Abstract
Background
Type 2 Gaucher disease is a rare and progressive subtype of this lysosomal storage disorder, marked by rapid, early-onset neurodegeneration. Distinguishing type 2 from types 1 and 3 Gaucher disease has remained challenging, due to the lack of a clear correlation between phenotype and enzymatic activity or genotype. β-glucocerebrosidase, the enzyme deficient in Gaucher disease, also has an essential role in maintaining epidermal permeability function, by regulating the ratio of ceramides to glucosylceramides in the stratum corneum of the skin.
Objectives
To further assess the diagnostic utility of epidermal evaluations in distinguishing patients with type 2 Gaucher disease in an expanded cohort.
Study design
Epidermal samples were evaluated from twenty children with type 2, three patients with type 3 Gaucher disease and two adults with type 1 Gaucher disease with different clinical manifestations and genotypes. Electron microscopy on ruthenium tetroxide post-fixed tissue was performed.
Results
Compared to controls and subjects with type 1 and type 3 Gaucher disease, only patients with type 2 Gaucher disease displayed characteristic electron dense, non-lamellar clefts and immature-lamellar membranes.
Conclusion
The appearance of characteristic alterations in epidermal ultrastructure provides an early and specific diagnostic tool to help in distinguishing type 2 from the other types of Gaucher disease.
Keywords: Gaucher disease, glucocerebrosidase, glucosylceramide, epidermis, acute neuronopathic, stratum corneum
1.Introduction
Gaucher disease, the inherited deficiency of the lysosomal enzyme β-glucocerebrosidase (E.C. GCase), is commonly divided into three subtypes based upon the presence and progression of neurological manifestations. Neurodegeneration in type 2 Gaucher disease is rapidly progressive and refractory to treatment.1, 2 Therefore, early differentiation of type 2 infants from more treatable infants affected with type 1 and 3 Gaucher disease is imperative. Beta-glucocerebrosidase plays an essential role in maintaining epidermal permeability function, including water homeostasis, by regulating the balance of ceramides to glucosylceramides in the stratum corneum of the skin.3, 4 A transgenic knock-out mouse model of Gaucher disease led to the appreciation of a distinct Gaucher phenotype, consisting of neonates presenting at or shortly after birth, with rapidly progressing fulminant disease, often associated with ichthyotic skin and/or hydrops fetalis.5, 6 The initial studies of human epidermal lipid content and cutaneous ultrastructure in all three types of Gaucher disease identified unique epidermal abnormalities specific only to type 2 disease. 5, 7 These alterations were consistently present, regardless of whether ichthyosis was clinically evident.8, 9 Electron microscopic examination of the skin from three patients with type 2 Gaucher disease showed immature, incompletely processed arrays of loosely-packed lamellar-body derived membranes, as compared to the fully-processed, orderly lamellar bilayers that typify normal skin. Notably, skin from patients with type 1 and type 3 Gaucher disease had a normal lamellar bilayer pattern, presumably preserved because of residual β-glucocerebrosidase activity.
These unique ultrastructural characteristics identified in patients with type 2 Gaucher disease were identical to the earlier epidermal ultrastructure findings in skin from type 2 (null-allele) Gaucher disease mice with a complete absence of epidermal β-glucocerebrosidase activity.6 These mice display dry, peeling skin and die in the first day of life, a clinical course resembling severely-affected human neonates with type 2 Gaucher disease.5 They also exhibit severely impaired barrier function, with markedly impaired transepidermal water loss and increased permeability.7 Their skin ultrastructure reveals immature, partially-processed lamellar bilayers, similar to that subsequently observed in type 2 Gaucher disease patients. In contrast, the ultrastructure of skin from heterozygous knock-out mice with residual β-glucocerebrosidase activity, as well as patients with type 1 and 3 Gaucher disease, showed orderly, normal lamellar membranes.7, 9
The relationship between the extent of residual enzyme activity and the structure and formation of epidermis was further demonstrated in topical inhibitor studies, which showed that abnormalities appear only when enzyme activity is less than 10 percent.7 In addition, lipid analyses showed structural changes corresponding to the altered stratum corneum glucosylceramide:ceramide ratio. Stratum corneum from type 2 Gaucher disease infants had increased levels of glucosylceramides and decreased ceramides, whereas patients with type 1 and 3 Gaucher disease had a lipid distribution showing a predominance of ceramides, comparable to that of normal individuals.7 These lipid abnormalities were identical to those observed in stratum corneum from the null-allele Gaucher mouse model, and in inhibitor-treated C57bl/6 normal mice.10, 11 The similar findings in patients and knock-out and pharmacological murine models of Gaucher disease, suggested that the unique epidermal ultrastructure in patients with type 2 Gaucher disease is related directly to reduced β-glucocerebrosidase activity and could provide a valuable prognostic tool to distinguish type 2 from the more treatable type 1 and 3 disease.9
More recently, several additional case reports have confirmed skin involvement in type 2 Gaucher disease,8, 12, 13 and the ultrastructural features in type 2 Gaucher disease have been characterized in more detail in comparison to other ichthyotic presentations.14 The lamellar membranes display an immature appearance, which is attributed to both persistence of glucosylceramides, and the failure to generate ceramides in the extracellular spaces of the stratum corneum. Because glucosylceramides are relatively non-polar glycosphingolipids, they form an electron-dense, non-lamellar phase that is interspersed within the partially processed lamellar membranes. Although a similar electron-dense non-lamellar phase feature occurs in recessive X-linked ichthyosis, it is interspersed among otherwise “mature” lamellar bilayers,14 and sufficient ceramides are generated in Niemann-Pick disease, types A and B, to generate mature lamellar bilayers.15, 16 Thus, in an appropriate clinical context, these features, coupled with normal-appearing lamellar body contents and a normal progression of corneodesmosome degradation, are characteristic of type 2 Gaucher disease.
We now present skin ultrastructural findings from a greatly expanded cohort of 22 patients with Gaucher disease in an effort to re-examine the prognostic utility of this method in distinguishing type 2 disease, and to determine whether the ultrastructural findings correlated with particular genotypes or patient phenotypes.
2. Methods
2.1 Patients
Samples from 22 patients with Gaucher disease were included in these studies. Twenty were classified as type 2 Gaucher disease, while two adult patients had type 1 Gaucher disease. Three previously described samples of patients with type 3 Gaucher disease are included for comparison. The range of presentations and clinical manifestations was vast. The specific case histories are summarized in Table 1. The diagnosis of Gaucher disease was established by enzymatic and/or genotypic analyses as previously described.17
Table 1.
| Case no. | Type of Gaucher Disease | Genotype | Age at diagnosis/biopsy | Background | Gender | Ichthyosis? | Neurological signs prior to biopsy | Age at Death |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | Q414X/L444P | 9 mos/9mos | Caucasian | F | No | Apneic spells, opthalmoplegia, developmental regression | 10 mos |
| 2 | 2 | L444P+D255H/L444P+D255H | 1 mo/2mos | Croatian | M | No | None | Alive |
| 3 | 2 | L444P/RecNciI | 1 mo/1 mo | African Am. | M | No | None | 6 mos |
| 4 | 2 | L444P/T323I | 6 mos/15 mos | Caucasian | F | No | Strabismus, hand tremors, clonus in feet, brainstem involvement | Alive |
| 5 | 2 | c.84GG/D409H | 5 mos/11 mos | Ashkenazi Jew/Caucasian | F | No | Abnormal eye saccades, delayed cognition, language, fine motor control | Alive |
| 6 | 2 | W184R/D409H | 5 mos/7 mos | Caucasian | F | No | Opisthotonos, hypertonia, increased gag and startle reflex, motor development delays | Alive |
| 7 | 2 | L444P/Y313H | 7 mos/8.5 mos | Caucasian | F | No | Oculomotor apraxia, developmental regression at 4 mos | 10 mos |
| 8 | 2 | IVS2+1G>A//RecNciI | Unknown/26 mos | African American | ? | No | Oculomotor apraxia, developmental regression | Unknown |
| 9 | 2 | L444P/? | Unknown/28 mos | Unknown | ? | No | Yes-not specified | Unknown |
| 10 | 2 | L444P/D380N | Unknown/18 mos | Unknown | ? | No | Yes-not specified | Unknown |
| 11 | 2 | L444P/IVS2+1G>T | 6 mos/8mos | Korean | M | No | Brainstem involvement, developmental regression | 9 mos |
| 12 | 2 | L444P+D255H/L444P+D255H | 8 mos/17 mos | Albanian | M | No | Brainstem involvement, developmental regression | 2 yr |
| 13 | 2 | L444P/Q414X | 2 mos/2 mos | Caucasian | M | No | No | 10 mos |
| 14 | 2 | IVS2+1G>A/F251L | 16 days/35 wk gestation | Asian | F | Yes | Low APGAR score, decreased spontaneous movement | 32 days |
| 15 | 2 | Y304C/L444P | 1 yr/3 yrs | Unknown | M | No | Yes-not specified | Unknown |
| 16 | 2 | K198E/K198E | Unknown/23 mos | Hispanic | F | Yes | Hypotonia, persistent myoclonic jerks, gaze abnormalities, developmental delays | Unknown |
| 17 | 2 | IVS2+1G>T/L444P | Unknown/ 8.5 mos | African/Filipino | F | Yes | Apnea, hypotonia, hyperreflexia, esotropia, | 8.5 mos |
| 18 | 2 | R257Q/L444P | Unknown/ 10 mos | Unknown | F | No | Extensor posturing of trunk and neck, dysconjugate and crossed gaze, developmental delay | Unknown |
| 19 | 2 | F213I/K74X | Unknown/ 5 yrs | Hispanic | M | No | Strabismus, opthalmoplegia, apneic spells, myoclonic seizures, developmental delay | 10 mos |
| 20 | 2 | Rec A/IVS10(+2) | Prenatal/20 wks gestation | Ashkenazi Jew | M | Yes | Hydrops, ascites and pleural effusions | 20 wk gestation |
| 21 | 1 | N370S/N370S | 20s/50yrs | Ashkenazi Jew | M | No | None | Alive |
| 22 | 1 | D409H/L444P | 19 yrs/48 yrs | Caucasian | F | No | Parkinsonism | 53yr |
| 23* | 3 | D409H/D409H | 12 yrs/12 yrs | Arab | M | No | Slowed horizontal saccades | Unknown |
| 24* | 3 | D409H/D409H | 4 yrs/19 yrs | Arab | M | No | Slowed horizontal saccades | Unknown |
| 25* | 3 | R463C/c.84insG | 2 yrs/15 yrs | Caucasian | M | No | Looped horizontal saccades, learning disabilities | Alive |
2.1Sources of human skin samples for ultrastructural studies
Skin samples were obtained from autopsies or punch biopsies from skin sites untreated with emollients. Samples were preserved in one-half strength Karnovsky's fixative before post-fixation and tissue processing for ultrastructural analysis (see below). Skin biopsy and autopsy samples were collected with informed consent under National Institutes of Health Institute Review Board-approved clinical protocols. Controls comprised both normal skin samples from the surgical margins of unaffected adults, and historical controls from previously-published studies.18
2.2.Electron Microscopy
Tissue samples were minced to <0.5 mm3, fixed in modified Karnovsky fixative overnight, rinsed in cacodylate buffer (pH 7.3), divided, and postfixed in either 0.5% ruthenium tetroxide or reduced 1% osmium.19 After fixation, all samples were dehydrated in graded ethanol series and embedded in an epoxy mixture. Ultrathin sections cut (Leica UltraCut E) and were examined with the use of an electron microscope (Zeiss 10A, Carl Zeiss, Thornwood NY) operated at 60V. Photos were taken with a Gatan BioScan digital camera (model 792).
Images were assessed by investigators blinded to clinical outcome for the following characteristics (Table 2): 1) Lamellar bilayer organization and maturity at ≥30 sites per patient sample, as revealed by ruthenium tetroxide after post- fixation. Lamellar bilayer structure was rated visually on a scale of 0, denoting a complete absence of mature bilayers, to ++, denoting fully processed, organized lamellar bilayers. 2) The presence of an electron-dense, non-lamellar phase separation, and 3) The appearance of lamellar body contents, corneodesmosomes, cornified envelopes (CE), and corneocyte lipid envelopes (CLE).
Table 2.
Ultrastructural findings in epidermis of affected patients
| Case no. | Type of Gaucher disease | Age at biopsy | ERT prior to biopsy | Maturity of lamellar membrane | Phase separation with electron dense phase | Lamellar body formation and contents |
|---|---|---|---|---|---|---|
| 1 | 2 | 9 mos | No | 0 | Present | Normal |
| 2 | 2 | 2 mos | No | 0 | Present | Normal |
| 3 | 2 | 1 mo | No | 0 | Present | Normal |
| 4 | 2 | 15 mos | Yes- for 7mos | + | Present | Normal |
| 5 | 2 | 11 mos | Yes | + | Present | Normal |
| 6 | 2 | 7 mos | Yes | 0 | Present | Normal |
| 7 | 2 | 8.5 mos | Yes-2-3 doses | + | Present | Normal |
| 8 | 2 | 26 mos | No | + | Present | Normal |
| 9 | 2 | 28 mos | Yes | + | Present | Normal |
| 10 | 2 | 18 mos | No | 0 | Present | Normal |
| 11 | 2 | 8 mos | No | + | Present | Normal |
| 12 | 2 | 17 mos | Yes- for 1 yr | 0 | Present | Normal |
| 13 | 2 | 2 mos | Yes | + | Present | Normal |
| 14 | 2 | 35 wk gestation | No | + | Present | Normal |
| 15 | 2 | 3 yrs | Yes-for 1 yr | + | Present | Normal |
| 16 | 2 | 23 mos | No | + | Present | Normal |
| 17 | 2 | 8.5 mos | No | 0 | Present | Normal |
| 18 | 2 | 10 mos | No | + | Present | Normal |
| 19 | 2 | 5 yrs | Yes- for 2-3 yrs | + | Present | Normal |
| 20 | 2 | 20 wk gestation | No | 0 | Present | Normal |
| 21 | 1 | Unk | Unknown | ++ | Not present | Normal |
| 22 | 1 | 48 yrs | Yes | ++ | Not present | Normal |
| 23* | 3 | 12 yrs | No | ++ | Not present | Normal |
| 24* | 3 | 19 yrs | No | ++ | Not present | Normal |
| 25* | 3 | 15 yrs | Yes | ++ | Not present | Normal |
Case previously published (Sidransky et.al, 1996).
3. Results
The ultrastructure of the skin samples from 20 patients with type 2 Gaucher disease, three patients with type 3 Gaucher disease and two patients with type 1 Gaucher disease were compared to normal adult samples. Ruthenium tetroxide post-fixation of all samples from patients with type 2 Gaucher disease showed characteristic electron dense, non-lamellar clefts, disrupting the architecture of the lamellar membranes. In addition, all patients showed some degree of immaturity of lamellar membranes, exhibited by the absence of lamellar bilayer unit structures that are seen in normal human samples (see Table 2). This likely resulted from the deficiency of β-glucocerebrosidase activity required to process lipids comprising the lamellar membrane. These findings resembled the three cases with type 2 Gaucher disease that were previously reported.9 Moreover, the skin ultrastructure of the two patients with type 1 Gaucher disease described in this report, similar to five previously described patients with type 1 and three with type 3 Gaucher disease ,9 displayed mature lamellar bilayer unit structure in broad arrays that fully engage the extracellular spaces, identical to findings in normal human skin. Interestingly, the morphology of the lamellar bodies in patient samples from type 2 Gaucher disease appeared normal, and did not differ from type 1 Gaucher disease subjects. Finally, the ultrastructure of corneodesmosomes, cornified envelopes, and the corneocyte lipid envelope in the samples from patients with Gaucher disease showed no abnormalities, and these features were indistinguishable from the morphology seen in normal human subjects. Together, these results suggest, that a deficiency of epidermal β-glucocerebrosidase leads to failure of lipid processing, with the appearance of a characteristic complex of changes that is potentially diagnostic of type 2 Gaucher disease.
Among the 20 evaluated patients with type 2 Gaucher disease, the associated clinical manifestations varied considerably. The age at the time of biopsy ranged from 20 weeks of gestation to 5 years, with the majority between ages 8-18 months. On skin biopsy the epidermal ultrastructure was already abnormal in three infants who subsequently developed the progressive neurologic deterioration typical of type 2 Gaucher disease. The degree of lamellar maturity did not correlate with the age at sampling, or the age at diagnosis. Clinically apparent skin abnormalities were only seen in five subjects, and again this finding was not related to maturity of the lamellae.
This study of infants with type 2 Gaucher disease, like other reported cases,17, 20, 21 also revealed considerable genotypic heterogeneity. Twenty different mutant alleles were identified, and only a few patients shared the same genotype. Two subjects were homozygous for a known complex mutation L444P+D255H,22 and three carried recombinant alleles.23 The early frameshift and splicing mutations c.84insG and IVS2+1G>A, presumably null alleles, were encountered in four cases. Mutation L444P, the first mutation identified in neuronopathic Gaucher disease was found in ten patients,24 none of whom were homozygous for the single point mutation. There was no correlation between mutations and the extent of structural abnormalities.
Enzyme replacement therapy for Gaucher disease, administered as an intravenous infusion, was used to treat eight of the twenty patients with type 2 Gaucher disease, and both of the patients with type 1 disease. The duration of the therapy prior to the time of biopsy ranged from several weeks to several years. It was not clear whether the treatment had any impact on maturity of the lamellar membranes, as all treated patients with type 2 Gaucher disease continued to display some degree of immaturity.
4. Discussion
Type 2 Gaucher disease is a rapidly progressive, early-onset neurodegenerative disorder,25 characterized by significant clinical variation, ranging from prenatal demise to survival for several years.26-28 It is associated with considerable genotypic heterogeneity, with over 50 known associated mutations.17, 29 Neither the genotype nor residual enzyme activity have consistently permitted a molecular based means to diagnose type 2 disease prior to the development of progressive neurological symptoms.28 Patients with type 1 and type 3 Gaucher disease respond well to enzyme replacement therapy, and, in symptomatic children, therapy should be begun as soon as possible. However, since type 2 Gaucher disease is associated with progressive neurologic deterioration that is largely refractory to current treatments, therapeutic decisions are less clear. Thus, clinicians faced with a young infant confront a diagnostic challenge, which impacts a patient's diagnosis, therapeutic decisions and family planning. Validating a method for early and pre-symptomatic identification of type 2 Gaucher disease could therefore assist greatly in therapeutic decision-making, and would be extremely helpful in counseling parents.
The use of ultrastructural examinations of the epidermis to identify type 2 Gaucher disease has been previously examined in two small patient cohorts.8, 9 In these preceding studies, and in one very recent study, electron dense lamellar, non-lamellar phase separation, immature lamellar bilayers and normal lamellar bodies differentiated patients with type 2 Gaucher disease from those with types 1 and 3.9, 30 Since a functionally null glucocerebrosidase mouse model for type 2 Gaucher disease exhibits the same morphological alterations, the absence of enzyme activity is likely responsible for this specific phenotype.
To ascertain the general utility and diagnostic specificity of electron microscopic (EM) analysis in Gaucher disease, we evaluated the skin ultrastructure of this larger cohort of patients with type 2 Gaucher disease, including patients at varying stages of clinical presentation. All 20 patients with type 2 Gaucher disease exhibited the same unique complex of skin ultrastructure abnormalities, regardless of clinical status, further validating that ultrastructure can be diagnostically helpful in the early identification of type 2 disease. Patients 2, 3, and 13 were biopsied prior to the development of neurologic signs or symptoms; yet importantly, their skin biopsies exhibited the same morphologic characteristics observed in biopsies obtained after the appearance of neurologic manifestations of type 2 Gaucher disease. These findings provide additional evidence that the characteristic epidermal ultrastructure abnormalities precede the clinical manifestations of neurodegeneration and can be diagnostically useful.8 A prospective study, with sequential skin biopsies, taken prior to, and during the development and progression of neurologic symptoms would provide additional support of this hypothesis. The inclusion of additional patients from all three types of Gaucher disease will help to truly validate this test. Moreover, the complex of electron dense non-lamellar, lamellar phase separation, immature lamellar bilayers, and normal lamellary body contents, coupled with normal corneodesmosome structure should readily distinguish Gaucher disease from recessive X-linked ichthyosis and Niemann-Pick disease. These two disorders display electron-dense phase separation, as in Gaucher disease, but lamellar membranes mature normally. In addition, the persistence of corneodesmosomes can distinguish recessive X-linked ichthyosis from both Gaucher disease and Niemann-Pick disease (Table 3).14
Table 3.
Differentiating features of disorders of lipid metabolism with electron-dense phase separation.
| Phase separation with electron dense phase? | Immature lamellar bilayers? | Mature lamellar bilayers? | Lamellar bodies | Corneodesmosomes | |
|---|---|---|---|---|---|
| X-linked ichthyosis | Yes | No | Yes (fragmented) | Normal | Abnormal-persistent in stratum corneum |
| Niemann-Pick disease | Yes | Some | Yes | Normal | Normal |
| Gaucher disease, type 2 | Yes | Yes | No | Normal | Normal |
| Gaucher disease, type 1 & 3 | No | No | Yes | Normal | Normal |
Since the deficiency of β-glucocerebrosidase results in the disruption of epidermal morphology by the buildup of accumulated, unprocessed glucosylceramide, we examined whether there was evidence that enzyme replacement therapy (ERT) affected epidermal structure. However, regardless of the mutations present, the age at biopsy and the ERT treatment regimens, the morphologic abnormities of the skin epidermis specific to type 2 Gaucher disease were persistent. This observation further validates the utility of epidermal ultrastructure examination as a diagnostic indicator of type 2 Gaucher disease, even if preemptive ERT has been administered. With the potential of future development of more effective treatments for type 2 Gaucher disease, tests enabling the early, presymptomatic identification of type 2 infants,,such as the analysis of cutaneous epidermal ultrastructure presented here, are urgently needed.
Highlights.
We explored the diagnostic utility of epidermal changes in distinguishing type 2 Gaucher disease. >Electron microscopy was performed on skin from 25 patients with Gaucher disease. >Only patients with type 2 Gaucher disease had distinct ultrastructural alterations. >These epidermal changes provide an early tool to identify type 2 Gaucher disease
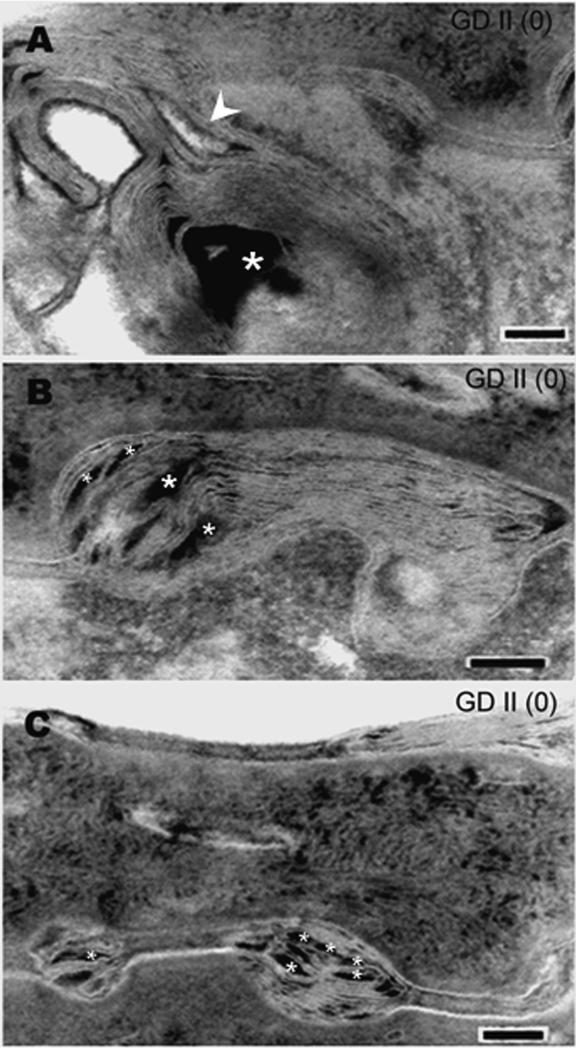
Figure 1.
(A, B, C): Examples of severely markedly abnormal lamellar bilayers , lacking any evidence of processing in epidermis from patients with type 2 Gaucher disease (GD II), grade (0). Asterisks indicate electron-dense non-lamellar phase; arrowhead depicts immature lamellar membranes. Ruthenium oxide post-fixation. Mag bars= 0.1μm. The features characteristic of type 2 Gaucher disease include: (1) Lamellar/non-lamellar phase separation with electron-dense nonlamellar phase; (2) Incompletely processed, immature lamellar membranes; (3) normal lamellar bodies (not shown).
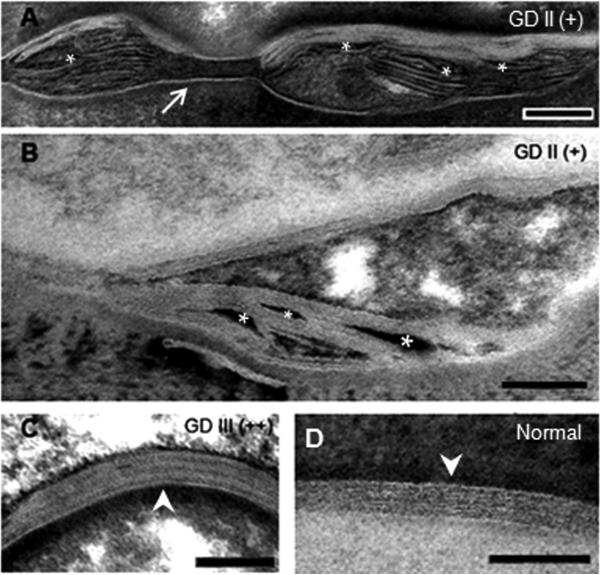
Figure 2.
The spectrum of lamellar processing in Gaucher disease. (A and B): Moderately processed, partially immature lamellar bilayers, grade (+). (C) Mature, fully processed lamellar membrane from a patient with type 3 Gaucher disease (GD III), grade (++). (D) Mature lamellar bilayer from normal human control. Asterisks indicate electron-dense non-lamellar phase; arrow depicts a normal corneodesmosome; arrowheads show normal lamellar membrane bilayer unit structure. Ruthenium oxide post-fixation. Mag bars=0.1 μm.
Acknowledgements
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health. The authors acknowledge the generosity of the many physicians who evaluated and referred these patients and those that contributed valuable tissues samples including Dr. Evelyn Carson, Dr Otto Schofer, Dr. Karin Juras, Dr. Leon Metley, Dr Hans Anderson, Dr. Gregory Pastores, Dr. Harvey Levy, and Dr. Cynthia Tifft
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts to disclose.
References
- 1.Prows CA, Sanchez N, Daugherty C, Grabowski GA. Gaucher disease: Enzyme therapy in the acute neuronopathic variant. Am J Med Genet. 1997;71:16–21. [PubMed] [Google Scholar]
- 2.Vellodi A, Tylki-Szymanska A, Davies EH, Kolodny E, Bembi B, Collin-Histed T, et al. Management of neuronopathic Gaucher disease: revised recommendations. J Inherit Metab Dis. 2009;32:660–4. doi: 10.1007/s10545-009-1164-2. [DOI] [PubMed] [Google Scholar]
- 3.Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res. 2008;49:697–714. doi: 10.1194/jlr.R800002-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi Y, Kriehuber E, Imokawa G, Elias PM, Holleran WM. beta-Glucocerebrosidase activity in mammalian stratum corneum. Journal of Lipid Research. 1999;40:861–9. [PubMed] [Google Scholar]
- 5.Sidransky E, Sherer DM, Ginns EL. Gaucher Disease in the Neonate - a Distinct Gaucher Phenotype Is Analogous to a Mouse Model Created by Targeted Disruption of the Glucocerebrosidase Gene. Pediatr Res. 1992;32:494–8. doi: 10.1203/00006450-199210000-00023. [DOI] [PubMed] [Google Scholar]
- 6.Tybulewicz VL, Tremblay ML, LaMarca ME, Willemsen R, Stubblefield BK, Winfield S, et al. Animal model of Gaucher's disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992;357:407–10. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- 7.Holleran WM, Ginns EI, Menon GK, Grundmann JU, Fartasch M, McKinney CE, et al. Consequences of beta-glucocerebrosidase deficiency in epidermis. Ultrastructure and permeability barrier alterations in Gaucher disease. J Clin Invest. 1994;93:1756–64. doi: 10.1172/JCI117160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holleran WM, Ziegler SG, Goker-Alpan O, Eblan MJ, Elias PM, Schiffmann R, et al. Skin abnormalities as an early predictor of neurologic outcome in Gaucher disease. Clin Genet. 2006;69:355–7. doi: 10.1111/j.1399-0004.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 9.Sidransky E, Fartasch M, Lee RE, Metlay LA, Abella S, Zimran A, et al. Epidermal abnormalities may distinguish type 2 from type 1 and type 3 of Gaucher disease. Pediatr Res. 1996;39:134–41. doi: 10.1203/00006450-199601000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Marsh NL, Elias PM, Holleran WM. Glucosylceramides stimulate murine epidermal hyperproliferation. J Clin Invest. 1995;95:2903–9. doi: 10.1172/JCI117997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanfer JN, Stephens MC, Singh H, Legler G. The Gaucher mouse. Prog Clin Biol Res. 1982;95:627–44. [PubMed] [Google Scholar]
- 12.Fujimoto A, Tayebi N, Sidransky E. Congenital Ichthyosis Preceding Neurologic Symptoms in 2 Sibs with Type-2 Gaucher-Disease. Am J Med Genet. 1995;59:356–8. doi: 10.1002/ajmg.1320590315. [DOI] [PubMed] [Google Scholar]
- 13.Plakkal N, Soraisham AS, Jirapradittha J, Pinto-Rojas A. Perinatal Lethal Gaucher Disease. Indian J Pediatr. 2011;78:106–8. doi: 10.1007/s12098-010-0247-2. [DOI] [PubMed] [Google Scholar]
- 14.Elias PM, Williams ML, Crumrine D, Schmuth M. Inherited clinical disorders of lipid metabolism. Curr Probl Dermatol. 2010;39:30–88. doi: 10.1159/000321084. [DOI] [PubMed] [Google Scholar]
- 15.Schmuth M, Man MQ, Weber F, Gao WN, Feingold KR, Fritsch P, et al. Permeability barrier disorder in Niemann-Pick disease: Sphingomyelin-ceramide processing required for normal barrier homeostasis. Journal of Investigative Dermatology. 2000;115:459–66. doi: 10.1046/j.1523-1747.2000.00081.x. [DOI] [PubMed] [Google Scholar]
- 16.Uchida Y, Hara M, Nishio H, Sidransky E, Inoue S, Otsuka F, et al. Epidermal sphingomyelins are precursors for selected stratum corneum ceramides. J Lipid Res. 2000;41:2071–82. [PubMed] [Google Scholar]
- 17.Stone DL, Tayebi N, Orvisky E, Stubblefield B, Madike V, Sidransky E. Glucocerebrosidase gene mutations in patients with type 2 Gaucher disease. Hum Mutat. 2000;15:181–8. doi: 10.1002/(SICI)1098-1004(200002)15:2<181::AID-HUMU7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 18.Elias PM, Fartasch M, Crumrine D, Behne M, Uchida Y, Holleran WM. Origin of the corneocyte lipid envelope (CLE): observations in harlequin ichthyosis and cultured human keratinocytes. J Invest Dermatol. 2000;115:765–9. doi: 10.1046/j.1523-1747.2000.00124-5.x. [DOI] [PubMed] [Google Scholar]
- 19.Hou SY, Mitra AK, White SH, Menon GK, Ghadially R, Elias PM. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991;96:215–23. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- 20.Mignot C, Doummar D, Maire I, De Villemeur TB. Grp FTGDS. Type 2 Gaucher disease: 15 new cases and review of the literature. Brain Dev-Jpn. 2006;28:39–48. doi: 10.1016/j.braindev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Tayebi N, Stone DL, Sidransky E. Type 2 Gaucher disease: An expanding phenotype. Mol Genet Metab. 1999;68:209–19. doi: 10.1006/mgme.1999.2918. [DOI] [PubMed] [Google Scholar]
- 22.Michelakakis H, Moraitou M, Dimitriou E, Santamaria R, Sanchez G, Gort L, et al. Homozygosity for the double D409H+H255Q allele in type II Gaucher disease. J Inherit Metab Dis. 2006;29:591. doi: 10.1007/s10545-006-0316-x. [DOI] [PubMed] [Google Scholar]
- 23.Tayebi N, Stubblefield BK, Park JK, Orvisky E, Walker JM, LaMarca ME, et al. Reciprocal and nonreciprocal recombination at the glucocerebrosidase gene region: Implications for complexity in Gaucher disease. Am J Hum Genet. 2003;72:519–34. doi: 10.1086/367850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuji S, Choudary PV, Martin BM, Stubblefield BK, Mayor JA, Barranger JA, et al. A Mutation in the Human Glucocerebrosidase Gene in Neuronopathic Gauchers-Disease. New Engl J Med. 1987;316:570–5. doi: 10.1056/NEJM198703053161002. [DOI] [PubMed] [Google Scholar]
- 25.Beutler E, Grabowski G. Gaucher disease. In: Scriver CR, Beaudet AL, Sly WS, Valle D, al e, editors. The metabolic & molecular bases of inherited disease. 8th ed. McGraw-Hill; New York: 2001. pp. 3635–68. [Google Scholar]
- 26.Ben Turkia H, Tebib N, Azzouz H, Abdelmoula MS, Ben Chehida A, Caillaud C, et al. Phenotypic continuum of type 2 Gaucher's disease: an intermediate phenotype between perinatal-lethal and classic type 2 Gaucher's disease. J Perinatol. 2009;29:170–2. doi: 10.1038/jp.2008.179. [DOI] [PubMed] [Google Scholar]
- 27.Goker-Alpan O, Schiffmann R, Park JK, Stubblefield BK, Tayebi N, Sidransky E. Phenotypic continuum in neuronopathic Gaucher disease: an intermediate phenotype between type 2 and type 3. J Pediatr. 2003;143:273–6. doi: 10.1067/S0022-3476(03)00302-0. [DOI] [PubMed] [Google Scholar]
- 28.Gupta N, Oppenheim IM, Kauvar EF, Tayebi N, Sidransky E. Type 2 Gaucher disease: phenotypic variation and genotypic heterogeneity. Blood Cells Mol Dis. 2011;46:75–84. doi: 10.1016/j.bcmd.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hruska KS, LaMarca ME, Scott CR, Sidransky E. Gaucher disease: Mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat. 2008;29:567–83. doi: 10.1002/humu.20676. [DOI] [PubMed] [Google Scholar]
- 30.Haverkaemper S, Marquardt T, Hausser I, Timme K, Kuehn T, Hertzberg C, et al. Congenital Ichthyosis in Severe Type II Gaucher Disease with a Homozygous Null Mutation. Neonatology. 2011;100:194–7. doi: 10.1159/000324116. [DOI] [PubMed] [Google Scholar]