Abstract
The link between social influence and drug abuse has long been established in humans. However, preclinical animal models of drug abuse have only recently begun to consider the role of social influence. Since social factors influence the initiation and maintenance of drug use in humans, it is important to include these factors in preclinical animal models. The current study examined the effects of the presence of a social partner on responding for sucrose pellets under various motivational conditions, as well as on d-amphetamine (AMPH) self-administration. Rats were trained to lever press for either sucrose pellets or AMPH (0.01 or 0.1 mg/kg/infusion unit dose). Following response stability, a novel same-sex conspecific was presented in an adjacent compartment separated by a clear Plexiglas wall, and responding for sucrose or AMPH reward was measured. Rats were allowed to restabilize, and were subsequently given an additional partner presentation. Presence of the social partner increased responding only during the first partner pairing in the AMPH 0.1 mg/kg/infusion unit dose, whereas inhibition of responding was observed during the first partner pairing during access to the 0.01 mg/kg/infusion unit dose. Under free feed conditions, inhibition of sucrose pellet responding was observed in the presence of the social partner, but this effect was attenuated under food restriction. In contrast, the results demonstrate social facilitation of AMPH self-administration at a high unit dose, thus extending the influence of social factors to an operant conditioning task. This model of social facilitation may have important implications as a preclinical model of social influence on drug abuse.
Keywords: Social Facilitation, d-Amphetamine, Self-Administration, Rat
The impact of social factors on drug abuse in humans is well documented (e.g., Kandel 1985). Peer influence has been identified as one of the most significant factors in the use of legal and illegal substances in adolescence (Glynn 1981; Kandel et al. 1978). Indeed, adolescence is a critical developmental period in which social interaction is high, and there is heightened risk taking and sensation seeking, thus rendering individuals more vulnerable to initiate use of alcohol and drugs (Spear 2000; Bava and Tapert 2010). According to Kandel (1985), adolescent use of drugs is subject to peer influence, with initiation of marijuana use being most heavily influenced by peers compared to other drugs of abuse.
Social influence also is a factor involved in increased risk taking, specifically in adolescence (Gardner & Steinberg 2005). In a study examining risk taking in adolescent versus adult human peer groups, Gardner and Steinberg (2005) found that adolescents are more likely to take risks and exhibit risky decision making in a driving task when in the presence of peers than when alone compared to their adult counterparts, thus supporting the notion that peer influence plays a role in adolescent risky behavior and impulsivity (Steinberg et al. 2008). Because impulsivity has been linked to drug use and abuse as both a determinant and a consequence (de Wit 2009), it is important to examine potential sources of increased impulsivity and risk taking in both clinical and preclinical drug abuse research.
Preclinical animal models of drug abuse have largely ignored social influence, until recently (see Newman et al. 2007; Fritz et al. 2011). Since social factors influence the initiation and maintenance of drug use in humans (Kandel 1973; 1985), it is important to include these factors in preclinical animal models of drug abuse. Similar to human adolescents, social interactions are rewarding in adolescent rats (Douglas et al. 2004). Recent research has demonstrated that social factors can enhance cocaine- and nicotine-induced conditioned place preference in rats (Thiel et al. 2008; 2009). According to Varlinskaya and Spear (2006), early adolescent rats exhibit higher levels of ethanol-induced facilitation of social behavior, and are less sensitive to the social suppressive effects of high doses of ethanol compared to their mid- or older adolescent counterparts. In addition, ethanol place aversion is attenuated in adult rats when they are given the opportunity to engage in social interaction (Gauvin et al. 1994). Furthermore, social play behavior (play fighting and social investigation) is facilitated following ethanol administration in adolescent rats (Varlinskaya & Spear 2009). Thus, the results from these studies suggest that drugs and social factors interact and contribute to each other's rewarding effects.
The phenomenon of social facilitation of learning a food-reinforced operant task is long established in preclinical research (see e.g., Zentall & Levine 1972). Social learning theory (Zajonc, 1965) suggests that social facilitation occurs because the presence of others induces a drive state that increases the probability of a dominant response. In principle, a dominant response can be defined as any response that an animal makes with a very high probability. Although in practice, a dominant response may not always be easy to define, the distinction is relatively easy to make in the context of an animal making an operant response. Thus, if one places a rat in an operant chamber, prior to training a rat to make a lever press response the dominant response may involve exploratory behavior, grooming, or sleeping. By definition the dominant response would not be lever pressing. However, following training, once the response has been well established and especially if lever pressing is reinforced on an intermittent schedule of reinforcement, it is likely that the dominant response would be lever pressing. Importantly, however, the presence of a conspecific is proposed to enhance lever press responding only in a well-trained animal (i.e., when lever pressing is the dominant response); in contrast, the presence of a conspecific can impair the acquisition of a non-dominant (i.e., to-be-learned) response (Zajonc 1965; Levine & Zentall, 1974).
To date, the impact of social factors during drug self-administration has not been demonstrated in an operant task. The current study examined the effects of a novel conspecific on responding for sucrose pellets and d-amphetamine (AMPH) infusions in adult rats. Rats were trained to respond for either sucrose pellets or AMPH, and then were introduced to a naïve conspecific in an adjacent compartment, separated by a clear Plexiglas wall. According to the predictions of Zajonc’s social facilitation theory, well-trained rats in the current study were hypothesized to increase responding for both sucrose and AMPH reward in the presence of a naïve conspecific.
EXPERIMENT 1
The purpose of the first experiment was to determine if the presentation of a naïve conspecific can facilitate responding for sucrose reward in free fed rats.
Method
Subjects
Seven male Sprague Dawley rats (250–275 g; Harlan, Indianapolis, IN) were single housed in a colony room held at a constant temperature. Light/dark phases were on a 12:12-hr cycle, and all experimentation occurred in the light phase. Rats had unlimited access to food and water in their home cage, were cared for in accordance with the 1996 National Institutes of Health Guide for the Care and Use of Laboratory Animals, and experimental protocols were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
Apparatus
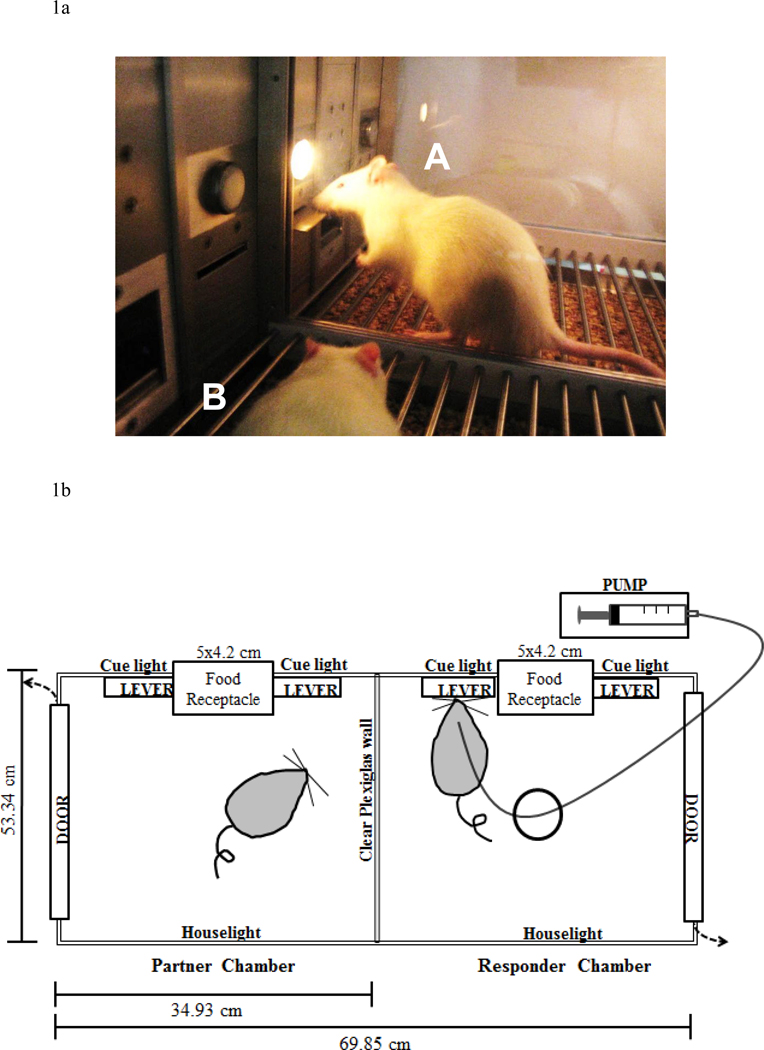
Two connected operant conditioning chambers (custom ordered; MED Associates, St. Albans, VT; see Figures 1a and 1b) were used. The design of the interconnected operant chambers was adapted from Akins, Klein, and Zentall (2002). The front and back walls of the operant chambers were made of aluminum, while the side walls and middle divider were made of Plexiglas. The entire apparatus measured 69.8 cm × 53.3 cm × 60.9 cm (length × width × height), and each individual chamber measured 34.9 cm long. There was a food tray (5×4.2 cm) located in the bottom-center of the front wall of both chambers. A retractable response lever was located on each side of the recessed food tray on the front wall of both chambers. A 28-V white cue light was located 6 cm above each response lever in both chambers. A houselight was mounted in the back wall of both chambers. All responses and scheduled consequences were recorded and controlled by a computer interface using Med-IV software.
Figure 1.
(a) Image of social self-administration chamber. Rats are separated by a Plexiglas divider. The responder rat (rat A) is trained in one compartment to lever press for either food or drug, while the other compartment is vacant until a partner pairing occurs. During a partner pairing, a naïve conspecific (rat B) is placed into the adjacent compartment. (b) Schematic of the social self-administration chamber.
Procedure
Training
Six rats were initially food restricted (15 g daily, given at end of each session) and trained to lever press on a fixed-ratio 1 (FR-1) schedule of reinforcement in one chamber of the connected two-chamber apparatus (the “responder” side of the operant chamber). Rats were given 32 trials per session, in which each response on one lever (active lever; position counterbalanced across rats) yielded the delivery of one sucrose pellet (F0042 sugar-based dustless precision pellets, 45 mg each, Bio-Serv, Frenchtown, New Jersey), followed by a 20-sec timeout period in which the levers remained extended. Responses during the timeout, as well as to the other lever (inactive lever), yielded no programmed consequence. The houselight was illuminated in the adjacent chamber throughout the duration of the experiment. Once responding on the active lever was acquired at an FR-1, the FR response requirement was gradually increased to an FR-5 schedule of reinforcement (with 32 trials per session). Once animals responded stably on FR-5 schedule (< 25% variability on the active lever across 3 sessions; criteria reached for each individual rat), rats were placed on free feed for the remainder of the experiment. Additionally, sessions were one hour in duration, rather than 32 trials, in which animals could earn as many reinforcers as possible within the time limit.
Partner Pairings
Once rats stabilized at an FR-5 schedule (< 25% variability on the active lever across 3 sessions) under free feed conditions, rats were given a partner pairing with a novel conspecific (matched for age, weight, and food restriction condition) in which the conspecific was placed into the adjacent compartment (the “partner” side of the operant chamber) for the duration of one session. Levers remained retracted for the conspecific during all partner pairing sessions. Rats were then given additional sessions without the partner until responding stabilized again to the same criteria. Following stability, rats were given a second partner pairing with the same conspecific.
Statistical Analyses
Data from baseline and partner pairing sessions were analyzed using repeated-measures analyses of variance (ANOVA). Data from the three sessions prior to each partner pairing were averaged and used as baseline, and baseline was recalculated for each additional partner pairing. Appropriate post-hoc analyses (paired samples t tests) were conducted to examine further differences between baseline and partner pairing sessions.
Results
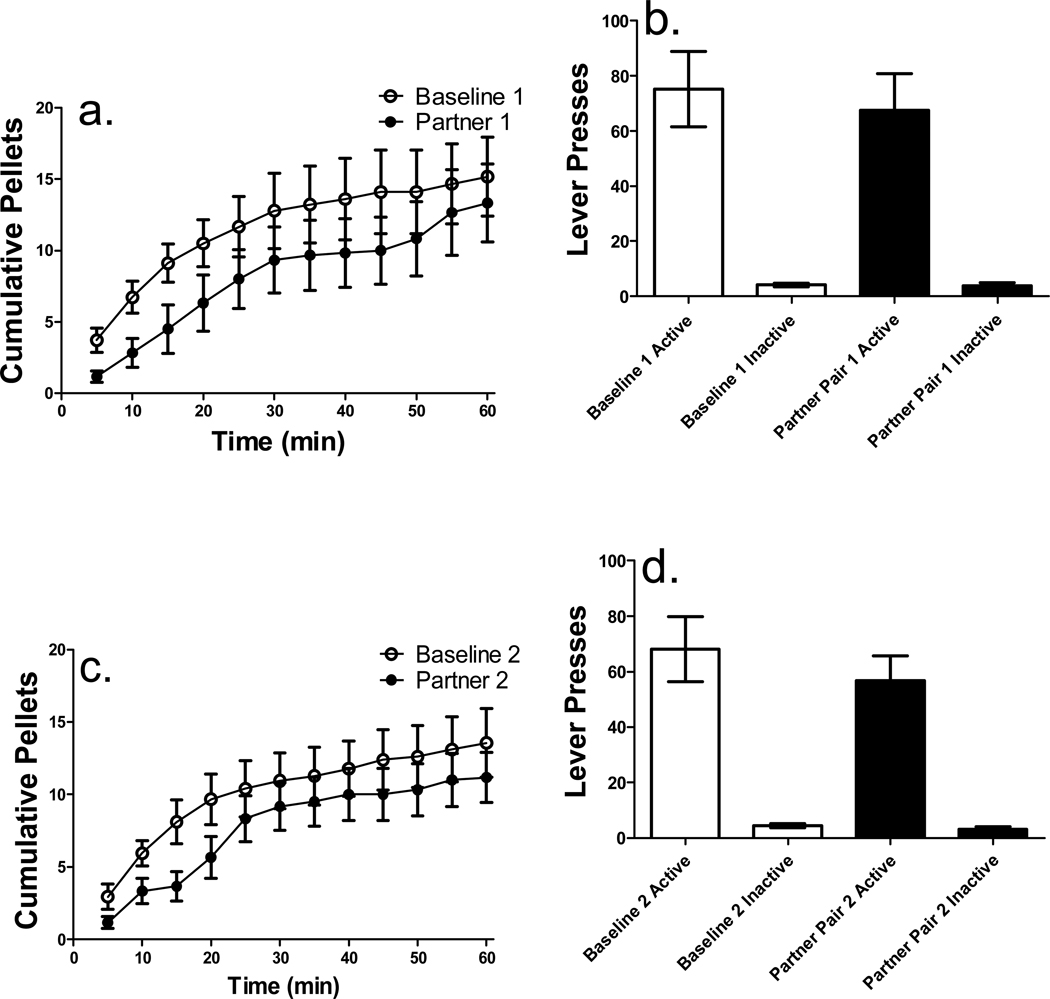
The three sessions in which rats reached stability criteria prior to each partner pairing were averaged, and used as the baseline comparison for each individual pairing (7 ± 1.10 sessions were needed to reach stability prior to the first partner pairing). Cumulative pellets earned on the first baseline and partner pairing sessions revealed that presentation of a novel conspecific under free feed conditions decreased sucrose pellet intake compared to baseline (see Figure 2a). A 2 × 12 (condition × interval) repeated measures ANOVA revealed main effects of condition [F(1, 5) = 6.81, p < .05] and time interval [F(11, 55) = 16.69, p < .001] for the first partner pairing. No significant interactions were found and thus no subsequent post-hoc comparisons were conducted.
Figure 2.
(a) Cumulative pellets earned under free feed conditions for the first baseline and partner pairing and (b) total active and inactive lever presses for the first baseline and partner pairing (n = 6). (c) Cumulative pellets earned for the second baseline and partner pairing and (d) total active and inactive lever presses for the second baseline and partner pairing. There was a significant main effect of lever. (*p < .01; **p < .001 baseline compared to partner pairing at each time point or active compared to inactive and baseline active compared to partner pairing active lever presses). Data expressed as mean ± SEM.
Total number of lever presses on both active and inactive levers for the first partner pairing revealed that active lever pressing was higher than inactive lever pressing on the first baseline and partner pairing sessions (see Figure 2b). A 2 × 2 (condition × lever) repeated-measures ANOVA revealed a significant main effect of lever [F(1, 5) = 28.57, p < .01], indicating that active lever pressing was higher than inactive lever pressing during the first partner pairing. No significant interaction was found, thus no post hoc analyses were conducted.
For the second partner pairing, presentation of the same conspecific as in the first partner pairing inhibited sucrose pellet intake (see Figure 2c for cumulative pellets earned during the second baseline and partner pairing sessions). A 2 × 12 (condition × interval) repeated measures ANOVA revealed significant main effects of condition [F(1, 5) = 5.52, p < .05] and time interval [F(11, 55) = 49.16, p < .001].
Total number of lever presses on both active and inactive levers for the second partner pairing is shown in Figure 2d. Active lever pressing was higher than inactive lever pressing on the second baseline and partner pairing sessions, as revealed by a 2 × 2 (condition × lever) repeated-measures ANOVA in which a significant main effect of lever [F(1, 5) = 25.62, p < .01] was found. No significant interaction was found, thus no post hoc analyses were conducted.
EXPERIMENT 2
The results from Experiment 1 provided no evidence for social facilitation in a food-reinforced operant task. To the contrary, the presence of a social partner tended to disrupt lever pressing on the active lever. The purpose of Experiment 2 was to increase the response rate in the operant task to determine if this would enhance the sensitivity to any disruptive effect of the social partner. In contrast to Experiment 1, the current experiment implemented a restricted food access regimen in order to increase hunger motivation during performance of the operant task.
Method
Subjects
Seven experimentally naive Sprague Dawley rats were used in Experiment 2. Conditions were identical to those used in Experiment 1, except rats were on food restriction throughout the experiment, receiving 15 g of supplemental food in their home cages after each session.
Apparatus
The same apparatus was used as in Experiment 1.
Procedure
The same procedure was used as in Experiment 1, except rats were maintained on food restriction for the duration of the experiment. As in Experiment 1, the same partner was used for both partner pairings.
Statistical Analyses
The same statistical analyses were conducted as in Experiment 1.
Results
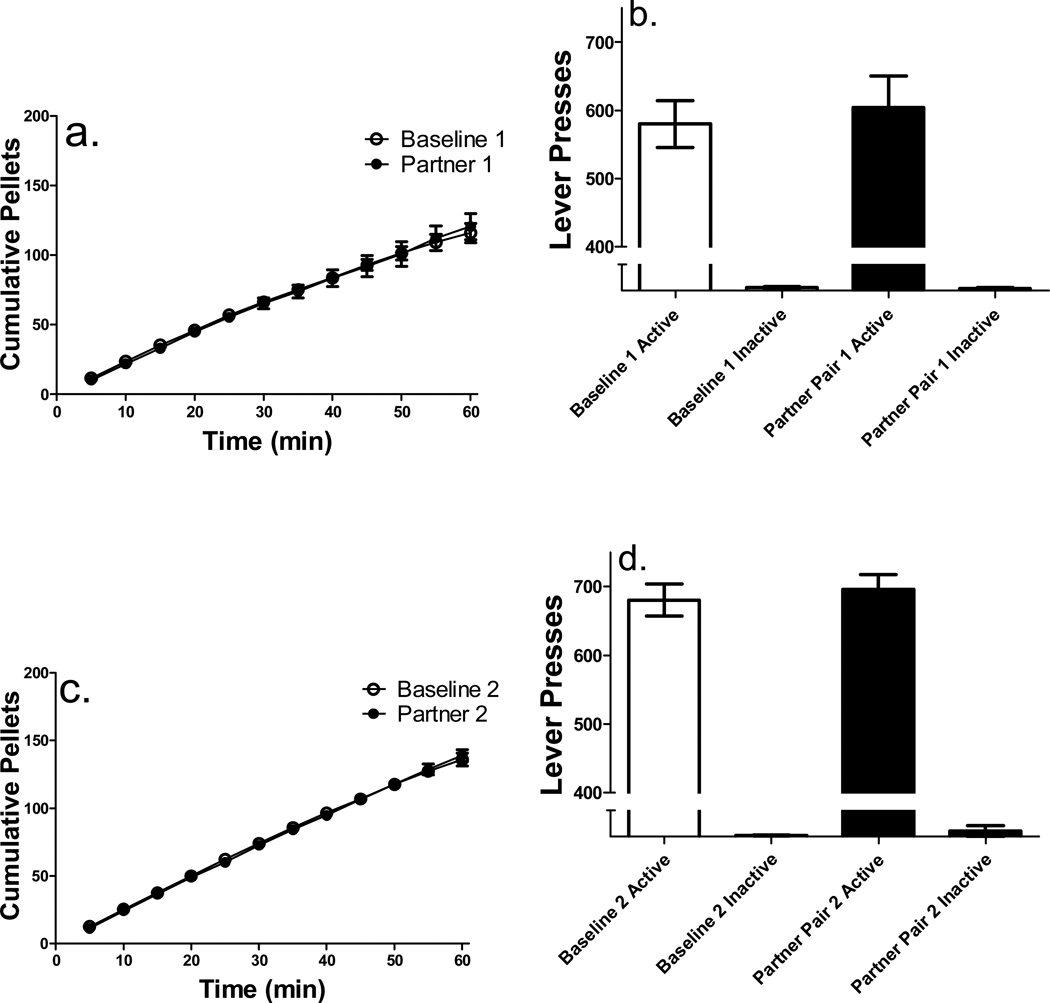
Similar to Experiment 1, the three sessions in which rats reached stability criteria prior to each partner pairing were averaged, and used as baseline for each individual pairing (11 ± 2.82 sessions were needed to reach stability prior to the first partner pairing). Cumulative pellets earned on the first baseline and partner pairing sessions under food restriction conditions revealed no difference in pellet intake, as shown in Figure 3a. A 2 × 12 (condition × interval) repeated-measures ANOVAs conducted on each partner pairing revealed only a significant main effect of time interval [F(11, 55) = 28.83, p < .001], and thus no post-hoc analyses were conducted.
Figure 3.
Cumulative pellets earned and total active and inactive lever presses under conditions of food restriction (n = 6). There was a significant main effect of lever. All details are as in Figure 2.
Total number of lever presses on both active and inactive levers for the first baseline and partner pairing sessions are shown in Figure 3b. A 2 × 2 (condition × lever) repeated-measures ANOVA revealed a significant main effect of lever [F(1, 5) = 429.45, p < .0001], indicating that active lever pressing was higher than inactive lever pressing during the first baseline and partner pairing sessions. No interactions were found, thus no subsequent post hoc analyses were conducted.
During the second partner pairing session under food restriction conditions, there was no difference from baseline in cumulative pellets earned (shown in Figure 3c). A 2 × 12 (condition × interval) repeated-measures ANOVAs conducted revealed only a significant main effect of time interval [F(11, 55) = 30.02, p < .001], thus no post hoc analyses were conducted.
Total number of lever presses on the active lever for both the second baseline and partner pairing sessions were higher than inactive lever presses (see Figure 3d). A 2 × 2 (condition × lever) repeated-measures ANOVA revealed a significant main effect of lever [F(1, 5) = 189.96, p < .0001], however, no interactions were found thus no post hoc analyses were conducted.
EXPERIMENT 3
The purpose of Experiment 3 was to determine if the presentation of a naïve conspecific can facilitate responding for AMPH (either 0.01 or 0.1 mg/kg/infusion, within subject) in a rat trained to self-administer AMPH.
Method
Subjects
Six experimentally naïve male Sprague Dawley rats were used in Experiment 3. Home cage conditions were identical to those used in Experiment 2.
Apparatus
The same apparatus was used as in Experiments 1 and 2, except drug reinforcer was delivered via a silastic tube from an infusion pump located outside of the responder side of the two-compartment operant chamber.
Procedure
A similar training procedure was used as in Experiments 1 and 2, except rats were maintained on an FR-1 schedule of reinforcement throughout the duration of Experiment 3. Rats were initially food trained under conditions of food restriction prior to surgery. Once rats acquired responding at an FR-1, rats were given unlimited access to food in their home cages for 3 days and then underwent jugular catheterization surgery.
Surgery
Rats were treated with the non-opioid analgesic carprofen (5 mg/kg, s.c.) prior to surgery for implantation of a chronic indwelling jugular catheter. While under anesthesia (100 mg/kg ketamine, 5 mg/kg diazepam, i.p.), a catheter was inserted into the jugular vein and exited via a metal cannula stabilized in a headmount made of dental acrylic. A silastic leash attached an infusion pump to the headmount during the self-administration sessions. Rats were given one week to recover from surgery, and a mixture of gentamicin (0.2 ml), heparin (0.6 ml), and saline (20 ml) was used to flush catheters daily.
Self-Administration Training
Following recovery from surgery, rats were trained to self-administer AMPH (either 0.01 or 0.1 mg/kg/infusion) on an FR-1 schedule of reinforcement (counterbalanced such that half of the rats were trained on the 0.01 mg/kg/infusion dose to stability first, and the other half were trained on the 0.1 mg/kg/infusion dose to stability first). Sessions began with the presentation of both the active and inactive levers. Responses on the active lever resulted in illumination of both cue lights and a 0.1 ml infusion of AMPH delivered across 5.9 sec, followed by a 20-sec time-out period in which both cue lights were illuminated. Responses to the inactive lever had no programmed consequence and responses on either lever during the time-out period also had no programmed consequence. Following the end of the time-out period, the cue lights turned off and responses on the active lever again resulted in an AMPH infusion.
Partner Pairings
Following response stability (< 25% variability across 3 sessions), rats were exposed to a novel conspecific in the connected chamber for one session (similar to Experiments 1 and 2), and responding was measured. Rats were allowed to reestablish a stable baseline, and were then given a second partner pairing. Following the second partner pairing, doses were switched such that rats receiving AMPH at a unit dose of 0.01 mg/kg/infusion initially were given AMPH at a unit dose of 0.1 mg/kg/infusion for the duration of the experiment, and rats receiving AMPH 0.1 mg/kg/infusion initially were given AMPH 0.01 mg/kg/infusion for the duration of the experiment. Rats were allowed to reestablish a stable baseline on the new dose (< 25% variability across 3 sessions), and were given a partner pairing with the same partner. Rats were again allowed to reestablish a stable baseline following the first partner pairing on the new dose, and were then given a second partner pairing.
Statistical Analyses
The same statistical analyses were used as in Experiments 1 and 2.
Results
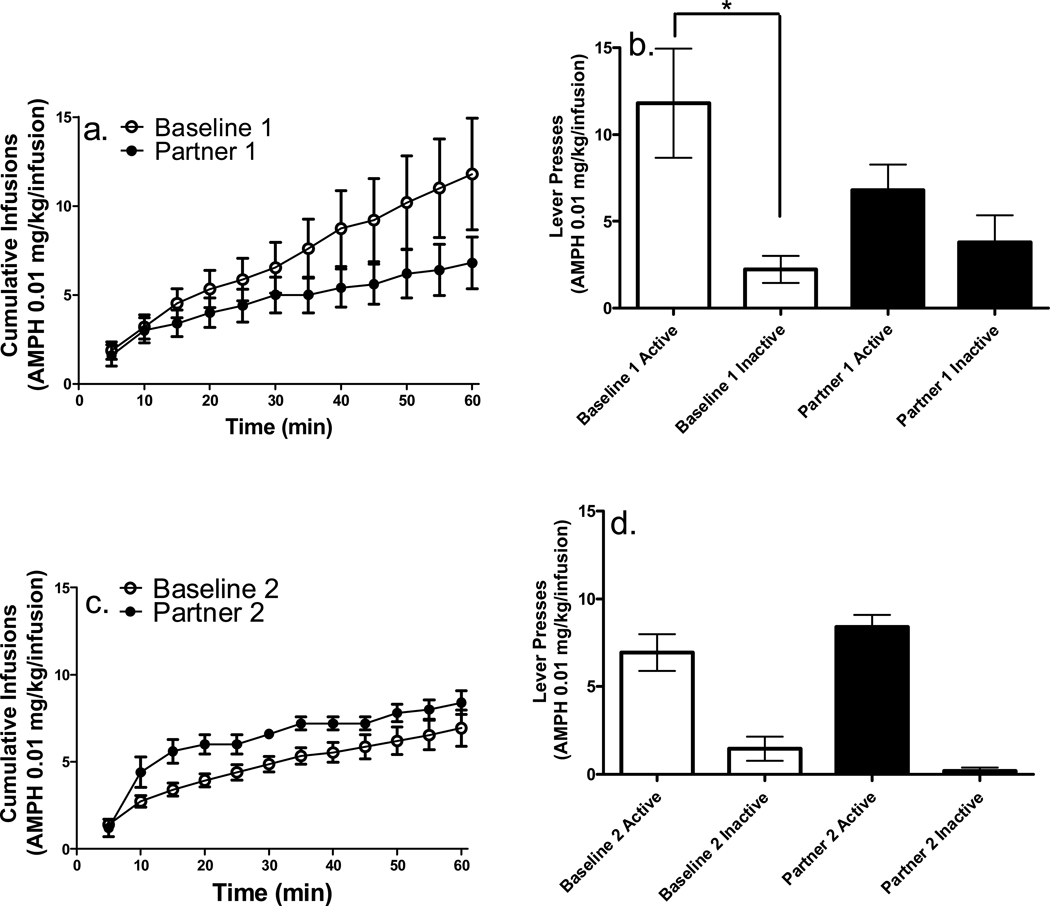
Similar to Experiments 1 and 2, the three sessions in which rats reached stability criteria prior to each partner pairing were averaged, and used as baseline for each individual pairing (12.4 ± 2.34 and 14 ± 3.77 sessions were needed to reach stability prior to the first partner pairing for the 0.01 and 0.1 mg/kg/infusion doses, respectively). Cumulative infusions earned on the first baseline and partner pairings for the 0.01 mg/kg/infusion AMPH unit dose are shown in Figure 4a. A 2 × 12 (condition × interval) repeated-measures ANOVA revealed a significant main effect of interval [F(11, 44) = 53.97, p < .0001], indicating an increase in number of infusions across the session. In addition, there was a significant condition × interval interaction [F(11, 44) = 2.20, p < .05], indicating that the number of AMPH infusions differed across the duration of the first baseline and partner pairing sessions. Although a significant interaction was found, post hoc analyses conducted at each time interval revealed that number of infusions earned during the first partner pairing were not significantly different from baseline at any time interval.
Figure 4.
Cumulative infusions and total active and inactive lever presses for the AMPH 0.01 mg/kg/infusion unit dose (n = 5; *p < .01, **p < .001). There was a significant main effect of lever. All other details are as in Figure 2.
Active lever presses were higher than inactive lever presses during the first baseline session, but not the first partner pairing session at the 0.01 mg/kg/infusion AMPH unit dose (Figure 4b). A 2 × 2 (condition × lever) repeated-measures ANOVA revealed a significant main effect of condition [F(1, 4) = 7.32, p < .05] and lever [F(1, 4) = 8.37, p < .05] for the first partner pairing, indicative of a suppression of active lever pressing during the first partner pairing session.
Cumulative infusions earned during the second baseline and partner pairing sessions for the 0.01 mg/kg/infusion AMPH unit dose revealed no differences in AMPH intake between the two conditions (Figure 4c). A 2 × 12 (condition × interval) repeated measures ANOVA conducted on the second partner pairing and baseline sessions revealed only a significant main effect of time interval [F(11, 44) = 35.58, p < .0001], thus no post-hoc comparisons were conducted.
For the second partner pairing at the 0.01 mg/kg/infusion AMPH unit dose, active lever presses were higher than inactive lever presses on both the second baseline and partner pairing sessions. Rate of responding on the active lever did not differ between the second baseline and partner pairing, indicative of an attenuation of the suppressive effect of the presentation of a conspecific on active lever pressing found during the first partner pairing session (Figure 4b). A 2 × 2 (condition × lever) repeated measures ANOVA revealed only a significant main effect of lever [F(1, 4) = 8.93, p < .05], thus no post-hoc analyses were conducted.
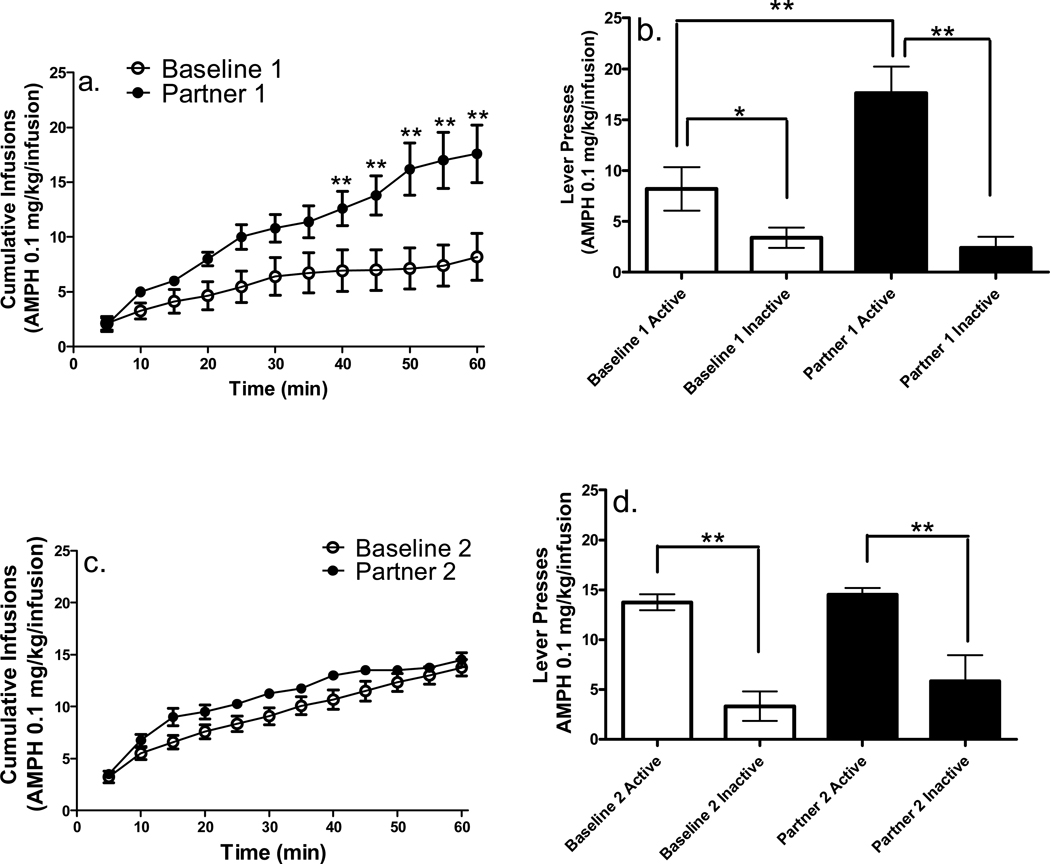
Cumulative infusions earned on the first baseline and partner pairing sessions for the 0.1 mg/kg/infusion AMPH unit dose revealed a facilitative effect on AMPH intake on the first partner pairing session compared to baseline (see Figure 5a). A 2 × 12 (condition × interval) repeated-measures ANOVA revealed a significant main effect of interval [F(11, 44) = 12.85, p < .0001], as well as an interval × condition interaction [F(11, 44) = 5.24, p < .0001]. Post-hoc analyses conducted to compare number of infusions earned on baseline and partner pairing conditions at each time interval revealed significantly higher number of infusions earned during the final 25 min of the first partner session compared to baseline.
Figure 5.
Cumulative infusions and total active and inactive lever presses for the AMPH 0.1 mg/kg/infusion unit dose(n = 5; *p < .01, **p < .001). All other details are as in Figure 2.
Active lever presses from the 0.1 mg/kg/infusion unit dose were higher than inactive lever presses during both the first baseline and partner pairing sessions (see Figures 5b). A 2 × 2 (condition × lever) repeated-measures ANOVA revealed a significant main effect of condition [F(1, 4) = 22.08, p < .01], lever [F(1, 4) = 54.19, p < .01], and condition × lever interaction [F(1, 4) = 12.36, p < .05] for the first partner pairing, indicating that active lever pressing was higher during the first partner pairing condition than the first baseline condition at the 0.1 mg/kg/infusion AMPH unit dose. Subsequent post-hoc analyses conducted on active and inactive lever presses from the first baseline and partner pairing session revealed that in each instance, active lever presses were significantly higher than inactive lever presses. In addition, active lever presses during the first partner pairing were significantly higher than the first baseline active lever presses, indicative of social facilitation.
Cumulative infusions earned during the second baseline and partner pairing sessions for the 0.1 mg/kg/infusion AMPH unit dose revealed no differences in intake between the two conditions (see Figure 5c), suggesting a potential transient property of the facilitative effect found during the first partner pairing at this dose. A 2 × 12 (condition × interval) repeated-measures ANOVA revealed only a significant main effect of interval [F(11, 44) = 13.52, p < .001], and thus no post hoc analyses were conducted.
For the second partner pairing, active lever presses were higher than inactive lever presses on both baseline and partner pairing sessions (Figure 5d). A 2 × 2 (condition × lever) repeated-measures ANOVA revealed only significant main effects of condition [F(1, 4) = 11.94, p < .05] and lever [F(1, 4) = 35.07, p < .01], thus no post hoc analyses were conducted.
Discussion
Social facilitation of AMPH self-administration was observed in the current report at the 0.1 mg/kg/infusion unit dose during the first partner pairing. Inactive lever presses were not influenced during the presentation of a conspecific, thus indicating that a general, nonspecific invigoration of responding was not likely responsible for the increase in responding on the active lever reinforced by AMPH. These results contrast with previous reports showing that presentation of novel inanimate stimuli or alternative non-drug reinforcers decrease stimulant self-administration in rats (Cain et al., 2004; Comer et al., 1996; Klebaur et al., 2001). However, results from the first partner pairing at the 0.1 mg/kg/infusion unit dose are consistent with social facilitation theory (Zajonc 1965), which postulates that the mere presence of a novel conspecific facilitates the emission of a well-trained, dominant response (in this case, responding on the active lever for a high unit dose of AMPH). During the second partner pairing, however, social facilitation was not observed. Thus, the ability of a conspecific to facilitate operant responding for AMPH may habituate following the first presentation of a particular conspecific.
At the 0.01 mg/kg/infusion unit dose of AMPH, social facilitation of active lever pressing was not observed. Previous research on the effect of inanimate novel stimuli on AMPH self-administration also found inhibition of AMPH self-administration at the 0.01 mg/kg/infusion dose (Cain et al. 2004). It is possible that the 0.01 mg/kg/infusion unit dose was below the threshold for facilitation of responding because the incentive motivation for this dose was surmounted by the social reward, thus leading to a loss of responding. Alternatively, the dose-dependent difference in social facilitation observed here may be reflect a rate dependency effect (Dews 1981; Odum et al. 2002), since the lower unit dose (0.01 mg/kg/infusion) produced lower rates of baseline responding compared to the higher unit dose (0.1 mg/kg/infusion). Since high rates of responding are generally more sensitive to disruption than low rates of responding, this could explain why presentation of the conspecific failed to produce social facilitation at the low unit dose of AMPH.
Although social facilitation was found at the high unit AMPH dose, this effect was not found with responding for sucrose reward. Under different conditions of motivation (free feed vs. food restriction), social facilitation was not observed in the current investigation. To the contrary, social stimuli disrupted food-reinforced behavior when rats were free feeding. This disruption, however, was overcome when the incentive was enhanced (i.e., palatable food when rats are hunger motivated). This finding contrasts with social facilitation theory (Zajonc 1965), as well as previous reports of social facilitation of feeding behavior in isolated versus paired rats (Harlow 1932), satiated chickens (Rajecki et al. 1976), and dog puppies (James 1953). Further, the results of the current investigation are inconsistent with previous research on social facilitation of responding for food reward under water restriction conditions in rats (Zentall & Levine 1972). Several procedural differences exist, however, between Zentall and Levine (1972) and the current report. For example, the schedules of reinforcement (FR-5 vs. continuous reinforcement schedules) and session length (30 vs. 60 minutes) varied, as well as different strains of rats (Sprague Dawley vs. Long Evans) were used in the two studies. In addition, Zentall and Levine (1972) used a stringent water restriction regimen (15 min access to water after each session). It is unclear to what extent these procedural differences contributed to the opposite results found in the two studies. However, it is possible that competition from the motive to affiliate may interfere with social facilitation of lever pressing unless the level of food or water motivation is quite high. Although this possibility may constrain social facilitation theory, it does not contrast with it. Moreover, the results from the current report are consistent with previous research in which novelty was found to disrupt ongoing motivated behavior (Cain et al. 2004).
Although the current work suggests that differences in ongoing behavior and reinforcer type used with the responder animal are important variables for demonstrating social facilitation of operant responding, future work should also investigate features of the conspecific partner. For example, future experimentation is needed to determine what features of the conspecific are critical, such as familiarity (novel vs. familiar partner), sex (same- vs. opposite sex), and social rank (dominant vs. subordinate).
Thus, to our knowledge, this is the first study to demonstrate social facilitation of AMPH self-administration in rats. Although social influence on drug use during self-administration is novel, previous research has found that social stress (attacks from aggressive males or lactating females) prior to drug self-administration increases acquisition of cocaine self-administration in rats (Haney et al. 1995). In addition, environmental enrichment with social cohorts and novel objects during development decreases AMPH self-administration compared to isolated rats (Bardo et al. 2001; Green et al. 2002), and protects against escalation of cocaine self-administration at low unit doses (Gipson et al. 2011). Thus, although previous research has examined the effects of prior social influence or social influence in a separate context on drug self-administration, this is the first demonstration of social influences during AMPH self-administration in rats. This operant model of social facilitation may have important implications as a preclinical assay of social factors involved in drug abuse. Additionally, social factors have a large impact on drug use and risk taking, especially during adolescence (Kandel 1985; Gardner & Steinberg 2005). Future research should examine social facilitation of drug self-administration during early, mid, and late adolescence in rats.
Acknowledgements
This work was supported by NIH grants F31 DA028018, DA05312, and T32 DA007304.
We would like to thank Kristin Alvers, Kate Fischer, Emily Denehy, William T. McCuddy, Luke Holderfield, and Dr. Carrie Wilmouth for technical assistance.
Footnotes
Disclosures
All authors contributed significantly to this work.
We have no financial conflicts of interest to disclose.
References
- Akins CK, Klein ED, Zentall TR. Imitative learning in Japanese quail (Coturnix japonica) using the bidirectional control procedure. Animal Learn Beh. 2002;30(3):275–281. doi: 10.3758/bf03192836. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Klebaur JE, Valone JM, Deaton C. Environmental enrichment decreases intravenous self administration of amphetamine in female and male rats. Psychopharmacology. 2001;155(3):278–284. doi: 10.1007/s002130100720. [DOI] [PubMed] [Google Scholar]
- Bava S, Tapert SF. Adolescent brain development and the risk for alcohol and other drug problems. Neuropsy Rev. 2010;20:398–413. doi: 10.1007/s11065-010-9146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain ME, Smith CM, Bardo MT. The effect of novelty on amphetamine self administration in rats classified as high and low responders. Psychopharmacology. 2004;176(2):129–138. doi: 10.1007/s00213-004-1870-2. [DOI] [PubMed] [Google Scholar]
- Comer SD, Lac ST, Wyvell CL, Carroll ME. Combined effects of buprenorphine and a nondrug alternative reinforce on IV cocaine self-administration in rats maintained under FR schedules. Psychopharmacology. 1996;125(4):355–360. doi: 10.1007/BF02246018. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Bio. 2009;14:22–23. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews PB. History and present status of the rate-dependency investigations. In: Thompson T, Dews PB, Barrett JE, editors. Advances in behavioral pharmacology. Vol. 3. 1981. pp. 111–118. [Google Scholar]
- Douglas LA, Varlinskaya EI, Spear LP. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev Psychobio. 2004;45(3):153–162. doi: 10.1002/dev.20025. [DOI] [PubMed] [Google Scholar]
- Fritz M, El Rawas R, Salti A, Klement S, Bardo MT, Kemmler G, Dechant G, Saria A, Zernig G. Reversal of cocaine-conditioned place preference and mesocorticolimbic Zif268 expression by social interaction in rats. Addiction Bio. 2011;16(2):273–284. doi: 10.1111/j.1369-1600.2010.00285.x. [DOI] [PubMed] [Google Scholar]
- Gardner M, Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: An experimental study. Dev Psy. 2005;41(4):623–635. doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Briscoe RJ, Goulden KL, Holloway FA. Aversive attributes of ethanol can be attenuated by dyadic social interaction in the rat. Alcohol. 1994;11(3):247–251. doi: 10.1016/0741-8329(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Beckmann JS, El-Maraghi S, Marusich JA, Bardo MT. Effect of environmental enrichment on escalation of cocaine self-administration in rats. Psychopharmacology. 2011;214(2):557–566. doi: 10.1007/s00213-010-2060-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn TJ. From family to peer: Transition of influence among drug using youth. J Youth Adolescence. 1981;9:449–465. doi: 10.1007/BF02088939. [DOI] [PubMed] [Google Scholar]
- Green T, Gehrke B, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Haney M, Maccari S, Le Moal M, Simon H, Piazza PV. Social stress increases the acquisition of cocaine self-administration in male and female rats. Brain Res. 1995;698(1–2):46–52. doi: 10.1016/0006-8993(95)00788-r. [DOI] [PubMed] [Google Scholar]
- Harlow HF. Social facilitation of feeding in the albino rat. J Gen Psy. 1932;41:211–221. [Google Scholar]
- Kandel DB. Adolescent marijuana use: Role of parents and peers. Science. 1973;181:1067–1070. doi: 10.1126/science.181.4104.1067. [DOI] [PubMed] [Google Scholar]
- Kandel DB. On processes of peer influences in adolescent drug use: A developmental perspective. Adv Alcohol Substance Abuse. 1985;4:139–163. doi: 10.1300/J251v04n03_07. [DOI] [PubMed] [Google Scholar]
- Kandel D, Kessler R, Margulies R. Adolescent initiation into stages of drug use: A developmental analysis. In: Kandel D, editor. Longitudinal Research on Drug Use: Empirical Findings and Methodological Issues. Washington DC: Hemisphere-Wiley; 1978. pp. 73–100. [Google Scholar]
- Klebaur JE, Bevins RA, Segar TM, Bardo MT. Individual differences in behavioral responses to novelty and amphetamine self-administration in male and female rats. Beh Pharm. 2001;12(2):267–275. doi: 10.1097/00008877-200107000-00005. [DOI] [PubMed] [Google Scholar]
- James WT. Social facilitation of eating behavior in puppies after satiation. J Comp Phys. 1953;46:427–428. doi: 10.1037/h0056028. [DOI] [PubMed] [Google Scholar]
- Levine JM, Zentall TR. Effect of conspecific's presence on deprived rats’ performance: Social facilitation vs. distraction/imitation. Anim Learn & Behav. 1974;2:119–122. [Google Scholar]
- Newman JL, Perry JL, Carroll ME. Social stimuli enhance phencyclidine (PCP) self administration in rhesus monkeys. Pharmacol Biochem Behav. 2007;87(2):280–288. doi: 10.1016/j.pbb.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum AL, Lieving LM, Schaal DW. Effects of d-amphetamine in a temporal discrimination procedure: selective changes in timing or rate dependency? J Exp Anal Beh. 2002;78:195–214. doi: 10.1901/jeab.2002.78-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajecki DW, Wilder DA, Kidd RF, Jaeger J. Social facilitation of pecking and drinking in “satiated” chickens. Learning Beh. 1976;4(1):30–32. [Google Scholar]
- Thiel KJ, Okun AC, Neisewander JL. Social reward-conditioned place preference: A model revieling an interaction between cocaine and social context rewards in rats. Drug Alcohol Dep. 2008;96(3):202–212. doi: 10.1016/j.drugalcdep.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology. 2009;204(3):391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobeh Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Dev Psy. 2008;44(6):1764–1778. doi: 10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E, Spear LP. Changes in sensitivity to ethanol-induced social facilitation and social inhibition from early to late adolescence. Annals New York Acad Sci. 2006;1021(1):459–461. doi: 10.1196/annals.1308.064. [DOI] [PubMed] [Google Scholar]
- Varlinskaya E, Spear LP. Ethanol-induced social facilitation in adolescent rats: role of endogenous activity at mu opioid receptors. Alcohol Clin Exp Res. 2009;33(6):991–1000. doi: 10.1111/j.1530-0277.2009.00920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajonc RB. Social facilitation. Science. 1965;149(3681):269–274. doi: 10.1126/science.149.3681.269. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Levine JM. Observational learning and social facilitation in the rat. Science. 1972;178(4066):1220–1221. doi: 10.1126/science.178.4066.1220. [DOI] [PubMed] [Google Scholar]