, was not affected by the Y7.43A mutation (post-hoc P value > 0.05). Representative competition binding curves for the PATs are shown in Figs. 5–7.
Fig. 5.
Representative radioligand competition displacement curves for the tertiary amine N(CH3)2-PAT at WT vs. S3.36A and Y7.43A (inset) point-mutated 5-HT2C receptors. Data show means and S.E.M. from an individual experiment of percent specific binding of [3H]-mesulergine. The curves for the point-mutated receptors are significantly (P < 0.05) shifted to the right compared to the WT receptor.
Fig. 7.
Representative radioligand competition displacement curves for the primary amine NH2-PAT at WT vs. S3.36A and Y7.43A (inset) point-mutated 5-HT2C receptors. Data show means and S.E.M. from an individual experiment of percent specific binding of [3H]-mesulergine. The curve for the S3.36A receptor is significantly (P < 0.05) shifted to the right, whereas the curve for the Y7.43A receptor is not different compared to the WT receptor.
3.2 Functional activity of ligands at S3.36A and Y7.43A point-mutated 5-HT2C receptors
WT, S3.36A and Y7.43A 5-HT2C receptors transiently expressed in HEK cells were examined for their ability to stimulate the phospholipase C signaling pathway by measuring the accumulation of [3H]-inositol phosphates from [3H]-myo-inositol following incubation of cells with either the primary amine agonist, 5-HT, the tertiary amine agonist, N(CH3)2-PAT, or the tertiary amine inverse agonist, mesulergine. Results from these studies are shown in Table 3, and include the calculated potency values, represented by half-maximal concentrations (EC50 values for agonists and IC50 values for inverse agonists), efficacy values, represented by maximal response levels (EMAX values for agonists and IMAX values for inverse agonists, representing percent change from basal counts per minute), as well as fold-differences in EC50 or IC50 values (the mean EC50/IC50 of a ligand at the point-mutated receptor divided by the mean EC50/IC50 of the ligand at the WT receptor) of ligands to alter 5-HT2C-receptor mediated inositol phosphate production.
Table 3.
EC/IC50 and E/IMAX values of ligands to activate/inactivate 5-HT2C receptor-mediated phospholipase C signaling. Functional assays were performed in HEK cells transiently expressing WT, S3.36A or Y7.43A 5-HT2C receptors. EMAX/IMAX values represent percent change from baseline. Data shown are means and S.E.M. (shown in parentheses) of at least 3 independent experiments performed with triplicates. EC/IC50 fold change represents the quantitative fold difference between EC/IC50 values of WT and S3.36A or WT and Y7.43A 5-HT2C receptors, respectively.
| LIGAND | WT 5-HT2C | S3.36A | Y7.43A | FOLD CHANGE |
|||
|---|---|---|---|---|---|---|---|
| EC/IC50 (nM) |
E/Imax | EC/IC50 (nM) |
E/Imax | EC/IC50 (nM) |
E/Imax | EC/IC50 | |
| Serotonin | 5.5 (1.3) | 204.7 (11.6) | 289.1 (40.4)a |
998.4 (137.1)b |
158.7 (19.9)a |
223.4 (22.7) | 52.6, 28.9 |
|
N(CH3)2- PAT |
61.1 (18.2) | 163.0 (9.7) | 682.5 (102.5)a |
588.0 (62.0) b |
519.7 (98.8)a |
180.8 (14.2) |
11.2, 8.5 |
| Mesulergine | 23.9 (8.3) | 82.9 (1.9) | 19.9 (8.2) | 152.0 (9.2)b | 38.9 (13.3) | 79.3 (2.5) | 0.8, 1.6 |
significantly different from WT 5-HT2C.
Results revealed that both the S3.36A and Y7.43A mutations led to significant decreases in the potency of 5-HT to stimulate production of [3H]-inositol phosphates (post-hoc P values < 0.05; F(2,7) = 29.1, P < 0.001). Paralleling the decrease in affinity of 5-HT at the point-mutated receptors, the potency of 5-HT at both point-mutated receptors was more than 25-times lower than its potency at the WT receptor. The potency of N(CH3)2-PAT to stimulate [3H]-inositol phosphate production also was significantly reduced at the point-mutated compared WT receptors (post-hoc P values < 0.05; F(2,6) = 23.0, P < 0.002). Like 5-HT, the potency of N(CH3)2-PAT at WT versus point-mutated receptors also paralleled its affinities for these receptors; relative to WT, both mutations led a greater than 8-fold reduction in potency of N(CH3)2-PAT. The potency of mesulergine was not significantly different between WT, S3.36A and Y7.43A receptors (F(2,8) = 0.9, P = 0.45), consistent with the lack of change in affinity of mesulergine at point-mutated compared to WT receptors.
The efficacy values of 5-HT were significantly different between receptors (F(2,8) = 44.6, P < 0.001). The EMAX of 5-HT at the WT receptor compared to the Y7.43A point-mutated receptor was not significantly different (post-hoc P value > 0.05). The EMAX value of 5-HT at the S3.36A point-mutated receptor, however, was significantly increased (nearly than 5-times higher) compared to the WT receptor (post-hoc P value < 0.05). Notably, the absolute values for basal and maximally stimulated [3H]-inositol phosphate accumulation (in counts per minute) from WT receptors was ~2500 and 5000, respectively, whereas, from S3.36A receptors, these values were ~800 and ~8000 (data not shown), suggesting the effect was not caused solely by reduced basal signaling of S3.36A receptors. The same trend was observed for N(CH3)2-PAT. Its efficacy was not different between WT and Y7.43A receptors (post-hoc P value > 0.05). At the S3.36A receptor, however, N(CH3)2-PAT had a significantly higher EMAX value than at the WT receptor (post-hoc P value < 0.05; F(2,6) = 81.0, P < 0.001). This was also observed in absolute values (data not shown). Relative to 5-HT, N(CH3)2-PAT behaved as a partial agonist at all three receptors. Mesulergine behaved as an inverse agonist at both the WT and Y7.43A receptors, decreasing basal signaling about 20 percent. At the S3.36A receptor, however, mesulergine behaved as a very low efficacy partial agonist, and this change from inverse agonism at WT and Y7.43A receptors to partial agonism at S3.36A receptors was statistically significant (Emax vs Imax values; post-hoc P values < 0.05; F(2,9) = 75.1).
3.3 Molecular models of ligand binding to WT and point-mutated 5-HT2C receptors١
3.3.1 5-HT and tryptamine
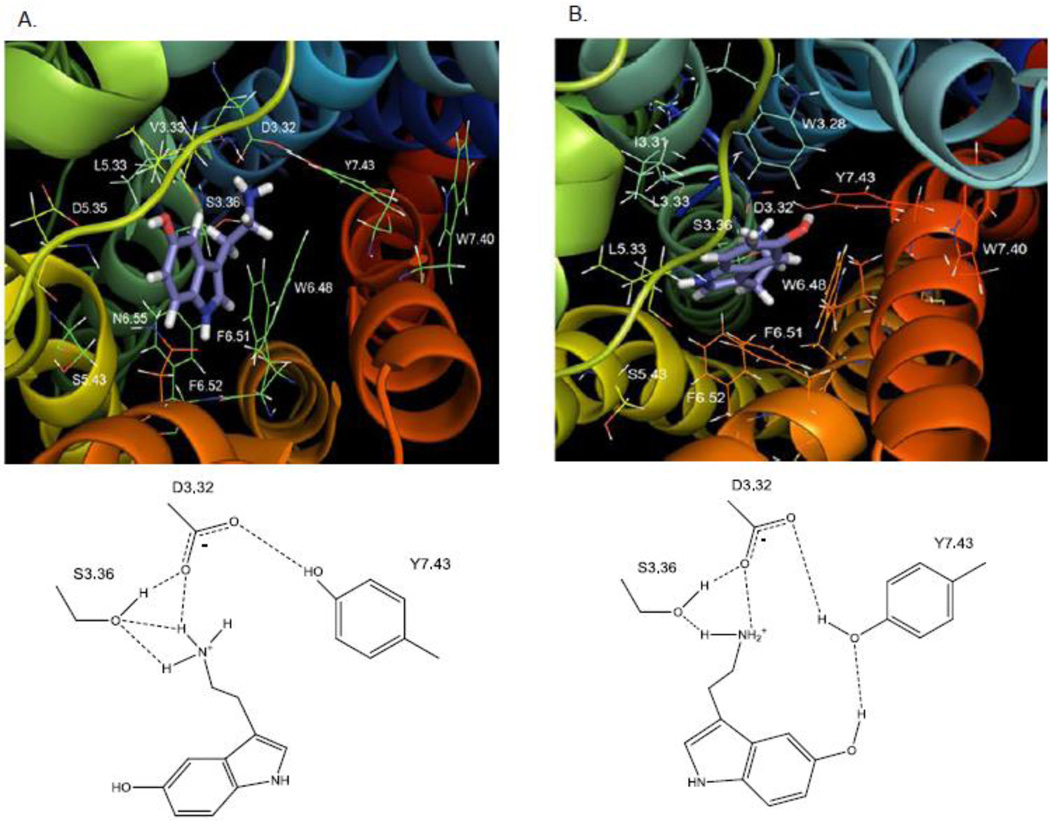
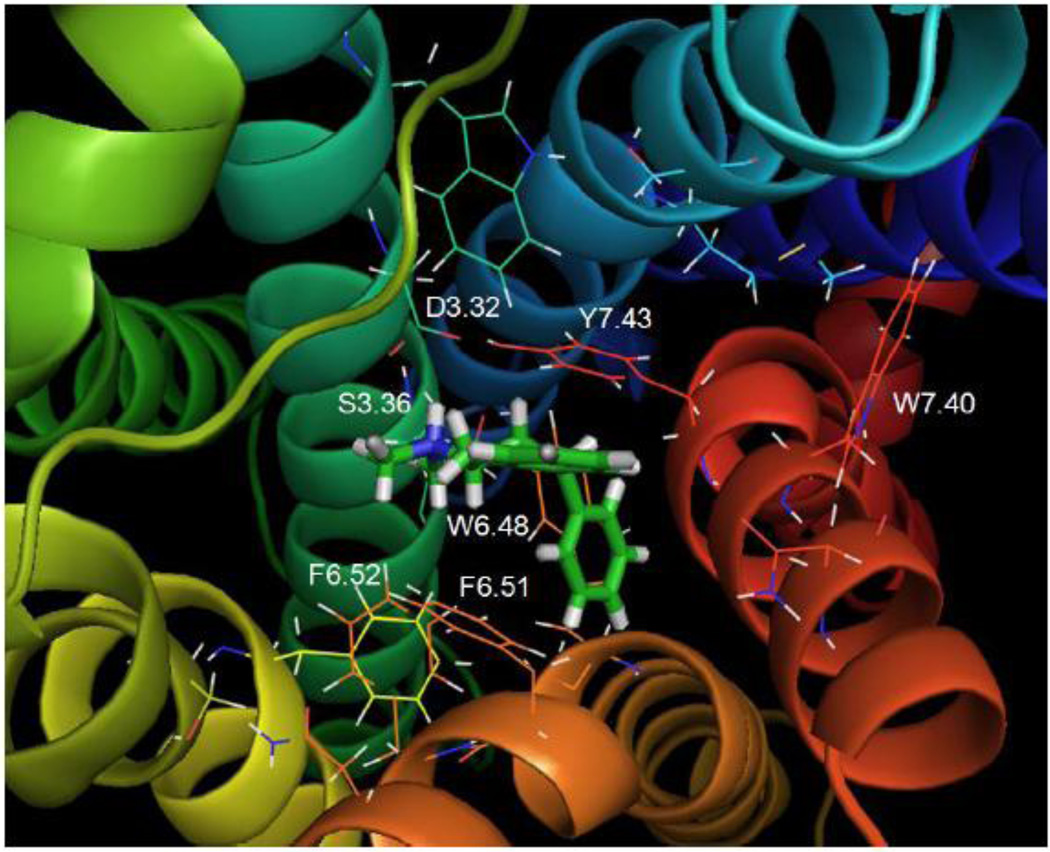
As shown in Fig. 8, ligand docking experiments revealed two interchangeable, low-energy poses for 5-HT in the putative binding pocket of the WT 5-HT2C receptor. Additional analyses using quantum mechanics calculations (described in Methods) indicated that the energy difference between structures representing modeling poses 1 and 2 was only about 34 Kcal/mol, suggesting both poses were plausible. Residues in the binding pocket in close proximity to serotonin in pose 1 (Fig. 8A) were: D3.32, V3.33, S3.36, L5.33, D5.35, W6.48, F6.51, F6.52, N6.55, V7.39, and Y7.43. Residues in close proximity to 5-HT in pose 2 (Fig. 8B) were: W3.28, I3.29, L3.31, D3.32, S3.36, L5.33, M6.47, W6.48, C6.49, V7.39, W7.40, and Y7.43. In both poses, the protonated amine group of 5-HT apparently acted as a hydrogen bond donor to the carboxylate side chain of D3.32 (1.85 Å, pose 1 and 2), to the hydroxyl group of S3.36 (2.20 Å, pose 1, and 2.08 Å, pose 2), and to the hydroxyl moiety of Y7.43 (3.05 Å pose 1, and 3.02 Å, pose 2). Consistent with these computational results, experimental results showed that the S3.36A point-mutated 5-HT2C receptor had a large reduction in affinity for 5-HT (Table 2). Also in both poses, binding of 5-HT facilitated a closer hydrogen bond interaction between the D3.32 carboxylate moiety and the Y7.43 para-hydroxy moiety (2.50 Å), whereas in the unbound (native) 5-HT2C and β2 adrenergic receptor models (not shown), the interaction between D3.32 and Y7.43 occurred over a longer distance (3.38 Å) and presumably was weaker.
Fig. 8. 5-HT.
A. Molecular model graphic pose 1 of 5-HT docked to the WT 5-HT2C receptor and directly underneath, schematic of pose 1. B. Molecular model graphic pose 2 of 5-HT docked to the WT 5-HT2C receptor and directly underneath, schematic of pose 2.
There were important differences between the two 5-HT poses. In pose 1 (Fig. 8A), the 5-HT indole moiety positioned close to transmembrane domain 6, near the indole of W6.48 (4.30 Å) and between the aromatic rings in F6.51 (3.81 Å) and F6.52 (4.61 Å), possibly forming π-π interactions. The 5-hydroxy group was near to N6.55 (4.28 Å) and D5.35 (5.31 Å), likely forming electrostatic interactions, but too far for hydrogen bonding. Finally, in pose 1, there was no apparent interaction of the 5-hydroxy group with residues in transmembrane domains 3 and 7 (e.g., D3.32, S3.36, and Y7.43). In pose 2 (Fig. 8B) the configuration of 5-HT was flipped relative to pose 1. The 5-HT indole moiety was oriented closer to transmembrane domain 7, and significantly, the 5-hydroxy group was capable of forming a hydrogen bond with Y7.43 (2.46 Å). Experimental evidence indicated that loss of this interaction and loss of the 5-HT binding-induced D3.32-Y7.43 interaction (described earlier) at the Y7.43A point-mutated 5-HT2C receptor resulted in a large reduction in 5-HT affinity (Table 2).
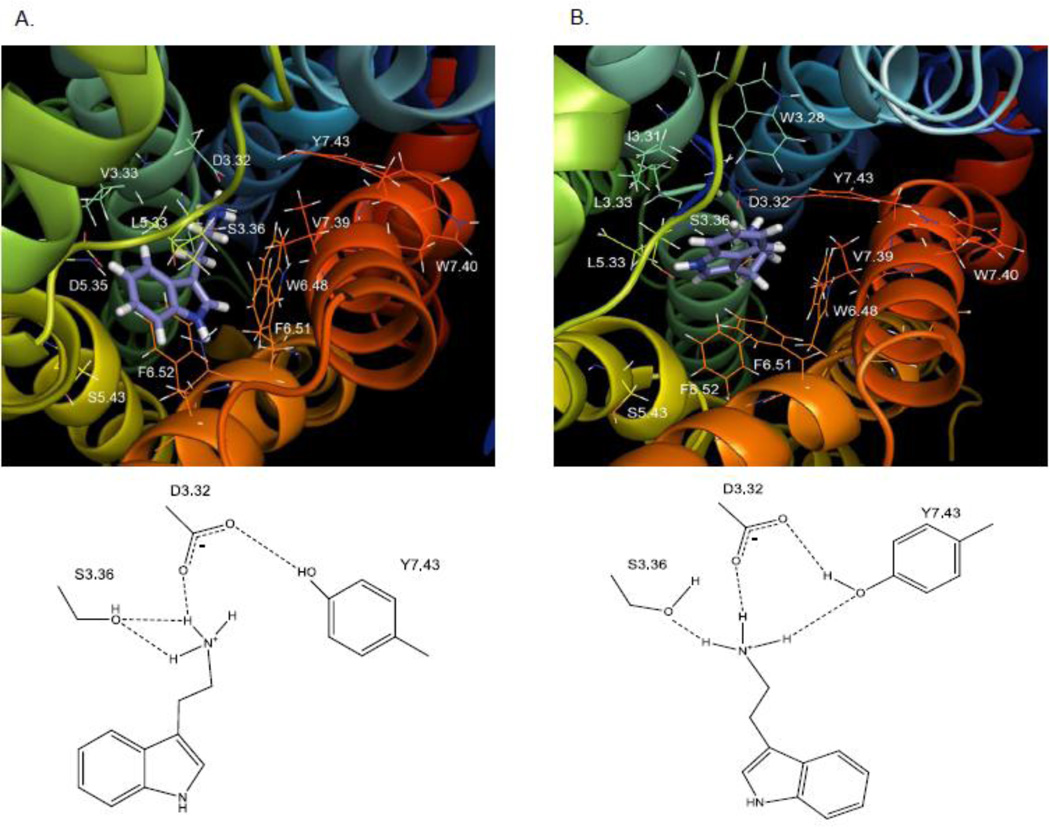
Docking results for tryptamine interactions at the 5-HT2C receptor also indicated two interchangeable, low-energy poses (Fig. 9). Like 5-HT, binding of tryptamine in both poses facilitated hydrogen bonding between the 5HT2C receptor residues D3.32 and Y7.43 (2.85 Å in pose 1 and 2.09 Å in pose 2). Also in pose 1 (Fig. 9A), the tryptamine protonated amine group was capable of forming a hydrogen bond with D3.32 (2.03 Å) and S3.36 (2.18 Å) but was much further away from Y7.43 (5.53 Å) than was the case for the 5-HT amine moiety; consistent with this binding mode is the much larger reduction in affinity for tryptamine at the S3.36A (~30-times) compared to the Y7.43A (~5-times) point-mutated receptors (Table 2). In pose 2 (Fig. 9B), the primary amine moiety of tryptamine docked similarly as in pose 1, and although the position of the aromatic indole moiety is shifted, there are no hydrogen bonding possibilities for the 5-position as is the case for 5-HT in pose 2 (Fig. 8B), which may explain the difference in degree of affinity loss between 5-HT (about 17-times less) and tryptamine (about 5-times less) at the Y7.43A point-mutated receptor relative to the WT receptor.
Fig. 9. Tryptamine.
A. Molecular model graphic pose 1 of tryptamine docked to the WT 5-HT2C receptor and directly underneath, schematic of pose 1. B. Molecular model graphic pose 2 of tryptamine docked to the WT 5-HT2C receptor and directly underneath, schematic of pose 2.
3.3.2 Lisuride and mesulergine
Fig. 10 shows the calculated lisuride pose docked closely to transmembrane domains 3, 5 and 6 at the WT 5-HT2C receptor. Similar to the binding of 5-HT, lisuride binding facilitated hydrogen bond formation between the 5-HT2C Y7.43 and D3.32 residues (1.52 Å), but loss of this hydrogen bond possibility at the Y7.43A point-mutated receptor did not strongly affect the affinity of lisuride, which was slightly enhanced at this receptor (Table 2). The protonated tertiary amine group of lisuride acted as a hydrogen bond donor to 5-HT2C D3.32 (1.56 Å), as was the case for the primary amine group of 5-HT. Unlike 5-HT, the lisuride amine moiety was too far from S3.36 (7.21 Å) and Y7.43 (3.95 Å) for significant hydrogen bonding interactions, consistent with experimental results indicating no change in affinity of lisuride at the S3.36A mutated receptor and only a minor change at the Y7.43A mutated receptor (Table 2). Although the phenyl ring of F6.52 is in a T-configuration for π-π stacking with respect to the lisuride indole system, the distance is 6.2 Å, preventing significant interactions. Overall, aromatic binding interactions between lisuride and 5-HT2C residues W6.48, F6.51, and F6.52 were not apparent. Studies docking lisuride at the S3.36A 5-HT2C and Y7.43A 5-HT2C receptor models (not shown) indicated the location of the ligand was similar to the orientation at the WT receptor. A notable difference was that the sterically small alanine residue allowed for increased binding pocket space in the point-mutated receptors. This was more apparent for the Y7.43A receptor due to the greater size differential between tyrosine and alanine compared to serine and alanine, i.e. the S3.36A receptor. In the Y7.43A mutant, lisuride was able to form a hydrogen bond with the carbonyl oxygen to the amide side chain in N5.66; this interaction was not present in the WT receptor, potentially explaining the slightly enhanced affinity of lisuride observed at the Y7.43A.
Fig. 10. Lisuride.
Lisuride docked to the WT 5-HT2C receptor.
Fig. 11 shows mesulergine docked closely to WT receptor transmembrane domains 3, 5 and 6, similar to 5-HT, tryptamine, and lisuride. In contrast to the 5-HT2C agonists, 5-HT, tryptamine, and lisuride, the inverse agonist mesulergine did not facilitate hydrogen bond formation between the 5-HT2C D3.32 and Y7.43 residues. In fact, binding of mesulergine increased the distance between these two residues (6.61 Å, compared to 3.38 Å in the unbound receptor). The protonated tertiary amine group of mesulergine apparently acted as a strong hydrogen bond donor to D3.32 (1.90 Å), consistent with experimental evidence that indicated no detectable binding of [3H]-mesulergine at the D3.32A point-mutated 5-HT2C receptor. The distance between the mesulergine tertiary amine moiety and S3.36 (8.68 Å) was too large and also the orientation did not allow for hydrogen bonding, consistent with experimental results that indicated no change in KD for [3H]-mesulergine at the S3.36A and WT receptors (Table 1). The aromatic indole group of mesulergine oriented parallel to the indole side chain of W6.48 (3.4Å), likely participating in forming π-π stacking interactions, however, it was relatively far away (5.40 Å) from Y7.43 and not in a suitable orientation for π-π or T-stacking interactions; experimental results indicated that the Y7.43A mutation did not significantly affect [3H]-mesulergine binding (Table 1).
Fig. 11. Mesulergine.
Mesulergine docked to the WT 5-HT2C receptor
3.3.3 Primary, secondary and tertiary amine PATs
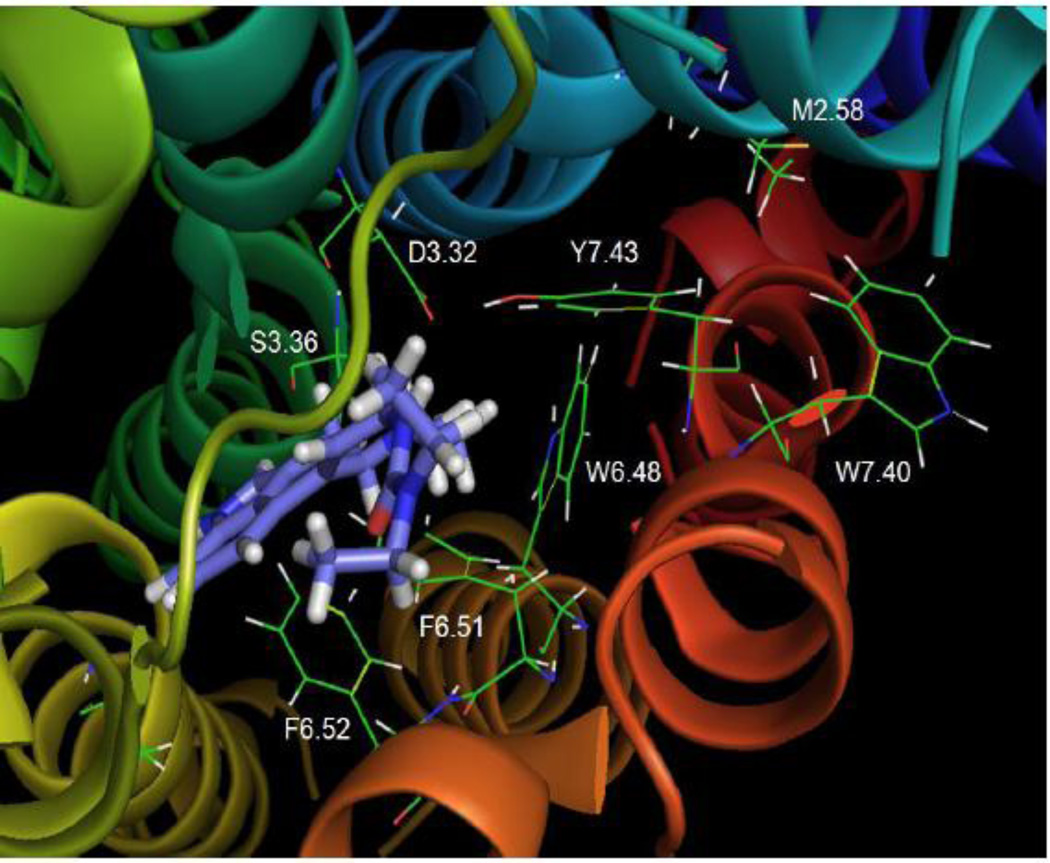
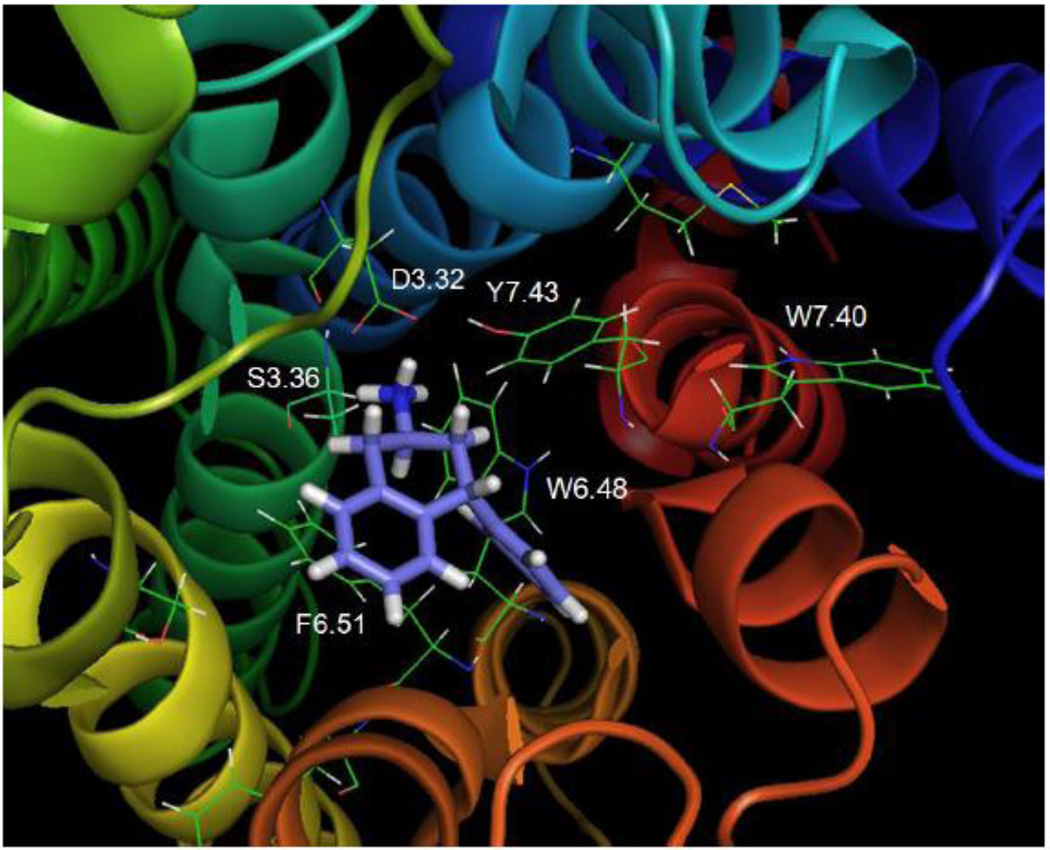
Fig. 12 shows that the tertiary amine analog, 5-HT2C agonist, N(CH3)2-PAT, docked at the WT receptor close to transmembrane domains 3, 6, and 7. Interestingly, binding of N(CH3)2-PAT did not alter the hydrogen bonding distance between Y7.43 and D3.32 (3.38 Å) that existed in the unbound 5-HT2C receptor and the β2 adrenergic receptor (not shown). This result contrasted with the binding of the other agonists (5-HT, tryptamine, and lisuride) that decreased the distance between these two residues, as well as, with the inverse agonist mesulergine that increased the distance.
Fig. 12. N(CH3)2-PAT.
N(CH3)2-PAT docked to the WT 5-HT2C receptor
The N(CH3)2-PAT protonated tertiary amine group appeared to form a tight hydrogen bond to D3.32 (1.84 Å), whereas weak hydrogen bonding was possible between the tertiary amine and S3.36 (3.27 Å) as well as Y7.43 (2.96 Å). The N(CH3)2-PAT tetrahydronaphthalene system aligned parallel to the aromatic ring of Y7.43, apparently forming π-π stacking binding interactions. These docking results were consistent with experimental results that indicated affinity of N(CH3)2-PAT was significantly diminished at the S3.36A and Y7.43A point-mutated 5-HT2C receptors (Table 2). The N(CH3)2-PAT pendant phenyl moiety oriented close to the W6.48, F6.51, and F6.52 aromatic amino acids in transmembrane domain 6, presumably forming aromatic π-π stacking binding interactions. The configuration of N(CH3)2-PAT docked in the β2 adrenergic receptor-based 5-HT2C model has some differences compared to the previously reported N(CH3)2-PAT docked in Rho-based 5-HT2C model (Booth , et al., 2009) . The main difference is the location of the pendant phenyl of N(CH3)2-PAT in the pocket; in contrast to the results described above for the N(CH3)2-PAT docked in β2 adrenergic receptor-based 5-HT2C model, in the Rho-based 5-HT2C model, the location of the PAT pendant phenyl moiety does not allow for interactions with W6.48, F6.51, and F6.52.
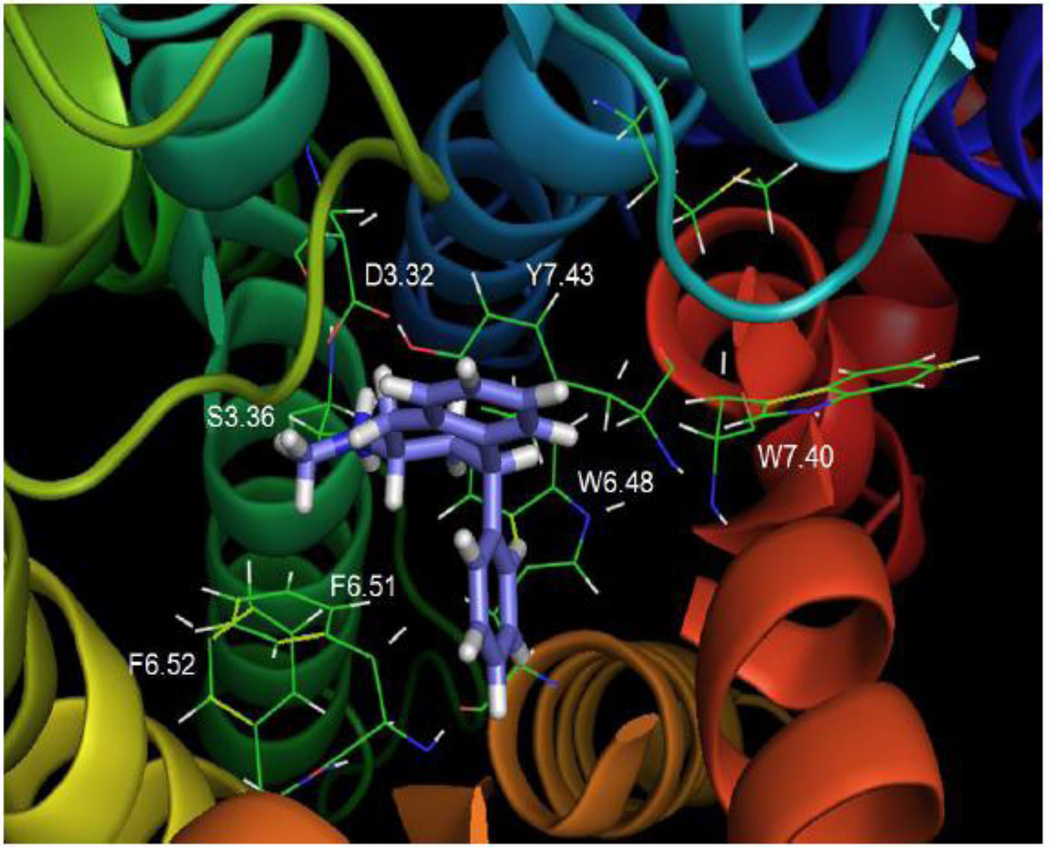
Fig. 13 shows that the secondary amine analog NH(CH3)-PAT bound similar to N(CH3)2-PAT, near transmembrane domains 3, 6, and 7, and formed a strong hydrogen bond to D3.32 (1.97 Å). Binding of NH(CH3)-PAT appeared to facilitate weak hydrogen bond interactions with 5-HT2C residues S3.36 (2.84 Å) and also with Y7.43 (2.78 Å). Furthermore, NH(CH3)-PAT binding interactions between the tetrahydronaphthalene system and pendant phenyl moieties and nearby 5-HT2C residues were similar to N(CH3)2-PAT. Experimental results indicated a significant loss of affinity of NH(CH3)-PAT at both the Y7.43A and S3.36A point-mutated receptors (Table 2), consistent with hydrogen bonding interactions between these residues and the PAT amine moieties.
Fig. 13. NH(CH3)-PAT.
NH(CH3)-PAT docked to the WT 5-HT2C receptor
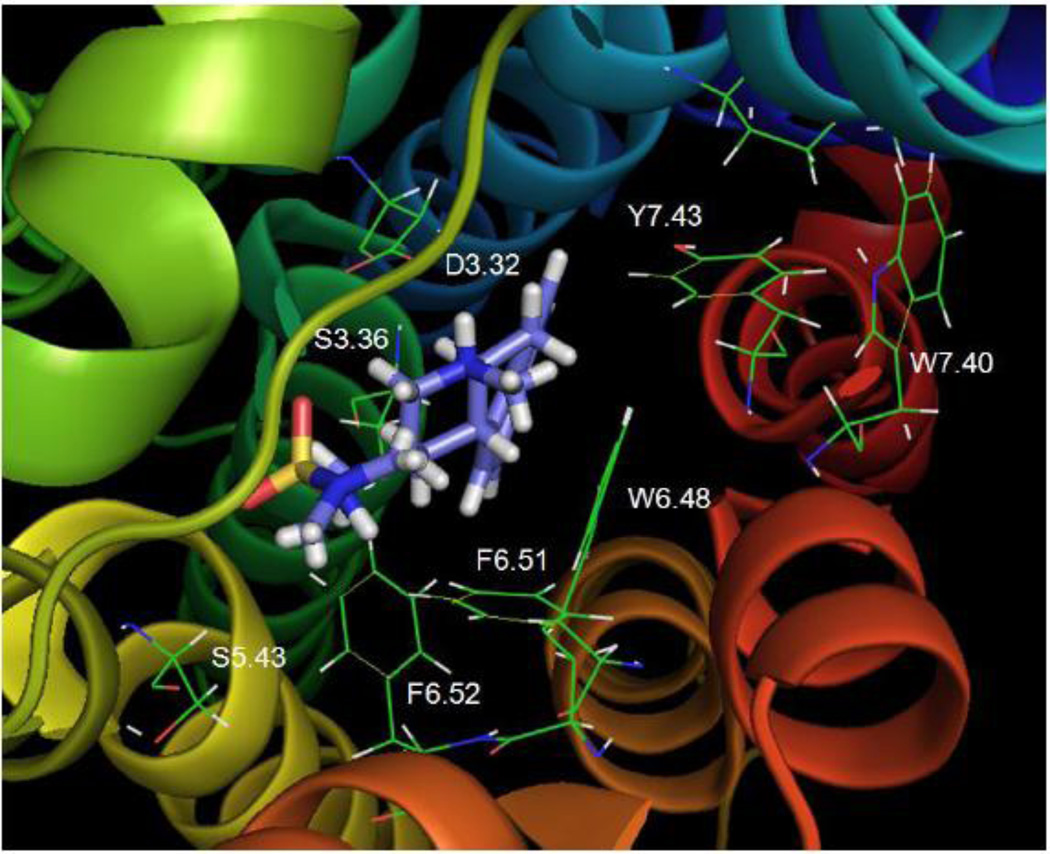
Fig. 14 shows the primary amine analog NH2-PAT bound close to transmembrane domains 3, 6, and 7. In particular, NH2-PAT formed a hydrogen bond with D3.32 (1.93 Å). Moreover, similar to the secondary and tertiary PATs, the pendant phenyl of NH2-PAT was in close proximity to W6.48. However, there was a difference in the overall position of NH2-PAT in the binding pocket compared to NH(CH3)-PAT and N(CH3)2-PAT that can be attributed to its lack of amine methyl substituents. Relative to NH2-PAT, the methyl groups on NH(CH3)-PAT and N(CH3)2-PAT increase amine basicity, likely enhancing electronic interactions with binding pocket residues to produce higher affinity (Table 2). Furthermore, there were weak interactions between the amine moiety of NH2-PAT and Y7.43 (3.70 Å), and the tetrahydronaphthalene moiety was not in a position suitable for π-π stacking interactions with the Y7.43 aromatic ring. Thus, mutation of the 5-HT2C Y7.43 residue to alanine did not affect NH2-PAT affinity (Table 2, Fig. 6). Meanwhile, the amine of NH2-PAT could form a hydrogen bond with S3.36 (2.62 Å), and the S3.36A point-mutated receptor showed about 6-times lower affinity for the ligand compared to the WT receptor (Table 2, Fig. 6). These data suggest that the hydrogen bond between the PAT primary amine moiety and S3.36 assumes added importance given the absence of compensatory π-π stacking between the NH2-PAT tetrahydronaphthalene moiety and Y7.43. Compared to 5-HT and tryptamine, however, NH2-PAT binds farther from the 5-HT2C S3.36 residue, and accordingly, its affinity was reduced to a lesser extent when S3.36 was mutated to alanine (Table 2).
Fig. 14. NH2-PAT.
NH2-PAT docked to the WT 5-HT2C receptor.
Fig. 6.
Representative radioligand competition displacement curves for the secondary amine NH(CH3)-PAT at WT vs. S3.36A and Y7.43A (inset) point-mutated 5-HT2C receptors. Data show means and S.E.M. from an individual experiment of percent specific binding of [3H]-mesulergine. The curves for the point-mutated receptors are significantly (P < 0.05) shifted to the right compared to the WT receptor.
4. Discussion
Measurements of ligand affinity, potency, and efficacy at WT compared to point-mutated 5-HT2C receptors, coupled with computational experiments and molecular modeling based on homology to the human β2 adrenergic receptor structure, indicated an important role for hydrogen bonding in ligand amine moiety interactions at the 5-HT2C-INI receptor. The molecular rigidity of the PAT chemical scaffold provided a known 3-dimensional arrangement of the aromatic ethylamine pharmacophore moiety to delineate the impact of amine substitution on interactions with the 5-HT2C receptor. This approach avoided the confounding entropy factors inherent in highly flexible tryptamine analogs that have been used in corresponding studies of the 5-HT2A receptor (Almaula et al., 1996; Ebersole et al., 2003). When the 5-HT2C S3.36 residue was mutated to alanine (S3.36A), affinity of the primary amine NH2-PAT (as well as the primary amines, 5-HT and tryptamine) was reduced to a greater extent than the secondary amine NH(CH3)-PAT, that in turn was reduced more than the tertiary amine N(CH3)2-PAT. Similarly, affinity of the tertiary amines lisuride and mesulergine were not reduced by the S3.36A mutation. The potencies for ligand modulation of 5-HT2C receptor-mediated phospholipase C signaling also were consistent with the corresponding ligand affinities at WT and S3.36A receptors, i.e., the potency of 5-HT was reduced more than N(CH3)2-PAT, yet the potency of mesulergine was unaffected by the mutation. Modeling and computational results indicated that in comparison to the secondary and tertiary amine ligands, primary amine ligands interact closely with the S3.36 residue.
Modeling results showed the methyl groups of the tertiary amine, N(CH3)2-PAT, form pi-pi interactions with the Y7.43 residue, which were lost when methyl groups were not present. Experimentally, N(CH3)2-PAT showed a greater loss in affinity relative to NH(CH3)-PAT at the Y7.43A point-mutated receptor, and NH2-PAT was unaffected by the mutation. Furthermore, the effect of the Y7.43A mutation on ligand affinity was consistent with its effect on ligand potency. Notably, tertiary amine substitution on the PAT scaffold provides enhanced basicity and increased electronic interactions with binding pocket residues that account for the higher affinity of N(CH3)2-PAT at the WT 5-HT2C receptor relative to the secondary and primary amines. Apparently, the increase in overall receptor binding interactions that accompanies increased basicity of tertiary amines can compensate for loss of hydrogen bonding between a primary amine and certain residues.
To our knowledge, this is the first report that utilized both mutagenesis experiments and molecular modeling based on the human β2 adrenergic receptor structure to estimate where the 5-hydroxy group of 5-HT binds at the human WT 5-HT2C receptor. Results indicated the 5-hydroxy group of 5-HT forms a hydrogen bond with the 5-HT2C receptor residue Y7.43. When this residue is mutated to alanine, 5-HT exhibits a much greater loss of affinity compared to tryptamine that lacks the 5-hydroxy group. In contrast, a computational study based on the structure of bovine rhodopsin suggested that the 5-hydroxy group of 5-HT interacts with 5-HT2C residues S3.39 and W6.48 (Bray and Goddard, 2008). There are some differences in the overall structure of the β2 adrenergic receptor-based 5-HT2C model used in the present studies relative to our previously reported rhodopsin-based 5-HT2C homology model (Booth et al., 2009). For example, there was a more pronounced kink in transmembrane domain 5 near P5.50 in the rhodopsin-based model, and a stronger helix kink in transmembrane domain 6 near P6.50 in the present model, among other differences. These variations may account for the differences in the interactions of 5-HT at the 5-HT2C receptor reported here relative to previous reports (Bray and Goddard, 2008). Comparative information for the 5-HT2A receptor, from mutagenesis studies and modeling based on bovine rhodopsin, suggests that the 5-hydroxy group of 5-HT interacts with 5-HT2A residues S5.43 and S5.46 (Braden and Nichols, 2007).
Although the 5-HT2 receptor family shares approximately 80% transmembrane sequence identity and a primary signal transduction pathway involving Gαq/11 proteins and activation of phospholipase C (Hoyer et al., 1994; Raymond et al., 2001), most 5-HT2 ligands demonstrate differential 5-HT2-subtype affinity and potency regarding 5-HT2-mediated intracellular signaling (Egan et al., 2000; Knight et al., 2004; Porter et al., 1999). Even the small, flexible 5-HT molecule distinguishes between 5-HT2A and 5-HT2C receptors such that its affinity and potency to activate phospholipase C signaling is at least two-times higher at 5-HT2C-INI compared to 5-HT2A receptors (Egan et al., 2000; Knight et al., 2004; Porter et al., 1999). These differences likely result from unique binding pocket conformations stabilized by a given ligand. Thus, the putatively subtle, structural differences between 5-HT2 receptor subtypes can be exploited to develop 5-HT2 subtype-specific agonists.
Interestingly, the current data show that the S3.36A point-mutation alters the phospholipase C signaling properties of the 5-HT2C-INI receptor. Notably, the efficacy of 5-HT, N(CH3)2-PAT, and mesulergine were not different at WT compared to Y7.43A receptors. At the S3.36A receptor, however, the efficacy of each ligand was potentiated in the direction of activation. Thus, the EMAX values for the agonists 5-HT and N(CH3)2-PAT were greatly enhanced at the S3.36A receptor (relative to the WT), and the inverse agonist (at WT 5-HT2C) mesulergine displayed low efficacy partial agonism at the S3.36A receptor. This phenomenon, at least for agonists at WT receptors, is not unusual for aminergic G protein-coupled receptors, including the 5-HT2A receptor (Almaula et al., 1996). Speculatively, the S3.36A receptor may reveal an active conformation that is not possible in the WT receptor and that can be stabilized by mesulergine.
Mutagenesis and modeling approaches are useful for predicting G protein-coupled receptor ligand affinity, however, molecular determinants governing ligand binding that lead to stabilization of agonist vs. inverse agonist or neutral antagonist conformation states are poorly understood. Even recent studies that report the active state β2 adrenergic receptor structure (Rasmussen et al., 2011; Rosenbaum et al., 2011) cannot predict unique functional outcomes that are dependent on the cellular milieu, as well as different receptor conformation(s) stabilized by different ligands. Indeed, a single G protein-coupled receptor can couple to multiple signaling pathways, and a ligand can modulate activity of each pathway with dissimilar potencies and efficacies (Kenakin, 1995; Urban et al., 2007). For example, it is known that 5-HT can activate 5-HT2C receptors linked to phospholipase C activation, as well as, 5-HT2C receptors linked to phospholipase A activation (Berg et al., 1999; Moya et al., 2007). The modeling results obtained here indicated that 5-HT can bind to the 5-HT2C receptor in at least two configurations, leading to speculation that individual ligand poses may stabilize a receptor conformation favoring a particular signaling pathway, e.g. biased agonism, and that agonist ligands with fewer feasible (or restricted) docking poses may be more selective for activating particular pathways. Notably, in both poses, 5-HT docked differently than the other 5-HT2C agonists tested, lisuride and N(CH3)2-PAT.
The current data support the hypothesis that different agonist ligands can stabilize different receptor binding pocket structures, suggesting a molecular basis for conformationally-selective receptor binding, activation, and type of signaling (Kenakin, 1995; Urban et al., 2007). N(CH3)2-PAT may demonstrate a unique 5-HT2C receptor functional profile compared to 5-HT and lisuride. N(CH3)2-PAT is known to be a functionally-selective ligand at the histamine H1 G protein-coupled receptor (Moniri et al., 2004) that is phylogenetically closely related to 5-HT2C receptors (Smit et al., 1999). Thus, N(CH3)2-PAT is an agonist with potency and efficacy similar to histamine regarding H1-mediated activation of adenylyl cyclase, but it is an antagonist (and inverse agonist) regarding H1-mediated activation of phospholipase C.
Finally, mesulergine, a WT 5-HT2C receptor inverse agonist (Berg et al., 1999; Moya et al., 2007), stabilized a 5-HT2C receptor binding pocket structure that was different from the conformation(s) stabilized by binding of the agonists 5-HT and lisuride. When mesulergine was docked to the WT 5-HT2C receptor, the distance between the D3.32 and Y7.43 side chains was about 6 Å, too far for a hydrogen bond. When 5-HT or lisuride was docked to the 5-HT2C receptor model, the distance between D3.32 and Y7.43 was about 2 Å, suggesting a strong hydrogen bond was formed. Thus, results here support the hypothesis that hydrogen bond formation between D3.32 and Y7.43 may be involved in 5-HT or lisuride-induced activation of the 5-HT2C receptor. This hypothesis is consistent with recent results from mutations of the M(3) muscarinic acetylcholine receptor (Han et al., 2005) and structural results using an engineered activated state of the β2 adrenergic receptor that showed activation is associated with inward movement of transmembrane domains 3 and 7 (Rasmussen et al., 2011; Rosenbaum et al., 2011).
In conclusion, drug discovery targeting 5-HT2C receptors has centered on agonists that produce anorexigenic effects to treat obesity (Rowland et al., 2008; Smith et al., 2010). 5-HT2C agonists with 5-HT2A receptor and 5-HT2B receptor inverse agonist properties, similar to N(CH3)2-PAT, may prove to be suitable medicines as they may not cause hallucinations (Nichols, 2004) or cardiopulmonary toxicity (Fitzgerald et al., 2000; Launay et al., 2002; Rothman et al., 2000) associated with activation of 5-HT2A and 5-HT2B receptors, respectively. Further clarifications of ligand molecular interactions that determine the uncommon pharmacology of PAT at 5-HT2 receptors will likely guide development of novel, clinically useful 5-HT2C-specific ligands.
Acknowledgement
This work was supported by National Institutes of Health grants DA023928, DA030989, and MH081193. The National Institutes of Health did not have a role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
The authors declare no conflicts of interest.
Molecular modeling figures are presented in color in the online version of this paper.
References
- Almaula N, Ebersole BJ, Zhang D, Weinstein H, Sealfon SC. Mapping the binding site pocket of the serotonin 5-Hydroxytryptamine2A receptor. Ser3.36(159) provides a second interaction site for the protonated amine of serotonin but not of lysergic acid diethylamide or bufotenin. J Biol Chem. 1996;271:14672–14675. doi: 10.1074/jbc.271.25.14672. [DOI] [PubMed] [Google Scholar]
- Arena Pharmaceuticals I. Lorcaserin hydrochloride (APD356) NDA 22–529 Briefing Document for FDA Advisory Committee Meeting; 2010. [Google Scholar]
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, Javitch JA. Activation of the beta 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001a;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- Ballesteros JA, Shi L, Javitch JA. Structural mimicry in G protein-coupled receptors: implications of the high-resolution structure of rhodopsin for structure-function analysis of rhodopsin-like receptors. Mol Pharmacol. 2001b;60:1–19. [PubMed] [Google Scholar]
- Berg KA, Stout BD, Cropper JD, Maayani S, Clarke WP. Novel actions of inverse agonists on 5-HT2C receptor systems. Mol Pharmacol. 1999;55:863–872. [PubMed] [Google Scholar]
- Booth RG, Fang L, Huang Y, Wilczynski A, Sivendran S. (1R, 3S)-(−)-trans-PAT: a novel full-efficacy serotonin 5-HT2C receptor agonist with 5-HT2A and 5-HT2B receptor inverse agonist/antagonist activity. Eur J Pharmacol. 2009;615:1–9. doi: 10.1016/j.ejphar.2009.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braden MR, Nichols DE. Assessment of the roles of serines 5.43(239) and 5.46(242) for binding and potency of agonist ligands at the human serotonin 5-HT2A receptor. Mol Pharmacol. 2007;72:1200–1209. doi: 10.1124/mol.107.039255. [DOI] [PubMed] [Google Scholar]
- Bray JK, Goddard WA., 3rd The structure of human serotonin 2c G-protein-coupled receptor bound to agonists and antagonists. J Mol Graph Model. 2008 doi: 10.1016/j.jmgm.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res. 2008;172:319–346. doi: 10.1016/S0079-6123(08)00916-3. [DOI] [PubMed] [Google Scholar]
- Bucholtz EC, Brown RL, Tropsha A, Booth RG, Wyrick SD. Synthesis, evaluation, and comparative molecular field analysis of 1-phenyl-3-amino-1,2,3,4-tetrahydronaphthalenes as ligands for histamine H(1) receptors. J Med Chem. 1999;42:3041–3054. doi: 10.1021/jm980428x. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Ebersole BJ, Visiers I, Weinstein H, Sealfon SC. Molecular Basis of Partial Agonism: orientation of Indoleamine Ligands in the Binding Pocket of the Human Serotonin 5-HT2A Receptor Determines Relative Efficacy. Molecular Pharmacology. 2003;63:36–43. doi: 10.1124/mol.63.1.36. [DOI] [PubMed] [Google Scholar]
- Egan C, Grinde E, Dupre A, Roth BL, Hake M, Teitler M, Herrick-Davis K. Agonist high and low affinity state ratios predict drug intrinsic activity and a revised ternary complex mechanism at serotonin 5-HT(2A) and 5-HT(2C) receptors. Synapse. 2000;35:144–150. doi: 10.1002/(SICI)1098-2396(200002)35:2<144::AID-SYN7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Fitzgerald LW, Burn TC, Brown BS, Patterson JP, Corjay MH, Valentine PA, Sun JH, Link JR, Abbaszade I, Hollis JM, Largent BL, Hartig PR, Hollis GF, Meunier PC, Robichaud AJ, Robertson DW. Possible role of valvular serotonin 5-HT(2B) receptors in the cardiopathy associated with fenfluramine. Mol Pharmacol. 2000;57:75–81. [PubMed] [Google Scholar]
- Han SJ, Hamdan FF, Kim SK, Jacobson KA, Bloodworth LM, Li B, Wess J. Identification of an agonist-induced conformational change occurring adjacent to the ligand-binding pocket of the M(3) muscarinic acetylcholine receptor. J Biol Chem. 2005;280:34849–34858. doi: 10.1074/jbc.M506711200. [DOI] [PubMed] [Google Scholar]
- Heller H, Schaefer M, Schulten K. Molecular Dynamics Simulation of a Bilayer of 200 Lipids in the Gel and in the Liquid-Crystal Phases. Journal of Physical Chemistry. 1993;97:8343–8360. [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- Jensen NH, Cremers TI, Sotty F. Therapeutic potential of 5-HT2C receptor ligands. ScientificWorldJournal. 2010;10:1870–1885. doi: 10.1100/tsw.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy. II. Agonist trafficking of receptor signals. Trends Pharmacol Sci. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, Bickerdike M. Pharmacological characterisation of the agonist radioligand binding site of 5-HT(2A), 5-HT(2B) and 5-HT(2C) receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:114–123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Kristiansen K, Dahl SG. Molecular modeling of serotonin, ketanserin, ritanserin and their 5-HT2C receptor interactions. Eur J Pharmacol. 1996;306:195–210. doi: 10.1016/0014-2999(96)00180-x. [DOI] [PubMed] [Google Scholar]
- Kristiansen K, Kroeze WK, Willins DL, Gelber EI, Savage JE, Glennon RA, Roth BL. A highly conserved aspartic acid (Asp-155) anchors the terminal amine moiety of tryptamines and is involved in membrane targeting of the 5-HT(2A) serotonin receptor but does not participate in activation via a "salt-bridge disruption" mechanism. J Pharmacol Exp Ther. 2000;293:735–746. [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Launay JM, Herve P, Peoc’h K, Tournois C, Callebert J, Nebigil CG, Etienne N, Drouet L, Humbert M, Simonneau G, Maroteaux L. Function of the serotonin 5-hydroxytryptamine 2B receptor in pulmonary hypertension. Nat Med. 2002;8:1129–1135. doi: 10.1038/nm764. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Serotonin 5-HT2C receptors as a target for the treatment of depressive and anxious states: focus on novel therapeutic strategies. Therapie. 2005;60:441–460. doi: 10.2515/therapie:2005065. [DOI] [PubMed] [Google Scholar]
- Moniri NH, Covington-Strachan D, Booth RG. Ligand-directed functional heterogeneity of histamine H1 receptors: novel dual-function ligands selectively activate and block H1-mediated phospholipase C and adenylyl cyclase signaling. J Pharmacol Exp Ther. 2004;311:274–281. doi: 10.1124/jpet.104.070086. [DOI] [PubMed] [Google Scholar]
- Motulsky HJ, Christopoulos A. A practical guide to curve-fitting. San Diego, CA: GraphPad Software, Inc.; 2003. Fitting models to biological data using linear and nonlinear regression. [Google Scholar]
- Moya PR, Berg KA, Gutierrez-Hernandez MA, Saez-Briones P, Reyes-Parada M, Cassels BK, Clarke WP. Functional selectivity of hallucinogenic phenethylamine and phenylisopropylamine derivatives at human 5-hydroxytryptamine (5-HT)2A and 5-HT2C receptors. J Pharmacol Exp Ther. 2007;321:1054–1061. doi: 10.1124/jpet.106.117507. [DOI] [PubMed] [Google Scholar]
- Muntasir HA, Takahashi J, Rashid M, Ahmed M, Komiyama T, Hossain M, Kawakami J, Nashimoto M, Nagatomo T. Site-directed mutagenesis of the serotonin 5-Hydroxytryptamine2c receptor: identification of amino acids responsible for sarpogrelate binding. Biol Pharm Bull. 2006;29:1645–1650. doi: 10.1248/bpb.29.1645. [DOI] [PubMed] [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–181. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, Adams DR, Sheardown MJ. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. Structure of a nanobody-stabilized active state of the beta(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, Burghammer M, Ratnala VR, Sanishvili R, Fischetti RF, Schertler GF, Weis WI, Kobilka BK. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature. 2007;450:383–387. doi: 10.1038/nature06325. [DOI] [PubMed] [Google Scholar]
- Raymond JR, Mukhin YV, Gelasco A, Turner J, Collinsworth G, Gettys TW, Grewal JS, Garnovskaya MN. Multiplicity of mechanisms of serotonin receptor signal transduction. Pharmacol Ther. 2001;92:179–212. doi: 10.1016/s0163-7258(01)00169-3. [DOI] [PubMed] [Google Scholar]
- Rosenbaum DM, Zhang C, Lyons JA, Holl R, Aragao D, Arlow DH, Rasmussen SG, Choi HJ, Devree BT, Sunahara RK, Chae PS, Gellman SH, Dror RO, Shaw DE, Weis WI, Caffrey M, Gmeiner P, Kobilka BK. Structure and function of an irreversible agonist-beta(2) adrenoceptor complex. Nature. 2011;469:236–240. doi: 10.1038/nature09665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Savage JE, Rauser L, McBride A, Hufeisen SJ, Roth BL. Evidence for possible involvement of 5-HT(2B) receptors in the cardiac valvulopathy associated with fenfluramine and other serotonergic medications. Circulation. 2000;102:2836–2841. doi: 10.1161/01.cir.102.23.2836. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Crump EM, Nguyen N, Robertson K, Sun Z, Booth RG. Effect of (−)-trans-PAT, a novel 5-HT2C receptor agonist, on intake of palatable food in mice. Pharmacol Biochem Behav. 2008;91:176–180. doi: 10.1016/j.pbb.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltzman AG, Morse B, Whitman MM, Ivanshchenko Y, Jaye M, Felder S. Cloning of the human serotonin 5-HT2 and 5-HT1C receptor subtypes. Biochem Biophys Res Commun. 1991;181:1469–1478. doi: 10.1016/0006-291x(91)92105-s. [DOI] [PubMed] [Google Scholar]
- Smit MJ, Hoffmann M, Timmerman H, Leurs R. Molecular properties and signalling pathways of the histamine H1 receptor. Clin Exp Allergy. 1999;29 Suppl 3:19–28. [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR. Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, Al-Shamma H, Smith B, Chalmers D, Behan D. Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther. 2008;325:577–587. doi: 10.1124/jpet.107.133348. [DOI] [PubMed] [Google Scholar]
- Urban JD, Clarke WP, von Zastrow M, Nichols DE, Kobilka B, Weinstein H, Javitch JA, Roth BL, Christopoulos A, Sexton PM, Miller KJ, Spedding M, Mailman RB. Functional selectivity and classical concepts of quantitative pharmacology. J Pharmacol Exp Ther. 2007;320:1–13. doi: 10.1124/jpet.106.104463. [DOI] [PubMed] [Google Scholar]