Abstract
Arabidopsis has inducible responses for tolerance of O2 deficiency. Plants previously exposed to 5% O2 were more tolerant than the controls to hypoxic stress (0.1% O2 for 48 h) in both roots and shoots, but hypoxic acclimation did not improve tolerance to anoxia (0% O2). The acclimation of shoots was not dependent on the roots: increased shoot tolerance was observed when the roots of the plants were removed. An adh (alcohol dehydrogenase) null mutant did not show acclimation of the roots but retained the shoot survival response. Abscisic acid treatment also differentiated the root and shoot responses; pretreatment induced root survival in hypoxic stress conditions (0.1% O2) but did not induce any increase in the survival of shoots. Cycloheximide blocked both root and shoot acclimation, indicating that both acclimation mechanisms are dependent on protein synthesis.
The supply of O2 to plant tissues may be restricted under certain environmental conditions (Hook and Crawford, 1978). When air spaces normally present in the soil become saturated with water, the root environment becomes hypoxic or anoxic as a result of O2 consumption by respiring roots and microorganisms and the insufficient diffusion of O2 through water (Armstrong, 1979). O2 deficiency is thought to be a major determinant in the adverse effects of waterlogging on crops and other plant species (Jackson et al., 1991). Plants have evolved inducible metabolic mechanisms to cope with these ephemeral, low-O2-stress conditions. When exposed to low-O2 conditions, plants switch to the expression of “anaerobic” polypeptides (Sachs et al., 1980, 1996). The induction of these proteins may be responsible for the tolerance to O2 deficiency that would otherwise be lethal. A number of anaerobic polypeptides have been identified as enzymes involved in glycolysis and ethanol fermentation (for a recent review, see Vartapetian and Jackson, 1997), and this supports the view that when O2 is limiting, oxidative catabolism of sugars is hindered and ethanolic fermentation acts as an alternative energy-producing pathway.
Ethanol is the main end product of anaerobic metabolism in plants (Smith and ap Rees, 1979; Good and Muench, 1993). Unlike lactate, which is also generated under O2 deficiency, ethanol is a relatively nontoxic end product (Jackson et al., 1982) and does not lead to the acidification of the cytoplasm, a major determinant in intolerance to O2 deficiency (Roberts et al., 1984, 1985). The induction of glycolytic enzymes probably reflects the need for increased glycolysis to compensate for the lower ATP yield of ethanol fermentation.
The importance of ethanol fermentation is supported by studies of adh (alcohol dehydrogenase) null mutants in a number of species (Schwartz, 1966; Harberd and Edwards, 1982; Jacobs et al., 1988; Matsumura et al., 1995), which report reduced tolerance to O2 deficiency in these plants.
Some plant tissues exposed to a period of mild hypoxia show more tolerance to subsequent hypoxic or anoxic stress than plants kept in fully aerated conditions before the stress (for review, see Drew, 1997; see also more recent work on tomato [Germain et al., 1997] and rice [Ellis and Setter, 1999]).
In this study we examined the survival of Arabidopsis plants after exposure to anoxic or hypoxic stress. Our results demonstrate that hypoxic pretreatment protects against hypoxic stress and that different mechanisms of acclimation to hypoxic stress are operative in root and shoot tissues.
MATERIALS AND METHODS
Plant Material
Arabidopsis ecotypes C24 and Bensheim were used, as well as an adh null mutant isolated in a Bensheim background (R002; Jacobs et al., 1988). This mutant has no ADH (EC 1.1.1.1) activity (Dolferus et al., 1997).
Plant Growth Conditions
Seeds were surface-sterilized by soaking in 70% ethanol for 30 s and in undiluted household bleach with a small amount of Triton X-100 for 15 min, followed by several washes in sterile, distilled water. All further manipulations were carried out under sterile conditions. The seeds were transferred individually on nylon mesh (425 μm) placed on the surface of medium containing Murashige and Skoog salts and nutrients (Murashige and Skoog, 1962) and 0.8% agar, with 48 seeds per Petri dish. The plates were placed at 4°C overnight to break dormancy and plants were then grown in a culture room (21°C, 16 h of light per day, 400 μmol m−2 s−1) for 3 weeks, during which time the roots grew through the nylon mesh into the agar medium.
Pretreatments with Hypoxia, ABA, and Cycloheximide
Three-week-old plants (root lengths, 2–3 cm) were transferred to plates containing 10 mL of liquid Murashige and Skoog medium by gently peeling the nylon mesh from the agar. For hypoxic pretreatment the plates were stacked without lids in autoclaved stainless-steel racks. The racks were placed in jars designed for growing anaerobic bacteria (Oxoid, Unipath Ltd., Basingstoke, Hampshire, UK); the medium was removed and replaced with 10 mL of liquid Murashige and Skoog medium that had been flushed to equilibrium with a gas containing 5% O2 (all gases were obtained as O2:N2 premixes from BOC gases). The jars were then sealed immediately (some contamination by atmospheric O2 may have occurred) and flushed with 5% O2 at an approximate rate of 1 L min−1 for 15 min, and then sealed and kept at 21°C in the dark for 48 h. In some experiments, 1 μg mL−1 or 10 μg mL−1 cycloheximide was added to the liquid medium at this stage (CX1-HPT and CX10-HPT, respectively). Controls for the pretreatments (NHPT) were left in the culture room during the pretreatment period, with the exception of the experiments on cycloheximide and the ABA treatments, in which the pretreatment controls were kept in aerated liquid Murashige and Skoog medium in the dark.
For pretreatments with ABA, plants grown on nylon mesh at the surface of 0.8% agar were transferred to liquid Murashige and Skoog medium 48 h before treatments. Six hours before treatment, 0, 10−4 m, 5 × 10−5 m, or 10−5 m ABA (cis-trans-ABA) was added to the medium. In the case of pretreatments with both hypoxia and ABA (ABA-HPT) plants were exposed to hypoxia as described above for 48 h before the stress-treatment phase; 6 h before treatment 5 × 10−5 m ABA was added to the medium, the jars were flushed with 5% O2, and then they were kept sealed for the remainder of the pretreatment period.
Treatments with Anoxia and Hypoxic Stress
The plates were stacked in stainless-steel racks, the liquid medium was removed from the plates, and the racks were placed in an anaerobic jar that had been flushed with argon for 5 min. Argon was flushed continuously until the jar was sealed to avoid contamination with atmospheric O2. After 5 min, the medium, which had been gased to equilibrium with N2 (anoxia) or 0.1% O2 (hypoxic stress), was injected into the Petri dishes within the jars using a 10-mL syringe fitted with a long-tip Pasteur pipette. The jars were sealed and flushed with 0.1% O2 or N2 at an approximate rate of 0.2 L min−1. The exhaust gases were flushed through a water trap and the O2 concentration was measured using an O2 electrode. Once the water reached the required O2 concentration, the jars were sealed and kept at 21°C in the dark with gentle orbital shaking for the duration of the treatment.
In all of the experiments pretreatment/treatment combinations were done in triplicate Petri dishes; replicates were allocated to separate treatment jars, and the positions of the Petri dishes within each jar were randomized.
Recovery Growth and Survival Scores
After treatment samples of 15 plants were taken from each plate and aligned on the surface of Murashige and Skoog medium with 1% agar in a 10- × 10-mm square Petri dish. The positions of the root tips were scored on the back of the plates with a scalpel blade. The recovery plates were incubated vertically in a culture room at 21°C under diffuse light for 2 to 3 weeks, after which the plates were photographed and survival was scored. During the recovery period plants that had been adversely affected by the stress showed chlorosis of leaf tissue. The extent of chlorosis was estimated by measuring chlorophyll content in a subsample of five plants per replicate according to the method of Porra et al. (1989). In more extreme cases the chlorosis included the center of the rosette containing the shoot meristem; in such cases no new leaves were produced during the recovery period and the shoot was scored as dead.
Root survival was based on growth during the recovery period. “Root survival” or “growth from existing root” was scored as the ability to initiate new growth from the root that was already present at the beginning of the recovery phase. In some experiments “root-tip survival” was scored as the ability of the root tip from the main root to extend beyond a mark scored at the beginning of the recovery phase.
RESULTS
Using an assay developed to measure tolerance to O2 deficiency in Arabidopsis, we investigated the effects of a pretreatment of mild hypoxia (5% O2) followed by a stress treatment of either 0.1% or 0% O2. In the recovery phase plants were returned to aerated conditions. The assay permitted us to monitor the recovery of both roots and shoots for all of the experimental treatments.
Hypoxic Pretreatment Enhances Tolerance to Hypoxic Stress
Three-week-old plants that had been exposed to hypoxia pretreatment (HPT, 5% O2 for 48 h) along with pretreatment controls (NHPT, normal atmosphere during the pretreatment period) were exposed to hypoxic stress (0.1% O2) for periods ranging from 6 to 48 h. Survival of shoot meristems and root tips was scored after a 2-week recovery period (Fig. 1A).
Figure 1.
Survival of HPT and NHPT plants after various times of hypoxic stress (A) and anoxia (B). Error bars represent the se. In this experiment only the survival of the existing root tip was scored; growth from lateral roots was not recorded. When the survival of the root system as a whole was scored after 48 h of hypoxic stress, survival in the HPT plants was 100%.
The survival of roots was not affected by hypoxic stress of up to 12 h. The roots of the NHPT plants were intolerant to longer treatments, with a significant decrease in survival after 24 h and death of all root tips after 36 or 48 h (Fig. 1A). The root tips of HPT plants were significantly more tolerant to the stress (Fig. 1A). Root survival declined somewhat but remained at more than 50% after 48 h.
The pretreatment by hypoxia also increased survival of shoots after hypoxic stress. The shoot meristem of HPT plants was highly tolerant; very few of these plants died, even after 48 h of hypoxic stress (Fig. 1A). In contrast, the shoot-meristem survival of NHPT plants was 50% after 24 h of hypoxic stress, and very few shoots survived a 48- h exposure.
Hypoxic Pretreatment Does Not Enhance Tolerance to Anoxia
In a second experimental design the O2 concentration during the treatment phase was 0% (anoxia). There was little difference between HPT and NHPT plants; both were highly intolerant of anoxia (Fig. 1B). Only a slight increase in survival over the pretreatment controls was seen in the pretreated plants after 12 h of stress. In both groups of plants, the survival of roots decreased to 0% after 24 h of anoxia, and that of the shoots after 36 h of anoxia. Even when anoxia was imposed in a stepwise mode (5% for 48 h, 0.1% for 24 h, and 0% for 24 h), all of the plants died (data not shown). Hypoxic pretreatment did not enable Arabidopsis plants to withstand a zero-O2 environment.
Roots Are Not Required for the Acclimation of Shoots
To determine whether the acclimation of shoots depended on the acclimation of roots, we investigated the effects of removing the roots of the plants either before the pretreatment or before the hypoxic-stress treatment. When the entire root system was removed by cutting just above the crown and the hypocotyl was placed in aerated Murashige and Skoog medium, shoots survived without apparent adverse effects and eventually grew roots de novo from the crown. Root removal before pretreatment had little effect on the ability of the shoots to acclimate: NHPT plants without roots showed a low percentage of survival after 48 h of hypoxic stress, and nearly all of the HPT plants without roots survived (Fig. 2B). The results were similar to those of control plants with an intact root system (Fig. 2A). Similar results were also obtained when the roots were removed between the pretreatment and the treatment (Fig. 2C).
Figure 2.
Shoot survival of plants with roots (A), with roots removed before pretreatment (B), or with roots removed before treatment (C). The pretreatment is indicated on the x axis, and was followed by 48 h of hypoxic stress. Error bars represent the se.
These observations indicate that shoot acclimation during the pretreatment and survival during the hypoxic stress are not dependent on events in roots.
Cycloheximide Blocks the Acclimation of Roots and Shoots
Because the synthesis of anaerobic proteins is likely to play an important role in the acclimation to low O2, we investigated the effects of applying the protein-synthesis inhibitor cycloheximide during the acclimation period. The presence of cycloheximide in concentrations similar to those used in our experiments has been shown to inhibit the induction of ADH by hypoxia in Arabidopsis (Hoeren et al., 1998).
To evaluate the possible toxicity of cycloheximide, plants were placed on recovery plates directly after cycloheximide pretreatment. In addition to root and shoot survival (Fig. 3, A and C), the chlorophyll content of the shoots and the fresh weight of the roots were also measured after the recovery period (Fig. 3, B and D).
Figure 3.
Shoot survival (A), shoot chlorophyll content (B), root survival (C), and root fresh weight (FW) (D) of plants that were given the pretreatment shown on the x axis, and were then either exposed to 48 h of hypoxic stress or were aligned on the recovery plates without a stress. Plants were pretreated with hypoxia only (HPT), with hypoxia with cycloheximide in the liquid medium (1 μg mL−1 cycloheximide [CX1-HPT] or 10 μg mL−1 cycloheximide [CX10-HPT]), or were not pretreated (NHPT). Error bars represent the se.
As in the previous experiments, the NHPT plants were intolerant to hypoxic stress: the shoots became chlorotic (Fig. 3B) and died (Fig. 3A), and their roots did not survive (Fig. 3C). The HPT plants were tolerant to the stress (>90% survival of stressed roots and shoots). Chlorosis occurred in small areas of the leaves; the chlorophyll content of the stressed HPT plants was 85% that of the HPT plants that were not subjected to hypoxic stress. The fresh weight of the roots of stressed HPT plants did not differ from that of unstressed HPT plants (Fig. 3D).
The presence of 1 or 10 μg mL−1 cycloheximide during the hypoxia pretreatment had no effect on plants that were not subsequently exposed to hypoxic stress. No death of either shoots or roots was observed, and the chlorophyll content and root fresh weight were not significantly decreased after exposure to hypoxia and cycloheximide compared with hypoxia alone. The CX1-HPT pretreatment caused a 40% increase in chlorophyll content and a 12% greater root fresh weight over the HPT controls. The reason for this stimulation is not known.
In contrast, the presence of cycloheximide during the hypoxia pretreatment dramatically decreased subsequent tolerance to hypoxic stress. In CX1-HPT plants both shoots and roots had reduced survival, and shoot chlorophyll content and root fresh weight were significantly lower than those of the HPT plants (Fig. 3). Survival values were further reduced at the higher concentration of cycloheximide.
Thus, application of cycloheximide in concentrations that did not affect survival of aerated plants completely blocked the ability of plants to acclimate to hypoxic stress during a pretreatment period, suggesting that protein synthesis is essential to the acclimation process.
An adh Null Mutant Shows Reduced Hypoxic Stress Tolerance in the Roots but Retains the Ability to Acclimate in the Shoots
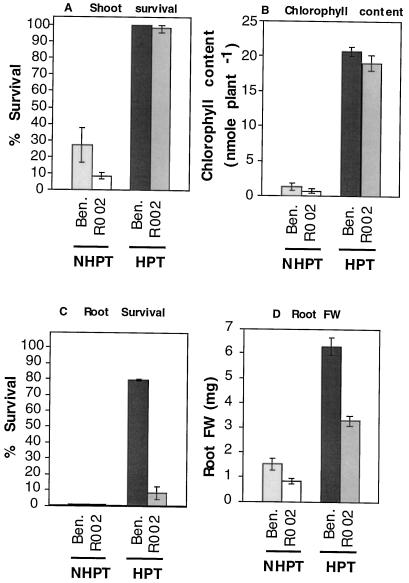
The tolerance of an adh null mutant (R002; Jacobs et al., 1988) was compared with that of the parental ecotype, Bensheim. Bensheim plants without pretreatment were intolerant to hypoxic stress and gave low survival of both roots and shoots, whereas plants pretreated with hypoxia were more tolerant to the stress (Fig. 4, compare NHPT [top left] versus HPT [top right]; see also Fig. 5). The response was similar to that of the C24 ecotype, described above. The shoots of the R002 mutants gave results similar to those of wild-type Bensheim shoots. In the NHPT controls shoots had a low frequency of survival and showed chlorosis after the recovery period (Fig. 4, bottom left, and Fig. 5, A and B). The pretreated R002 mutants were similar to the pretreated wild type in that their survival was close to 100% and the chlorophyll content of the wild type and mutant shoots was not significantly different (Fig. 5, A and B).
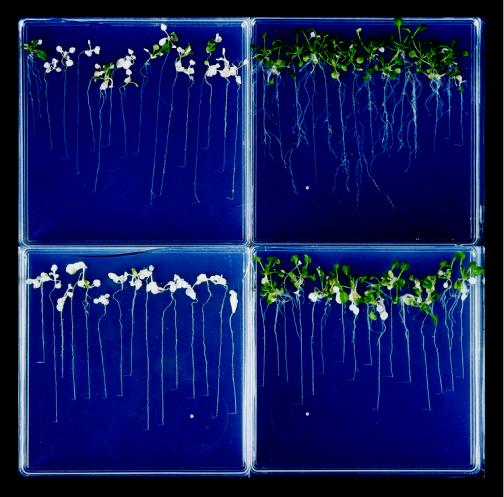
Figure 4.
Vertical recovery plates 2 weeks after a 48-h hypoxic stress treatment. Top left, NHPT Bensheim; top right, HPT Bensheim; bottom left, NHPT adh null mutant R002; and bottom right, HPT adh null mutant R002.
Figure 5.
Recovery scores of Bensheim (Ben.) and the adh null mutant (R002), HPT, and NHPT (as indicated on the x axis) after 48 h of hypoxic stress. A, Shoot survival; B, shoot chlorophyll content; C, percentage of plants showing growth from the existing root; D, root fresh weight (FW). Error bars represent the se.
In contrast, the roots of the mutant were less tolerant than those of the wild type (Fig. 4, compare HPT Bensheim [top right] and HPT R002 [bottom right]). The proportion of HPT R002 showing recovery growth from the existing root was 5%, as opposed to 75% in the HPT wild type (Fig. 5C). Some root growth was observed in most HPT R002 plants, but this originated exclusively from the crown of the plant (Fig. 4, bottom right). This adventitious growth of new roots is probably a consequence of the shoot survival rather than true tolerance of the roots (adventitious roots grew from the hypocotyl of plants after roots were completely removed; data not shown). Because of the initiation of new roots from the crown of the plants, the fresh weight of HPT R002 roots was intermediate between that of HPT Bensheim and that of NHPT plants (Fig. 5D).
ABA Application Induces Tolerance to Subsequent Hypoxic Stress in Roots but Not in Shoots
The previous experiment demonstrated the importance of ADH in root survival during hypoxic stress. We then investigated whether exposure to ABA could substitute for hypoxic acclimation, since ABA is known to induce ADH activity in Arabidopsis roots (Dolferus et al., 1994). ABA increased the survival of roots (Fig. 6C); the frequency of root survival in plants exposed to the hormone before hypoxic stress was 65% to 90%, significantly higher than for the NHPT plants (15%) and comparable to that of the HPT plants (70%). The fresh weight of roots exposed to ABA and hypoxic stress was twice that of the NHPT plants, but significantly lower than that of the HPT plants (Fig. 6D). It is possible that this reduction in root weight reflects the death of the shoots in the ABA-pretreated plants (see below).
Figure 6.
Recovery scores of plants that were given the pretreatment indicated on the x axis followed by 48 h of hypoxic stress. A, Shoot survival; B, shoot chlorophyll content; C, root survival; D, root fresh weight (FW). Error bars represent the se.
There was almost no survival of the shoots of plants exposed to the hormone, and these showed complete chlorosis after the recovery period, with chlorophyll content similar to that of NHPT plants. The chlorophyll content of HPT and ABA-HPT was not significantly different (Fig. 6B). Thus, the application of ABA did not enhance tolerance of the shoots.
There was no evidence for an additive effect of ABA and hypoxic pretreatment: there was no difference between HPT and ABA-HPT plants in either root survival or fresh weight after recovery (Fig. 6, C and D). A significant proportion of ABA-HPT shoots survived (Fig. 6A), although the percentage was lower than in the HPT shoots.
DISCUSSION
Hypoxia Pretreatments Induce Hypoxic Stress Tolerance in Arabidopsis
Plants have evolved the ability to adapt to adverse environmental conditions, including O2 deficiency. The acclimation to O2 shortage has been demonstrated in several species (for review, see Drew, 1997). The roots of aerated plants were able to tolerate O2 deficiency for only a relatively short time, whereas roots that had been given a hypoxic pretreatment were able to survive for much longer.
We found similar responses in Arabidopsis plants. Plants grown in aerated conditions died rapidly when exposed to a strong hypoxic stress, with death of roots and shoots occurring after 12 to 36 h of stress treatment (Fig. 1A). In contrast, plants that were previously treated under mild hypoxia showed much greater tolerance to the same stress (Fig. 1A).
These results demonstrate that Arabidopsis has evolved mechanisms for tolerating an extreme O2 shortage and that these mechanisms are induced by low O2 concentrations. The pretreatment we imposed lasted 48 h, but shorter pretreatment times may be sufficient to elicit the acclimation response. We found that a 24-h pretreatment was equally effective (data not shown).
Because survival after hypoxic stress was scored after a recovery period, there is a possibility that the tolerance mechanisms induced by the pretreatment are effective after the return to aerated conditions rather than during the hypoxic stress period itself. Postanoxic injury does have severe effects on plant survival (Pfister-Sieber and Brändle, 1994), and mechanisms for tolerating this stress have been reported (Monk et al., 1987).
Arabidopsis plants, regardless of whether they were subjected to hypoxic pretreatment, were highly intolerant to anoxia (Fig. 1B). The strict anoxic treatment imposed on plants in our experiments, with manipulations under argon and incubation in jars designed for strict anaerobes, provided extreme conditions of O2 deficiency, and Arabidopsis may not have evolved mechanisms for tolerating such an extreme stress. It may also be the case that one of the essential components of the acclimation of Arabidopsis is dependent on at least some molecular O2.
Arabidopsis Has Inducible Hypoxic Stress Tolerance in the Shoots
Okimoto et al. (1980) found no evidence for anaerobic protein synthesis in mature maize leaves, and the anaerobic induction of genes such as ADH is root specific (Freeling and Bennett, 1985). In Arabidopsis ADH is mainly induced in roots, with only low levels of induction in leaves (Dolferus et al., 1994).
We found that hypoxic pretreatment led to the induction of hypoxic stress tolerance in the shoots of mature Arabidopsis plants, and that the improved tolerance of shoots is not a consequence of the improved root survival (Fig. 2). Even when roots were removed before the pretreatment with hypoxia, shoots were able to acclimate. These results demonstrate that Arabidopsis plants have mechanisms for sensing and adapting to O2 deficiency in shoots and in roots. The acclimation of shoots was inhibited by cycloheximide (Fig. 3), suggesting that protein synthesis may be necessary for the increase in survival.
Low O2 is known to affect the shoots of other species. In particular, rice can become totally submerged during monsoons, causing the shoots to be exposed to severe O2 shortage (Setter et al., 1989). The shoots of rice seedlings exposed to hypoxia have been shown to tolerate subsequent anoxia better than NHPT controls (Ellis and Setter, 1999). Other plants may also be affected by hypoxic conditions in the shoots as a result of ice encasement (Andrews and Pommeroy, 1979).
We present two lines of evidence to suggest that the stress tolerance in shoots involves mechanisms that are different from those operating in roots. First, the ability to tolerate hypoxic stress in roots and shoots could be separated genetically; an adh null mutant had a reduced ability to tolerate hypoxic stress in the roots but retained the ability to acclimate in the shoots. Second, treatment with ABA increased the tolerance of hypoxic stress of the roots but had no effect on the tolerance of the shoots.
Ethanol Fermentation Is Required for Hypoxic Stress Tolerance in the Roots but Not in the Shoots
The importance of ethanol fermentation during anaerobic stress has been demonstrated in a number of species using adh null mutants. Reduced tolerance to anaerobic stress has been reported in adh null mutants of maize (Schwartz, 1966), barley (Harberd and Edwards, 1982), and rice (Matsumura et al., 1995). In Arabidopsis, Jacobs et al. (1988) reported that the seeds of the adh null mutant R002 had a reduced ability to germinate under O2 deprivation. In this paper we report that the roots of this mutant were much more sensitive to hypoxic stress than the wild type (Figs. 4 and 5D). This result is consistent with the hypothesis that ethanol fermentation is an essential component of root survival under limiting O2 conditions, presumably by allowing for continued glycolytic flux and energy production (this possibility has been discussed critically in several reviews: Perata and Alpi, 1993; Drew et al., 1994; Ricard et al., 1994; Drew, 1997).
Alternatively, hypoxic-stress sensitivity in the adh null mutants could be attributable to the accumulation of a substrate of ADH, acetaldehyde, which is highly toxic to plant cells (Perata et al., 1992). In our case this explanation is unlikely, because the plants were grown in a nonstagnant liquid medium with a large area of contact with the atmosphere (more than 6 cm2 mL−1 of culture solution), allowing for the escape of this extremely volatile compound.
In contrast, the shoots of the R002 mutant retained the ability to acclimate (see Figs. 4 and 5, A and B). Improved tolerance in the adh1 null mutants after hypoxia pretreatment has been reported in maize roots (Johnson et al., 1994), but in that case the improved tolerance could be attributed to the induction of a second maize ADH gene. In Arabidopsis there is a single ADH gene (Arabidopsis has a second ADH-like gene that is not involved in the production of ethanol [Dolferus et al., 1997]). R002 mutants have no detectable ADH activity (Dolferus et al., 1997) and are unable to catalyze the conversion of acetaldehyde to ethanol in vitro (data not shown) and hence can be considered “ethanol fermentation null mutants.” Therefore, the acclimation in the shoots of the adh null mutant must involve a mechanism independent of ethanol fermentation. It is possible that shoots produce another end product besides ethanol, such as Ala or lactate (Xia and Saglio, 1992), in proportions that would avoid cytoplasmic acidosis (Menegus et al., 1989).
We observed that ABA application was able to substitute for hypoxic pretreatment in roots. Exposure of plants to this hormone increased root survival after hypoxic stress (Fig. 6C). Similar results were obtained by Hwang and VanToai (1991), who found that ABA induced anoxia tolerance in maize root tips. These investigators also reported that ABA-induced anaerobic tolerance was inhibited by cycloheximide, suggesting that ABA leads to the synthesis of proteins that confer tolerance to anoxia. It seems likely that hypoxia and ABA lead to the induction of the same proteins in roots.
The treatment of plants with ABA had no effect on the subsequent hypoxic stress tolerance of shoots (Fig. 6, A and B). It is unlikely that the lack of response in the shoots was a consequence of the root application of this hormone. The ABA treatments we performed were identical to those used by De Bruxelles et al. (1996), who showed that ABA levels in Arabidopsis exposed to the hormone in liquid root medium were high in both roots and shoots (De Bruxelles, 1996).
Our results suggest that, in contrast to the roots, the proteins leading to anaerobic tolerance in the shoots are not inducible by ABA.
CONCLUSIONS
We have demonstrated the existence of adaptive mechanisms for survival under hypoxia in Arabidopsis roots and shoots. Both shoot and root tolerance were inhibited by the protein-synthesis inhibitor cycloheximide applied during the acclimation period. We found evidence that ethanol fermentation was essential in roots but not in shoots. Roots and shoots also differed in their response to ABA, which induced tolerance only in roots. Further work is required to elucidate the nature of acclimation to low O2 in the shoots. In roots, our results demonstrate the importance of ethanol fermentation. The conditions that induced the tolerance in roots (hypoxia and ABA) have previously been shown to induce ADH (Jarillo et al., 1993; De Bruxelles et al., 1996). However, it is very unlikely that the induction of ADH alone accounts for the increase of survival after hypoxia pretreatment (Johnson et al., 1994); other genes with similar responses to ADH are probably also involved. Transgenic plants with altered levels of ethanol fermentation and glycolytic enzymes will lead to an understanding of the regulation and importance of this pathway during anaerobic stress.
ACKNOWLEDGMENT
We thank Dr. Rudy Dolferus for the adh null mutant used in this study and for helpful discussions.
Abbreviations:
- ADH
alcohol dehydrogenase
- HPT
hypoxically pretreated
- NHPT
not hypoxically pretreated
LITERATURE CITED
- Andrews CJ, Pommeroy MK. Toxicity of anaerobic metabolites accumulating in winter wheat seedlings during ice encasement. Plant Physiol. 1979;64:120–125. doi: 10.1104/pp.64.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong W. Aeration in higher plants. Adv Bot Res. 1979;7:225–232. [Google Scholar]
- De Bruxelles GL (1996) Involvement of ABA in environmental stress responses and expression of the Arabidopsis Alcohol dehydrogenase gene. PhD thesis. Australian National University, Canberra
- De Bruxelles GL, Peacock WJ, Dennis ES, Dolferus R. Abscisic acid induces the alcohol dehydrogenase gene in Arabidopsis. Plant Physiol. 1996;111:381–391. doi: 10.1104/pp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolferus R, de Bruxelles GL, Dennis ES, Peacock WJ. Regulation of the Arabidopsis Adh gene by anaerobic and other environmental stresses. Ann Bot. 1994;74:301–308. [Google Scholar]
- Dolferus R, Osterman JC, Peacock WJ, Dennis ES. Cloning of the Arabidopsis and rice formaldehyde dehydrogenase genes: implications for the origin of plant ADH enzymes. Genetics. 1997;146:1131–1141. doi: 10.1093/genetics/146.3.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew MC. Oxygen deficiency and root metabolism: injury and acclimation under hypoxia and anoxia. Annu Rev Plant Physiol Plant Mol Biol. 1997;48:223–250. doi: 10.1146/annurev.arplant.48.1.223. [DOI] [PubMed] [Google Scholar]
- Drew MC, Cobb BG, Johnson JR, Andrews D, Morgan PW, Jordan W, He CJ. Metabolic acclimation of root tips to oxygen deficiency. Ann Bot. 1994;74:281–286. [Google Scholar]
- Ellis MH, Setter TL (1999) Hypoxia induces anoxia tolerance in completely submerged rice seedlings. J Plant Physiol (in press)
- Freeling M, Bennett DC. Maize Adh1. Annu Rev Genet. 1985;19:297–323. doi: 10.1146/annurev.ge.19.120185.001501. [DOI] [PubMed] [Google Scholar]
- Germain V, Ricard B, Raymond P, Saglio PH. The role of sugars, hexokinase, and sucrose synthase in the determination of hypoxically induced tolerance to anoxia in tomato roots. Plant Physiol. 1997;114:167–175. doi: 10.1104/pp.114.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good AG, Muench DG. Long-term anaerobic metabolism in root tissue. Metabolic products of pyruvate metabolism. Plant Physiol. 1993;101:1163–1168. doi: 10.1104/pp.101.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harberd NP, Edwards KJR. The effect of a mutation causing alcohol dehydrogenase deficiency on flooding tolerance in barley. New Phytol. 1982;90:631–644. [Google Scholar]
- Hoeren FU, Dolferus R, Wu Y, Peacock WJ, Dennis ES. Evidence for a role of AtMYB2 in the induction of the Arabidopsis alcohol dehydrogenase gene (ADH1) by low oxygen. Genetics. 1998;149:479–490. doi: 10.1093/genetics/149.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hook DD, Crawford RMM (1978) Plant Life in Anaerobic Environments. Ann Arbor Science, Ann Arbor, MI
- Hwang S-Y, VanToai TT. Abscisic acid induces anaerobiosis tolerance in corn. Plant Physiol. 1991;97:593–597. doi: 10.1104/pp.97.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Davies DD, Lambers H (eds) (1991) Plant Life under Oxygen Deprivation: Ecology, Physiology and Biochemistry. SPB Academic, The Hague, The Netherlands
- Jackson MB, Herman B, Goodenough A. An examination of the importance of ethanol in causing injury to flooded plants. Plant Cell Environ. 1982;5:163–172. [Google Scholar]
- Jacobs M, Dolferus R, Van Den Bosshe VB. Isolation and biochemical analysis of ethyl methyl sulfonate induced alcohol dehydrogenase null mutants of Arabidopsis thaliana (L.) Heynh. Biochem Genet. 1988;26:102–112. doi: 10.1007/BF00555492. [DOI] [PubMed] [Google Scholar]
- Jarillo JA, Leyva A, Salinas J, Martinez-Zapater JM. Low temperature induces the accumulation of alcohol dehydrogenase mRNA in Arabidopsis thaliana, a chilling-tolerant plant. Plant Physiol. 1993;101:833–837. doi: 10.1104/pp.101.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JR, Cobb G, Drew MC. Hypoxic induction of anoxia tolerance in roots of Adh1 null Zea mays L. Plant Physiol. 1994;105:61–67. doi: 10.1104/pp.105.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura H, Takano T, Yoshida KT, Takeda G. A rice mutant lacking alcohol dehydrogenase. Breed Sci. 1995;45:365–367. [Google Scholar]
- Monk L, Fagerstedt KV, Crawford RMM. Superoxide dismutase as an anaerobic polypeptide. A key factor in recovery from oxygen deprivation in Iris pseudacorus? Plant Physiol. 1987;85:1016–1020. doi: 10.1104/pp.85.4.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for the growth and bioassay with tobacco tissue culture. Physiol Plant. 1962;15:473–497. [Google Scholar]
- Menegus F, Cattaruzza L, Chersi A, Fronza G. Differences in the anaerobic lactate-succinate production and in the changes of cell sap pH for plants with high and low resistance to anoxia. Plant Physiol. 1989;90:29–32. doi: 10.1104/pp.90.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okimoto R, Sachs MM, Porter EK, Freeling M. Patterns of polypeptide synthesis in various maize organs under anaerobiosis. Planta. 1980;150:89–94. doi: 10.1007/BF00385619. [DOI] [PubMed] [Google Scholar]
- Perata P, Alpi A. Plant responses to anaerobiosis. Plant Sci. 1993;93:1–17. [Google Scholar]
- Perata P, Vernieri P, Armellini D, Bugnoli M, Tognoni F, Alpi A. Immunological detection of acetaldehyde-protein adducts in ethanol-treated cells. Plant Physiol. 1992;98:913–918. doi: 10.1104/pp.98.3.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister-Sieber M, Brändle R. Aspects of plant behaviour under anoxia and post-anoxia. Proc R Soc Edinb. 1994;102B:313–324. [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann TE. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls A and B extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta. 1989;975:384–394. [Google Scholar]
- Ricard B, Couée I, Raymond P, Saglio PH, Saint-Ges V, Pradet A. Plant metabolism under hypoxia and anoxia. Plant Physiol Biochem. 1994;32:1–10. [Google Scholar]
- Roberts JKM, Andrade FH, Anderson IC. Further evidence that cytoplasmic acidosis is a determinant of flooding intolerance in plants. Plant Physiol. 1985;77:492–494. doi: 10.1104/pp.77.2.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JKM, Callis J, Jardetzky O, Walbot V, Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci USA. 1984;81:6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs MM, Freeling MM, Ikomoto R. The anaerobic proteins of maize. Cell. 1980;20:761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Sachs MM, Subbaiah CC, Saab IN. Anaerobic gene expression and flooding tolerance in maize. J Exp Bot. 1996;47:1–15. [Google Scholar]
- Schwartz D. An example of gene fixation resulting from selective advantage in suboptimal conditions. Am Nat. 1966;103:4798. [Google Scholar]
- Setter TL, Greenway H, Kupkanchankul T. Submergence of rice. Aust J Plant Physiol. 1989;16:265–278. [Google Scholar]
- Smith AM, ap Rees T. Pathways of carbohydrate fermentation in the roots of marsh plants. Planta. 1979;146:327–334. doi: 10.1007/BF00387805. [DOI] [PubMed] [Google Scholar]
- Vartapetian BB, Jackson MB (1997) Plant adaptations to anaerobic stress: a review. Ann Bot 79 (suppl A): 3–20
- Xia J-H, Saglio PH. Lactic acid efflux as a mechanism of hypoxic acclimation of maize root tips to anoxia. Plant Physiol. 1992;100:40–46. doi: 10.1104/pp.100.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]