Abstract
Objective
In men with prostate cancer, androgen deprivation reduces insulin sensitivity; however, the relative roles played by testosterone and estradiol are unknown. To investigate the respective effects of these hormones on insulin sensitivity in men, we employed a model of experimental hypogonadism with or without hormone replacement.
Design
Placebo-controlled, randomized trial.
Participants
22 healthy male volunteers, 18–55 years old.
Methods
Following screening, subjects received the gonadotropin releasing hormone antagonist acyline plus one of the following for 28 days: Group 1, placebo transdermal gel and placebo pills; Group 2, transdermal testosterone gel 10g/day plus placebo pills; Group 3, transdermal testosterone gel 10 g/day plus the aromatase inhibitor anastrozole 1 mg/day to normalize testosterone while selectively reducing serum estradiol. Fasting insulin, glucose, adipokines and hormones were measured bi-weekly.
Results
With acyline administration, serum testosterone was reduced by >90% in all subjects in Group 1. In these men, mean fasting insulin concentrations were significantly increased compared with baseline (p=0.02) at 28 days, despite stable body weight and no changes in fasting glucose concentrations. Decreased insulin sensitivity also was apparent in the insulin sensitivity indices HOMA-IR (p=0.03) and QUICKI (p=0.04). In contrast, in Groups 2 and 3, testosterone concentrations remained in the physiologic range, despite significant reduction in mean estradiol in Group 3. In these groups, no significant changes in insulin sensitivity were observed.
Conclusions
Acute testosterone withdrawal reduces insulin sensitivity in men independent of changes in body weight, whereas estradiol withdrawal has no effect. Testosterone appears to maintain insulin sensitivity in normal men.
Keywords: testosterone, insulin resistance, leptin, adiponectin, metabolism
Introduction
Manipulation of testosterone has a number of important clinical applications in men including androgen deprivation therapy (ADT) for the treatment of prostate cancer, androgen replacement in hypogonadal men, and the experimental use of exogenous testosterone for male contraception. In addition, non-medical androgen use is increasing, likely due to the well-recognized effects of exogenous testosterone on body composition.1 Recent cross-sectional data indicate that long-term ADT in men with advanced prostate cancer significantly increases the risk of type 2 diabetes mellitus, cardiovascular disease, and the metabolic syndrome in older men,2–4 but the relationships between testosterone and these conditions in younger men are less well characterized.
ADT in older men with advanced prostate cancer results in decreased lean body mass and increased fat mass,5 while testosterone replacement in older men confers the opposite effects over time.6 In men undergoing ADT, changes in body composition are associated with reductions in insulin sensitivity observed within 12 weeks of initiating therapy.5, 7 Similarly, in men with idiopathic hypogonadotropic hypogonadism (IHH), withdrawal of physiologic testosterone replacement leads to significant decrements in insulin sensitivity within 2 weeks.8 Interestingly, these changes were noted even before alterations in body composition were observed, suggesting testosterone may exert direct effects on metabolic regulators or signaling, rather than by effecting changes in body composition. Testosterone has been shown to improve insulin sensitivity in hypogonadal men,9 as well as in men with both hypogonadism and diabetes.10 The mechanisms whereby androgens may exert metabolic effects have not been fully elucidated, although prospective studies suggest sex steroids may modulate adipokine secretion in men.11 Circulating adipokines such as adiponectin and leptin appear to be important regulators of insulin sensitivity.12 Moreover, the relative contributions of testosterone versus its active metabolite estradiol on metabolism have not been differentiated in most intervention studies to date. Importantly, men deficient in aromatase, the enzyme responsible for the conversion of testosterone to estradiol, have severe metabolic derangements including insulin resistance and dyslipidemia that are corrected by the administration of estradiol.13
We sought to better define the physiologic relationships between testosterone and estradiol and insulin sensitivity in men. Since existing interventional data might be confounded by the specific populations studied (men with prostate cancer, congenital hypogonadism, or pre-existing diabetes), we performed a placebo-controlled intervention trial in young-middle aged, healthy men. We hypothesized that testosterone withdrawal would acutely decrease insulin sensitivity and modify serum adipokine concentrations and that selective replacement with testosterone would reverse these effects. Furthermore, we hypothesized that suppressing estradiol while normalizing testosterone would not impair the ability of testosterone administration to improve insulin sensitivity.
Methods
Subjects
Men ages 18–55 were recruited through advertisements. Study participants had no chronic medical or reproductive conditions, were taking no medications and had normal baseline physical examinations, serum chemistries, complete blood counts, gonadotropins, and total testosterone levels (10.4–34.7 nmol/L). Exclusion criteria included a history of prostate cancer, breast cancer, or benign prostatic hypertrophy; a prostate-specific antigen (PSA)>3.0 μg/L; regular use of testosterone, anabolic steroids, or drugs known to affect steroid metabolism within the prior year; clinically significant, untreated sleep apnea; hematocrit>55%; diabetes or severe obesity (BMI >35) or an abnormal digital rectal exam.
Drug Assignment
All subjects received the gonadotropin releasing hormone (GnRH) antagonist acyline (300 mcg/kg) by subcutaneous injection on Day 0 and Day 14 of treatment. Acyline dramatically suppresses serum concentrations of gonadotropins and testosterone, with castrate levels of testosterone achieved within 24 hours of administration, an effect that lasts for 14 days.14 The first 8 enrolled subjects were assigned to Group 1 and received daily placebo transdermal gel and daily oral placebo pills for 28 days. The next 16 enrolled subjects were randomly assigned to Groups 2 and 3 by a random number sequence. In addition to acyline, subjects in Group 2 received 10 grams of 1% transdermal T gel daily (Testim, Auxilium Pharmaceuticals, New Jersey) and daily placebo pills for 28 days. Subjects in Group 3 received 10 grams of 1% transdermal testosterone gel daily and 1 mg of oral anastrozole daily (Arimidex, AstraZeneca, Wilmington, DE) for 28 days.
Study Protocol
All study visits were performed at the University of Washington Medical Center where the Institutional Review Board approved all study procedures. Written informed consent was obtained prior to any study procedures in all cases. Following screening, randomized subjects returned on Days 0, 14, 28, and 56 (follow-up) for study visits which included a physical examination, a fasting blood draw, and adverse event monitoring. A 2-week supply of each of the study drugs was dispensed on Days 0 and 14. Compliance with the study medications was assessed by analysis of completed drug logs and returned medications on Days 14 and 28. Blood for the measurement of serum luteinizing hormone (LH), follicular stimulating hormone (FSH), estradiol, total testosterone, sex-hormone binding globulin (SHBG), glucose, and insulin levels was obtained at each study visit. Adiponectin, leptin, and ghrelin levels were measured at baseline and on Day 28. The measurements of fasting insulin and glucose were subsequently used to calculate indices of insulin resistance and sensitivity, namely the homeostasis model of insulin resistance (HOMA-IR) and the quantitative insulin sensitivity check index (QUICKI), according to published formulas.15, 16
Laboratory Assessments
Safety laboratory assessments including serum chemistries, complete blood counts, liver function tests, and fasting glucose were measured by the clinical laboratory at the University of Washington Medical Center. For all study endpoints, serum was stored at −80 C°until completion of the study, and assays were run in a single batch for all study participants. Serum LH and FSH were measured by immunofluorometric assay, and testosterone, estradiol, and SHBG were measured by radioimmunoassay.17 Fasting insulin was measured with a Tosoh AIA 1800 auto-analyzer, with each batch of samples analyzed with quality control standards. The coefficients of variation for high and low insulin level quality controls are 2.5% and 3.0%, respectively. Adiponectin and leptin were measured by radioimmunoassay (Millipore, Inc., Billerica, MA) with intra-assay co-efficients of variation of 6.2 and 3.7%, respectively.17 Retinol-binding protein 4 (RBP4) in serum was quantified using immunonephelometry on a Siemens BN-II automated clinical instrument (N Latex Retinol Binding Protein). Monocyte chemoattractant protein-1 (MCP-1) was measured with a Quantikine ELISA kit (R&D Systems, Minneapolis, MN) according to manufacturer’s instructions.
Statistical Analysis
Because Group 1 was completely recruited before subjects were recruited for Groups 2 and 3, statistical analyses were limited to changes from baseline within a given group, and between-group comparisons were not performed. Comparison of results from the end of treatment and recovery with baseline were made using a Wilcoxon sign-rank test without corrections for multiplicity. Correlations were performed using Spearman’s technique. Statistical analyses were performed using STATA version 10 (College Park, TX, USA). For all comparisons, a p-value <0.05 was considered significant.
Results
Study participants
Thirty-one volunteers were recruited, 27 met all screening criteria, and 25 initiated treatment. One subject withdrew after the first study visit due to soreness at the acyline injection site, 1 subject lost interest after Week 2, and a third started a new job that conflicted with study participation and withdrew prior to Day 28. Twenty-two subjects completed all study procedures, 8 in Group 1, 6 in Group 2, and 8 in Group 3. There were no serious adverse events. Six subjects, 4 in Group 1 and 2 in Group 3, complained of low libido during treatment, and 3 subjects in Group 1 complained of hot flashes and 2 of fatigue. All of these adverse effects had resolved by the Day 56 visit without intervention. There were no clinically significant changes in liver function tests, blood chemistries, or blood counts in any of the subjects. At baseline, subjects in all study groups were healthy, normotensive, eugonadal men. The average age among the participants was 30.0 ± 10.5 years, with mean ages of 38.5 ± 10.6, 24.3 ± 4.0 and 25.8 ± 8.4 years for Group 1, 2, and 3 subjects, respectively. Subjects’ average body mass index (BMI) was 25.5 ± 3.0 kg/m2. Baseline characteristics including weight, gonadotropins, serum sex steroids, fasting glucose, fasting insulin, HOMA-IR, and QUICKI are shown in Figure 1 and Table 1.
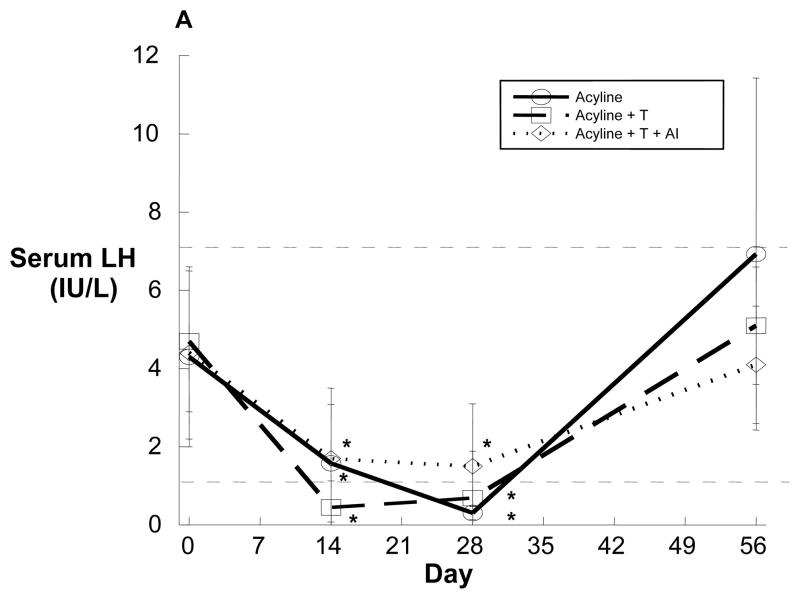
Figure 1.
Serum luteinizing hormone (A), testosterone (B) and estradiol (C) over time in healthy young men administered the GnRH antagonist acyline and placebo testosterone (solid line, n=8), acyline and testosterone (broken line, n=6) or acyline, testosterone and the aromatase inhibitor anastrozole (dotted line, n=8). Normal ranges are designated by the thin dotted lines. Values are expressed as means ± standard deviation (SD). *p<0.05 compared with baseline.
Table 1.
Body weight, indices of insulin sensitivity, and MCP-1 values by treatment group expressed as means (SD) *p<0.05 compared with baseline.
| Group 1 (n=8) | Group 2 (n=6) | Group 3 (n=8) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Day | 0 | 28 | 56 | 0 | 28 | 56 | 0 | 28 | 56 |
| Weight (kg) | 87(7.3) | 87(7.2) | 88(7.8) | 90(14) | 90(13) | 88(14) | 78(9.4) | 78(10) | 79(10) |
| BMI (kg/m2) | 26(2.6) | 26(2.4) | 27(2.8) | 27(3.3) | 27(3.0) | 26(3.2) | 23(2.3) | 24(2.3) | 24(2.3) |
| Glucose (mmol/L) | 5.3(0.2) | 5.2(0.4) | 5.2(0.3) | 4.8(0.2) | 5.0(0.4) | 4.8(0.2) | 5.1(0.3) | 5.0(0.3) | 5.2(0.3) |
| Insulin (pmol/L) | 54(26) | 69(25)* | 54(26) | 65(28) | 59(26) | 64(27) | 50(16) | 42(23) | 50(16) |
| HOMA-IR | 1.8(0.9) | 2.4(1.0)* | 2.2(0.9) | 2.0(0.9) | 1.9(0.9) | 1.9( 0.9) | 1.6(0.6) | 1.4(0.8) | 1.7(0.8) |
| QUICKI | 0.36 (0.03) | 0.34* (0.03) | 0.35 (0.03) | 0.35 (0.02) | 0.35 (0.02) | 0.35 (0.02) | 0.36 (0.02) | 0.38 (0.02) | 0.36 (0.02) |
| MCP-1 (ng/L) | 359 (134) | 441* (196) | 415* (129) | 507 (173) | 472 (245) | 481 (213) | 485 (220) | 457 (206) | 444 (243) |
Hormone concentrations
Administration of the potent GnRH antagonist acyline significantly reduced serum LH in all groups (Figure 1A). In Group 1, mean total testosterone concentrations were below 5 nmol/L on Day 14 and testosterone suppression was maintained through Day 28 (Day 28 mean: 0.8 ± 0.8 nmol/L, Figure 1B). Similarly, in Group 1, mean serum estradiol levels decreased substantially from baseline, and suppression persisted for the 4 week treatment duration (Day 28 mean: 31.9 ± 11.2 pmol/L, Figure 1C). In contrast to Group 1, subjects in Groups 2 and 3 who received testosterone gel had normal serum testosterone concentrations (Figure 1B). These testosterone concentrations did not differ significantly from baseline values. In Group 3, the Day 14 mean testosterone level appeared to rise above baseline; however, this resulted from an unusually high, unexplained testosterone level in 1 subject that normalized without intervention by Day 28. Despite this, no statistically significant differences in serum testosterone were evident between any study timepoints. Group 2 subjects maintained normal serum estradiol levels throughout the study period, while subjects in Group 3 receiving an aromatase inhibitor had significant and sustained reductions in estradiollevels similar to that observed in Group 1 (Figure 1C). There were no significant changes in concentrations of SHBG in any group during the study (Group 1 Day 0 mean: 35 ± 17 nmol/L, Day 28 mean: 38 ± 16 nmol/L, p=0.5), implying that changes in total steroid hormone concentrations were commensurate with changes in free hormone concentrations.
Insulin sensitivity
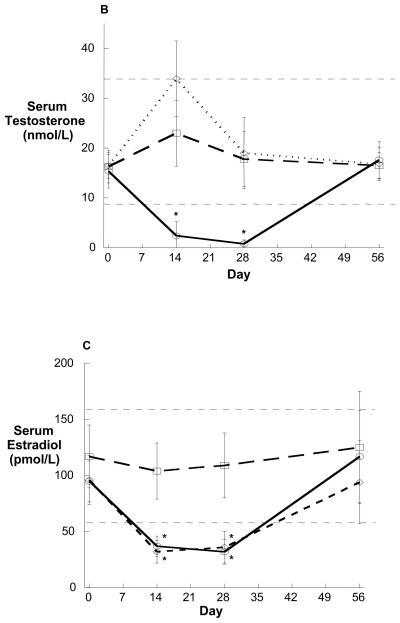
At baseline, all subjects had normal fasting plasma glucose and insulin concentrations and normal insulin sensitivity as assessed by the HOMA-IR and QUICKI (Table 1). During treatment, fasting glucose did not differ significantly from baseline in any treatment group. On Day 14, fasting insulin concentrations remained similarly unchanged (Group 1 Day 0 v. 14, p=0.82). However, by Day 28, subjects in Group 1 experienced a significant increase in fasting insulin concentration with mean levels increasing from 53.8 ± 26.4 pmol/L to 68.8 ± 25.5 pmol/L (p=0.02). Despite no significant change in fasting glucose (Table 1, Figure 2A), an increase in insulin concentration occurred in 7 of 8 subjects in this group (Figure 2B). This finding, suggestive of reduced insulin sensitivity, was corroborated by significant changes in both the HOMA-IR and QUICKI in Group 1 (Table 1). Notably, the reduced insulin sensitivity observed in Group 1 was not associated with any significant changes in BMI or body weight during treatment (Table 1). On Day 56, after recovery of endogenous sex hormones, fasting insulin, HOMA-IR, and QUICKI returned to baseline (Table 1). In contrast to the significant increase in insulin resistance observed in Group 1, no changes in insulin concentration, HOMA-IR or QUICKI were observed among subjects in Groups 2 and 3. Similarly, no significant changes in BMI or body weight occurred in these groups (Table 1).
Figure 2.
Serum insulin (A) and glucose (B) in eight healthy young men administered the GnRH antagonist acyline and placebo testosterone gel and placebo anastrozole. Note the preservation of normal glucose concentrations by the significantly increased concentrations of serum insulin. The group mean is depicted in solid black.
Adipokines and other circulating mediators
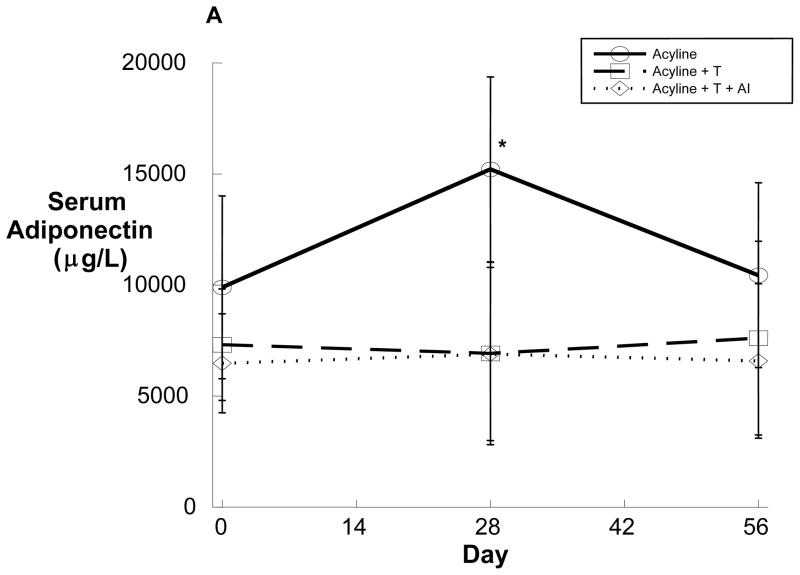
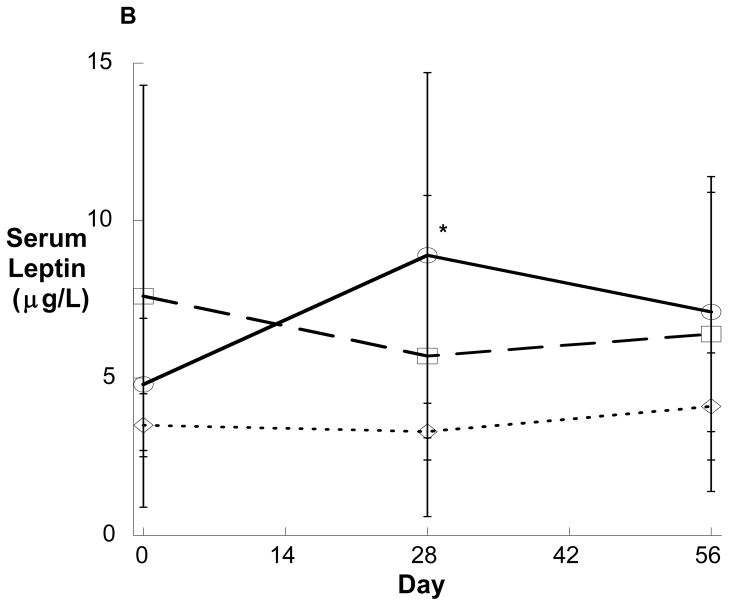
To determine the effects of acute sex steroid withdrawal on adipokine secretion, serum adiponectin and leptin levels were obtained on Days 0 and 28 in all groups. In Group 1, concentrations of both serum leptin and serum adiponectin increased significantly during treatment (Figure 3), an effect that was lost after one month of recovery. In contrast to Group 1, no changes in serum adipokines were observed in Groups 2 or 3 during treatment (Figure 3). Changes in serum adiponectin observed among Group 1 subjects strongly correlated with increases in HOMA-IR (R=0.750, p=0.032) and negatively correlated with changes in QUICKI (R=−0.728, p=0.041). In contrast, the changes in serum leptin did not correlate with changes in fasting insulin, HOMA-IR, or QUICKI (data not shown).
Figure 3.
Serum adiponectin (A) and leptin (B) over time in healthy young men administered the GnRH antagonist acyline and placebo testosterone (solid line, n=8), acyline and testosterone (broken line, n=6) or acyline, testosterone and the aromatase inhibitor anastrozole (dotted line, n=8). Values are expressed as means ± SD. *p<0.05 compared with baseline.
To further explore androgen-dependent effects on potential mediators of insulin resistance, we measured serum levels of RBP4, ghrelin, and MCP-1, as each has been reported to correlate with insulin resistance18–20. In Group 1, no changes in fasting ghrelin (Day 0 mean: 16 ± 8.7 ng/L, Day 28 mean: 13 ± 7.6 ng/L) or RBP4 (Day 0 mean: 4.6 ± 1.0 mg/L, Day 28 mean: 4.6 ± 0.9 mg/L) were observed with treatment. However, a significant increase in serum MCP-1 was observed exclusively in Group 1 subjects and, further, appeared sustained after normalization of endogenous sex steroid production (Table 1).
Discussion
In this work, we present data on the acute metabolic effects of sex steroid withdrawal in young, healthy, eugonadal men. Our data demonstrate that short-term experimental hypogonadism confers a reduction in insulin sensitivity in the absence of changes in body weight. This reduction in insulin sensitivity was associated with significant and substantial increases in both adiponectin and leptin. Moreover, none of these effects were observed in subjects who experienced a selective decline in serum estradiol, suggesting that the observed changes were attributable specifically to testosterone withdrawal. Use of the GnRH antagonist acyline enabled characterization of short-term sex steroid withdrawal without the confounding effects of a transient, early rise in sex steroids as is often observed with use of GnRH agonists for medical castration. These results support a direct role for testosterone in modulating insulin sensitivity and adipokine secretion in men.
Consistent with our results, older men undergoing ADT for the treatment of advanced prostate cancer exhibit decreases in insulin sensitivity manifesting as increased fasting insulin concentrations in the setting of euglycemia.7 Similarly, ADT worsens glycemic control in men with diabetes,3 and androgen withdrawal increases insulin resistance acutely in men with IHH.8 In contrast to our results, Rabiee and colleagues did not observe acute changes in insulin sensitivity in a recent study of sex steroid deprivation in 8 healthy young men.21 These discrepant findings might be a function of the different methods employed for assessing insulin sensitivity. Our findings of a selective increase in fasting insulin suggest a phenotype specifically of hepatic insulin resistance, as higher insulin concentrations are required to suppress basal hepatic glucose production (HGP). The study by Rabiee and colleagues employed the hyperinsulinemic-euglycemic clamp but utilized a labeled glucose tracer to expressly assess HGP in only 3 study subjects. Moreover, clamp data reflect hepatic insulin sensitivity only at low infusion rates,22 whereas higher insulin infusion rates primarily assess skeletal muscle insulin sensitivity.22 Particularly given the small sample size of both studies, our respective results therefore do not necessarily conflict and rather may implicate testosterone specifically in acute modulation of hepatic insulin resistance. Notably, previous studies employing the euglycemic clamp have demonstrated positive associations between testosterone production and insulin sensitivity.23 Finally, the nearly uniform increase in fasting insulin concentration evident across Group 1 subjects strongly supports the validity of our findings. Nonetheless, the apparent discrepancy clearly mandates further investigation and underscores the need to interrogate the tissue-specific effects of testosterone on insulin sensitivity. Of note, Rabiee and colleagues evaluated body composition and found no changes with acyline administration over 4 weeks.21 We cannot exclude the possibility that changes in body composition might also contribute to the observed increase in insulin resistance despite our observations of weight maintenance in this study.
One mechanism by which androgens might modulate insulin sensitivity is by altering adipokine concentrations, either directly or secondarily by affecting fat mass.24, 25 Our data demonstrate that testosterone withdrawal is associated with acute changes in both leptin and adiponectin levels, effects that occurred despite the absence of changes in body weight. Previous prospective studies similarly suggest that testosterone modulates adipokine secretion,26, 27 but studies to date have not clearly distinguished the relative contributions of testosterone versus its active metabolite estradiol to this regulation. Estradiol may have substantial metabolic effects in men as rare males deficient in aromotase, the enzyme responsible for the conversion of testosterone, exhibit reduced insulin sensitivity, increased adiposity, and dyslipidemia, all of which improve with estradiol replacement.13 Our results strongly suggest that the acute effects of sex steroid manipulation on serum adipokines are mediated specifically by changes in androgens rather than estradiol, as no changes were evident in the study group selectively deficient in estradiol. Thus, our data provide novel and direct evidence that androgens are the predominant sex steroid determinant of adiponectin and leptin secretion in men.
Interestingly, although androgen withdrawal increased insulin resistance and leptin, changes that generally occur in tandem, the concurrent rise in adiponectin in this setting is surprising. Generally, adiponectin is characterized by a strong inverse correlation with both fat mass and insulin resistance.12 This paradoxical rise is, however, consistent with in vitro data indicating a direct effect of testosterone on adiponectin secretion.24, 28, 29 In humans, sex steroid withdrawal clearly increases adiponectin concentrations,11, 17 and in recent randomized trials of hypogonadal men with diabetes, testosterone replacement reduced adiponectin levels though improved glucose control.27 Further, the implicated suppressive effect of testosterone on adiponectin secretion might explain inconsistencies in clinical observations regarding the relationship between testosterone and insulin resistance;30 whereas testosterone reduces adiposity, a concurrent decrease in adiponectin could produce a counteracting effect on insulin sensitivty.
The observed increase in leptin is consistent with data from previous studies demonstrating elevated leptin levels in men with hypogonadism and decreased serum leptin with testosterone replacement.26 Notably, this decrease occurs even after short-term testosterone replacement, suggesting that testosterone also might modulate leptin production directly.26 In vitro data provide evidence that androgens suppress leptin secretion, and withdrawal of this suppressive effect may underlie the observed increment in serum leptin.25 However, prior clinical studies, like ours, do not distinguish definitively between the primary effects of testosterone and those conferred indirectly through altered adiposity and adipose tissue remodeling. Additional studies are needed to better discriminate primary and secondary effects of testosterone on adipokine secretion in vivo.
The present study did not demonstrate any changes in ghrelin or RBP4 levels consequent to sex steroid withdrawal. Ghrelin has been associated with differential testosterone exposure,31, 32 and both ghrelin and RBP4 have been associated with obesity and insulin resistance in some, but not all, human studies.18, 19, 32–34 As previous studies have proposed that RBP4 correlates with adiposity specifically rather than insulin resistance, the lack of change in RBP4 is consistent with the absence of change in mean body weight and presumably fat mass observed during the study. Mediators associated with immune system activation variably have been associated with obesity and insulin resistance, and MCP-1 particularly has been implicated in these states.20 In our study, acute testosterone deprivation was associated with an increase in MCP-1 that appeared sustained even subsequent to sex steroid normalization. To our knowledge, this is the first report of changes in MCP-1 as a consequence of androgen manipulation in healthy men. Interestingly, in a mouse model, MCP-1 infusion was sufficient to induce insulin resistance in the absence of obesity.35 Further investigation is needed to identify the specific cell types in which MCP-1 production might be androgen-responsive. Similarly, additional studies are needed to determine whether MCP-1 might play a causative role in the observed increase in insulin resistance.
The major limitations of our study are the small sample size and the pattern of drug assignment. As this was a pilot study, Group 1 enrollment was completed first to determine whether any treatment effects were evident. Subsequently, Groups 2 and 3 were assigned in randomized fashion to provide controls. Our conclusions also are limited in part by the absence of body composition data; thus, we cannot exclude the possibility that changes in insulin sensitivity or adipokines resulted from changes in body fat distribution. Finally, the changes observed in fasting insulin concentration were relatively modest, suggesting the importance of additional, more sensitive metrics of insulin sensitivity such as euglycemic clamp data in future trials.
Conclusions
Our findings collectively underscore the complexity of the role of sex steroids in metabolic regulation. Importantly, sole inclusion of healthy men avoids potential confounders in previous studies including co-morbidities or baseline abnormalities in gonadal function. Moreover, the short study duration promotes the disentangling of sex steroids’ direct metabolic effects from those conferred indirectly by changes in body weight. The findings strongly suggest that testosterone indeed exerts direct effects on insulin sensitivity and adipokine secretion that are independent of effects on body weight. Given the absence of significant metabolic changes in either group receiving exogenous testosterone, these data delineate a clear role for testosterone in modulation of adipokine secretion and insulin sensitivity. The results of the present study specifically indicate short-term metabolic effects of androgen withdrawal in young, healthy men. Additional investigation is needed to determine whether the increases in insulin resistance associated with androgen deprivation vary as a function of time, degree of testosterone withdrawal, or patient age and to elucidate further the mechanisms that underlie our observations.
Supplementary Material
Acknowledgments
Sources of Support: This work was supported by the National Institute of Health through the Eunice Kennedy Shriver National Institute of Child Health and Human Development cooperative agreement U54 HD42454 as part of the Cooperative Contraceptive Research Centers Program, and by the Diabetes and Endocrinology Research Center Grant DK017047 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Rubinow is supported, in part, by grant #T32DK007247 from National Institute of Diabetes, Digestive and Kidney Diseases, a division of the National Institute of Health. Dr. Hoofnagle receives support from Nutrition and Obesity Research grant P30DK035816. Transdermal and placebo testosterone gel was provided by Auxilium (Malvern, PA) who did not otherwise provide support nor any input into the study design, analyses or manuscript. The authors have no additional sources of financial support to disclose.
Reference List
- 1.Basaria S. Androgen abuse in athletes: detection and consequences. J Clin Endocrinol Metab. 2010;95:1533–1543. doi: 10.1210/jc.2009-1579. [DOI] [PubMed] [Google Scholar]
- 2.Keating NL, O’Malley AJ, Freedland SJ, et al. Diabetes and cardiovascular disease during androgen deprivation therapy: observational study of veterans with prostate cancer. J Natl Cancer Inst. 2010;102:39–46. doi: 10.1093/jnci/djp404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derweesh IH, Diblasio CJ, Kincade MC, et al. Risk of new-onset diabetes mellitus and worsening glycaemic variables for established diabetes in men undergoing androgen-deprivation therapy for prostate cancer. BJU Int. 2007;100:1060–1065. doi: 10.1111/j.1464-410X.2007.07184.x. [DOI] [PubMed] [Google Scholar]
- 4.Braga-Basaria M, Dobs AS, Muller DC, et al. Metabolic syndrome in men with prostate cancer undergoing long-term androgen-deprivation therapy. J Clin Oncol. 2006;24:3979–3983. doi: 10.1200/JCO.2006.05.9741. [DOI] [PubMed] [Google Scholar]
- 5.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–4267. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 6.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Lee H, Nathan DM. Insulin sensitivity during combined androgen blockade for prostate cancer. J Clin Endocrinol Metab. 2006;91:1305–1308. doi: 10.1210/jc.2005-2507. [DOI] [PubMed] [Google Scholar]
- 8.Yialamas MA, Dwyer AA, Hanley E, et al. Acute sex steroid withdrawal reduces insulin sensitivity in healthy men with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2007;92:4254–4259. doi: 10.1210/jc.2007-0454. [DOI] [PubMed] [Google Scholar]
- 9.Simon D, Charles MA, Lahlou N, et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care. 2001;24:2149–2151. doi: 10.2337/diacare.24.12.2149. [DOI] [PubMed] [Google Scholar]
- 10.Jones TH, Arver S, Behre HM, et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 study) Diabetes Care. 2011;34:828–837. doi: 10.2337/dc10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith MR, Lee H, Fallon MA, et al. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–322. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316:129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Simpson ER, Jones ME. Of mice and men: the many guises of estrogens. Ernst Schering Found Symp Proc. 2006:45–67. doi: 10.1007/2789_2006_016. [DOI] [PubMed] [Google Scholar]
- 14.Herbst KL, Coviello AD, Page S, et al. A single dose of the potent gonadotropin-releasing hormone antagonist acyline suppresses gonadotropins and testosterone for 2 weeks in healthy young men. J Clin Endocrinol Metab. 2004;89:5959–5965. doi: 10.1210/jc.2003-032123. [DOI] [PubMed] [Google Scholar]
- 15.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 16.Katz A, Nambi SS, Mather K, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 17.Page ST, Herbst KL, Amory JK, et al. Testosterone administration suppresses adiponectin levels in men. J Androl. 2005;26:85–92. [PubMed] [Google Scholar]
- 18.Graham TE, Yang Q, Bluher M, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 19.Purnell JQ, Weigle DS, Breen P, et al. Ghrelin levels correlate with insulin levels, insulin resistance, and high-density lipoprotein cholesterol, but not with gender, menopausal status, or cortisol levels in humans. J Clin Endocrinol Metab. 2003;88:5747–5752. doi: 10.1210/jc.2003-030513. [DOI] [PubMed] [Google Scholar]
- 20.Rull A, Camps J, Alonso-Villaverde C, et al. Insulin resistance, inflammation, and obesity: role of monocyte chemoattractant protein-1 (or CCL2) in the regulation of metabolism. Mediators Inflamm. 2010;2010:1–11. doi: 10.1155/2010/326580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabiee A, Dwyer AA, Caronia LM, et al. Impact of acute biochemical castration on insulin sensitivity in healthy adult men. Endocr Res. 2010;35:71–84. doi: 10.3109/07435801003705601. [DOI] [PubMed] [Google Scholar]
- 22.Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 23.Pitteloud N, Hardin M, Dwyer AA, et al. Increasing insulin resistance is associated with a decreasein Leydig cell testosterone secretion in men. J Clin Endocrinol Metab. 2005;90:2636–2641. doi: 10.1210/jc.2004-2190. [DOI] [PubMed] [Google Scholar]
- 24.Nishizawa H, Shimomura I, Kishida K, et al. Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51:2734–2741. doi: 10.2337/diabetes.51.9.2734. [DOI] [PubMed] [Google Scholar]
- 25.Wabitsch M, Blum WF, Muche R, et al. Contribution of androgens to the gender difference in leptin production in obese children and adolescents. J Clin Invest. 1997;100:808–813. doi: 10.1172/JCI119595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jockenhovel F, Blum WF, Vogel E, et al. Testosterone substitution normalizes elevated serum leptin levels in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2510–2513. doi: 10.1210/jcem.82.8.4174. [DOI] [PubMed] [Google Scholar]
- 27.Lanfranco F, Zitzmann M, Simoni M, et al. Serum adiponectin levels in hypogonadal males: influence of testosterone replacement therapy. Clin Endocrinol (Oxf) 2004;60:500–507. doi: 10.1111/j.1365-2265.2004.02007.x. [DOI] [PubMed] [Google Scholar]
- 28.Kalinchenko SY, Tishova YA, Mskhalaya GJ, et al. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: the double–blinded placebo-controlled Moscow study. Clin Endocrinol (Oxf) 2010;73:602–612. doi: 10.1111/j.1365-2265.2010.03845.x. [DOI] [PubMed] [Google Scholar]
- 29.Blouin K, Nadeau M, Perreault M, et al. Effects of androgens on adipocyte differentiation and adipose tissue explant metabolism in men and women. Clin Endocrinol (Oxf) 2010;72:176–188. doi: 10.1111/j.1365-2265.2009.03645.x. [DOI] [PubMed] [Google Scholar]
- 30.Saad F, Gooren LJ. The role of testosterone in the etiology and treatment of obesity, the metabolic syndrome, and diabetes mellitus type 2. J Obes. 2011;2011:1–10. doi: 10.1155/2011/471584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gambineri A, Pagotto U, De Lasio R, et al. Short-term modification of sex hormones is associated with changes in ghrelin circulating levels in healthy normal-weight men. J Endocrinol Invest. 2005;28:241–246. doi: 10.1007/BF03345380. [DOI] [PubMed] [Google Scholar]
- 32.Pagotto U, Gambineri A, Pelusi C, et al. Testosterone replacement therapy restores normal ghrelin in hypogonadal men. J Clin Endocrinol Metab. 2003;88:4139–4143. doi: 10.1210/jc.2003-030554. [DOI] [PubMed] [Google Scholar]
- 33.Kloting N, Graham TE, Berndt J, et al. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Santoro N, Perrone L, Cirillo G, et al. Variations of retinol binding protein 4 levels are not associated with changes in insulin resistance during puberty. J Endocrinol Invest. 2009;32:411–414. doi: 10.1007/BF03346477. [DOI] [PubMed] [Google Scholar]
- 35.Tateya S, Tamori Y, Kawaguchi T, et al. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology. 2010;151:971–979. doi: 10.1210/en.2009-0926. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.