Abstract
High levels of impulsivity can increase the vulnerability for development of alcohol dependence. Moreover, impulsivity is considered to be a predictor of poor treatment outcomes. Few studies, however, have directly examined the genetics of impulsivity in alcohol-dependent patients. We analyzed the relationships between a well-recognized genetic marker of serotonin activity and levels of impulsivity as measured by both the Barratt Impulsiveness Scale (BIS-11) and the stop-signal task among 304 alcohol-dependent patients. The stop-signal task was used as an independent, objective method of estimating the level of behavioral impulsivity, and the BIS-11 as a self-report measure of global impulsivity. Blood was collected and analyzed for the T102C (rs6313) polymorphism in the serotonin type 2A receptor gene (HTR2A). Our results indicate a significant association between high levels of behavioral impulsivity and the C/C genotype of rs6313 in alcohol-dependent patients. The CC genotype has been previously found to be associated with a reduction in 5HT2A receptors in the central nervous system. These results support the hypothesis that genetic factors are important determinants of behavioral impulsivity in alcohol-dependent patients, and that the serotonin system plays an important role in establishing its level.
Keywords: alcohol dependence, genetic polymorphism, HTR2A, impulsivity, serotonin
INTRODUCTION
According to Moeller et al. (Moeller et al., 2001), impulsivity may be defined as “a predisposition toward rapid, unplanned reactions to internal or external stimuli without regard to the negative consequences of these reactions”. Current research concentrates on impulsivity as a multidimensional construct, distinguishing at least two types of impulsivity: behavioral, which can be defined as an impaired ability to stop an initiated response, and cognitive, which means impairment in weighing the consequences of one’s behavior and therefore in delaying gratification (Arce and Santisteban, 2006). Recently, de Wit has proposed a third type of impulsivity, attentional, which means an impairment in the ability to maintain focus on a specific task, that may also be important in substance use disorders (de Wit, 2009).
It has been established that high levels of impulsivity may increase the vulnerability to develop alcohol dependence (Lejuez et al., 2010). Impulsivity is also considered to be a predictor of poor outcomes following treatment for alcohol dependence (Rubio et al., 2008). Moreover, alcohol consumption may increase levels of impulsivity in alcohol-dependent and other individuals by disrupting frontal lobe functioning (Lejuez et al., 2010; Petry, 2001). Results of experimental studies indicate that different neurophysiologic pathways underlie different types of impulsivity, implicating that these are separate and independent phenomena (Evenden, 1999). Recent studies have provided a novel perspective suggesting that alcohol craving and level of behavioral impulsivity may be controlled by the same brain areas (Li et al., 2009), consistent with a close relationship between this type of impulsivity and alcohol dependence.
Impulsivity is regarded as a potential endophenotype (i.e., an intermediate phenotype between genes and behavioral symptoms) in alcohol dependence. The heritability of impulsivity has been confirmed in animal as well as human subjects (Condon et al., 2008), accounting for about 45% of the variance.
To date, few studies have investigated the associations between levels of impulsivity and polymorphisms of candidate genes (Bjork et al., 2002; Preuss et al., 2001a; Stoltenberg et al., 2006). However, most of these studies did not employ measures of impulsivity per se, but rather analyses of aggressive or suicidal behaviors, which are often but not always associated with high levels of impulsivity. Findings suggest that functional polymorphisms in genes associated with serotonin activity may be a key to understanding the mechanisms underlying impulsivity. Specifically, genetic and biochemical analyses support the hypothesis that lower levels of serotonin activity may lead to higher levels of impulsivity (Congdon et al., 2008; Linnoila et al., 1993). Current studies also indicate that the serotonin system may exert its effects on impulsivity through dopamine and glutamate as well as GABA neurotransmission (Higgins et al., 2003; Le et al., 2008; Winstanley et al., 2005). In particular, the serotonin type 2A gene (HTR2A) polymorphism T102C/rs6313 has been shown to play a significant role not only in alcohol dependence, but also in other behavioral manifestations of impulsivity including suicidality (Bjork et al., 2002; do Prado-Lima et al., 2004; Hwu and Chen, 2000; Preuss et al., 2001a). Two studies applied to direct measures of impulsivity. According to Bjork et al. (Bjork et al., 2002), the C/C genotype was associated with significantly higher behavioral impulsivity in healthy subjects as measured by a continuous performance task. Preuss et al. (2001a) confirmed a relationship between variation in the HTR2A gene and self-assessed impulsivity in alcohol-dependent individuals. This work referred to a functional polymorphism, A1438G, in the promoter region, which is in linkage disequilibrium with T102C polymorphism.
Although impulsivity is considered to be an important characteristic of alcohol dependence and clinical observations strongly support the hypothesis of a genetic influence, few studies have examined the genetics of impulsivity in alcohol-dependent individuals directly. This study investigated associations between a specific genetic marker of serotonin function and global as well as behavioral impulsivity.. We selected the HTR2A polymorphism because of evidence for its significance in different behavioral manifestations of impulsivity, particularly alcohol dependence. Since previous data showed decreased serotonin levels associated with increased impulsivity, we hypothesized that the T allele of the HTR2A gene polymorphism (rs6313), which is associated with a higher number of the receptors in the central nervous system, would be associated with lower levels of impulsivity in alcohol-dependent individuals.
MATERIALS AND METHODS
Subjects
As previously described in relation to suicidality (Wojnar et al., 2008), participants consisted of patients entering abstinence-based, drug-free alcohol treatment programs in residential treatment centers (270 patients) and outpatient clinics (34 patients) in Warsaw, Poland. Participation was confidential and voluntary; all patients were informed about the protocol of the study and gave written informed consent. The study was approved by the Institutional Review Board of the University of Michigan Medical School and the Bioethics Committee at the Medical University of Warsaw. Only patients with a current DSM-IV diagnosis of alcohol dependence as assessed clinically and confirmed with the MINI International Neuropsychiatric Interview (Sheehan et al., 1998), were eligible. Individuals under 18 years of age, with active withdrawal or psychotic symptoms, as well as agitated patients were excluded from the study. All subjects scored 28 or higher on the Mini-Mental State Exam (Folstein et al., 1975).
Procedures
Participants completed a questionnaire that included information about demographics, alcohol problems and impulsivity. The stop-signal task was performed in the presence of a trained member of the research team who provided instructions and any necessary help during the test. Blood samples for genetic analysis were collected in 7.5 ml EDTA-lined vacutainers by a nurse and DNA was extracted in the Psychopharmacology Laboratory at the Department of Psychiatry of Medical University of Warsaw and independently at the Molecular & Behavioral Neuroscience Institute of the University using the Puregene (now Qiagen) kit.
Measures
I. The level of impulsivity was measured by the Barratt Impulsiveness Scale and the stop-signal task.
The Barratt Impulsiveness Scale (BIS-11) (Patton et al., 1995) is a subjective measure of impulsivity. A Polish version of BIS-11 was used, translated from the original version according to methodology described by Wojnar et al. (Wojnar et al., 2008). The self-administered questionnaire consists of 30 statements describing thoughts and behaviors related to different dimensions of impulsivity. Patients rated how well each statement referred to them using responses of rarely, never, sometimes, or always. The total score of the BIS-11 was used as a measure of global impulsivity, as it estimates all component aspects of impulsivity. Six basic factors of impulsivity distinguished in the BIS-11 are an attention factor, cognitive instability, motor impulsivity, perseverance, self-control, and cognitive complexity. The Cronbach’s alpha coefficient for the 30-item total BIS-11 score was .83, suggesting good internal consistency.
The stop-signal task provides an objective measure of estimating the level of behavioral impulsivity (Logan et al., 1984). It is administered via a computer program in which the ability to stop a response that has already been started is evaluated. During this two-alternative choice reaction time task, participants saw an X or an O on a computer screen and responded rapidly with one of two keys on the computer keyboard (the „m” and „v” keys, using the left and right index fingers). On some trials a tone (the Stop Signal) sounded shortly after the X or O appeared, indicating that participants should withhold the response. After two practice blocks of 32 trials each, four blocks of 64 trials were administered. The total time for the test is 20 minutes. For analysis, the final three blocks were averaged.
The most reliable estimates of stop signal reaction time (StopRT) are obtained with a response-reaction time tracking methodology (Band et al., 2003) where the delay between the visual stimulus and the warning tone is varied to maintain 50% success rate at withholding the response. Therefore the time between the presentation of the X or O was varied systematically depending on whether or not the participant was successful in stopping the prior trial that contained a Stop Signal. The onset of the Stop Signal was increased by 50 msec if there was a successful inhibition of the response and decreased by 50 msec if a response was made in spite of the Stop Signal. This procedure results in equal inhibition or stopping rates across participants (50%) but yields a measure of the time taken to inhibit an ongoing-response (StopRT). A quantitative model of reaction time (RT) processes enables calculation of each participant’s speed of stopping or inhibiting a response (StopRT) by subtracting average stop signal delay from average RT to the trials without a stop signal (GoRT) (Logan et al., 1984). GoRT trials with incorrect responses were not included in the calculation of GoRT. StopRT is a useful indicator of executive functions: long stop reaction time means difficulties in inhibiting the reaction (pushing the button even with a short interval between visual stimulus and the Stop Signal). Longer StopRTs indicate higher levels of behavioral impulsivity.
II. The history of alcohol problems was evaluated using a modified version of the Substance Abuse Outcomes Module (Smith et al., 1996) including age at onset of alcohol problems, duration of alcohol dependence, family history of alcohol problems and previous alcohol treatment history.
Genotyping
The single nucleotide polymorphism, rs6313 (T102C), in the HTR2A gene (13q14 – q21) (Erdmann et al., 1996) was analyzed, resulting in three genotypes: T/T, C/T, and C/C. DNA was amplified by means of PCR. In Warsaw, rs6313 was analyzed in all patients using a Real-Time polymerase chain reaction method (RT-PCR). RT-PCR was performed by custom genetic analysis services, the LightCycler® 480 instrument available from Roche Applied Science, using Simple Probes, LightCycler® 480 Probes Master Mix and universal cycling conditions. LightSNiP (SimpleProbe) assays for SNPs were designed by TIB-MolBiol (Berlin, Germany). At the University of Michigan, HTR2A genotyping procedures for the present sample were previously reported in Wojnar et al. (2009). About 200 of the 304 genotypes were determined by both methods, and genotyping was 100% concordant between them.
Statistical analysis
Statistical analysis was performed using Statistica Software, version 9.0. The analyses focused on examining associations between the genetic polymorphism and the levels of total, behavioral, and cognitive impulsivity in alcohol-dependent patients. Dependent variables were stop reaction time and total BIS-11 score.. All continuous data were tested for normal distribution using the Kolmogorov-Smirnov test. For parametric variables, data were presented as arithmetic means and standard deviations (mean ± SD). For non-parametric variables, data were presented as median and quartiles (25; 75).
Polesskaya and Sokolov (2002) found a linear (additive) relationship between genotype and mRNA expression in healthy controls with the CC genotype having the least expression, the TT genotype having the most expression, and the CT genotype having intermediate expression. Less mRNA expression might result in fewer 5-HT2A receptors, less 5-HT neurotransmission, and greater impulsivity. Therefore, we hypothesized a linear (additive) relationship between genotype and impulsivity. To test genetic associations between particular genotypes and the level of impulsivity, therefore, linear regression analysis was performed after coding the HTR2A C102T genotype as an interval variable with CC = 1, CT = 2, and TT = 3. Most geneticists prefer linear regression as a first test of genetic association to determine the amount of variance in a quantitative trait explained by a genetic polymorphism (e.g., Solé et al. 2006). This is because it makes the least number of assumptions. It assumes an additive effect without dominance or hyperdominance, for example. In contrast, an analysis of variance (ANOVA) tests for many more genetic models—e.g., recessive, dominant, hyperdominant—and, thus uses more degrees of freedom, which reduces power. If the linear regression was significant, then an ANOVA was also performed.
RESULTS
Demographic and clinical characteristics
The sample consisted of 304 patients (26% female) with a mean age of 43.5 ± 9.7 years. All patients were Caucasian. The median (interquartile range) education level was 12 (11;14) years, which corresponds to the last level of secondary school in Poland. At the time of study entry, 61% of patients were unemployed. About 47% of the subjects participated previously in at least one alcohol treatment program during their lifetime, and 63.8% reported alcohol problems in at least one parent. The mean duration of alcohol dependence in the study group was 19.21 ± 9.86 years with a median age of onset of drinking problems being about 21 (18;28) years. There were no significant differences between the level of behavioral (StopRT, p = 0.24) as well as global impulsivity (as measured by BIS, p = 0.81) between male and female individuals. Similarly we observed no significant association between impulsivity and treatment setting, neither for behavioral impulsivity (p = 0.37) nor for global impulsivity (p = 0.72).
Genetic analysis
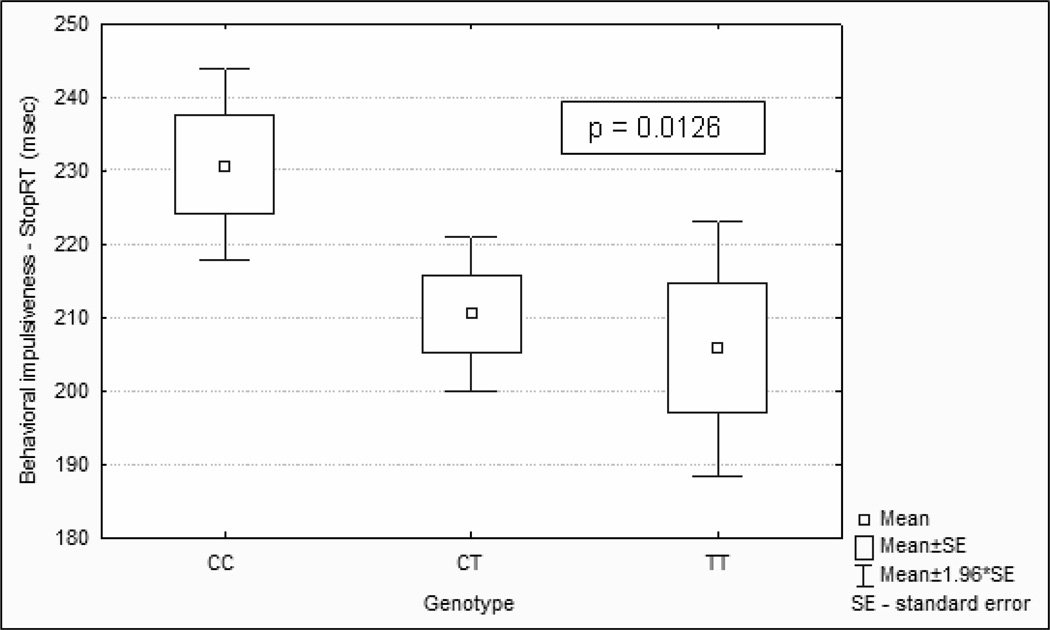
The distribution of analyzed genotypes and alleles is presented in Table 1. The genotypes were not in Hardy-Weinberg equilibrium (HWE, p = 0.017) due to a lower than expected number of TT homozygotes. The statistical analysis revealed a nominally significant association between the HTR2A polymorphism and behavioral impulsivity as measured by the stop-signal task (linear regression: beta = 0.16; F = 6.31; df = 1,247; p = 0.0126; adjusted R2 = 0.025). An ANOVA comparing the three genotypes on the StopRT was also significant (F = 3.605, df = 2, p = 0.028), with an increase in p value expected due to the increased degrees of freedom. Patients with the common C/C genotype had the longest stop-reaction times (highest levels of behavioral impulsivity), whereas subjects with the rarer T/T genotype had the shortest StopRT or lowest levels of behavioral impulsivity (see Table 2 and Fig. 1). Patients homozygous for allele C were significantly more impulsive than subjects having at least one T allele in T102C polymorphism (t = 2.661; df = 247; p = 0.008) with small to medium effect size (Cohen’s d = 0.35). There was no significant association between the HTR2A polymorphism and the level of total impulsivity as measured by the BIS-11 (p = 0.669).
Table 1.
Distribution of HTR2A genotypes and alleles in the T102C (rs6313) polymorphism in the group of alcohol-dependent patients.
| Polymorphism HTR2A rs6313 |
Genotype N (%) | Allele Frequency | |||
|---|---|---|---|---|---|
| C/C | C/T | T/T | C | T | |
| Men | 80 (38.3) | 109 (52.1) | 20 (9.6) | 0.64 | 0.36 |
| Women | 19 (25.3) | 44 (58.7) | 12 (16.0) | 0.55 | 0.45 |
| Total | 99 (34.9) | 153 (53.9) | 32 (11.2) | 0.62 | 0.38 |
HTR2A – serotonin type 2A receptor gene
There were no significant differences in the distribution of the genotypes (χ2 = 5.11; p = 0.07) and alleles (χ2 = 1.68; p = 0.19) in the HTR2A T102C (rs6313) polymorphism between men and women.
Table 2.
Relationships between different types of impulsivity and HTR2A gene (rs6313) polymorphism.
| HTR2A rs6313 polymorphism | Stop reaction time (ms) | Global impulsivity (BIS-11) |
|---|---|---|
| CC | 230.86 ± 61.72 | 72.14 ± 10.14 |
| CT | 210.50 ± 61.42 | 70.99 ± 9.68 |
| TT | 205.77 ± 48.28 | 71.53 ± 10.01 |
| p | 0.0126 | 0.669 |
HTR2A – serotonin type 2A receptor gene, BIS-11 – Barratt Impulsivity Scale.
The values presented are arithmetic means and standard deviations (mean ± SD).
Linear regression, p-values < 0.05 are bolded.
When analyzes were made in the more homogenous group of only male patients, the association between HTR2A polymorphism and behavioral impulsivity remained significant (linear regression: beta = 0.16; F = 4.84; df = 1,185; p = 0.0289; adjusted R2 = 0.020). An ANOVA comparing three genotypes was in this case not significant (F = 2.658, df = 2, p = 0.073), but was significant for the comparison of the StopRT between subjects with or without a T allele (F = 5.183, df = 1, p = 0.024).
DISCUSSION
Our results indicate that high levels of behavioral impulsivity (measured by the stop-signal task) in alcohol-dependent patients may be associated with the C/C genotype in T102C HTR2A polymorphism, which is considered to be a genetic marker of lower central serotonin activity. Individuals with the C/C genotype in the T102C HTR2A polymorphism were significantly more impulsive than patients with either the T/T or T/C genotype who had comparable levels of behavioral impulsivity. Thus, the T allele may be protective in a dominant fashion.
Although this is to our knowledge the first report showing an association between the T102C HTR2A polymorphism with impulsivity in alcohol-dependent patients, our results are consistent with the literature. In the study of healthy subjects by Bjork et al. (Bjork et al., 2002), the C/C genotype was also associated with significantly higher behavioral impulsivity as measured by a continuous performance task. Preuss et al. (Preuss et al., 2001a) confirmed a relationship between variation in the HTR2A gene and self-assessed impulsivity in alcohol-dependent individuals, but this work referred to a different and non-functional polymorphism. In other studies, not aimed directly towards impulsivity, but rather its behavioral manifestations, the significance of C/C genotype was also observed. In these studies, the C/C genotype was associated with aggressive behavior in male alcoholics (Hwu et al., 2000), more frequent nicotine dependence (do Prado-Lima et al., 2004), and more frequent suicidal ideation in individuals with a major depressive episode (Du et al., 2000). At least two studies, however, did not support the significance of the T102C polymorphism. Preuss et al. (Preuss et al., 2000) did not observe relationship between the HTR2A genotype and the number of suicide attempts in alcohol-dependent individuals. Similarly, Bondy et al. (Bondy et al., 2000) did not find an association between the T102C polymorphism and suicide ideation or attempts. On the other hand, recent studies revealed significantly lower density of 5-HT2A receptors in single photon emission computed tomography (SPECT) scans of patients with a history of at least one suicide attempt (Audenaert et al., 2002).
In addition to Preuss et al (Preuss et al., 2001a), a Polish study by Samochowiec et al. (Samochowiec et al., 1999) found an association between impulsivity in alcohol-dependent patients with comorbid antisocial personality and the monoamine oxidase A gene. Thus, their results may be interpreted in the context of a personality disorder for which impulsivity is a prominent symptom. Other studies pertaining to a relationship between gene polymorphisms and impulsivity in alcohol-dependent individuals revealed a possible influence of serotonin transporter gene (SLC6A4) (Preuss et al., 2001b).
The concept of impulsivity as an endophenotype in alcohol dependence comes mainly from clinical observations that high levels of impulsivity are associated with a family history of alcohol dependence and earlier development of the disorder (Lykouras et al., 2004). Our research provides more direct evidence supporting this concept. Our study revealed an association between an HTR2A polymorphism and impulsivity as measured by the stop-signal task but not the BIS-11. Unlike the BIS-11, which measures a person’s perception of their impulsive tendencies, the stop-signal task is an independent, manipulation-free method of assessing the level of impulsivity that reflects real-time brain activity. The stop signal task, therefore, is arguably closer to genetic activity and a better candidate for an endophenotype than a self-administered questionnaire. Current functional magnetic resonance imaging (fMRI) studies have shown that high levels of impulsivity as measured by different questionnaires correlated with activity in different brain areas (Congdon et al., 2008). Results of our study are consistent with the hypothesis that impulsivity is a multidimensional construct, with each dimension reflected in different measures and biological correlates.
The polymorphism analyzed in the present study, T102C in the HTR2A gene is synonymous, i.e. it does not change the amino acid sequence (Polesskaya and Sokolov, 2002). It is, however, associated with the quantity of the mRNA and protein made. Pollesskaya and Sokolov (2002) observed that the T allele was associated with a higher number of 5-HT2A receptors in the central nervous system. Mechanisms underlying these expression differences remain unclear, although it has been speculated that the T102C polymorphism affects the stability of the respective mRNA (Polesskaya and Sokolov, 2002). Another hypothesis concerns potential methylation differences in the promoter region of HTR2A gene (Polesskaya et al., 2006).
Summarizing this theoretical explanation, individuals with the C/C genotype in the HTR2A T102C polymorphism probably have significantly lower expression of the gene. Thus, alcohol-dependent patients with higher levels of behavioral impulsivity may be those with lower serotonergic activity, as has been shown consistently in the “pre-genetic” literature (Harrison et al., 1997; Linnoila et al., 1993).
Although the magnitude of the difference in Stop Reaction Time between the CC vs CT/TT genotypes is not large (21 msec), this difference may still be clinically relevant. The StopRT is calculated by subtracting the average Stop Signal Delay from the average GoRT. Thus, it is a measure of one cognitive process, rather than a measure of overt reaction time which would include several cognitive processes as well as the motor response time. Indeed, studies of other clinical groups (e.g., schizophrenia, children with sleep problems) have shown differences in SSRT of about the same magnitude as in the current study (Wong et al., 2010, Thakkar et al., 2011).
Our study has some limitations, which have to be noted. The small sample size was mentioned above. In addition, most of the sample consisted of men (74%) and was recruited from residential treatment centers. According to the rules of those treatment programs as well as qualification criteria for outpatient facilities patients had to be free from all psychotropic drugs, could not have severe depressive symptoms, and were required to participate in meetings of Alcoholics Anonymous. Thus, our sample may not fully represent the population of alcohol-dependent individuals. Moreover, patients entered the study at different stages of abstinence. These differences may have potentially influenced stop reaction times due to a potential relationship between levels of impulsivity (especially behavioral) and duration of abstinence (Wilhelm et al., 2007).
Our genotype frequencies deviate significantly from HWE. Since we achieved 100% concordance rate between two different genotyping procedures performed in blinded fashion on two continents, genotyping error is not a likely explanation for this deviation. In addition, when the same assay was run by the same technique in a population sample (data not published), the genotypes were in HWE. Deviation from HWE here may thus be due to ascertainment bias due to the characteristics of the clinical sample used for this study. It is plausible that more of the least impulsive TT individuals were not enrolled to our study because they are more likely to abstain from alcohol and therefore do not come for the addiction treatment, thus leading to the observed deviation from HWE. Another possibility is that TT homozygosity correlates with some other variable in our sample (e.g., education, employment or marital status) that is skewed in alcoholics when compared to the general population.
In conclusion, our results indicate that high levels of behavioral impulsivity (but not self-assessed impulsivity) in alcohol-dependent patients are associated with a genetic marker of lower serotonin activity (the C/C genotype in the T102C HTR2A polymorphism). Further studies should be performed to replicate this finding in larger, more representative samples and to examine relationships between impulsivity and other serotonin-related genetic polymorphisms in alcohol-dependent patients.
Figure 1.
Association of T allele in HTR2A (rs6313) gene polymorphism and level of behavioral impulsivity in alcohol-dependent patients.
Acknowledgments
We would like to thank all members of the research team in Poland (especially, Anna Wnorowska, MD; Anna Klimkiewicz, MD; Katarzyna Kositorna, MS; Maciej Kopera, MD; Aleksandra Konopa, MD; Elzbieta Wozny, PhD; Malgorzata Abramowska, MSc, Julia Pupek, MD; Piotr Serafin MD; Izabela Nowosad MD) as well as the medical staff and patients at “Kolska”, “Pruszkow”, “Petra” and “Solec” Addiction Treatment Centers in Warsaw for their support of this research.
Funding Source
This study was supported by the Polish Ministry of Science and Higher Education grant NN405357239, the Fogarty International Center/NIDA International Substance Abuse Research Program grant D43-TW05818, the Fogarty International Center/NIAAA International Collaborative Alcohol & Injury Research Training Program grant D43-TW007569 and NIAAA grant R21 AA016104.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors
Marcin W, KB, JG and MB designed the study and wrote the protocol. AJ, Marcin W, KB and JSM managed the literature search. Malgorzata W, JŁ, HM, ES and MB designed and directly supervised genotyping. AJ, KB and Marcin W performed all statistical analyses and summarized the results. Marcin W, AJ, JG, ES and MB analyzed and interpreted clinical and genotyping results. AJ, JSM and Marcin W conducted the stop-signal task and data collection. AJ, MW and KB wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Arce E, Santisteban C. Impulsivity: a review. Psicothema. 2006;18:213–220. [PubMed] [Google Scholar]
- Audenaert K, Goethals I, Van Laere K, Lahorte P, Brans B, Versijpt J, et al. SPECT neuropsychological activation procedure with the Verbal Fluency Test in attempted suicide patients. Nuclear Medicine Communications. 2002;23:907–916. doi: 10.1097/00006231-200209000-00015. [DOI] [PubMed] [Google Scholar]
- Band GP, van der Molen MW, Logan GD. Horse-race model simulations of the stop-signal procedure. Acta Psychologica. 2003;112:105–142. doi: 10.1016/s0001-6918(02)00079-3. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Moeller FG, Dougherty DM, Swann AC, Machado MA, Hanis CL. Serotonin 2a receptor T102C polymorphism and impaired impulse control. American Journal of Medical Genetics. 2002;114:336–339. doi: 10.1002/ajmg.10206. [DOI] [PubMed] [Google Scholar]
- Bondy B, Kuznik J, Baghai T, Schule C, Zwanzger P, Minov C, et al. Lack of association of serotonin-2A receptor gene polymorphism (T102C) with suicidal ideation and suicide. American Journal of Medical Genetics. 2000;96:831–835. doi: 10.1002/1096-8628(20001204)96:6<831::aid-ajmg27>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Congdon E, Canli T. A neurogenetic approach to impulsivity. Journal of Personality. 2008;76:1447–1484. doi: 10.1111/j.1467-6494.2008.00528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- do Prado-Lima PA, Chatkin JM, Taufer M, Oliveira G, Silveira E, Neto CA, et al. Polymorphism of 5HT2A serotonin receptor gene is implicated in smoking addiction. American Journal of Medical Genetics, Part B: Neuropsychiatric Genetics. 2004;128B:90–93. doi: 10.1002/ajmg.b.30004. [DOI] [PubMed] [Google Scholar]
- Du L, Bakish D, Lapierre YD, Ravindran AV, Hrdina PD. Association of polymorphism of serotonin 2A receptor gene with suicidal ideation in major depressive disorder. American Journal of Medical Genetics. 2000;96:56–60. doi: 10.1002/(sici)1096-8628(20000207)96:1<56::aid-ajmg12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Erdmann J, Shimron-Abarbanell D, Rietschel M, Albus M, Maier W, Korner J, et al. Systematic screening for mutations in the human serotonin-2A (5-HT2A) receptor gene: identification of two naturally occurring receptor variants and association analysis in schizophrenia. Human Genetics. 1996;97:614–619. doi: 10.1007/BF02281871. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berlin) 1999;146:348–361. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Harrison AA, Everitt BJ, Robbins TW. Central 5-HT depletion enhances impulsive responding without affecting the accuracy of attentional performance: interactions with dopaminergic mechanisms. Psychopharmacology (Berlin) 1997;133:329–342. doi: 10.1007/s002130050410. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Enderlin M, Haman M, Fletcher PJ. The 5-HT2A receptor antagonist M100,907 attenuates motor and 'impulsive-type' behaviours produced by NMDA receptor antagonism. Psychopharmacology (Berlin) 2003;170:309–319. doi: 10.1007/s00213-003-1549-0. [DOI] [PubMed] [Google Scholar]
- Hwu HG, Chen CH. Association of 5HT2A receptor gene polymorphism and alcohol abuse with behavior problems. American Journal of Medical Genetics. 2000;96:797–800. doi: 10.1002/1096-8628(20001204)96:6<797::aid-ajmg20>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Li Z, Fletcher PJ. Intra-median raphe nucleus (MRN) infusions of muscimol, a GABA-A receptor agonist, reinstate alcohol seeking in rats: role of impulsivity and reward. Psychopharmacology (Berlin) 2008;195:605–615. doi: 10.1007/s00213-007-0943-4. [DOI] [PubMed] [Google Scholar]
- Lejuez CW, Magidson JF, Mitchell SH, Sinha R, Stevens MC, de Wit H. Behavioral and biological indicators of impulsivity in the development of alcohol use, problems, and disorders. Alcoholism: Clinical and Experimental Research. 2010;34:1334–1345. doi: 10.1111/j.1530-0277.2010.01217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Luo X, Yan P, Bergquist K, Sinha R. Altered Impulse Control in Alcohol Dependence: Neural Measures of Stop Signal Performance. Alcoholism: Clinical and Experimental Research. 2009;33:740–750. doi: 10.1111/j.1530-0277.2008.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, George T, Higley D. Impulse control disorders. International Clinical Psychopharmacology. 1993;8 Suppl 1:53–56. doi: 10.1097/00004850-199309001-00008. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. Journal of Experimental Psychology. Human Perception and Performance. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- Lykouras L, Moussas G, Botsis A. Examination of type I/type II alcoholism typology in a Greek hospital treatment population. European Psychiatry. 2004;19:214–218. doi: 10.1016/j.eurpsy.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. American Journal of Psychiatry. 2001;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. Journal of Clinical Psychology. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Petry NM. Delay discounting of money and alcohol in actively using alcoholics, currently abstinent alcoholics, and controls. Psychopharmacology (Berlin) 2001;154:243–250. doi: 10.1007/s002130000638. [DOI] [PubMed] [Google Scholar]
- Polesskaya OO, Aston C, Sokolov BP. Allele C-specific methylation of the 5-HT2A receptor gene: evidence for correlation with its expression and expression of DNA methylase DNMT1. Journal of Neuroscience Research. 2006;83:362–373. doi: 10.1002/jnr.20732. [DOI] [PubMed] [Google Scholar]
- Polesskaya OO, Sokolov BP. Differential expression of the "C" and "T" alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. Journal of Neuroscience Research. 2002;67:812–822. doi: 10.1002/jnr.10173. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Koller G, Bahlmann M, Soyka M, Bondy B. No association between suicidal behavior and 5-HT2A-T102C polymorphism in alcohol dependents. American Journal of Medical Genetics. 2000;96:877–878. [PubMed] [Google Scholar]
- Preuss UW, Koller G, Bondy B, Bahlmann M, Soyka M. Impulsive traits and 5-HT2A receptor promoter polymorphism in alcohol dependents: possible association but no influence of personality disorders. Neuropsychobiology. 2001a;43:186–191. doi: 10.1159/000054888. [DOI] [PubMed] [Google Scholar]
- Preuss UW, Koller G, Soyka M, Bondy B. Association between suicide attempts and 5-HTTLPR-S-allele in alcohol-dependent and control subjects: further evidence from a German alcohol-dependent inpatient sample. Biological Psychiatry. 2001b;50:636–639. doi: 10.1016/s0006-3223(01)01196-9. [DOI] [PubMed] [Google Scholar]
- Rubio G, Jimenez M, Rodriguez-Jimenez R, Martinez I, Avila C, Ferre F, et al. The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcoholism: Clinical and Experimental Research. 2008;32:1681–1687. doi: 10.1111/j.1530-0277.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- Samochowiec J, Lesch KP, Rottmann M, Smolka M, Syagailo YV, Okladnova O, et al. Association of a regulatory polymorphism in the promoter region of the monoamine oxidase A gene with antisocial alcoholism. Psychiatry Research. 1999;86:67–72. doi: 10.1016/s0165-1781(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59 Suppl 20:22–33. [PubMed] [Google Scholar]
- Smith GE, Ross RL, Rost KM. Psychiatric outcomes module: substance abuse outcomes module (SAOM) In: Sederer LI, Dickey B, editors. Outcome assessment in clinical practice. Baltimore, MD: Williams and Wilkins; 1996. pp. 85–88. [Google Scholar]
- Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–1929. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- Stoltenberg SF, Glass JM, Chermack ST, Flynn HA, Li S, Weston ME, et al. Possible association between response inhibition and a variant in the brain-expressed tryptophan hydroxylase-2 gene. Psychiatric Genetics. 2006;16:35–38. doi: 10.1097/01.ypg.0000176528.30362.34. [DOI] [PubMed] [Google Scholar]
- Thakkar KN, Schall JD, Boucher L, Logan GD, Park S. Response inhibition and response monitoring in a saccadic countermanding task in schizophrenia. Biological Psychiatry. 2011;69:55–62. doi: 10.1016/j.biopsych.2010.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcoholism: Clinical and Experimental Research. 2007;31:1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Robbins TW. Interactions between serotonin and dopamine in the control of impulsive choice in rats: therapeutic implications for impulse control disorders. Neuropsychopharmacology. 2005;30:669–682. doi: 10.1038/sj.npp.1300610. [DOI] [PubMed] [Google Scholar]
- Wojnar M, Ilgen MA, Jakubczyk A, Wnorowska A, Klimkiewicz A, Brower KJ. Impulsive suicide attempts predict post-treatment relapse in alcohol dependent patients. Drug and Alcohol Dependence. 2008;97:268–275. doi: 10.1016/j.drugalcdep.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojnar M, Brower KJ, Strobbe S, Ilgen MA, Matsumoto H, Nowosad I, Sliwerska E, Burmeister M. Association between Val66Met Brain-Derived Neurotrophic Factor (BDNF) Gene Polymorphism and Post-Treatment Relapse in Alcohol Dependence. Alcoholism: Clinical and Experimental Research. 2009;33:693–702. doi: 10.1111/j.1530-0277.2008.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong MM, Brower KJ, Nigg JT, Zucker RA. Childhood sleep problems, response inhibition, and alcohol and drug outcomes in adolescence and young adulthood. Alcoholism: Clinical and Experimental Research. 2010;34:1033–1044. doi: 10.1111/j.1530-0277.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]