Abstract
Background
KCC2, a neuronal-specific K-Cl cotransporter, is involved in pain perception physiology through its effects on postsynaptic inhibition in spinal cord neurons. We injected a newly identified, highly potent and selective inhibitor of KCC2 (D4), an inactive structural variant (D4.14), and the Na-K-2Cl cotransporter (NKCC1) inhibitor, bumetanide, into the intrathecal space of mice to measure their effect on heat-evoked nociceptive responses.
Methods
Commercially available intrathecal catheters were modified and surgically placed into two cohorts of 10 mice. After recovery from the procedure, the mice were injected with D4, D4.14, and bumetanide through this catheter. Nociceptive measurements (hotplate assay, tail flick assay) were performed after injection of each of the test drugs and compared to vehicle controls.
Results
Two mice in each cohort were omitted due to postprocedure complications. There was a statistically significant decrease (P < 0.01) in withdrawal latency after injection of the active KCC2 inhibitor but not after injection of the inactive compound (P = 0.78), as measured by hotplate assay at 55°C. Injection of bumetanide significantly increased withdrawal latency (P = 0.02) at the same temperature. These results were confirmed using tail flick assays performed at 49°C.
Conclusions
Inhibition of KCC2 by D4 led to decreased heat-evoked withdrawal latency in mice, as measured by hotplate and tail flick assays, while inhibition of NKCC1 by bumetanide resulted in increased response latencies to heat stimuli as measured by both of these nociceptive tests.
INTRODUCTION
Neurons located in the dorsal horn of the spinal cord process sensory information coming from the periphery and relay this information to the brain through ascending fibers. This information is modulated in part by gamma-aminobutyric acid A receptors located in postsynaptic relay neurons as well as in the presynaptic terminals of sensory afferent fibers (presynaptic). A neuronal-specific K-Cl cotransporter (KCC2) expressed in spinal cord neurons, drives the intracellular Cl− concentration below its electrochemical equilibrium potential, thereby strengthening gamma-aminobutyric acid A hyperpolarizing responses and postsynaptic inhibition (1-3). Similarly, a Na-K-2Cl cotransporter (NKCC1) expressed in afferent neurons, drives the intracellular Cl− concentration above its equilibrium potential, thereby facilitating primary afferent depolarization and presynaptic inhibition (4-6). These two cotransporters are critical in gating sensory information from the peripheral to the central nervous system.
Several studies have examined the role of KCC2 in nociception. Whereas one study showed hyposensitivity of KCC2 hypomorphic mice to tactile and thermal stimulation (7), several other studies demonstrated that decreased KCC2 expression in the spinal cord is associated with increased nociception (1, 8-12). However, because of unanticipated effects due to possible compensatory mechanisms, a complementary pharmacological approach would be useful. This is now possible, as we recently identified, using a high throughput screening strategy, novel compounds that inhibit KCC2 with high potency and selectivity (13).
In this study, we took advantage of one of these compounds (called D4) and one of its inactive structural variants (D4.14), and developed an intrathecal catheter delivery method to assess the effect of pharmacological inhibition of KCC2 on heat-evoked nociceptive responses in mice. We also used bumetanide, a highly selective inhibitor of NKCC1, to test the effect of pharmacological inhibition of this transporter on nociception.
MATERIAL AND METHODS
Catheter Modification
The intrathecal catheter (catalog# 0007743, Alzet Osmotic pump, Cupertino, CA) was modified by trimming the thinnest portion of the catheter to a length of 1.0 cm and trimming the thickest portion the catheter to a length of 0.3 cm. The length of the thin portion was selected since the distance from the superficial muscle to the intrathecal space was measured to be approximately 0.5 cm. Since the catheter is inserted at the L5-L6 intervertebral space, this length places the tip of the catheter at the L3-L4 level. The thick end of the catheter was shortened as much as possible to decrease the chance of removal while still being functional. A 0.2 cm portion of the thick tubing was fastened to the distal end of the middle portion of the catheter using epoxy (Figure 1). This extra tubing acted as a tether for the fascial suture and was placed to decrease the chance of catheter withdrawal. After modification, the guiding wire was reinserted into the catheter. The nature of the polyurethame material in the catheter permitted sterilization by autoclaving. The volume within the modified catheter was determined to be 1.3 μL by calculating the difference in weight between a catheter filled with distilled water and the same dry catheter.
Fig. 1.
Intrathecal catheter before and after modification. The thickest and thinnest portions of the catheter were trimmed to 0.3 cm (a) and 1.0 cm (b), respectively. A 0.2 cm section of the thick tubing was added to the distal end of the middle portion to act as an anchor to the fascial suture (c). The catheter volume decreased from 8.0 μl to 1.3 μl with the modification. Note the Teflon-coated metal stylet (arrowhead) used for easier placement.
Animal Studies
All animal procedures and experiments were approved by the Vanderbilt University Institutional Animal Care and Use Committee and were in compliance with United States Public Health Service Policy on Humane Care and Use of Laboratory Animals. Two cohorts of 10 mice (65-day old C57BL/6J males) were used for our experiments.
Catheter Insertion
The intrathecal catheter procedure was adapted from Wu et al (14). Anesthesia was induced with 5% isoflurane, using a Vapomatic vaporizer (A.M. Bickford Inc., Wales Center, N.Y.). The anesthesia was maintained with 3-5% isoflurane by titrating the concentration to both the respiratory rate and response to surgical stimuli. The lower backs of the mice were shaved using electric clippers, and prepped successively with betadine and 70% ethanol (Figure 2A). Postoperative analgesia was provided by injecting ketoprofen (10 mg/kg) subcutaneously before incision. The pelvic girdle was then palpated and a 1-cm midline incision was made directly above the cephalad border of the girdle. The skin was then retracted to expose the lower lumbar vertebrae. The mouse was held firmly by the pelvic girdle in one hand while gently flexing its spine, and the intervertebral space was accessed through an opening in the overlying muscle created by a 22-gauge needle (Figure 2B). The catheter was then placed in a paravertebral fashion by pushing into one side of the L5-L6 process at an angle of approximately 20-30 degrees above the vertebral column and 20-30 degrees to the midline of the vertebra. When the sign of dural penetration (sudden movement of the tail or hindlimb) was observed, the guidewire was withdrawn to avoid nerve damage by the metal wire. The thinnest portion of the catheter was then inserted entirely into the intrathecal space and the catheter was secured by one fascial suture (4.0 polypropylene, Ethicon, San Angelo, TX) using the fabricated tether (Figure 2C). The skin incision was then closed with the catheter exiting the most caudal aspect of the incision (Figure 2D). The end of the catheter was then sealed with a small piece of transparent film dressing (Tegaderm™, 3M, St. Paul, MN).
Fig. 2.
Placement of the intrathecal catheter. After prepping the surgical area (A), the L5-L6 intervertebral space was located with a 22-gauge needle (B). The catheter was secured to the overlying muscle with one suture using the restraint fashioned from the extra portion of thick tubing (C). The skin closure only required one or two sutures due to the small incision and an extra suture was used at the proximal end of the catheter to limit animal contact (D).
One day after implantation, ketoprofen (10 mg/kg) was given subcutaneously, the catheter dressing was removed, and 2.5 μL of 2% lidocaine (Hospira, Inc., Lake Forest, IL) followed by 5 μL of normal saline was injected through the catheter. Immediate motor paralysis of the hindlimbs was taken as correct placement of the intrathecal catheter.
Timeline
After surgery, the mice were allowed to recover for two days before testing. Before each test, the animals were transferred to a behavioral unit and allowed to habituate for a minimum of 1 h. First, mice were injected with vehicle and a baseline measurement was performed at the appropriate time point. Second, the same mice were injected with a test drug and the behavioral measurement was repeated at the same time point. The mice were then allowed to recover for 24 h to ensure redistribution and metabolism of the test drug. The following baseline measurement was used to confirm complete elimination of prior drug effect. For the time course experiment, D4 was injected and repeated behavioral tests were performed at specific time points.
Nociceptive Measurements
Hotplate assay was performed by placing the mice individually on a platform maintained at 52-55°C (Hotplate Analgesia Meter, Columbus Instruments, Columbus, OH). A plastic cylinder 15-cm in diameter and 20-cm high confined the mouse to the surface of the hotplate. The time necessary for the mouse to respond to the thermal stimulus (hindpaw fluttering, licking, or withdrawal) was measured with a stopwatch. After the initial response or the maximum cut-off time of 15 sec, the mice were removed from the hotplate and returned to the home cage. A minimum recovery period of 1 h was implemented between hotplate assay sessions.
For the tail flick assay, the mouse was gently restrained using a 50 ml polypropylene conical tube and the distal 2-cm of their tail was inserted into a thermostatically controlled water bath. The latency to withdraw or flick the tail was recorded using a stopwatch. The tests were performed at a temperature of 49°C, with a minimum recovery period of 1 h between trials. A maximum cut-off time of 20 sec was implemented to prevent permanent injury.
Activity of novel compounds and bumetanide on KCC2
KCC2 over-expressing HEK293 cells (13) were grown in 10-cm plastic Petri dishes in a 37°C incubator, 95% air and 5% CO2. The dishes contained 10 ml DMEM/F12 (Invitrogen, Carlsbad, CA), supplemented with 10% fetal bovine serum (Atlanta Biological, Lawrencewill, GA), 50 units/ml penicillin, 50 pg/ml streptomycin (Invitrogen), and 2 μg/ml puromycin (Sigma, St. Louis, MO). Confluent dishes were trypsinized and resuspended in identical culture medium. A homogenous cell suspension was plated on polylysine-coated 35-mm plastic dishes (2 ml/dish) and placed in the 37°C incubator. Two hours later, the medium from 3 dishes was aspirated and replaced with 1 ml of an HEPES-buffered isosmotic saline containing 100 μM ouabain (Na/K pump inhibitor), and 500 μM N-ethylmaleimide (K-Cl cotransporter activator), at room temperature for 15 min preincubation. The medium was then aspirated and replaced with 1 ml of saline containing 100 μM ouabain, 1 μCi/ml 86Rb, in the presence or absence of drugs (0.1- 30 μM) for a 15-min uptake period. Next, the radioactive medium was aspirated and the cells washed three times with ice-cold saline. Eight groups of 3 dishes (triplicate measurements) were staggered every 2 minutes to allow precise uptake measurements. At the end of the experiment, the cells were lysed with 500 μl 0.25N NaOH for 1 h, and neutralized with 250 μl glacial acetic acid. A 300 μl aliquot per dish was used for β-scintillation counting and a 30 μL aliquot was used for Bradford protein assay (BioRad, Hercules, CA). KCC2-mediated K+ influx was expressed as pmole K+ mg protein−1 min−1. Data were fitted, by nonlinear regression sigmoidal dose-response, using Prizm 3.0 (GraphPad, San Diego, CA).
Drugs
The synthesis of D4 (PubChem, CID7211972) was previously described in Delpire et al. (13). Active and inactive variants of D4 were synthesized by the Vanderbilt Institute of Chemical Biology, Chemical Synthesis Core, Vanderbilt University, Nashville, TN. Bumetanide and DIOA (2-[[(2S)-2-butyl-6,7-dichloro-2-cyclopentyl-1-oxo-3H-inden-5-yl]oxy]) were obtained from Sigma (St. Louis, MO). All drugs were resuspended in dimethylsulfoxide as 50 mM stock solutions, diluted in saline to appropriate concentration, and filtered sterilized using Millipore Millex 0.22 mm syringe filters (Millipore, Bedford, MA).
Statistical Analysis
All statistics were performed using Instat 3.01 (GraphPad, San Diego, CA). Because all data passed the normality test with P > 0.05 using the method of Kolmogorov and Smirnov, t tests instead of nonparametric tests were used. Because the behavior was recorded for each mouse before and after drug injection, our data are first presented as paired values and analyzed using paired t tests. To account for repeated measure differences that go in opposite directions, our data are also presented as averages and analyzed using unpaired t tests. The two statistical analyses reach similar conclusions. Finally, to ensure consistency between sessions, we analyzed our baseline data using repeated measure ANOVA and found no significant differences between sessions (P > 0.5). Repeated measure ANOVA was also used to analyze the time course data.
RESULTS
The purpose of this study was to develop a method to deliver small molecules to the subarachnoid space of the spinal cord of a mouse, and measure their effect on heat-evoked nociceptive responses. The biological targets were two cation-chloride cotransporters, KCC2 and NKCC1. In a previous study, we identified a highly potent and selective inhibitor of KCC2 (D4), and identified several putative structural variants with little to no effect on the cotransporter (13). To demonstrate the potency of D4 on KCC2, and the absence of inhibitory effect of D4.14 (a structurally related variant of D4) and of bumetanide on KCC2, we provide concentration response curves for all three compounds on KCC2-mediated K+ influx, as measured in HEK293 cells (Figure 3). Note the half maximum inhibition of KCC2 by D4 in the submicromolar range and the complete absence of inhibition of D4.14 and bumetanide at concentrations up to 30 μM.
Fig. 3.
Concentration-response curves for test drugs in HEK293 cells over-expressing KCC2. D4 suppresses more than 50% of KCC2 activity at a concentration lower than 1 μM. Bumetanide and D4.14 have no effect on KCC2 function at similar concentrations. It should be noted that bumetanide inhibits KCC2 at higher concentrations which are not shown on this graph (EC50 = 655 μM (Ref 13)). The chemical structures of D4 and D4.14 are illustrated to show that they differ only in an extra methylene group separating the phenyl and pyridazine rings and a repositioning of the methyl group on the thiazol ring (arrows).
Intrathecal catheters were successfully placed in ten mice using the procedure described in the Materials and Methods section. Gross motor function was examined one day after catheter implantation by observing for any gait dysfunction, weakened hindlimb withdrawal reflex, or limited toe spread. One mouse was noted to have significant left hindlimb paresis and was euthanized. In addition, the catheter of another mouse had been displaced overnight and was nonfunctional. This mouse was also euthanized.
For the initial hotplate assay, a temperature of 52°C was selected based on the statistically significant response observed between wild-type and SPAK knockout mice (15). For this experiment, the mice were slowly injected with 8 μl saline containing dimethyl sulfoxide (as vehicle for drugs) and tested at two time points: 20 min and 80 min after intrathecal injection. These two time points were chosen based on the time effect of furosemide and bumetanide on capsaicin-induced nociceptive response (16), and the 1 h recovery period between hotplate assay sessions. After baseline measurements, the same mice were injected with 8 μl of the active KCC2 inhibitor (D4), resulting in a final drug concentration of 20 μM, and the assays performed again at the two time points. As seen in Figure 4A, there was no statistical difference in the pain response times between the vehicle and D4, 20 min after injection. However, there was a small (13%) but statistically significant decrease in withdrawal latency in mice injected with D4 after 80 min. Based on these results, we increased the hotplate temperature to 55°C and omitted the 20-min measurement. Increasing the hotplate temperature had been shown in previous work to lead to more significant differences in pain latencies (5, 15).
Fig. 4.
A) Effect of D4 on nociception as measured by the hotplate assay at 52°C. Panels on the left show paired individual test measurements after 20 min and 80 min vehicle or D4 injection. There was no statistical difference (P = 0.83, paired t test, 7 degree freedom) between vehicle and D4 at 20 min after injection. At 80 min after D4 injection, the difference in withdrawal latency became statistically significant (P = 0.04, paired t test, 7 degree freedom). Panels on the right show means and SEM (n = 8). Unpaired t tests were also used to compare consolidated data, yielding P = 0.91 at 20 min and P = 0.14 at 80 min (14 degrees of freedom). ns = not statistically significant; * = statistically significant. B) Effect of D4 (active compound) and D4.14 (inactive compound) on nociception as measured by the hotplate assay at 55°C. Panels on the left show paired individual test measurements after 80 min vehicle, D4, and D4.14 injections. At 80 min after D4 injection, the difference in response latency was highly statistically significant (P = 0.009, paired t test, 7 degree freedom), while D4.14 did not have an effect on pain perception (P = 0.78, paired t test, 7 degree freedom). Panels on the right show means and SEM (n = 8). Unpaired t tests were also used to compare consolidated data, yielding P = 0.007 for D4 and P = 0.8 at 80 min for D4.14 (14 degrees of freedom). ns = not statistically significant; ** = highly statistically significant.
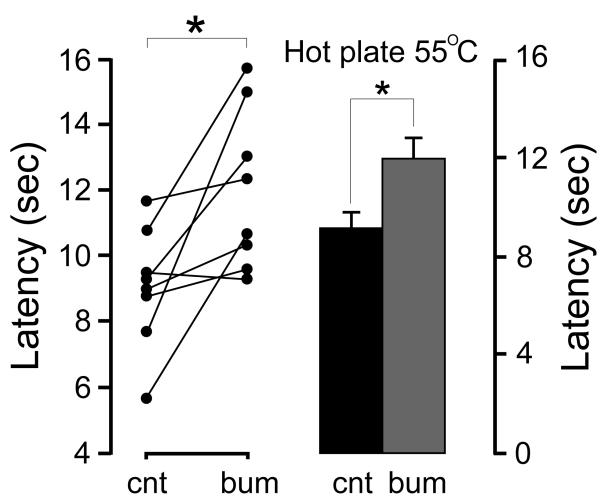
When measured at 55°C, we observed a larger decrease in withdrawal latency (28%) after D4 injection, compared to vehicle (Figure 4B). Importantly, there was no appreciable difference in pain withdrawal after injection of the inactive KCC2 compound (Figure 4B). To determine the duration of the D4 effect, we performed a time course experiment using a separate cohort of animals. After injection of the KCC2 inhibitor, the hotplate assay was performed after 50, 110, 170, and 290 min. Figure 5A shows these time points as well as the 20 and 80 min time points of the previous experiment. As seen in the Figure, the D4 effect is no longer observed after 3 and 6 h. Next, in order to assess the potency of D4 in vivo, we diluted the compound to reach final concentrations of 10, 5, 2.5, and 1 μM. As seen in Figure 5B, there was no statistical difference at 1□M, whereas the effect became statistically significant at 2.5 μM and higher. We also tested DIOA, a known KCC2 inhibitor, at a concentration of 10 μM and observed no decrease in withdrawal latency (9.93 ± 2.07 versus 10.35 ± 2.21, P = 0.46). To address the participation of the Na-K-2Cl cotransporter in nociception, we tested the effect of 20 μM bumetanide. At this concentration, the loop diuretic inhibits NKCC1 with high selectivity, without affecting the activity of KCC2 (Figure 3). Bumetanide significantly increased withdrawal latency, compared to vehicle (Figure 6). To allow ample time for drug redistribution and metabolism, we waited approximately 24 h between injections of the test drugs. Individual control times were compared to one another to ensure consistent baseline pain response before injection of test drug. Statistical analysis of the baseline measurements showed no significant difference between them.
Fig. 5.
A) Time course of D4 effect. Latency to respond to noxious 55°C heat stimulus was measured at various time points ranging from 20 to 300 min after D4 injection. As boxed data points are from the first cohort of mice, there was ample time between hotplate sessions. Repeated measure ANOVA showed significant differences between data points (P = 0.0255), (*) Tukey-Kramer Multiple Comparisons Test with P < 0.05. B) D4 effect at different concentrations. Latency to respond to 55°C heat stimulus was measured at various D4 concentrations ranging from 1 to 20 μM. Squares represent vehicle injection, whereas circles represent drug injection. Data points are mean ± SEM (n = 8 mice). The boxed data point is from the first cohort of mice. Paired t-tests provided P values of 0.2962, 0.0045, 0.0044, 0.0027, 0.0086, for 1, 2.5, 5, 10, and 20 μM, respectively. (**) P < 0.01.
Fig. 6.
Effect of bumetanide on nociception as measured by hotplate assay at 55°C. The panel on the left shows paired individual test measurements after 80 min vehicle and bumetanide injections. At 80 min after bumetanide injection, the difference in withdrawal latency was statistically significant (P = 0.02, paired t test, 7 degrees of freedom). The panel on the right shows means and SEM (n = 8). An unpaired t test was also used to compare the consolidated data, yielding P = 0.02 for bumetanide (14 degrees of freedom). ns = not statistically significant; * = statistically significant.
To provide an independent measure of heat-evoked nociception, we next used tail flick assays. These assays were performed at a temperature of 49°C. This temperature was selected based on previous studies showing a baseline latency of 3-4 sec at a temperature of 52°C (15). Because we were anticipating shorter latencies upon KCC2 inhibition, we slightly decreased the bath temperature to obtain more appropriate baseline times. While performing these assays, we noted that one of the intrathecal catheters leaked during injection. This mouse was subsequently euthanized, decreasing the total number of animals to seven. Similar to the hotplate assay results, we observed a statistically significant decrease in the pain response time after injection of D4, and no statistical difference in withdrawal latency after injection of the inactive compound (Figure 7A). Finally, we observed a small but statistically significant increase in withdrawal latency after injection of the NKCC1 inhibitor, bumetanide (Figure 7B). As for the hotplate assays, a 24 h recovery period was used between drug injections to allow for drug clearance, and there was no statistical difference between the different baseline measurements.
Fig. 7.
A) Effect of D4 (active compound) and D4.14 (inactive compound) on nociception as measured by the tail flick assay at 49°C. Panels on the left show paired individual test measurements after 80 min vehicle, D4, D4.14, bumetanide injections. At 80 min after D4 injection, the difference in withdrawal latency was statistically significant (P = 0.02, paired t test, 6 degrees of freedom), while D4.14 did not have an effect on pain perception (P = 0.42, paired t test, 6 degrees of freedom). Panels on the right show means and SEM (n = 7). Unpaired t tests were also used to compare consolidated data, yielding P = 0.02 for D4 and P = 0.97 at 80 min for D4.14 (13 and 12 degrees of freedom, respectively). B) Effect of bumetanide on nociception as measured by the tail flick assay at 49°C. The panel on the left shows paired individual test measurements after 80 min vehicle and bumetanide injections. At 80 min after bumetanide injection, the withdrawal latency was reduced, although not quite statistically significant (P = 0.059, paired t test, 6 degrees of freedom). The panel on the right shows means and SEM (n = 7). An unpaired t test was also used to compare the consolidated data, yielding P = 0.046 for bumetanide (12 degrees of freedom), which was in this case statistically significant. ns = not statistically significant; * = statistically significant.
DISCUSSION
The present study describes a simple surgical procedure which allows repeated delivery of small molecules in the intrathecal space of mice. The study also uses this methodology to address the role of the neuronal-specific K-Cl cotransporter (KCC2) on nociception, through the injection of a novel KCC2 inhibitory compound. We chose to place a catheter instead of repeated needle injection to minimize traumatic injury and guarantee that all injections were placed in the same location within the intrathecal space. The intrathecal catheter procedure was simplified from the method of Wu et al. (14). The authors had demonstrated that the placement of a catheter in the L5-L6 interspace did not produce morphological changes under light microscopy for up to 12 days. First, we used a commercially available catheter that allows easy modification, resulting in reproducible results. Second, we performed only a small incision, reducing postoperative pain and possibility of complications. Third, by not tunneling the catheter, we were able to shorten it and decrease the dead-space volume. Fourth, by using Tegaderm to sterilely cap the catheter instead of repeatedly melting and cutting the tip, we were able to keep the outside portion small. Only one animal out of 10 displaced the catheter immediately after the procedure. Therefore, this minimally invasive procedure shortened the recovery time and allowed for testing 2 days postimplantation.
The placement of the catheter facilitated the delivery of compounds to the subarachnoid space of the spinal cord and the testing of a novel small molecule that inactivates KCC2 but not NKCC1. Pharmacological separation of KCC2 and NKCC1 activities was difficult in the past due to the fact that high concentrations of furosemide inhibited both transport mechanisms. In this study, we tested the effect of D4 (PubChem, CID7211972), at a final concentration of 20 μM, as well as an inactive variant of the compound, to serve as control, on the response of the mouse to nociceptive stimuli. The final drug concentration was estimated based on a cerebrospinal fluid volume of 35-40 μl in an adult mouse (17-19). Using ion fluxes in KCC2-overexpressing HEK293 cells, we show complete inhibition of KCC2 with 20 μM D4, whereas no effect of D4.14 or bumetanide at that concentration. As D4.14 is structurally related to D4, but inactive, this compound constitutes a perfect negative control for this experiment. Furthermore, as bumetanide does not affect KCC2 at 20 μM, but completely inhibits NKCC1 at that concentration (for review see (20)), the use of D4 and bumetanide can nicely separate the functional activities of KCC2 and NKCC1. We therefore tested the effect of D4, D4.14, and bumetanide on nociception using hotplate and tail flick assays. Both tests yielded similar data with D4 and bumetanide resulting in opposite effects on the latency to respond to the heat stimuli, while D4.14 showed no effect. The potency of D4 was demonstrated by diluting the compound to a concentration of 2.5 μM and still observing the effect. The decreased response latency to the noxious stimuli upon KCC2 inhibition is consistent with previous data showing increased nociception associated with reduction in KCC2 expression (1, 8-12), with the exception of one study using KCC2 hypomorphic mice, which showed increased latency to heat stimulus or a hyposensitivity (7). In that particular case, however, KCC2 was genetically manipulated from conception, and compensatory mechanisms at play during development could have affected the nociceptive response of these animals. We also tested the effect of DIOA at 10 μM. Although this compound in vitro inhibits K-Cl cotransport in the low micromolar range, we did not observe any significant effect. This lack of effect could be due to a reduced potency of the alkaloic acid or to the nonselectivity of this compound. Indeed, DIOA inhibits not only K-Cl cotransport, but also organic solute transport (21), K+ (22) and Cl− (23) conductances, as well as a K+(Na+)/H+ exchanger (24). Therefore, the different effect of DIOA and D4 found in this study highlights the research benefits of using an agent with improved potency and specificity. The lack of a DIOA effect could also have been due to the possible inhibition of NKCC1 (13), which could negate the effect of KCC2 inhibition. In fact, opposite to KCC2 inhibition, we observed increased response latency to the heat stimuli upon NKCC1 inhibition with low dose of bumetanide. These bumetanide data are consistent with previous data showing decreased nociception associated with the knockout of NKCC1 (5, 25), and the antinociceptive effect of NKCC1 inhibitors in a formalin model of tissue injury-induced pain (16). The data are also consistent with a previous study showing that spinal administration of bumetanide reduces dorsal root reflex activity, mechanical allodynia, and hyperalgesia produced by intradermal injection of capsaicin (26).
The tail flick and hotplate assays are two methods of assessing pain response in mice that have been well validated and standardized (27-31). Both of these tests are based on spinal reflexes that require segmental connections and a propriospinal connection from dorsal horn neurons to ventral motor neurons (28, 30). Latencies in these reflexes are dependent on the activation time of cutaneous thermal nociceptors, the conduction time of this impulse through dorsal horn neurons and the central nervous system, and the time for impulse transmission through ventral horn neurons with activation of effector muscles. In addition, supraspinal centers modulate these reflexes through excitatory or inhibitory effects on dorsal horn interneurons. Since KCC2 is expressed in postsynaptic relay neurons which are intrinsic to these reflex arcs (2, 3), these two pain sensitivity tests are appropriate for measuring KCC2 activity. It should be noted that since the mice are restrained during the tail flick assay, and restraint is a stressful event (32, 33), additional factors (e.g., hormonal, psychological) might modulate the nociceptive response. In this case, because the genetic background of all mice is identical, this variable should similarly affect all mice in this cohort. Because the hotplate assay does not have this complication, it should be preferred when selecting only one paradigm for screening of drug effects on nociception.
In summary, inhibition of the K-Cl cotransporter, KCC2, led to decreased heat-evoked response latency in mice, indicating nociceptive hypersensitivity, while inhibition of the Na-K-2Cl cotransporter, NKCC1, resulted in increased response latencies to heat stimuli, indicating hyposensitivity to pain.
Acknowledgments
Funding: NIH grant GM74771 to Eric Delpire
Footnotes
The authors declare no conflicts of interest.
Reprints will not be available from the authors.
DISCLOSURES:
Name: Thomas M Austin, M.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Thomas M Austin has seen the original study data, reviewed the analysis of the data, and approved the final manuscript.
Name: Eric Delpire, Ph.D.
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript.
Attestation: Eric Delpire has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files.
This manuscript was handled by: Quinn H. Hogan, MD
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thomas M Austin, Department of Anesthesiology, Vanderbilt University Medical School, Nashville, TN
Eric Delpire, Departments of Anesthesiology and Molecular Physiology and Biophysics, Vanderbilt University Medical School, Nashville, TN
REFERENCES
- 1.Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–42. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- 2.Boulenguez P, Liabeuf S, Bos R, Bras H, Jean-Xavier C, Brocard C, Stil A, Darbon P, Cattaert D, Delpire E, Marsala M, Vinay L. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat Med. 2010;16:302–7. doi: 10.1038/nm.2107. [DOI] [PubMed] [Google Scholar]
- 3.Stil A, Jean-Xavier C, Liabeuf S, Brocard C, Delpire E, Vinay L, Viemari JC. Contribution of the potassium-chloride co-transporter KCC2 to the modulation of lumbar spinal networks in mice. Eur. J. Neurosci. 2011;33:1212–22. doi: 10.1111/j.1460-9568.2010.07592.x. [DOI] [PubMed] [Google Scholar]
- 4.Alvarez-Leefmans FJ. Chloride transporters in presynaptic inhibition, pain and neurogenic inflammation. In: Alvarez-Leefmans FJ, Delpire E, editors. Physiology and Pathology of Chloride Transporter and Channels in the Nervous System: From molecules to diseases. Academic Press; London: 2009. pp. 439–70. [Google Scholar]
- 5.Sung K-W, Kirby M, McDonald MP, Lovinger DM, Delpire E. Abnormal GABAA-receptor mediated currents in dorsal root ganglion neurons isolated from Na-K-2Cl cotransporter null mice. J. Neurosci. 2000;20:7531–8. doi: 10.1523/JNEUROSCI.20-20-07531.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delpire E, Austin TM. Kinase regulation of Na+-K+-2Cl− cotransport in Primary afferent neurons. J Physiol. 2010;588:3365–73. doi: 10.1113/jphysiol.2010.190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tornberg J, Voikar V, Savilahti H, Rauvala H, Airaksinen MS. Behavioural phenotypes of hypomorphic KCC2-deficient mice. Eur. J. Neurosci. 2005;21:1327–37. doi: 10.1111/j.1460-9568.2005.03959.x. [DOI] [PubMed] [Google Scholar]
- 8.Mantyh PW, Hunt SP. Setting the tone: superficial dorsal horn projection neurons regulate pain sensitivity. Trends Neurosci. 2004;27:582–4. doi: 10.1016/j.tins.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Nomura H, Sakai A, Nagano M, Umino M, Suzuki H. Expression changes of cation chloride cotransporters in the rat spinal cord following intraplantar formalin. Neurosci. Res. 2006;56:435–40. doi: 10.1016/j.neures.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Liu L-Y, Xu T-L. Reduced potassium-chloride co-transporter expression in spinal cord dorsal horn neurons contributes to inflammatory pain hypersensitivity in rats. Neuroscience. 2008;152:502–10. doi: 10.1016/j.neuroscience.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 11.Jolivalt CG, Lee CA, Ramos KM, Calcutt NA. Allodynia and hyperalgesia in diabetic rats are mediated by GABA and depletion of spinal potassium-chloride co-transporters. Pain. 2008;140:48–57. doi: 10.1016/j.pain.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miletic G, Miletic V. Loose ligation of the sciatic nerve is associated with TrkB receptor-dependent decreases in KCC2 protein levels in the ipsilateral spinal dorsal horn. Pain. 2008;137:532–9. doi: 10.1016/j.pain.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delpire E, Days E, Mi D, Lewis M, Kim K, Lidnsley C, Weaver CD. Small molecule screen identifies inhibitors of the neuronal K-Cl cotransporter KCC2. Proc Natl Acad Sci U S A. 2009;106:5383–8. doi: 10.1073/pnas.0812756106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu WP, Xu XJ, Hao JX. Chronic lumbar catheterization of the spinal subarachnoid space in mice. J Neurosci Methods. 2004;133:65–9. doi: 10.1016/j.jneumeth.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Geng Y, Byun N, Delpire E. Behavioral Analysis of Ste20 Kinase SPAK Knockout Mice. Behavioural Brain Res. 2010;208:377–82. doi: 10.1016/j.bbr.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granados-Soto V, Arguelles CF, Alvarez-Leefmans FJ. Peripheral and central antinociceptive action of Na–K–2Clcotransporter blockers on formalin-induced nociception in rats. Pain. 2005;114:231–8. doi: 10.1016/j.pain.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 17.Rudick RA, Zirretta DK, Herndon RM. Clearance of albumin from mouse subarachnoid space: a measure of CSF bulk flow. J Neurosci Methods. 1982;6:253–9. doi: 10.1016/0165-0270(82)90088-7. [DOI] [PubMed] [Google Scholar]
- 18.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:1–32. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Partdridge WM. Transnasal and intraventricular delivery Peptide Drug Delivery to the Brain. Raven Press; New York: 1991. p. 357. [Google Scholar]
- 20.Russell JM. Sodium-Potassium-Chloride Cotransport. Physiol. Rev. 2000;80:211–76. doi: 10.1152/physrev.2000.80.1.211. [DOI] [PubMed] [Google Scholar]
- 21.Culliford SJ, Bernhardt I, Ellory JC. Activation of a novel organic solute transporter in mammalian red blood cells. J Physiol. 1995;489(Pt 3):755–65. doi: 10.1113/jphysiol.1995.sp021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lauf PK, Misri S, Chimote AA, Adragna NC. Apparent intermediate K conductance channel hyposmotic activation in human lens epithelial cells. Am. J. Physiol. Cell Physiol. 2008;294:C820–C32. doi: 10.1152/ajpcell.00375.2007. [DOI] [PubMed] [Google Scholar]
- 23.Svoboda N, Pruetting S, Grissmer S, Kerschbaum HH. cAMP-dependent chloride conductance evokes ammonia-induced blebbing in the microglial cell line, BV-2. Cell Physiol Biochem. 2009;24:53–64. doi: 10.1159/000227813. [DOI] [PubMed] [Google Scholar]
- 24.Weiss E, Lang HJ, Bernhardt I. Inhibitors of the K+(Na+)/H+ exchanger of human red blood cells. Bioelectrochemistry. 2004;62:35–40. doi: 10.1016/j.bioelechem.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 25.Laird JM, Garcia-Nicas E, Delpire EJ, Cervero F. Presynaptic inhibition and spinal pain processing in mice: a possible role of the NKCC1 cation-chloride co-transporter in hyperalgesia. Neurosci. Lett. 2004;361:200–3. doi: 10.1016/j.neulet.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 26.Valencia-de Ita S, Lawand NB, Lin Q, Castaneda-Hernandez G, Willis WD. Role of the Na+-K+-2Cl− cotransporter in the development of capsaicin-induced neurogenic inflammation. J. Neurophysiol. 2006;95:3553–61. doi: 10.1152/jn.01091.2005. [DOI] [PubMed] [Google Scholar]
- 27.Dewey WL, Snyder JW, Harris LS, Howes JF. The effect of narcotics and narcotic antagonists on the tail-flick response in spinal mice. J Pharm Pharmacol. 1969;21:548–50. doi: 10.1111/j.2042-7158.1969.tb08312.x. [DOI] [PubMed] [Google Scholar]
- 28.Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- 29.Dourish CT, O’Neill MF, Coughlan J, Kitchener SJ, Hawley D, Iversen SD. The selective CCK-B receptor antagonist L-365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur J Pharmacol. 1990;176:35–44. doi: 10.1016/0014-2999(90)90129-t. [DOI] [PubMed] [Google Scholar]
- 30.Karl T, Pabst R, von Horsten S. Behavioral phenotyping of mice in pharmacological and toxicological research. Exp Toxicol Pathol. 2003;55:69–83. doi: 10.1078/0940-2993-00301. [DOI] [PubMed] [Google Scholar]
- 31.Gustafsson LL, Post C, Edvardsen B, Ramsay CH. Distribution of morphine and meperidine after intrathecal administration in rat and mouse. Anesthesiology. 1985;63:483–9. doi: 10.1097/00000542-198511000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Flint MS, Carroll JE, Jenkins FJ, Chambers WH, Han ML, Baum A. Genomic profiling of restraint stress-induced alterations in mouse T lymphocytes. J Neuroimmunol. 2005;167:34–44. doi: 10.1016/j.jneuroim.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Dhabhar FS, Miller AH, Stein M, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]