Abstract
Teenage suicide is a major public health concern, but its neurobiology is not well understood. Proinflammatory cytokines play an important role in stress and in the pathophysiology of depression—two major risk factors for suicide. Cytokines are increased in the serum of patients with depression and suicidal behavior; however, it is not clear if similar abnormality in cytokines occurs in brains of suicide victims. We therefore measured the gene and protein expression levels of proinflammatory cytokines interleukin (IL)-1β, IL-6, and tissue necrosis factor (TNF)-α in the prefrontal cortex (PFC) of 24 teenage suicide victims and 24 matched normal control subjects. Our results show that the mRNA and protein expression levels of IL-1β, IL-6, and TNF-α were significantly increased in Brodmann area 10 (BA-10) of suicide victims compared with normal control subjects. These results suggest an important role for IL-1β, IL-6, and TNF-α in the pathophysiology of suicidal behavior and that proinflammatory cytokines may be an appropriate target for developing therapeutic agents.
Keywords: Cytokines, Teenage suicide, Postmortem brain, TNF-α, IL-1β, IL-6
1. Introduction
A relationship between the immune system and pathogenesis of depression has been suggested in patients with depressive illness. Patients with depression have been found to have abnormal inflammatory pathways. These suggestions are based on the observation that proinflammatory cytokines, which are released from immune cells as a result of inflammation or stress, are abnormal in the serum of patients with depression (Maes, 1995; O'Brien et al., 2004; Pandey & Dwivedi, 2007; Schiepers et al., 2005). Similarly, altered levels of cytokines have also been reported in patients with bipolar illness and schizophrenia (Ganguli et al., 1994; Monteleone et al., 1997). The other evidence of the involvement of cytokines in depression is derived from the studies reporting that the administration of interferons (IFN) or other cytokines induces depression in patients with chronic hepatitis C, multiple sclerosis, or some forms of cancer (Bonaccorso et al., 2002a; Bonaccorso et al., 2002b; Capuron et al., 2003; Capuron et al., 2000). The other psychiatric symptoms produced by the administration of IFN-α are cognitive changes that involve verbal memory, cognitive speed, and executive function—often termed as sickness behavior.
Stressful events have also been shown to cause changes in immune function. Stress increases susceptibility to infection and autoimmune disease and several studies indicate stress- related immunosuppression (Leonard, 2000). Also, a number of studies suggest that exposure to stressful life events causes impairment in various aspects of cellular immune function (Connor & Leonard, 1998; Maes et al., 1998). Stress-induced alterations in the function of the hypothalamic-pituitary-adrenal (HPA) axis result in altered cytokine levels and functions, providing further evidence for stress-related changes in cytokines (Leonard, 2006). Exposure to acute and repeated stress have been shown to cause changes in the levels of cytokines in the rat brain (Miyahara et al., 2000; Nguyen et al., 1998).
Although the role of cytokines and immune dysregulation has been studied in great detail in patients with mood disorders and schizophrenia, their role in suicide is less clear. Since both depression and stress are major risk factors for suicide, it is quite likely that abnormalities of proinflammatory cytokine may be associated with the pathophysiology of suicide. There is also some direct and indirect evidence suggesting a relationship between immune dysregulation and suicide. Steiner et al. (2008) have found increased microgliosis in the postmortem brain of suicide victims with affective disorders and schizophrenia compared with normal control subjects. Goodwin and Eaton (2005a) found a significant association between asthma and increased suicidal ideation and suicide attempts among adults in the community. Goodwin et al. (2005b) also found that youth who are hospitalized for asthma have higher than expected levels of suicidal ideation. That an abnormality in cytokines may be associated with suicidal behavior is supported by a recent report by Tonelli et al. (2008) that found increased mRNA expression of IL-4 and IL-3 in the PFC of female suicide victims and IL-13 in male suicide victims compared with normal control subjects.
Janelidze et al. (2011) determined the levels of cytokines in plasma of suicidal and non-suicidal depressed patients and found that the levels of proinflammatory cytokines IL-6 and TNF-α were significantly higher in suicide attempters compared with non-suicidal depressed patients. Taken together, these studies suggest that cytokines may be abnormal in suicide.
Although abnormal levels of cytokines are observed in the serum of patients with depression or suicide (Pandey et al., 2007; Schiepers et al., 2005), it is not clear if there are also abnormal levels of cytokines in the brain of depressed and/or suicide subjects. The immunological aspects of the neurobiology of suicide have been reported (Steiner et al., 2008), but the cytokines in the brain of suicide victims or subjects with depression have not been systematically studied. In order to examine the role of proinflammatory cytokines in suicide, we determined the gene and protein expression levels of IL-1β, IL-6, and TNF-α—the proinflammatory cytokines—in the PFC of teenage suicide victims and normal control subjects.
2. Methods
2.1 Acquisition of human postmortem brain samples
Brain tissues were obtained from the Maryland Brain Collection at the Maryland Psychiatric Research Center, Baltimore, Maryland, in collaboration with the Office of the Chief Medical Examiner of the State of Maryland. Tissue samples were obtained from 24 teenage suicide victims and from 24 teenage control subjects (Table 1). Toxicological data were obtained by analysis of urine and blood samples from these subjects. All procedures were approved by the University of Maryland Institutional Review Board.
Table 1.
Characteristics of Teenage Suicide and Normal Control Subjects
| Patient No/Sex/Age,y/Race | PMI, h | Brain pH | Cause of Death | Drug Toxicity | Psychiatric Diagnosis |
|---|---|---|---|---|---|
| CONTROL GROUP* | |||||
| 1/M/19/B | 6 | 6.14 | GSW | None | Normal |
| 2/M/16/B | 6 | 6.54 | GSW | None | Normal |
| 3/M/16/B | 8 | 5.64 | GSW | None | Normal |
| 4/M/19/B | 12 | 5.9 | GSW | None | Normal |
| 5/M/13/W | NA | 5.19 | Accident | None | Normal |
| 6/M/17/B | 11 | 6.52 | GSW | None | Normal |
| 7/M/16/W | 10 | 5.42 | Stabbing | None | Normal |
| 8/M/17/B | 10 | 5.9 | GSW | None | Normal |
| 9/M/13/B | 22 | 6.07 | GSW | None | Normal |
| 10/M/14/B | 18 | 5.73 | GSW | None | Normal |
| 11/M/18/B | 27 | 6.09 | Drowning | None | Normal |
| 12/M/16/W | 21 | 5.97 | Accidental hanging | None | Normal |
| 13/F/18/W | 35 | 5.99 | Multiple injuries | None | Normal |
| 14/F/17/B | 26 | 6.23 | Multiple injuries | None | Normal |
| 15/F/19/W | 30 | 6.2 | Cardiac arrhythmia | None | Normal |
| 16/F/18/B | 16 | 6.6 | MVA | None | Normal |
| 17/M/13/B | 20 | 6.6 | Drowning | None | Normal |
| 18/M/19/B | 16 | 6.6 | Congenital heart disease | None | Normal |
| 19/F/16/W | 24 | 6.71 | Myocarditis | None | Normal |
| 20/M/15/W | 16 | 6.5 | Cardiac arrhythmia | None | Normal |
| 21/M/15/W | 21 | 6.37 | PE/DVT | Cyclobenzaprine | Normal |
| 22/F/16/W | 20 | 6.89 | MVA | None | Normal |
| 23/F/13/B | 23 | 6 | Hanging | Not yet done | |
| 24/M/18/W | 19 | 5.80 | Complications of Morbid Obesity | Morphine (Rx) and bupivicaine | Normal |
| SUICIDE GROUP+ | |||||
| 1/F/15/W | 7 | 5.48 | GSW | Ethanol | Alcohol abuse |
| 2/M/20/W | 32 | 6.41 | Hanging | Ethanol | Alcohol abuse |
| 3/M/12/B | 10 | 5.91 | Hanging | None | Major depression |
| 4/M/15/W | 11 | 5.33 | Asphyxia | None | Major depression |
| 5/F/15/W | 17 | 5.58 | Drug overdose | Imipramine, Desipramine | Major depression, Hyperactivity attention deficit disorder |
| 6/M/15/W | 27 | 6.08 | GSW | Pseudoephedrine, Phenylpropanolamine | Adjustment disorder, Major depression |
| 7/M/18/W | 17 | 6.3 | Hanging | None | Major depression - single episode |
| 8/M/19/W | 18 | 6.2 | CO intoxication | Ethanol, CO | Major depression, Ethanol abuse, Polysubstance abuse |
| 9/F/15/W | 20 | 6.59 | Hanging | None | Major depression - single episode, Ethanol abuse |
| 10/M/17/W | 23 | 6.66 | Hanging | Ethanol | Major depression - single episode, Ethanol abuse |
| 11/M/13/W | 18 | 6 | Hanging | Ritalin | Hyperactivity attention deficit disorder |
| 12/F/17/W | 25 | 5.55 | Drug overdose | Verapamil | Adjustment disorder |
| 13/F/16/W | 33 | 6.61 | GSW | None | Adjustment disorder |
| 14/M/16/W | 24 | 6.81 | Hanging | None | Adjustment, Conduct disorders |
| 15/F/15/W | 21 | 6.48 | Hanging | None | Adjustment disorder with depressed mood |
| 16/F/15/W | 20 | 6.1 | Hanging | None | Borderline personality disorder |
| 17/M/19/W | 15 | 6.9 | GSW to chest | Fluoxetine | Dissociative disorder, Substance abuse (kind unclear), PTSD |
| 18/F/15/W | 11 | 6.44 | Drug overdose | Phenylpropanolamine Cholorophenylamine, Codeine, Salicylate, Acetaminophen | No mental disorder |
| 19/M/17/A | 7 | 5.9 | GSW | Ethanol | No mental illness |
| 20/M/16/H | 20 | 6.17 | Hanging | None | No mental illness |
| 21/F/16/W | 18 | 6.31 | GSW | Amitriptyline | No mental illness |
| 22/M/15/W | 16 | 6.18 | Hanging | None | Normal |
| 23/M/14/B | 22 | 6.44 | Hanging | None | No mental illness |
| 24/F/17/W | 24 | 6.49 | Diphenhydramine overdose | Diphenhydramine, Citalopram | Dx (not enough info.) |
Abbreviations: M, male; F, female; B, black; W, white; A, Asian; H, Hispanic; ACSVD, atherosclerotic cardiovascular disease; GSW, gunshot wound; MVA, motor vehicle accident; NA, not available; PE/DVT, pulmonary embolism, deep vein thrombosis; PMI, postmortem interval
Mean ± SD age was 16.29 ± 2.03 years; PMI, 18.13 ± 7.67 hours; and brain pH, 6.15 ± 0.43, 17 male, 7 female
Mean ± SD age was 15.92 ± 1.86 years; PMI, 19.00 ± 6.85 hours; and brain pH, 6.21 ± 0.44, 14 male, 10 female
All subjects in this study were diagnosed using the Schedule for Clinical Interviews for the DSM-IV (SCID) (First et al., 1997). The SCID was administered by a trained interviewer using a family member as an informant and included a review of all obtainable medical and psychiatric records. The SCID diagnoses are validated by two trained psychiatrists. This has been found to be a very accurate way to make diagnoses (Ramirez Basco et al., 2000). The protocol was approved by the University of Illinois at Chicago Institutional Review Board.
Family members gave permission for the use of brain tissue for research and for clinical records to be obtained from mental health treatment providers when there was a prior history of mental health treatment, or suicide attempts. Two senior psychiatrists provided independent DSM-IV diagnoses. Similarly, normal controls were verified as free from mental illnesses using such consensus diagnostic procedures.
2.2 Determination of cytokines in the brain
2.2.1 Determination of mRNA levels using real time PCR
2.2.1.1 RNA extraction and reverse transcription
Total RNA was extracted from 100 mg of tissue using the TRIZOL reagent (Invitrogen) as per manufacturer's instructions and treated with DNAse 1 (Invitrogen, USA). The RNA yield was determined by absorbance at 260 nm using NanoDrop®ND-1000 (NanoDrop Technologies, Montchanin, DE, USA). RNA qualitywas assessed using Agilent Bioanalyzer 2100 (Agilent) and only samples with 28S/18S ratios >1.2 and RIN ≥ 7.0 were included.
Expression levels of mRNA were determined using a two-step real-time RT-PCR (qRT-PCR) method. 1ug of total RNA was reverse transcribed using 50ng random hexamers, 2mM dNTP mix, 10 units ribonuclease inhibitor, and 200 units MMLV-reverse transcriptase enzyme in a final reaction volume of 20 μl.
2.2.1.2 Relative real-time PCR
Real-time PCR was performed with MX3005p sequence detection system (Agilent) using specific TaqMan gene expression assays (Applied Biosystems, Foster City, CA) and TaqMan Universal PCR MasterMix, with UNG (Applied Biosystems) according to the manufacturer’s instructions. The TaqMan assay IDs were as follows: ACTB, Hs99999903; PPIA, Hs99999904_m1; IL1B, Hs00174097_m1; TNF-alpha, Hs00174128_m1; IL6, Hs00985641_m1. The stability and optimal number of housekeeping genes was determined using geNORM version 3.4 (PrimerDesign Ltd, UK) according to the manufacturer's instructions (Vandesompele et al., 2002). This comparison identified β-actin and cyclophilin A (PPIA) as the most stable housekeeping genes. PCR efficiency after 5-log dilution series was 92–105%. Both β-actin and PPIA had similar amplification efficiencies as the target genes and were run in parallel. For each primer/probe set, the PCR reaction is carried out using 10 μl of cDNA diluted 1:10 fold and includes a non-RT and no template control. All measurements were performed in triplicates. The amount of target gene mRNA normalised to β-actin and PPIA and relative to the control reference samples is expressed as 2−(ΔΔCt).
2.2.2 Determination of protein levels using ELISA
The cytosol fraction from the brain tissue samples for the determination of cytokines was prepared by homogenizing 100 mg of tissue in 0.6 ml of buffer containing 100 mM Tris (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium decanoate, 0.1% SDS, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 5 μg/ml aprotinin, leupeptin, pepstatin, and 100 mM sodium orthovanadate. The homogenate was centrifuged at 15,000 g for 10 min at 4°C, and the supernatant was used for all the assays. Protein concentration was determined according to the method of (Lowry et al., 1951).
Levels of proinflammatory cytokines were determined in aliquots of the cytosol fraction by enzyme-linked immunosorbent assay (ELISA) using commercially available Quantakine® kits for human IL-1β, human IL-6, and human TNF-α purchased from R & D Systems, Minneapolis, MN.
2.3 Statistical analysis
Statistical differences in age, postmortem interval (PMI), and the cytokine levels between normal controls and suicide victims were evaluated by Student’s t-test. The relationships between age, gender, PMI, pH of the brain, race, and the cytokine levels were determined by Pearson product-moment correlation analysis.
3. Results
The demographic and clinical characteristics of the teenage suicide victims (n = 24) and normal control subjects (n = 24) are given in Table 1. The age range of the teenage suicide victims and normal control subjects was 12 to 20 years. There were no significant differences in the mean age or the mean PMI between the suicide victims and normal control subjects.
3.1 Protein expression of TNF-α, IL-1β, and IL-6 in PFC (BA-10) of teenage suicide victims and normal control subjects
Using the ELISA method, we determined the protein expression levels of TNF-α, IL-1β, and IL-6 in the BA-10 of suicide victims and normal control subjects. We observed that the mean protein expression of TNF-α and IL-1β was significantly higher in BA-10 of teenage suicide victims compared with the normal control subjects (Fig. 1). The protein expression levels of IL-6 were also increased at a p level of 0.06 in BA-10 of teenage suicide victims compared with controls (Fig.1). Although the increase was 208%, it just missed the level of significance. This may be due to large standard deviations in both groups.
Figure 1.
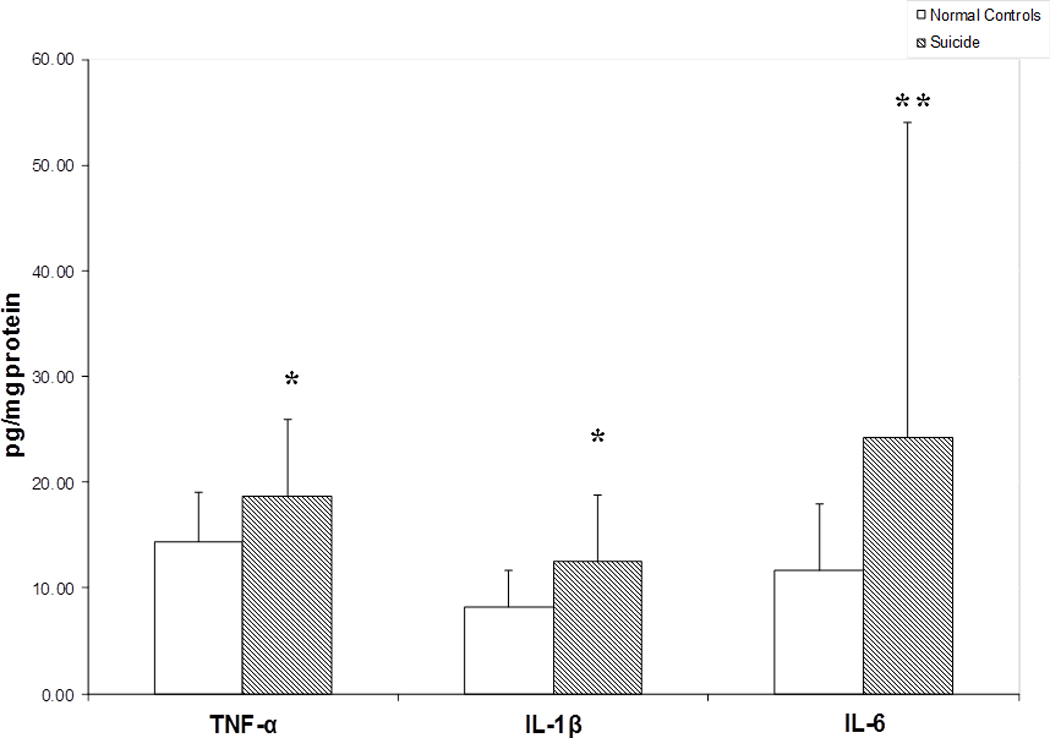
Protein levels of proinflammatory cytokines in the postmortem brain of teenage suicide victims and normal control subjects. Mean ± SD values of TNF-α, IL-1β and IL-6 in the prefrontal cortex (BA-10) of normal control subjects (n = 24) and teenage suicide victims (n = 24).
* p< 0.01
**p< 0.06
3.2 Protein expression of TNF-α and IL-1β in PFC (BA-8) of teenage suicide victims and normal control subjects
In order to examine if the increase protein expression levels of proinflammatory cytokines observed in BA-10 was reproducible and/or abnormal in another area of the PFC, we also determined the protein expression levels of the proinflammatory cytokines IL-1β and TNF-α in BA-8 (Fig. 2), and found that the results were very similar to those observed in BA-10. The levels of TNF-α and IL-1β were again significantly higher in BA-8 of suicide victims compared with normal control subjects (Fig. 2).
Figure 2.
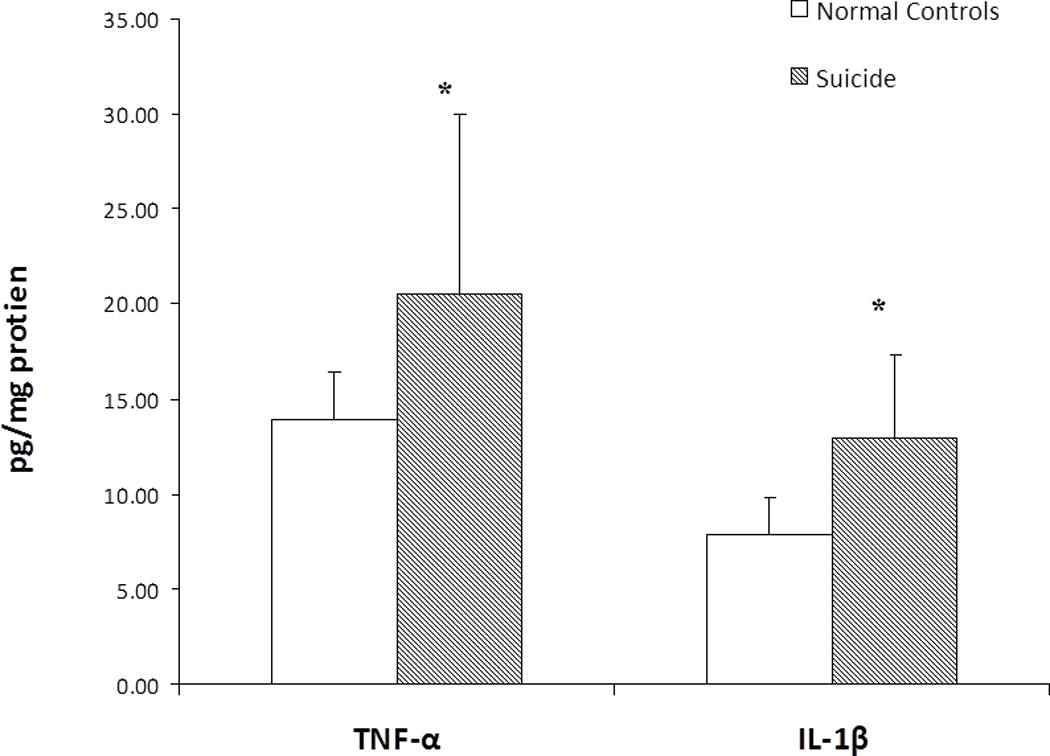
Protein levels of proinflammatory cytokines in the postmortem brain of teenage suicide victims and normal control subjects. Mean ± SD values of TNF-α and IL-1β in the prefrontal cortex (BA- 8) of normal control subjects (n = 24) and teenage suicide victims (n = 24).
* p< 0.01
3.3 mRNA levels of IL-1β, IL-6 and TNF-α in PFC (BA-10) of teenage suicide victims and normal control subjects
Since we observed an increase in the protein expression levels of the proinflammatory cytokines in the PFC of suicide victims, we then examined if there are similar changes in the gene expression levels of the proinflammatory cytokines in suicide victims. We therefore determined the mRNA levels of IL-1β, IL-6, and TNF-α in the PFC (BA-10) of suicide victims and normal control subjects. We found that the mRNA levels of IL-1β, IL-6 and TNF-α were significantly higher in BA-10 of suicide victims compared to normal controls, as shown in Fig. 3.
Figure 3.
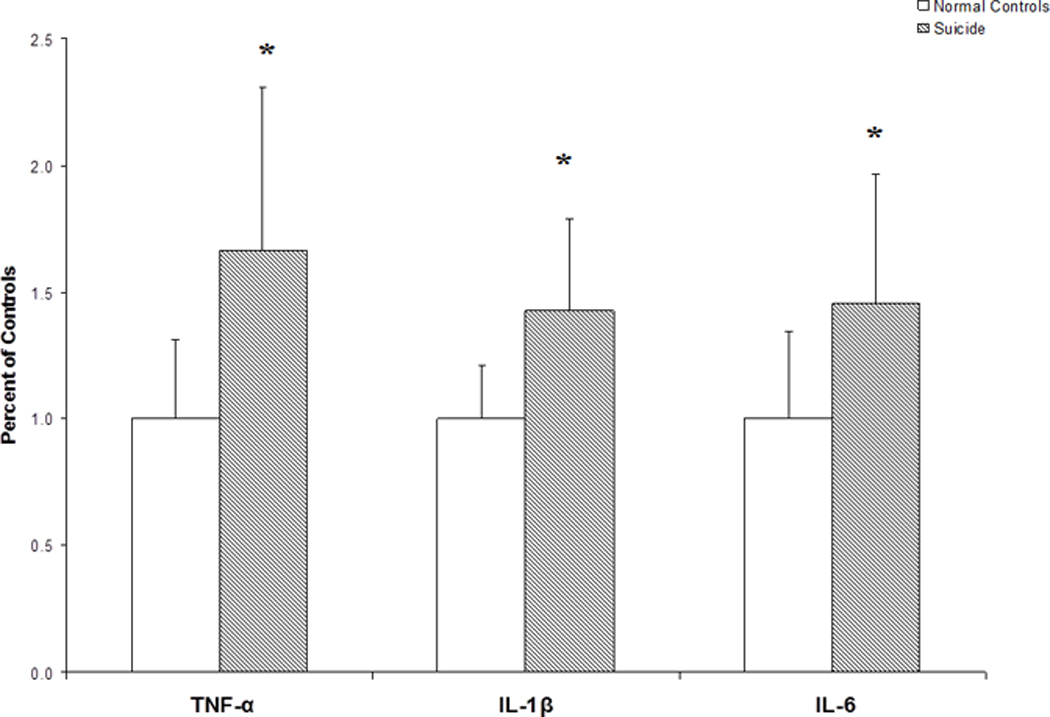
mRNA levels of proinflammatory cytokines in the postmortem brain of teenage suicide victims and normal control subjects. Mean ± SD values of TNF-α, IL-1β and IL-6 in the prefrontal cortex (BA-10) of normal control subjects (n = 24) and teenage suicide victims (n = 24).
* p< 0.01
3.4 Effects of confounding variables
To rule out the possibility that the observed increase in the levels of proinflammatory cytokines in the PFC of suicide victims is related to the covariance, we examined the effects of age, gender, PMI and pH of brain on these parameters. There was no significant correlation of age gender, PMI or pH of the brain with protein or mRNA expression levels of either TNF-α, IL-1β or IL-6.
Since it has been shown that antidepressant treatment may cause changes in the levels of proinflammatory cytokines, we have also compared the protein and mRNA levels of these cytokines in the 4 suicide victims who had a history of antidepressant treatment and who had antidepressant present in their blood at time of death. The protein and mRNA levels of either TNF-α, IL-1β, or IL-6 were not significantly different in those antidepressants-treated suicide victims (n = 4) compared with those suicide cases with no antidepressant treatment (n = 20).
In fact, there was still a significant increase (p < 0.001) in the protein and mRNA levels of TNF-α and IL-1β, and in the mRNA levels of IL-6 in teenage suicide subjects excluding these 4 cases compared with controls. After the exclusion of the four antidepressant-treated suicide victims, the protein levels of IL-6 remain higher in teenage suicide group compared with the control group at the level of p = .07.
4. Discussion
In this study we observed that the protein and mRNA levels of the proinflammatory cytokines, TNF-α, IL-1β, and IL-6 were significantly increased in the PFC (BA-10) of teenage suicide victims compared with normal control subjects.
The interleukin-1 (IL-1) family has at least three proteins known as IL-1α, IL-1β, and IL-1-RA. Both IL-1α and IL-β are agonist and are believed to exert similar effects in the periphery. IL-1-RA is a specific endogenous receptor antagonist that blocks the actions of both IL-1α and IL-1β, but has no known agonist activity (Dinarello & Thompson, 1991). It appears that only IL-β is induced in the brain in response to injury or infection, or in sores (Rothwell, 1997a; b; Rothwell et al., 1997; Rothwell & Luheshi, 1994). IL-1 has several actions on the brain. For example, IL-1β or TNF-α injected into the brain produces anxiogenic-like effects, fever, sickness behavior, and alterations in sleep and the neuroendocrine parameters (Anisman et al., 2005; Connor et al., 1998).
TNF also exists in α and β forms and these proteins are derived from two different genes (Fears et al., 1992a; Fears et al., 1992b; Munoz-Fernandez & Fresno, 1998). TNF-β shares biological activities and membrane receptors with TNF-α but is a molecule produced from a different gene. There are two types of TNF receptors and both exhibit affinity for the two forms of TNF.
If an increase in in proinflammatory cytokines is associated with the pathophysiology of suicide, is it possible to treat suicidal behavior by reducing the levels of these cytokines? Both preclinical and clinical studies with depressed patients provide strong evidence in support of this possibility.
For example, the physiological effects of TNF-α are mediated through its two receptors, known as TNF-R1 and TNF-R2. Simen et al. (2006) studied the effect of the deletion of these receptors in the knock-out mice on behaviors related to depression. They found that deletion of either TNF-R1 or TNF-R2 leads to an antidepressant-like response in the forced swim test, and that mice lacking TNF-R2 demonstrated hedonic response in a sucrose drinking test compared with the wild-type litter mates. In addition, deletion of TNF-R1 leads to decreased fear conditioning. These investigators suggested that TNF-α can induce depression-like symptoms and that blocking or removal of the receptors for TNF which mediate these behavioral effects can result in improvement of depression-like behaviors.
Koo and Duman (2008) studied the role of IL-1β as a mediator of the antineurogenic and anhedonic effects of stress. They found that blockade of IL-1β receptor, IL-1RI, by using either an inhibitor or IL-1RI null mice blocks the antineurogenic effect of stress and blocks the anhedonic behavior caused by chronic stress. These results again suggest that IL-1β is involved in antineurogenic and depression-like behavior caused by stress (Koo et al., 2008).
Clinical evidence in support of this hypothesis has been provided by Tyring et al. (2006) who studied 618 patients with moderate to serious psoriasis, a skin disorder associated with substantial psychological and emotional effects, including depression. These patients received double-blind treatment with placebo or 50 mg twice-weekly etanercept, a soluble TNF-α receptor that prevents TNF-α-mediated cellular responses by competitively inhibiting the interaction of TNF-α with cell surface receptors and it has also been shown to be effective in patients with psoriasis. They found that a large portion of patients who received etanercept had at least a 50% improvement in the Hamilton rating scale for depression (Ham-D) or Beck depression inventory (BDI) at week 12 compared with the placebo group. This study provides strong clinical evidence that reducing or blocking the effects of TNF-α could result in improving depression.
The other indirect evidence supporting the hypothesis that a decrease in cytokine levels may be related to treatment response is provided by studies of Tuglu et al. (2003) that determined the TNF-α and C-reactive protein (CRP) levels before and post-treatment in 26 patients with major depressive disorders (MDD). They observed that the levels of TNF-α and CRP, which were significantly higher in MDD patients than in normal controls, decreased significantly after treatment.
The other issue is the relationship between the immune system and the brain. It is well known now that the immune system does not operate in isolation but is in communication with the brain. The presence of cytokines, which serve key roles in the generation of the immune response in brain, is now quite well established (Hopkins & Rothwell, 1995; Schobitz et al., 1994a; Schobitz et al., 1993; Schobitz et al., 1994b). The receptors for IL-1, TNF-α and IL-6 have been observed in a number of brain regions. They are found on both glia as well as in the neurons (Hopkins et al., 1995; Schobitz et al., 1994a; Schobitz et al., 1993; Schobitz et al., 1994b). Many of the effects of infection, such as fever, disturbed sleep, decreased food and water intake, or decreased social interactions are also observed after intracerebroventricular administration of these cytokines.
Although an association between immune dysregulation and suicide has been suggested by several studies, there are only a few studies of cytokines in general, and TNF-α, IL-1β, and IL-6 in particular, in the serum of suicidal patients. Nassberger and Traskman-Bendz (1993) reported increased levels of soluble IL-2 receptors in the serum of suicide attempters. Lindqvist et al. (2009) determined the levels of several cytokines in the CSF and plasma of patients who attempted suicide and normal control subjects. They found that levels of IL-6 were significantly higher in suicide attempters than in controls. Janelidze et al. (2011) determined the plasma levels of IL-2, IL-6 and TNF-α in suicidal patients, non-suicidal depressed patients and normal healthy control subjects. They found increased levels of TNF-α and IL-6 and decreased levels of IL-2 in suicide attempters compared with normal controls. There is only one study of cytokines in the blood of adolescent suicidal patients by Gabbay et al. (2009). They found decreased plasma levels in adolescent suicidal patients with MDD, whereas IFN-γ was increased in both suicidal and non-suicidal MDD patients.
Shelton et al. (2011) conducted gene expression profiling studies of a number of pro- and anti-inflammatory cytokines in the postmortem brain (BA-10) of MDD subjects and found upregulation of a variety of pro- and anti-inflammatory cytokines, including IL-1α, in the MDD subjects compared with controls. Tonelli et al. (2008) determined the mRNA expression of TNF-α, IL-1β, and IL-4,5,6 and 13 in the postmortem brain (BA-11) of suicide and control subjects. They found increased expression of IL-4 in female suicide victims and IL-13 in male suicide victims but no significant change in the expression of TNF-α either in male or in female suicide victims.
Steiner et al. (2008) have also observed increased microglial density in the PFC of suicide victims. Since microglial activation might result in increased levels of cytokines, these appear to be consistent with our findings. In a recent study, Patersson et al. (2006) have reported increased levels of TNF-α mRNA in the PFC of schizophrenic subjects.
Our current study of IL-1β, IL-6, and TNF-α clearly indicates a significant increase in the cytokines in the PFC of teenage suicide victims compared with normal control subjects. Our studies are not comparable to those reported by Tonelli et al. (2008) because we determined the cytokines in the PFC of teenage suicide subjects as opposed to the adult suicide subjects studied by them. It is quite possible that the pathophysiology of teenage suicide and the role of cytokines in teenage suicide may differ from that in adults as reported by Tonelli et al. (2008).
These proinflammatory cytokines may produce their physiological and behavioral effects through various mechanisms, and one of the mechanisms suggested by which they can produce their effects is through its interaction with the serotonergic systems (Myint & Kim, 2003; Wichers & Maes, 2002; Wichers et al., 2007; Yamada et al., 2000). TNF-α can alter the availability of serotonin (5HT) (Yamada et al., 2000) and also can affect the 5HT transporter (Tsao et al., 2006).
Besides causing the inhibition of neurite outgrowth and branching, as suggested earlier (Neumann et al., 2002), proinflammatory cytokines may also cause the inhibition of adult neurogenesis (Neumann et al., 2002). Thus, changes in neuronal density and synaptic plasticity observed in depressed and/or suicidal patients may be related to the effects of TNF-α or IL-1β on neurogenesis.
In summary, the observed increases of proinflammatory cytokines in the postmortem brain of teenage suicide victims suggest that TNF-α, IL-1β or IL-6 are associated with the neurobiology of suicide and that targeting these cytokines may help in developing new therapies for the treatment of suicidal behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anisman H, Merali Z, Poulter MO, Hayley S. Cytokines as a precipitant of depressive illness: animal and human studies. Current Pharmaceutical Design. 2005;11:963–972. doi: 10.2174/1381612053381701. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Biondi M, Grimaldi F, Ippoliti F, Maes M. Depression induced by treatment with interferon-alpha in patients affected by hepatitis C virus. Journal of Affective Disorders. 2002a;72:237–241. doi: 10.1016/s0165-0327(02)00264-1. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M. Increased depressive ratings in patients with hepatitis C receiving interferon-alpha-based immunotherapy are related to interferon-alpha-induced changes in the serotonergic system. Journal of Clinical Psychopharmacology. 2002b;22:86–90. doi: 10.1097/00004714-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. The American Journal of Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Early depressive symptoms in cancer patients receiving interleukin 2 and/or interferon alfa-2b therapy. Journal of Clinical Oncology. 2000;18:2143–2151. doi: 10.1200/JCO.2000.18.10.2143. [DOI] [PubMed] [Google Scholar]
- Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sciences. 1998;62:583–606. doi: 10.1016/s0024-3205(97)00990-9. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Thompson RC. Blocking IL-1: interleukin 1 receptor antagonist in vivo and in vitro. Immunology Today. 1991;12:404–410. doi: 10.1016/0167-5699(91)90142-G. [DOI] [PubMed] [Google Scholar]
- Fears R, Ferres H, Glasgow E, Standring R, Hogg KJ, Gemmill JD, Burns JM, Rae AP, Dunn FG, Hillis WS. Monitoring of streptokinase resistance titre in acute myocardial infarction patients up to 30 months after giving streptokinase or anistreplase and related studies to measure specific antistreptokinase IgG. British Heart Journal. 1992a;68:167–170. doi: 10.1136/hrt.68.8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fears R, Hearn J, Standring R, Anderson JL, Marder VJ. Lack of influence of pretreatment antistreptokinase antibody on efficacy in a multicenter patency comparison of intravenous streptokinase and anistreplase in acute myocardial infarction. American Heart Journal. 1992b;124:305–314. doi: 10.1016/0002-8703(92)90591-i. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) Arlington, Virginia, USA: American Psychiatric Publishing, Inc.; 1997. [Google Scholar]
- Gabbay V, Klein RG, Guttman LE, Babb JS, Alonso CM, Nishawala M, Katz Y, Gaite MR, Gonzalez CJ. A preliminary study of cytokines in suicidal and nonsuicidal adolescents with major depression. Journal of Child and Adolescent Psychopharmacology. 2009;19:423–430. doi: 10.1089/cap.2008.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguli R, Yang Z, Shurin G, Chengappa KN, Brar JS, Gubbi AV, Rabin BS. Serum interleukin-6 concentration in schizophrenia: elevation associated with duration of illness. Psychiatry Research. 1994;51:1–10. doi: 10.1016/0165-1781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Eaton WW. Asthma, suicidal ideation, and suicide attempts: findings from the Baltimore epidemiologic catchment area follow-up. American Journal of Public Health. 2005a;95:717–722. doi: 10.2105/AJPH.2003.019109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin RD, Messineo K, Bregante A, Hoven CW, Kairam R. Prevalence of probable mental disorders among pediatric asthma patients in an inner-city clinic. The Journal of Asthma. 2005b;42:643–647. doi: 10.1080/02770900500264770. [DOI] [PubMed] [Google Scholar]
- Hopkins SJ, Rothwell NJ. Cytokines and the nervous system. I: Expression and recognition. Trends in Neurosciences. 1995;18:83–88. [PubMed] [Google Scholar]
- Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in the blood may distinguish suicide attempters from depressed patients. Brain, Behavior and Immunity. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE. Stress, depression and the immune system. Stress Medicine. 2000;16:133–137. [Google Scholar]
- Leonard BE. HPA and immune axes in stress: involvement of the serotonergic system. Neuroimmunomodulation. 2006;13:268–276. doi: 10.1159/000104854. [DOI] [PubMed] [Google Scholar]
- Lindqvist D, Janelidze S, Hagell P, Erhardt S, Samuelsson M, Minthon L, Hansson O, Bjorkqvist M, Traskman-Bendz L, Brundin L. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biological Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Progress in Neuro-psychopharmacology & Biological Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10:313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- Miyahara S, Komori T, Fujiwara R, Shizuya K, Yamamoto M, Ohmori M, Okazaki Y. Effects of repeated stress on expression of interleukin-6 (IL-6) and IL-6 receptor mRNAs in rat hypothalamus and midbrain. Life Sciences. 2000;66:PL93-8. doi: 10.1016/s0024-3205(99)00626-8. [DOI] [PubMed] [Google Scholar]
- Monteleone P, Fabrazzo M, Tortorella A, Maj M. Plasma levels of interleukin-6 and tumor necrosis factor alpha in chronic schizophrenia: effects of clozapine treatment. Psychiatry Research. 1997;71:11–17. doi: 10.1016/s0165-1781(97)00036-x. [DOI] [PubMed] [Google Scholar]
- Munoz-Fernandez MA, Fresno M. The role of tumour necrosis factor, interleukin 6, interferon-gamma and inducible nitric oxide synthase in the development and pathology of the nervous system. Progress in Neurobiology. 1998;56:307–340. doi: 10.1016/s0301-0082(98)00045-8. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Medical Hypotheses. 2003;61:519–525. doi: 10.1016/s0306-9877(03)00207-x. [DOI] [PubMed] [Google Scholar]
- Nassberger L, Traskman-Bendz L. Increased soluble interleukin-2 receptor concentrations in suicide attempters. Acta Psychiatrica Scandinavica. 1993;88:48–52. doi: 10.1111/j.1600-0447.1993.tb03412.x. [DOI] [PubMed] [Google Scholar]
- Neumann H, Schweigreiter R, Yamashita T, Rosenkranz K, Wekerle H, Barde YA. Tumor necrosis factor inhibits neurite outgrowth and branching of hippocampal neurons by a rho-dependent mechanism. The Journal of Neuroscience. 2002;22:854–862. doi: 10.1523/JNEUROSCI.22-03-00854.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Deak T, Owens SM, Kohno T, Fleshner M, Watkins LR, Maier SF. Exposure to acute stress induces brain interleukin-1beta protein in the rat. The Journal of Neuroscience. 1998;18:2239–2246. doi: 10.1523/JNEUROSCI.18-06-02239.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SM, Scott LV, Dinan TG. Cytokines: abnormalities in major depression and implications for pharmacological treatment. Human Psychopharmacology. 2004;19:397–403. doi: 10.1002/hup.609. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y. The role of cytokines in depression. In: Plotnikoff NP, Faith RE, Murgo AJ, Good RA, editors. Cytokines, stress and immunity. Boca Raton, Florida: CRC Press, Taylor & Francis Group; 2007. pp. 51–66. [Google Scholar]
- Paterson GJ, Ohashi Y, Reynolds GP, Pratt JA, Morris BJ. Selective increases in the cytokine, TNFalpha, in the prefrontal cortex of PCP-treated rats and human schizophrenic subjects: influence of antipsychotic drugs. Journal of Psychopharmacology. 2006;20:636–642. doi: 10.1177/0269881106062025. [DOI] [PubMed] [Google Scholar]
- Ramirez Basco M, Bostic JQ, Davies D, Rush AJ, Witte B, Hendrickse W, Barnett V. Methods to improve diagnostic accuracy in a community mental health setting. The American Journal of Psychiatry. 2000;157:1599–1605. doi: 10.1176/appi.ajp.157.10.1599. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Cytokines and acute neurodegeneration. Molecular Psychiatry. 1997a;2:120–121. doi: 10.1038/sj.mp.4000223. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ. Sixteenth Gaddum Memorial Lecture December 1996. Neuroimmune interactions: the role of cytokines. British Journal of Pharmacology. 1997b;121:841–847. doi: 10.1038/sj.bjp.0701248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Loddick SA, Stroemer P. Interleukins and cerebral ischaemia. International Review of Neurobiology. 1997;40:281–298. doi: 10.1016/s0074-7742(08)60724-2. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Luheshi G. Pharmacology of interleukin-1 actions in the brain. Advances in Pharmacology. 1994;25:1–20. doi: 10.1016/s1054-3589(08)60428-7. [DOI] [PubMed] [Google Scholar]
- Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2005;29:201–217. doi: 10.1016/j.pnpbp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Schobitz B, De Kloet ER, Holsboer F. Gene expression and function of interleukin 1, interleukin 6 and tumor necrosis factor in the brain. Progress in Neurobiology. 1994a;44:397–432. doi: 10.1016/0301-0082(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Schobitz B, de Kloet ER, Sutanto W, Holsboer F. Cellular localization of interleukin 6 mRNA and interleukin 6 receptor mRNA in rat brain. The European Journal of Neuroscience. 1993;5:1426–1435. doi: 10.1111/j.1460-9568.1993.tb00210.x. [DOI] [PubMed] [Google Scholar]
- Schobitz B, Sutanto W, Carey MP, Holsboer F, de Kloet ER. Endotoxin and interleukin 1 decrease the affinity of hippocampal mineralocorticoid (type I) receptor in parallel to activation of the hypothalamic-pituitary-adrenal axis. Neuroendocrinology. 1994b;60:124–133. doi: 10.1159/000126742. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Claiborne J, Sidoryk-Wegrzynowicz M, Reddy R, Aschner M, Lewis DA, Mirnics K. Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Molecular Psychiatry. 2011 doi: 10.1038/mp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biological Psychiatry. 2006;59:775–785. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, Bernstein HG, Bogerts B. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. Journal of Psychiatric Research. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Tonelli LH, Stiller J, Rujescu D, Giegling I, Schneider B, Maurer K, Schnabel A, Moller HJ, Chen HH, Postolache TT. Elevated cytokine expression in the orbitofrontal cortex of victims of suicide. Acta Psychiatrica Scandinavica. 2008;117:198–206. doi: 10.1111/j.1600-0447.2007.01128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Chen CC, Bai CH, Wu SR. Cytokines and serotonin transporter in patients with major depression. Progress in Neuro-psychopharmacology # biological psychiatry. 2006;30:899–905. doi: 10.1016/j.pnpbp.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. The International Journal of Neuropsychopharmacology. 2002;5:375–388. doi: 10.1017/S1461145702003103. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Kenis G, Koek GH, Robaeys G, Nicolson NA, Maes M. Interferon-alpha-induced depressive symptoms are related to changes in the cytokine network but not to cortisol. Journal of Psychosomatic Research. 2007;62:207–214. doi: 10.1016/j.jpsychores.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Yamada K, Iida R, Miyamoto Y, Saito K, Sekikawa K, Seishima M, Nabeshima T. Neurobehavioral alterations in mice with a targeted deletion of the tumor necrosis factor-alpha gene: implications for emotional behavior. Journal of Neuroimmunology. 2000;111:131–138. doi: 10.1016/s0165-5728(00)00375-1. [DOI] [PubMed] [Google Scholar]


