Abstract
More than several hundreds of millions of people will be diabetic and obese over the next decades in front of which the actual therapeutic approaches aim at treating the consequences rather than causes of the impaired metabolism. This strategy is not efficient and new paradigms should be found. The wide analysis of the genome cannot predict or explain more than 10–20% of the disease, whereas changes in feeding and social behavior have certainly a major impact. However, the molecular mechanisms linking environmental factors and genetic susceptibility were so far not envisioned until the recent discovery of a hidden source of genomic diversity, i.e., the metagenome. More than 3 million genes from several hundreds of species constitute our intestinal microbiome. First key experiments have demonstrated that this biome can by itself transfer metabolic disease. The mechanisms are unknown but could be involved in the modulation of energy harvesting capacity by the host as well as the low-grade inflammation and the corresponding immune response on adipose tissue plasticity, hepatic steatosis, insulin resistance and even the secondary cardiovascular events. Secreted bacterial factors reach the circulating blood, and even full bacteria from intestinal microbiota can reach tissues where inflammation is triggered. The last 5 years have demonstrated that intestinal microbiota, at its molecular level, is a causal factor early in the development of the diseases. Nonetheless, much more need to be uncovered in order to identify first, new predictive biomarkers so that preventive strategies based on pre- and probiotics, and second, new therapeutic strategies against the cause rather than the consequence of hyperglycemia and body weight gain.
Keywords: Gut microbiota dysbiosis, Prebiotic and probiotic, Inflammation, Type 2 diabetes, Obesity, High throughput sequencing
Introduction
Metabolic diseases such as diabetes and obesity are becoming a social problem of utmost importance for all countries. Their impact on developing countries such as South Asia is even more dramatic since, besides being affected by the highest growing rate, the social system can certainly not afford the corresponding expenses. Therefore, the disease is poorly treated and pathological complications are blooming. Indeed, a major outcome linked to the occurrence of metabolic diseases is the rapid increase in cardiovascular events leading to death [1]. Over the last decade, diabetes has been the cause of lethal cardiovascular events that have progressed the most, since a 62% increase has been quantified [2, 3]. Its progression is much higher than the risk allocated to cholesterol or even hypertension. Therefore, in Western countries, where metabolic diseases are installed, as well as in eastern countries, where diabetes and obesity are strongly emerging, there is a crucial need to identify first risk factors of diabetes and obesity and second new therapeutic targets. Consequently, preventive strategies, most likely based on a change in dietary habits or the use of food complements prebiotic and probiotic, could be massively launched. Secondly, therapeutic treatments that aim at treating the cause rather than the consequence of an increased body weight or glycemia are needed. To this aim, a new paradigm of metabolic troubles is now emerging, resulting in the revision of the underlying causal factors.
Diabetes and obesity result from genetic and environmental factors. It can be estimated that point mutations accounts for less than 10% of the overall metabolic phenotype. The low impact of genetics on metabolic diseases is further reinforced by the growing incidence of diabetes and obesity over the last decades. The incidence of type 2 diabetes reaches 4–5% in Europe, 8–10% in the USA and more in South Asia [4]. These numbers have more than doubled over the last 20 years. Therefore, one can suggest that even if genetic analyses provide the basis for such epidemic, changes in our genome cannot be solely responsible [5]. Numerous other hypotheses have been proposed. First, epigenetic non-coded factors generate a new promising era of hypotheses that would need to be studied and that would not depend on genomic players. Second, more realistic is the impact of a change in feeding habits and social behavior that are certainly important causes of the growing incidence of metabolic diseases. However, both factors cannot either explain the overall epidemic. Linked to the environmental hypothesis, recent evidences have brought up the metagenome hypothesis. The latter is defined as the overall bacterial genome, whereas the expression of the corresponding gene represents the metatranscriptome. This prokaryotic genome, next to our eukaryotic one, has emerged thanks to recent advances of high throughput sequencing technologies allowing to obtain millions reads over a short period of time and avoiding the need for laboratory cultivation of gut bacteria [6], even if a recent study seems to reverse this concept [7]. The recent advances in DNA sequencing technology have allowed the collection of high-dimensional data from human-associated microbial communities on an unprecedented scale [8]. In addition, tremendous efforts have been made in bioinformatic analyses allowing the encoding and the deciphering of all sequences. Humans host different metagenomes from multiple locations such as skin, lungs, vagina, mouth, even if the intestine hosts the most [9, 10]. In fact, the human gut hosts 100 trillion microorganisms, encompassing up to thousand of species at an average concentration of 1014 per ml and weighing in average 1.5 kg [11]. The importance of this metagenome resides in its gene repertoire, 100 times superior than our eukaryotic nuclear genome [12], providing hence a huge genetic diversity susceptible to convey a tremendous amount of functions.
An important concept is that all mammalians were born sterile, without any flora. Following the first hours, days, and weeks, the mother’s and the environmental flora colonized the overall body of the new born in a specific order [13]. The initial infant gut microbiota is a simple structure usually dominated by Bifidobacteria, and through a series of successions and replacements, it shifts to a more complex adult pattern [14–16]. The microbiota also undergoes substantial changes at the extremes of life, in infants and older people, the ramifications of which are still being explored [17]. However, the adult intestinal microbiota has been shown to be relatively stable over time [18] and is sufficiently similar between individuals to allow identification of a core microbiome comprising 66 dominant operational taxonomic units (OTUs) that corresponds to 38% of sequence reads from 17 individuals [19]. Eventually, the core microbiota changes to become distinct in elderly subjects from that observed for younger adults, with a greater proportion of Bacteroides spp. and distinct abundance patterns of Clostridium groups. Ecological rules govern the shape of microbial diversity throughout the life. This suggests that each member can interact in a perfect mutualistic symbiosis with each other and defines a steady microbiota [9, 10, 20]. However, before reaching an ideal microbial ecology the microbes interact with the host. The interaction of epithelial cells with microbes and components released by microbes, including their metabolites, is a key mediator of the cross-talk between the epithelium and other cell types [21]. The exchanges between bacteria and epithelium may differ in the small and large intestine because of anatomical differences and the extent to which the secreted mucus layer covers the epithelium. An important feature is that the mucosa is free of bacteria, however, this does not rule out that bacterial fragments might diffuse throughout the mucosa to bind receptors at the surface of epithelial intestinal cells [22]. These bacterial to host interaction helps to maturate the intestinal epithelial layer, the mucosal innate immune system, the enteric nervous system, as well as the intestinal vascular system [21]. Hence, intestinal microbiota has a strong impact on the control of numerous major physiological functions. Whether the first years of life can impact adult physiological functions is suspected. An interesting caveat is that modern standards of hygiene [23] and/or the switch from breast feeding to baby bottle may have altered transmission mechanisms [13, 24]. Similar hypotheses could be raised regarding premature neonates [25]. Altogether it is conceivable that the early intestinal microbial colonization at birth may impact the occurrence of metabolic diseases [26]. Recent data suggested that the Bifidobacterial count in fecal samples during infancy, as assessed by FISH with flow cytometry, was higher in children remaining normal weight over a 7-year follow-up study [26]. Conversely, the microbiota aberrancy during infancy in children becoming overweight was associated with a greater number of Staphylococcus aureus.
Once adult microbiota established, actors such as antibiotics, prebiotics, and probiotics could modulate its ecological architecture. However, although some plasticity does exist, these effects are always reversible, suggesting that a tight host-to-microbiota relationship has been established during the neonatal life where the host shapes the microbiota and vice versa [27]. The corresponding mechanisms could be linked to the maturation of the immune system [28] where some bacterial species such as the segmented filamentous bacteria can largely induce the coordinated maturation of T cells, whose responses are induced by the whole microbiota. The innate immune system, as a first line of defense, keeps sterile the intestinal mucosal layer through the phagocytosis of invading pathogenic bacteria. Secondarily, B lymphocytes will secrete IgA into the mucosa that will be specifically directed against invasive pathogens [29–31]. Eventually, the synthesis by intestinal epithelial cells of lectin will provide bedding for commensal mucosal bacteria and will regulate the secretion of defensin. The latters, natural antibiotics secreted by the Paneth cells, will select some bacterial genera and species that will remain close to the intestinal mucosa.
Considering all the above options, it remains to determine the major factors responsible for the epidemic of metabolic diseases which is rapidly outgrowing. Recently, intestinal microbiota ecology has been shown to shape metabolic diseases itself such as obesity [32] and diabetes [33]. High throughput sequencing analyses have shown a change in some of the major phyla that will be described in this review according to the phenotype. However, in humans, a major question remains as to whether intestinal microbiota is the consequence or the cause of the observed phenotype. Although studies using germ-free mice demonstrated the causal role of intestinal microbiota in triggering metabolic impairments, it still remains to demonstrate whether the genetic background may influence the development of a specific microbiota.
That the diet could be a major regulator factor in shaping gut microbiota and, hence, its relationship with host metabolism, can be logically assumed, given the major role of gut microbiota in digestion [34]. In fact, a comparative study of multiple dietary habits such as herbivore, omnivore and carnivore has shown that the acquirement of a new diet is sufficient to radically modify gut microbiota, acting as an evolutionary trigger of new species [35]. In fact, both host diet and phylogeny shape bacterial variety, which increases from carnivore to herbivore to omnivore. In addition, a modern lifestyle shifts gut microbiota of humans closer to the one of omnivorous primates [35]. Therefore, it goes without saying that our intestinal microbiota feeds on the type of food that is absorbed by changing its ecological structure. Each individual bacterium lives in mutualistic ecology with the others. Therefore, an excess or lack of nutrient may change the metabolic activity of a given bacterium, which will no longer or excessively produce such a metabolite essential or deleterious for the neighboring bacteria. The major example is oxygen that is been used by the aerobes from the upper intestinal tract inducing a deep anaerobic state in the distal intestine allowing hence strict anaerobes to survive. The use of prebiotics such as non-digestible dietary polysaccharides as substrates for genera like Bifidobacterium will favor their growth and their anti-inflammatory function [36]. Similarly, a fat-enriched diet, which is widely used to induce metabolic diseases, strongly impacts the development of diabetes and obesity.
The molecular mechanisms through which a given intestinal microbiota induces metabolic diseases will be discussed in this review. Briefly they are linked to an increased energy harvesting and the triggering of the low-grade inflammatory status characterizing insulin resistance and obesity [37, 38]. The bacterial molecules responsible for the triggering of theses physiological functions are currently been discovered. This will certainly lead to the identification of new therapeutic strategies for the treatment but also for the prevention of metabolic diseases. A second outcome will be the identification of biomarkers able to predict the development of diabetes and over weight in absence of any risk factor. This is envisioned since intestinal microbiota is causal for the development of metabolic diseases.
Altogether, the new “microbiota to host paradigm” for the control of metabolic disease is promising. It involves transversal fields of investigation such as microbiology, immunology, metabolism, and bioinformatics. Joining the different pieces of the puzzle should lead to innovating preventive and therapeutic strategies that involve pharmacological, prebiotic, and probiotic developments as well as synbiotic approach [39].
Changes in microbiota during metabolic diseases
During the past ∼160 million years, mammals have co-evolved with a vast and diverse microbial community that colonizes our cutaneous and mucosal surfaces. Most of these microorganisms reside within our gastrointestinal tract, and their constituency is determined by host phylogeny and diet [40, 41]. Since 0.3% of our eukaryotic genome is modified over 1 million years, it can be calculated that 50–55% of our genome has evolved in a tight relationship with the microbial community generating strong genetic dependency. Beside this very long-term genetic evolution, over the last decade, a major observation has been that metabolic diseases are accompanied by a change in intestinal microbiota composition in animals and humans [32, 42, 43]. The development of high throughput sequencing technologies, such as pyrosequencing, has allowed the analysis of feces from obese patients during body weight loss. Briefly, this technology sequences millions of 16S rRNA gene fragments per run. The 16S rRNA is composed of 9 hyper-variable regions (V1–V9) [44, 45] that represent the target for the amplification-based sequencing. The degree of diversity or homology is proportional to the genetic distance when plotted onto a phylogenic tree. Hence, these characteristics allow the identification of bacterial Phyla, Classes, Orders, Families, Genera and Species when compared with a naïve data basis [46]. The longer the sequence the more precise is the analysis. The use of tags to identify different samples allows the analysis of several samples at once, reducing hence the influence of sequencing differences in the efficacy. However, this strategy reduces as well the depth of the analysis by limiting the number of reads per sample. The sequence reads are assigned to the NCBI non-redundant, Clusters of Orthologous Groups (COG) [47], or Kyoto Encyclopedia of Genes and Genomes (KEGG) [48] databases. Altogether, on a general basis, the averaging results from all data sets have been reported as follows: 94% of the tags assigned to the non-redundant database were bacterial, 3.6% were eukaryotic (0.29% Mus musculus; 0.36% fungal), 1.5% were Archaea (1.4% Euryarcheota; 0.07% Crenarcheota), and 0.61% were viral (0.57% double stranded DNA viruses) [49]. First analyses using this technique have been performed on obese patients followed up during 1 year of restriction calories diets in order to reduce their body weight. Gordon’s group initially showed that the obese patients are characterized by changes in the relative abundance of the two dominant bacterial divisions, the Bacteroidetes and the Firmicutes [32, 50]. They showed a reduced Bacteriodetes/Firmicutes ratio in obese patients which evolved toward that of lean patients during weight loss. Thus, obesity is associated with phylum-level changes in the microbiota and reduced bacterial diversity [32, 43, 51]. Therefore, a change in intestinal microbiota was linked to the obese phenotype. Two hypotheses could be raised based on these results. The obese phenotype would be secondary to the microbiota or the contrary. To answer this question, the fecal microbial communities of adult female monozygotic and dizygotic twin pairs concordant for leanness or obesity, and their mothers have been studied [51]. The results reveal that the human gut microbiome is shared among family members, but that each person’s gut microbial community varies in the specific bacterial lineages present, with a comparable degree of co-variation between adult monozygotic and dizygotic twin pairs [51]. These results demonstrate that a diversity of organismal assemblages can, however, yield a core microbiome at a gene level and that deviations from this core are associated with different physiological states, for example, obese versus lean.
It is noteworthy that this technology evolves rapidly and that new generations of sequencing with different bioinformatic analyses allow an even faster analysis of the microbiome. These techniques uses the Illumina GAIIx platform to sequence a diverse array of samples at a depth averaging of several million reads per sample which is continuously increasing [52]. The data demonstrate an excellent consistency in taxonomic recovery and recapture diversity patterns that were previously reported on the basis of meta-analysis of many studies from the literature. The use of this technique has confirmed the existence of the above-reported core microbiome [51].
Recent data characterized intestinal microbiota in type 2 diabetic patients. The authors described that the proportions of phylum Firmicutes and class Clostridia were significantly reduced in the diabetic group compared to the control group [33]. Furthermore, the ratios of Bacteroidetes to Firmicutes as well as the ratios of Bacteroides-Prevotella group to C. coccoides-E. rectale group correlated positively and significantly with plasma glucose concentration but not with BMIs. Therefore, bacterial sequences, specific for type 2 diabetes rather than obesity, can be considered as signatures of hyperglycemic syndrome.
Older data reported changes in intestinal microbiota related to different metabolic phenotypes. Thirty years ago, it was observed during gastric bypass surgery, a surgical method now widely performed to treat diabetes and morbid obesity [53], a change in intestinal microbiota as observed by culture-based methods [54]. Furthermore, in animal models of obesity, induced by brain lesion of the ventromedial hypothalamus, a change of intestinal microbiota was also observed suggesting an important impact of the brain on the control of intestinal microbiota [55]. At that time, cultivation-based technologies showed changes in enterocci and lactobacilli.
The use of metagenomic sequences should now help to precisely define the role of the brain on the control of microbiota. Along the same line of observation, the genetic ablation of the leptin receptor gene in mice harbors a microbiota that possesses a significantly higher percentage of Firmicutes, and a correspondingly lower percentage of Bacteroidetes, than their wild-type littermates [49]. This data set demonstrates that leptin certainly regulates gut microbiota. The molecular relays linking leptin to the microbiome are unknown but the data suggest that its action would be through either its central effect or via the induction of obesity. Furthermore, it has been recently shown a relationship between gut microbiota and brain development since germ-free mice displayed increased motor activity and reduced anxiety, when compared with specific pathogen-free (SPF) mice with a normal gut microbiota [56]. In particular, the authors showed that the microbial colonization initiates signaling mechanisms capable to affect neuronal circuits involved in motor control and anxiety control.
Another hypothesis would be linked to the role played by hormones on the immune system which by itself can shape intestinal microbiota as shown for type 1 diabetes models, where the immune system is impaired and in other instances as well [57–59]. Indeed, one potential outcome of the adaptive coevolution of humans and bacteria is the development of commensal relationship [60]. An important mechanism involves the secretion of antimicrobial molecules belonging to the defensin family or members of the RNAse family such as angiogenin [58, 59]. These molecules, produced by intestinal Paneth cells, are secreted into the gut lumen and have bactericidal activity against intestinal microbes. Their expression is induced by Bacteroides thetaiotaomicron, a predominant member of the gut microflora. Mice deleted from the corresponding genes have different microbiota, revealing a mechanism whereby intestinal commensal bacteria influence gut microbial ecology and shape innate immunity [58]. The immune system can then regulate the secretion of defensin and shapes the microbial community [61]. Therefore, one might suggest that in metabolic diseases, the important role played by inflammation [37, 38, 62], mostly due to an impaired immune system, could differently shape intestinal microbiota.
Diet and nutritional status are amongst the most important modifiable determinants of human health. Furthermore, a change in feeding habits is most likely the most prevalent factor susceptible to induce metabolic diseases. Many studies have reported direct links between diet and the structure of the gut microbiome in mouse models. One recent example observed that microbiome structure rapidly shifts in response to a change from a low-fat, plant-based diet to a high-sugar, high-fat diet, modifying both the available metabolic pathways and actual gene expression [63, 64]. Furthermore, the change in feeding habits was associated with an increased intestinal permeability to LPS leading to a state of insulin resistance [3, 65]. This latter study further demonstrated that the change in intestinal microbiota was also associated with a different susceptibility to antibiotic treatment [65]. The high-fat diet-fed mice were more sensitive to the antibiotic treatment suggesting that the new microbial ecology that follows the dietary treatment was very fragile. Over the last decades, the proportion of fat in diet has mostly replaced that of dietary fibers, reducing hence the prebiotic effect of the latters. Therefore, intestinal microbiota has, at least in part, changed in response to the new feeding habit. Hence, new antigenic determinants and risk factors for the immunomodulation and the occurrence of diabetes could be related to the change in microbiome. This deeply influenced the structure of the microbiota within a single day, changed the representation of metabolic pathways in the microbiome, and altered microbiome gene expression. Humanized mice fed the Western diet have increased adiposity. This trait is transmissible via microbiota transplantation. It is noteworthy that in human studies where most of the analyses have been made from fecal flora, the results represent most likely 5–20% of the overall intestinal microbiota. Therefore, numerous bacterial species are yet to be described.
The treatment of metabolic disease is challenging. Bariatric surgery is currently the only available treatment for morbid obesity that consistently achieves and sustains substantial weight loss [8, 53]. It is becoming a widely used procedure even for diabetes and obese patient with moderate excessive body weight. Various surgical procedures have been developed over the last 50–60 years. The Roux-en-Y gastric bypass (RYGB) involves creating a small (about 15–30 mL) gastric pouch from the bottom of the stomach; then, the distal stomach and proximal small intestine are bypassed by attaching the distal end of the mid-jejunum to the proximal gastric pouch (creating the Roux limb) and then reattaching the biliary and pancreatic limb at a specific location along the Roux limb [53, 66]. This very efficient procedure allows the remission from diabetes and then a major reduction in body weight. A major consequence is the rapid change in enteroendocrine functions, such as the secretion of gut hormones such as GLP-1, whose origin is yet unknown [67]. However, it is also associated with a drastic modification of microbiota, which could be the cause of the enteroendocrine change [68, 69]. Specifically, the Firmicutes were dominant in normal-weight and obese individuals but significantly decreased in post-gastric-bypass individuals, who had a proportional increase in Gammaproteobacteria. Interestingly, numbers of the H2-producing family of Prevotellaceae were highly enriched in obese subjects as well as the Archaea members of the order Methanobacteriales, which are H2-oxidizing methane producing bacteria. The role is unknown but could be related to a mechanism important for increasing energy uptake by the human large intestine in obese persons. Interestingly, obese and diabetic patients were also characterized by a reduction in the anti-inflammatory bacterium F. prausnitzii [68] when compared with obese patients. Some molecules issued from intestinal microbiota have been characterized using NMR-based metabolomic analyses [70]. Gut flora-derived metabolites such as hippuric acid, trigonelline, 2-hydroxyisobutyrate, and xanthine contributed most to the classification model and were responsible for the discrimination between obese and lean individuals. Moreover, the typical obese metabotype (contraction for metabolic phenotype) is lost after weight loss induced by bariatric surgery. The causal role of these molecules could not be demonstrated but it can at least be suggested that they do represent biomarkers.
Mechanisms through which intestinal microbiota may lead to the development of hyperglycemia and fat storage
The storage hypothesis
Intestinal microbes utilize nutrients and produce metabolites that influence a wide range of human metabolic phenotypes, including susceptibility to conditions such as obesity [3, 42, 49, 71], insulin resistance [3], metabolic syndrome [89], liver steatosis [72, 73]. Using metagenomic and biochemical analyses, Gordon’s group demonstrated that these changes affect the metabolic potential of the gut microbiota from obese mice [43]. The “obese microbiome” has an increased capacity to harvest energy from the diet [49]. However, this mechanism was contested by other studies which suggested that the relationship between the microbial composition and energy harvesting capacity is more complex than previously considered [74]. However, a major point was that this trait is transmissible since colonization of germ-free mice with the microbiota from obese mice resulted in a significantly greater increase in total body fat (up to 40%) than colonization with the microbiota from a lean mouse [49, 71]. Consequently, in contrast to mice with a gut microbiota, germ-free animals are protected against the obesity that develops after consuming a Western-style, high-fat, sugar-rich diet [71]. Their persistent lean phenotype is associated with increased skeletal muscle and liver levels of phosphorylated AMP-activated protein kinase (AMPK) and its downstream targets involved in fatty acid oxidation such as the acetylCoA carboxylase and carnitine-palmitoyltransferase. Furthermore the germ-free mice have increased expression of the intestinal fasting-induced adipocyte factor (FIAF) that induces the peroxisomal proliferator-activated receptor gamma coactivator-1alpha (Pgc-1alpha). Thus, the authors suggested that germ-free mice were protected from diet-induced obesity by two independent but complementary mechanisms that result in increased fatty acid metabolism.
The inflammatory hypothesis
Metabolic diseases are characterized by a low-grade inflammation where the role of the innate and adaptive immune systems is of major importance [37, 38, 62, 75–78]. However, the origin of the factors triggering inflammation before the onset of obesity or diabetes remains unknown. We first proposed that the lipopolysaccharides (LPS) which are highly inflammatogenic component of the cell wall of the Gram-negative bacteria were causally involved in the onset of the low-grade inflammation in response to a fat-enriched diet [3]. Bacterial fragments are recognized by Toll-like receptors (TLRs) that are a conserved family of integral membrane pattern-recognition receptors that have a crucial role in the innate immune system, which is the early host defense against invading pathogens but are also required for intestinal homeostasis [79]. Other intracellular receptors, such as the Nucleotide-binding Oligomerization Domain (NOD)-like Receptors (NLRs) to bacterial DNA or NOD1 and NOD2, to other fragments such as peptidoglycans, are involved in innate immunity [80, 81] and could be considered as target to control inflammation. Mice fed a high-fat diet for a short period of 2 weeks where characterized by a moderate 2–3-fold increase in blood LPS defined as metabolic endotoxemia [3]. It remains within this range, i.e., very far from what observed during an acute infection. Therefore, on the basis of this steady high concentration of plasma LPS, adipose tissue, liver and muscle inflammation developed [3, 82]. The causality of this bacterial factor was demonstrated since a continuous low-rate infusion of LPS induced most of the early factors recapitulating metabolic syndrome and conversely mice deleted from the LPS receptor TLR4, or part of TLR4 machinery such as CD14, resisted the occurrence of the disease [83]. Furthermore, adipocytes treated with LPS developed inflammation [84]. Numerous data suggested that the original site of inflammation is indeed the adipose tissue but recent studies, using conventional and germ-free mice, suggested that it could be localized into the intestine where HF Western diet and gut bacteria interact to promote intestinal inflammation, which contributes to the progression of obesity and insulin resistance [85]. The role of intestinal microbiota was further demonstrated since a chronic antibiotic treatment reduced the intensity of the disease in high-fat diet and ob/ob mice [65, 86]. Plasma LPS concentration could also be considered as a risk factor since it was present in excess in the blood of apparently healthy patients feeding more fat than carbohydrate or proteins [82]. The increased plasma LPS concentration could be acutely induced by a single absorption of lipid in human [87] and in mice [3] and seems to depend on an increased intestinal permeability through a GLP-2 dependent mechanism [88]. Other evidences showed that the TLR5 receptor was conversely protecting against metabolic syndrome since mice genetically deficient in TLR5 exhibited hyperphagia and developed hallmark features of metabolic syndrome [89]. TLR5 initially helps defend against infection. It was here shown that TLR5 controls hyperphagia, hyperlipidemia, hypertension, insulin resistance, and increased adiposity through the mechanisms requiring inflammation. A further phenotype was that the metabolic features correlated with changes in the composition of the gut microbiota. The metabolic phenotype was transmissible since the transplantation of the microbiota from TLR5-deficient to germ-free mice resulted in obesity and reduced insulin sensitivity. These findings further demonstrate that the modulation of the immune system affects host metabolism by altering gut microbiota.
Taken into account what reported above, a key matter is to understand how bacterial fragments and LPS reach the target organs and trigger inflammation. LPS molecules are carried into the blood mostly by lipoproteins where in the liver they have been proposed to induce hepatitis [90]. Moreover, an antibacterial treatment reduced the diseases [91]. Therefore, it has been suggested that LPS can be absorbed by the intestine during the synthesis of chylomicrons [92, 93] then exchanged with other lipoproteins [94] that can be chronically transported toward target tissues such as liver [95] or blood vessels [96] and trigger inflammation. However, in an acute situation, a flash administration of lipoproteins can buffer plasma LPS and reduce its impact on the acute phase inflammation [97, 98]. No hypothesis is so far available for other bacterial fragments.
Altogether, the huge diversity of intestinal microbiota allows multiple hypotheses regarding the molecular mechanisms responsible for metabolic diseases. Certainly, inflammation must be taken into account. One could also suggest that food intake and energy storage must be involved in the picture.
Gut microbiota and lipid metabolism
Conclusions from the previous paragraph suggest that intestinal microbiota, which strongly influences fat storage in white adipose tissue, may as well tightly regulate lipid metabolism and its consequences on cardiovascular diseases. This hypothesis is easily intuitive since the intestine is the entry door of lipid. Microbiota, although present at low concentration in the duodenum and jejunum (104–105 cell/ml), where most of the lipids are absorbed, would be informing the intestinal cells with lipid metabolites. Otherwise an excess of lipids, hence not absorbed, would be feeding the microbiota present in the large intestine which would produce informative metabolites as well. Evidences showed that conventional mice bearing a normal microbiota were characterized by increased production of energy metabolites, e.g., pyruvic, citric, fumaric, and malic acid, when compared with germ-free mice [99]. Conversely, plasma levels of cholesterol and a number of lipid species in the serum triglycerides and phosphatidylcholine were reduced by the microbiome whereas they were increased in the tissue such as the adipose tissue and the liver. This suggested that the clearance of lipids was increased by the microbiota. The mechanisms remain unknown but are most likely due to the change in the bacterial genera and the corresponding microbiome, present or not within the flora from the diabetic or obese animals. Even intestinal cholesterol metabolism is affected by the intestinal microbiota. In germ-free rats, hepatic microsomal hydroxylation of steroid hormones and of lithocholic acid is more efficient than in the conventional counterparts [100]. A precise example is the discovery of a Bacteroidetes D8, a cholesterol-reducing bacterium of human intestinal origin, which was isolated from a senior male volunteer, with a high capacity to reduce luminal cholesterol to coprostanol [101]. Several decades ago, the role of intestinal microbiota on the control of lipid metabolism was also indirectly suggested since it was shown that biliary acids can be metabolized by the flora [102]. In germ-free rats, the amount of urobilin and stercobilin is almost negligible which shows that bilirubin is reduced to urobilins by the intestinal microbial flora exclusively. The colonization with different bacterial strains showed that the metabolism of biliary acids was differently affected by the type of intestinal microbiota. In all instances, monocolonization was not sufficient to fully restore a normal bile acid metabolization.
Surprisingly, the impact of gut microbiota on systemic metabolism (Fig. 1) has been shown even in relation to lipid homeostasis in not-metabolically active organs such as the eye [103]. In a recent study, Oresic et al. compared the lipid structure of eyes issued from germ-free and conventional mice. The authors analyzed both lens and retina lipidome by Mass Spectrometry (MS) performed in ion-negative mode (ESI-), and a total of 140 and 276 lipids were, respectively, detected. The main finding was the microbiota-driven reduction in overall phosphatidylcholines, which suggested an increased exposition to oxidative stress in conventional mice when compared with germ-free mice.
Fig. 1.
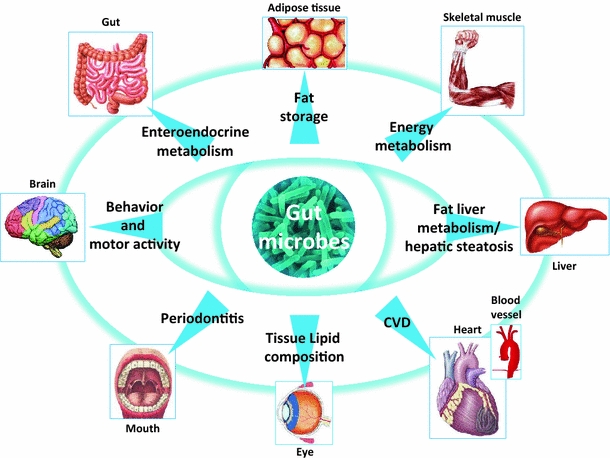
Multiple-sited impact of gut microbiota on whole host metabolism. Gut microbes have been shown or proposed to have an impact on adipose tissue and liver fat storage, skeletal muscle energy metabolism, fat liver metabolism and hepatic steatosis, atherosclerosis and cardiovascular diseases (CVD), tissue lipid composition in the retina lens, periodontitis, behavior and motor activity, and enteroendocrine metabolism. The precise bacteria involved remained to be determined and should be the basis of present and future discoveries
However, in the quest of mechanisms linking gut microbiota, lipid metabolism and vascular diseases recent finding demonstrate strong molecular hypotheses [104]. Before, discussing these issues, it is noteworthy that cardiovascular diseases have been linked to infection for several decades by augmenting pro-atherosclerotic changes in vascular cells [105]. A microbiome has been found in atherosclerotic plaques since bacterial DNA can be identified in more than 50% of all plaques [106] and its origin could be intestinal or oral [107]. The vascular risk was indeed increased in population studies where the plasma concentration of LPS was increased (Fig. 2) [104, 108, 109]. Conversely, anti-LPS molecules such as soluble CD14 protected against aortic stiffness and hence an impaired vascular function [110]. Therefore, antibiotic therapies may have some positive impact on vascular function [111]. The molecular control of the generation of atherosclerotic plaques by factors from intestinal original has been recently shown in a study where the authors used a metabolomics approach to generate unbiased small-molecule metabolic profiles in plasma that predict risk for CVD. Three metabolites of the dietary lipid phosphatidylcholine—choline, trimethylamine N-oxide (TMAO) and betaine—were identified and then shown to predict risk for CVD in an independent large clinical cohort. Dietary supplementation and germ-free mouse studies confirmed a critical role for dietary choline and gut flora in TMAO production. It was linked to macrophage cholesterol accumulation and foam cell formation. Hence, the role of monocytes/macrophages is important in low-grade inflammatory diseases since an increased number favors coronary collateral growth in type 2 diabetic patients [112]. Eventually the suppression of intestinal microflora in atherosclerosis-prone mice inhibited dietary-choline-enhanced atherosclerosis. Therefore, microbiota from intestinal or oral origin is now certainly recognized as a risk and a causal factor of the cascade of events leading to atherosclerosis. An interesting hypothesis would be that the microbiome could control host gene expression via miRNA [113]. Comparative profiling of miRNA expression using miRNA arrays from conventional and germ-free mice revealed that mmu-miR-665, which was dysregulated during colonization, down-regulated Abcc3 expression by directly targeting the Abcc3 3′-UTR [113]. The role of miRNA on endothelial metabolism has been shown elsewhere [114] and therefore one could suggest that intestinal microbiota could regulate endothelial function and human atherosclerotic lesions [114]. However, the overall control of inflammation mediated by the innate and adaptive immune system can certainly regulate the aggressiveness of the gut flora. Consequently, the truth might rely on a set of markers associating gut microbiota as a risk factor, the regulatory role of the immune system and the genetic background of the individual.
Fig. 2.
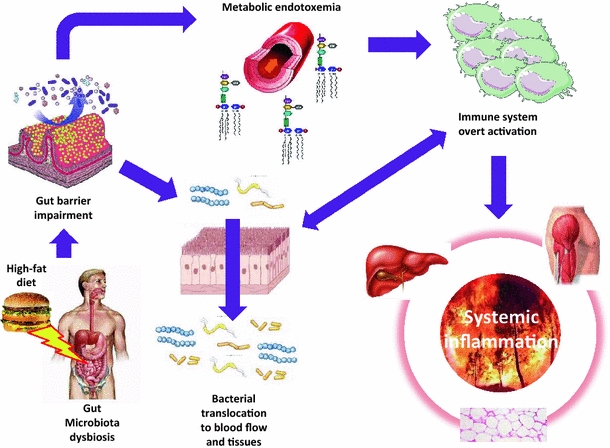
The inflammatory burn: gut microbiota dysbiosis and the origin of metabolic impairments. The origin of metabolic diseases is multifactorial but the impact of deleterious feeding habits is certainly the major factor responsible. This directly modifies intestinal ecology and we first showed that upon an increased intestinal permeability it led to an increased circulating concentration of LPS from Gram-negative bacteria of intestinal origin [3, 82] called metabolic endotoxemia. The inflammatory factors LPS and other bacterial fragments can translocate toward target tissues such as the blood, the liver, and the adipose depots or the arterial wall to interfere with cells from the immune system to generate the chronic low-grade inflammation required for the development of metabolic and cardiovascular diseases
Gut microbiota as a regulator of hepatic steatosis
Hepatic steatosis is one of the major complications of abdominal obesity, insulin resistance and type 2 diabetes [115–117]. About 60–80% of these patients developed a stage of the disease ranging from 1 to 5 where the lowest limit corresponds to the accumulation of lipid and then to inflammation, apoptosis, fibrosis, liver failure or cancer. It is now becoming a priority for Europe through the launching of European programs to fund research. The argument that intestinal microbiota would be involved in the triggering of hepatic steatosis originates from observations that conventionalized animals have 40% more body fat than germ-free animals [42]. Several molecular mechanisms have been proposed. An increased intestinal production of short chain fatty acids would be providing more energy to the liver [49, 71]. The genetic background provides a susceptibility to the development of hepatic steatosis such as in the SV129S6 mouse [72]. In this instance, the multivariate statistical modeling of the metabolomic spectra from urine samples have shown that the genetic predisposition of the mouse strain to liver steatosis is associated with disruptions of choline metabolism [72]. It was demonstrated that the symbiotic gut microbiota converted the choline into methylamines leading to low circulating levels of plasma phosphatidylcholine and high urinary excretion of methylamines (dimethylamine, trimethylamine, and trimethylamine N-oxide), which reduces the bioavailability of choline and mimics the effect of choline-deficient diets, causing NAFLD [72, 73]. However, this model does not involve a key feature which is that the liver disease is associated with a state of inflammation. The molecular inflammatory mechanism could be attributed to plasma LPS which is increased in the patients with cirrhosis and in the hepatoportal vein following alcohol consumption [3, 118, 119]. This mechanism was dependent on TLR4 and initiates inflammation [120]. However, in absence of alcohol consumption, plasma LPS concentration moderately increases in the blood of mice which develop a fatty liver such as the high-fat diet-fed mice [3]. This process characterizes a metabolic endotoxemia and is considered as a triggering factor of inflammation and metabolic diseases. Metabolic endotoxemia was linked to an increased intestinal permeability [65]. The treatment of the mice fed a fat-enriched diet with antibiotics reduced the metabolic endotoxemia and the accumulation of fat in the liver and the local inflammatory status [65, 86]. In addition, to the inflammatory mechanism, it has been proposed that intestinal microbiota would be increasing fat accumulation in the liver through a mechanism involving the regulation of FIAF [42, 71]. This member of the angiopoietin-like family of proteins is selectively suppressed in the intestinal epithelium of normal mice by intestinal microbiota. FIAF is a circulating lipoprotein lipase inhibitor and its suppression is essential for the microbiota-induced deposition of triglycerides in adipocytes. Indeed, in the obese diabetic ob/ob mouse, the corresponding microbiome has an increased capacity to harvest energy from the diet further fueling the liver with carbon residues generated through the bacterial fermentation of non-digestible fibers. Therefore, upon a change of intestinal microbiota, it is suggested that both increased dietary fiber fermentation, and the induction of metabolic endotoxemia would lead to accumulate lipids in liver and trigger inflammation. This hypothesis would fit with the rapid development of hepatic steatosis corroborating the change in feeding habits and hence of intestinal microbiota.
Gut microbiota and type 1 diabetes
Type 1 diabetes is an autoimmune disease which is due to the specific destruction of the endocrine insulin secreting pancreatic beta cells by T lymphocytes [121]. Consequently, a progressive but absolute insulinopenic state occurs within the following months and years. This mechanism is irreversible but could be prevented by immunosuppressive agents when used early enough [122, 123]. The antigens targeted by the immune system have been partly described and are related to the recognition of Glutamic Acid Decarboxylase (GAD)-64 proteins. T lymphocytes invade the pancreatic islets as described by an insulitis, and progressively destroy the insulin secreting cells only [121]. What remains unknown is the process that prevents from the proper destruction of the autoreacting T lymphocytes or the system that allows the autoimmune reaction by misrecognition of the self-antigens such as GAD64. One could suggest that the maturation of T lymphocytes would be impaired. Recent data showed the critical role of the gastrointestinal microbiota in the protection or the triggering of type 1 diabetes [57, 124]. The animal models suitable for such studies are the non-obese diabetic (NOD) mouse strain, or the BB rat. In both models, the target pancreatic insulin producing beta cells are attacked and destroyed by activated immune cells, leading to type-1 diabetes. The discovery of the role of intestinal microbiota came from the hygienic hypothesis [23] following the observation that the incidence of spontaneous T1D in the NOD mouse colony can be affected by the microbial environment in the animal housing facility [125] or by exposure to microbial stimuli such as injection with mycobacterium or various microbial products [126, 127]. In human, the incidence of type 1 diabetes has increased during the past several decades in developed countries where environmental conditions have dramatically changed [128–131]. This hypothesis suggests that bacterial antigens would be presented by the innate immune system to the T lymphocytes very early in life supporting the notion that immunostimulation can benefit the maturation of the postnatal immune system [132, 133]. Consequently, in case of misrecognition of the bacterial antigen, the adaptive immune system will be exacerbating its aggressiveness against the pancreatic cells and destroy them. Consequently, the recognition of bacterial determinants from intestinal microbiota would be a triggering factor of autoimmune disease. The Toll-like receptors (TLRs) are innate pattern-recognition receptors involved in host defense that control over commensal bacteria and maintain tissue integrity [79, 134]. The corresponding signaling molecule is MyD88 adaptor, therefore mice lacking this molecular mechanisms were protected against insulitis [57]. This is dependent on commensal microbes because germ-free MyD88 knockout mice develop robust diabetes.
The type of intestinal microbiota is important. That from NOD mice is most likely inducing diabetes since the colonization of these mice with a microbiota from non-type 1 diabetic mice prevented the incidence of diabetes in germ-free NOD mice. Therefore, both the quality of the intestinal microbiota and the activation of the innate immune system control the aggressiveness of T lymphocytes and consequently the development of autoimmune diseases. In the Biobreeding rat model of type 1 diabetes, it has been found that the Lactobacillus species present in feces were negatively correlated with type 1 diabetes development [135]. Precisely, two species the L. johnsonii and L. ruteri prevented the effect on type 1 diabetes development [135]. It was suggested that two strains of bacteria induced changes in the intestinal mucosal protein and oxidative stress response leading to low level of IFNγ. Consequently, observations following the administration of antibiotics in type 1 diabetic Biobreeding rat models showed that the occurrence of the disease was reduced, which has strengthened the hypothesis that a specific intestinal microbiota could induce autoimmunity [136].
Altogether engineering intestinal microbiota by the mean of prebiotics, probiotics, and food complement, or by bacterial-derived immunotherapeutic strategies could beneficiate the prevention of type 1 diabetes (Fig. 3).
Fig. 3.
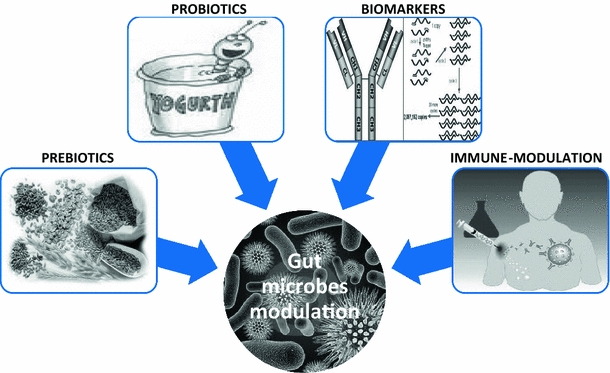
Therapy strategies challenging gut microbes. The discovery of the role of intestinal microbiota on the control of metabolic diseases opens numerous therapeutic strategies such as prebiotics, probiotics, and immune modulation. It also allows the generation of biomarker strategies to set predictive profiles, to classify and to stratify the patients and the corresponding metabolic and cardiovascular diseases
Gut microbiota and periodontitis
Cross-sectional studies suggest a strong association between systemic diseases and oral infection, such as periodontal disease [137–140], which is considered the sixth complication of diabetes mellitus [141, 142]. Periodontitis is a common chronic multifactorial infection characterized by an inflammatory reaction against a specific mouth microbiota. The latter sets a complex biofilm that is mainly composed of Gram-negative bacteria in the subgingival microenvironment [143, 144]. Based on this aspect, Saito et al. [145] hypothesized that the specific correlation between the periopathogens and the development of obesity might also be associated with changes in plasma LPS concentration. Importantly, the endotoxin from the Gram-negative pathogenes could be responsible of remote effects of periodontal disease on systemic health [145]. LPS release in systemic circulation directly impacts organs such as liver, lung, adipose tissue, skeletal muscle, and heart [146]. The challenge of this bacterial antigen at multiple organ sites is able to trigger an inflammatory reaction, resulting in insulin resistance, finally leading to metabolic impairments [38]. With regard to another chronic disease associated with a low-grade inflammation such as atherosclerosis, recent data demonstrate that bacteria from the oral cavity may correlate with disease markers of the vascular disease [107]. By targeting the 16S rRNA V1–V2 region by pyrosequencing and by applying qPCR analysis, data identified shared sequences belonging to the Veillonella and Streptococcus groups of sequences between oral and atherosclerotic plaque samples within the same individuals. Moreover, in this study, principal component analyses suggested that the abundance of Fusobacterium, one of the first colonizers in periodontal plaque, may directly impact the levels of total cholesterol and LDL. This evidence shows the translocation of oral bacterial into systemic circulation. Hence, in light of the above reported results, many studies have indicated that treating periodontal disease by reducing oral bacterial pathogens [147–149] may improve metabolic control in diabetic patients.
The use of Pre-, Pro-, and Synbiotic to prevent or treat metabolic troubles
Facing the epidemic of obesity and diabetes, one needs to recognize that preventive strategies must be used. So far, appropriate feeding habits are only scarcely applied by individuals whereas more and more teenagers are under the influence of fat- and sugar-enriched diet. Hence, in front of this social problem new strategies should be envisioned.
One way would be to rehabilitate our intestinal microbiota by the mean of pre- or pro-biotics. With regard to prebiotic there is now much interest in manipulation of the microbiota composition in order to improve the potentially beneficial aspects. The prebiotic approach dictates that non-viable food components are specifically fermented in the colon by indigenous bacteria thought to be of positive value, e.g., bifidobacteria, lactobacilli [150, 151]. Any food ingredient that enters the large intestine is a candidate prebiotic [152]. Most current attention and success have been derived using non-digestible oligosaccharides derived from fructose, xylose, soya, galactose, glucose, and mannose with different efficacy on metabolic diseases. The mechanisms of action remain unclear but could be related to the regulation of intestinal mucosal biology where the intestinal mucosa was characterized by higher villi, deeper crypts, increased number of goblet cells and a thicker mucus layer on the colonic epithelium [153]. The inhibition of intestinal permeability to agents such as LPS has been proposed recently through a mechanism that might be involving the secretion of enteric peptide such as the glucagon-like peptide 2 [88]. Eventually, intestinal enteroendocrine functions could also be targeted by prebiotics [154]. Fructoligosaccharides increase the production of glucagon-like peptide 1 that could favor insulin secretion and activate the gut-brain axis for the control of glucose metabolism [155].
Other strategy involves the use of bacteria, i.e., probiotic to restore a healthy intestinal microbiota. Several genera are currently used and amongst them the most common are Bifidobacteria and Lactobacilli with different benefit for health. The identified mechanisms of action are numerous [156]. They seem to be at least in part related to the modification of the adhesion of certain bacterial strains to the mucosa [157], the influence of the enteric immune system through the production of IgA [158] or the induction of anti-inflammatory molecules such as Il10 [159–161], or the regulation of intestinal permeability [162]. A recent evidence has clearly demonstrated that a specific strain, i.e., the B. longum is able to reduce inflammation of the intestine by producing high amount of acetate from the fermentation of dietary fibers [163]. Acetate interacts with the enteric immune system to favor the synthesis of regulatory T lymphocytes. Other mechanisms might involve the production of single-bacterial molecules such as the polysaccharide A from Bacteroides fragilis which is required to suppress pro-inflammatory interleukin-17 production by intestinal immune cells [164]. The metabolic consequences of the use of probiotics are numerous. Most of them involve the reduction in body weight [165], non-alcoholic hepatic steatosis [166–168], or glycemia and insulin resistance [169, 170].
To the light of the beneficial effects of pre- and pro-biotics, a new strategy combining both approaches is now raising and defined as “synbiotic.” Understanding the mutualistic connection between gut microbiota and host physiology for the control of metabolism is crucial in the quest for mechanisms which are responsible of the dramatic raise of cardiovascular diseases [2, 3]. In this scenario, very recently a synbiotic strategy has been shown capable of ameliorating the lipid profile of hypercholesterolemic men and women. In this study, patients have been given a combination of Lactobacillus acidophilus CHO-220 and inulin [150, 151] or a placebo, for 12 weeks. Despite the lack of effect on body weight and energy intake, the synbiotic treatment reduced both total and LDL plasma cholesterol via a mechanism involving lipid transporters [39]. Therefore, since triglycerides concentration in lipoproteins is considered as a main risk factors of atherosclerosis, the authors suggested the atheropreventive role of the synbiotic strategy.
Conclusions: therapeutic and preventing present and future avenues
We are now facing a new era during which we will have to understand the role of a new organ rich of more than 3 million genes: gut microbiota. It is doubtless that this metagenome will be the basis of many new therapeutic approaches to treat and prevent metabolic diseases and the corresponding cardiovascular consequences. By understanding this new ecology strategies based on prebiotics, probiotics, even targeted antibiotics could be envisioned. The identification of the eukaryotic genes regulated by gut microbiota will also be considered as new targets against which pharmaceutical companies should be able to design compounds. Eventually, gut microbiota is considered as a large set of antigens and some of them could serve as the basis of immunotherapeutical strategies to prevent or to treat. Diagnostic kits to identify patients at risk or to classify and stratify the diseases will be helpful for the clinician to better adapt the therapeutic strategy.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Pantalone KM, Kattan MW, Yu C, Wells BJ, Arrigain S, Jain A, et al. The risk of developing coronary artery disease or congestive heart failure, and overall mortality, in type 2 diabetic patients receiving rosiglitazone, pioglitazone, metformin, or sulfonylureas: a retrospective analysis. Acta Diabetol. 2009;46(2):145–154. doi: 10.1007/s00592-008-0090-3. [DOI] [PubMed] [Google Scholar]
- 2.Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher-than-optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368(9548):1651–1659. doi: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 3.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 4.WHO . Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. Geneva: WHO; 2004. [Google Scholar]
- 5.Ruchat SM, Elks CE, Loos RJ, Vohl MC, Weisnagel SJ, Rankinen T, et al. Association between insulin secretion, insulin sensitivity and type 2 diabetes susceptibility variants identified in genome-wide association studies. Acta Diabetol. 2009;46(3):217–226. doi: 10.1007/s00592-008-0080-5. [DOI] [PubMed] [Google Scholar]
- 6.Amann RI, Zarda B, Stahl DA, Schleifer KH. Identification of individual prokaryotic cells by using enzyme-labeled, rRNA-targeted oligonucleotide probes. Appl Environ Microbiol. 1992;58(9):3007–3011. doi: 10.1128/aem.58.9.3007-3011.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodman AL, Kallstrom G, Faith JJ, Reyes A, Moore A, Dantas G, et al. Extensive personal human gut microbiota culture collections characterized and manipulated in gnotobiotic mice. Proc Natl Acad Sci USA. 2011;108(15):6252–6257. doi: 10.1073/pnas.1102938108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knights D, Costello E, Knight R. Supervised classication of human microbiota. FEMS Microbiol Rev. 2010;35:343–359. doi: 10.1111/j.1574-6976.2010.00251.x. [DOI] [PubMed] [Google Scholar]
- 9.Dethlefsen L, Eckburg PB, Bik EM, Relman DA. Assembly of the human intestinal microbiota. Trends Ecol Evol. 2006;21(9):517–523. doi: 10.1016/j.tree.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 10.Dethlefsen L, McFall-Ngai M, Relman DA. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature. 2007;449(7164):811–818. doi: 10.1038/nature06245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 14.Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002;68(1):219–226. doi: 10.1128/AEM.68.1.219-226.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer C, Bik EM, DiGiulio DB, Relman DA, Brown PO. Development of the human infant intestinal microbiota. PLoS Biol. 2007;5(7):e177. doi: 10.1371/journal.pbio.0050177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoetendal EG, Rajilic-Stojanovic M, de Vos WM. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut. 2008;57(11):1605–1615. doi: 10.1136/gut.2007.133603. [DOI] [PubMed] [Google Scholar]
- 17.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, de Weerd H, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4586–4591. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zoetendal EG, Akkermans AD, De Vos WM. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl Environ Microbiol. 1998;64(10):3854–3859. doi: 10.1128/aem.64.10.3854-3859.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tap J, Mondot S, Levenez F, Pelletier E, Caron C, Furet JP, et al. Towards the human intestinal microbiota phylogenetic core. Environ Microbiol. 2009;11(10):2574–2584. doi: 10.1111/j.1462-2920.2009.01982.x. [DOI] [PubMed] [Google Scholar]
- 20.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124(4):837–848. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Wells JM, Rossi O, Meijerink M, van Baarlen P. Epithelial crosstalk at the microbiota-mucosal interface. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4607–4614. doi: 10.1073/pnas.1000092107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci USA. 2008;105(39):15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277–2284. doi: 10.2337/dc10-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asquith MT, Harrod JR. Reduction of bacterial contamination in banked human milk. J Pediatr. 1979;95(6):993–994. doi: 10.1016/S0022-3476(79)80291-7. [DOI] [PubMed] [Google Scholar]
- 25.Rotimi VO, Olowe SA, Ahmed I. The development of bacterial flora of premature neonates. J Hyg (Lond) 1985;94(3):309–318. doi: 10.1017/S0022172400061532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalliomaki M, Collado MC, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87(3):534–538. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 27.Manichanh C, Reeder J, Gibert P, Varela E, Llopis M, Antolin M, et al. Reshaping the gut microbiome with bacterial transplantation and antibiotic intake. Genome Res. 2010;20(10):1411–1419. doi: 10.1101/gr.107987.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science. 2010;328(5986):1705–1709. doi: 10.1126/science.1188454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28(6):740–750. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cerutti A. Immunology. IgA changes the rules of memory. Science. 2010;328(5986):1646–1647. doi: 10.1126/science.1192488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 33.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Serino M, Luche E, Chabo C, Amar J, Burcelin R. Intestinal microflora and metabolic diseases. Diabetes Metab. 2009;35(4):262–272. doi: 10.1016/j.diabet.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008;320(5883):1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenburg ED, Zheng H, Joglekar P, Higginbottom SK, Firbank SJ, Bolam DN, et al. Specificity of polysaccharide use in intestinal Bacteroides species determines diet-induced microbiota alterations. Cell. 2010;141(7):1241–1252. doi: 10.1016/j.cell.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shoelson S, Lee J, Goldfine A. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 39.Ooi LG, Ahmad R, Yuen KH, Liong MT. Lactobacillus acidophilus CHO-220 and inulin reduced plasma total cholesterol and low-density lipoprotein cholesterol via alteration of lipid transporters. J Dairy Sci. 2010;93(11):5048–5058. doi: 10.3168/jds.2010-3311. [DOI] [PubMed] [Google Scholar]
- 40.Ley R, Hamady M, Lozupone C, Turnbaugh P, Ramey R, Bircher J, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 42.Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101(44):15718–15723. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102(31):11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baker GC, Smith JJ, Cowan DA. Review and re-analysis of domain-specific 16S primers. J Microbiol Methods. 2003;55(3):541–555. doi: 10.1016/j.mimet.2003.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Neefs JM, Van de Peer Y, De Rijk P, Chapelle S, De Wachter R. Compilation of small ribosomal subunit RNA structures. Nucleic Acids Res. 1993;21(13):3025–3049. doi: 10.1093/nar/21.13.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang Q, Garrity G, Tiedje J, Cole J. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Mering C, Jensen LJ, Snel B, Hooper SD, Krupp M, Foglierini M, et al. STRING: known and predicted protein–protein associations, integrated and transferred across organisms. Nucleic Acids Res. 2005;33(Database issue):D433–D437. doi: 10.1093/nar/gki005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32(Database issue):D277–D280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 50.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444(7122):1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 51.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–484. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Caporaso JG, Lauber CL, Walters WA, Berg-Lyons D, Lozupone CA, Turnbaugh PJ, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buchwald H, Avidor Y, Braunwald E, Jensen MD, Pories W, Fahrbach K, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292(14):1724–1737. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 54.Bjorneklett A, Viddal K, Midtvedt T, Nygaard K. Intestinal and gastric bypass. Changes in intestinal microecology after surgical treatment of morbid obesity in man. Scand J Gastroenterol. 1981;16:681–687. doi: 10.3109/00365528109182030. [DOI] [PubMed] [Google Scholar]
- 55.Nishizawa Y, Imaizumi T, Tanishita H, Yano I, Kawai Y, Mormii H. Relationship of fat deposition and intestinal microflora in VMH rats. Int J Obes. 1988;12(2):103–110. [PubMed] [Google Scholar]
- 56.Heijtz RD, Wang S, Anuar F, Qian Y, Bjorkholm B, Samuelsson A, et al. Normal gut microbiota modulates brain development and behavior. Proc Natl Acad Sci USA. 2011;108(7):3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wen L, Ley R, Volchkov P, Stranges P, Avanesyan L, Stonebraker A, et al. Innate immunity and intestinal microbiota in the development of type 1 diabetes. Nature. 2008;455(7216):1109–1113. doi: 10.1038/nature07336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hooper LV, Stappenbeck TS, Hong CV, Gordon JI. Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat Immunol. 2003;4(3):269–273. doi: 10.1038/ni888. [DOI] [PubMed] [Google Scholar]
- 59.Hooper LV, Gordon JI. Commensal host-bacterial relationships in the gut. Science. 2001;292(5519):1115–1118. doi: 10.1126/science.1058709. [DOI] [PubMed] [Google Scholar]
- 60.Bry L, Falk PG, Midtvedt T, Gordon JI. A model of host-microbial interactions in an open mammalian ecosystem. Science. 1996;273(5280):1380–1383. doi: 10.1126/science.273.5280.1380. [DOI] [PubMed] [Google Scholar]
- 61.Schenk M, Mueller C. The mucosal immune system at the gastrointestinal barrier. Best Pract Res Clin Gastroenterol. 2008;22(3):391–409. doi: 10.1016/j.bpg.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 62.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41(10):1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 63.Cani PD, Delzenne NM, Amar J, Burcelin R. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 2008;56(5):305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 64.Turnbaugh P, Ridaura V, Faith J, Rey F, Knight R, Gordon J. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6–14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 66.Bukoff M, Carlson S. Diet modifications and behavioral changes for bariatric gastric surgery. J Am Diet Assoc. 1981;78(2):158–161. [PubMed] [Google Scholar]
- 67.Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes. 1999;48(1):86–93. doi: 10.2337/diabetes.48.1.86. [DOI] [PubMed] [Google Scholar]
- 68.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–3057. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106(7):2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Calvani R, Miccheli A, Capuani G, Tomassini Miccheli A, Puccetti C, Delfini M, et al. Gut microbiome-derived metabolites characterize a peculiar obese urinary metabotype. Int J Obes (Lond) 2010;34(6):1095–1098. doi: 10.1038/ijo.2010.44. [DOI] [PubMed] [Google Scholar]
- 71.Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA. 2007;104(3):979–984. doi: 10.1073/pnas.0605374104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dumas ME, Barton RH, Toye A, Cloarec O, Blancher C, Rothwell A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin-resistant mice. Proc Natl Acad Sci USA. 2006;103(33):12511–12516. doi: 10.1073/pnas.0601056103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spencer M, Hamp T, Reid R, Fischer L, Zeisel S, Fodor A. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;140(3):976–986. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, et al. Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut. 2010;59(12):1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 75.Caspar-Bauguil S, Cousin B, Galinier A, Segafredo C, Nibbelink M, Andre M, et al. Adipose tissues as an ancestral immune organ: site-specific change in obesity. FEBS Lett. 2005;579(17):3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 76.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112(12):1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15(8):921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Fritz JH, Le Bourhis L, Sellge G, Magalhaes JG, Fsihi H, Kufer TA, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26(4):445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 81.Kufer TA, Sansonetti PJ. Sensing of bacteria: NOD a lonely job. Curr Opin Microbiol. 2007;10(1):62–69. doi: 10.1016/j.mib.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 82.Amar J, Burcelin R, Ruidavets J, Cani P, Fauvel J, Alessi M, et al. Energy intake is associated with endotoxemia in apparently healthy men. Am J Clin Nutr. 2008;87:1219–1223. doi: 10.1093/ajcn/87.5.1219. [DOI] [PubMed] [Google Scholar]
- 83.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, et al. C3H/HeJ mice carrying a toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50(6):1267–1276. doi: 10.1007/s00125-007-0654-8. [DOI] [PubMed] [Google Scholar]
- 84.Song MJ, Kim KH, Yoon JM, Kim JB. Activation of toll-like receptor 4 is associated with insulin resistance in adipocytes. Biochem Biophys Res Commun. 2006;346(3):739–745. doi: 10.1016/j.bbrc.2006.05.170. [DOI] [PubMed] [Google Scholar]
- 85.Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5(8):e12191. doi: 10.1371/journal.pone.0012191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Membrez M, Blancher F, Jaquet M, Bibiloni R, Cani PD, Burcelin RG, et al. Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice. Faseb J. 2008;22(7):2416–2426. doi: 10.1096/fj.07-102723. [DOI] [PubMed] [Google Scholar]
- 87.Laugerette F, Vors C, Geloen A, Chauvin MA, Soulage C, Lambert-Porcheron S et al (2010) Emulsified lipids increase endotoxemia: possible role in early postprandial low-grade inflammation. J Nutr Biochem 22(1):53–59 [DOI] [PubMed]
- 88.Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O et al (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58(8):1091–1103 [DOI] [PMC free article] [PubMed]
- 89.Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, et al. Metabolic syndrome and altered gut microbiota in mice lacking toll-like receptor 5. Science. 2010;328(5975):228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Black DD, Tso P, Weidman S, Sabesin SM. Intestinal lipoproteins in the rat with D-(+)-galactosamine hepatitis. J Lipid Res. 1983;24(8):977–992. [PubMed] [Google Scholar]
- 91.Pappo I, Becovier H, Berry EM, Freund HR. Polymyxin B reduces cecal flora, TNF production and hepatic steatosis during total parenteral nutrition in the rat. J Surg Res. 1991;51(2):106–112. doi: 10.1016/0022-4804(91)90078-Z. [DOI] [PubMed] [Google Scholar]
- 92.Harris HW, Brady SE, Rapp JH. Hepatic endosomal trafficking of lipoprotein-bound endotoxin in rats. J Surg Res. 2002;106(1):188–195. doi: 10.1006/jsre.2002.6413. [DOI] [PubMed] [Google Scholar]
- 93.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res. 2009;50(1):90–97. doi: 10.1194/jlr.M800156-JLR200. [DOI] [PubMed] [Google Scholar]
- 94.Rensen PC, Oosten M, Bilt E, Eck M, Kuiper J, Berkel TJ. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats in vivo. J Clin Invest. 1997;99(10):2438–2445. doi: 10.1172/JCI119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kumwenda ZL, Wong CB, Johnson JA, Gosnell JE, Welch WJ, Harris HW. Chylomicron-bound endotoxin selectively inhibits NF-kappaB activation in rat hepatocytes. Shock. 2002;18(2):182–188. doi: 10.1097/00024382-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 96.Westerterp M, Berbee JF, Pires NM, van Mierlo GJ, Kleemann R, Romijn JA, et al. Apolipoprotein C-I is crucially involved in lipopolysaccharide-induced atherosclerosis development in apolipoprotein E-knockout mice. Circulation. 2007;116(19):2173–2181. doi: 10.1161/CIRCULATIONAHA.107.693382. [DOI] [PubMed] [Google Scholar]
- 97.Thabut D, Tazi KA, Bonnefont-Rousselot D, Aller M, Farges O, Guimont MC, et al. High-density lipoprotein administration attenuates liver proinflammatory response, restores liver endothelial nitric oxide synthase activity, and lowers portal pressure in cirrhotic rats. Hepatology. 2007;46(6):1893–1906. doi: 10.1002/hep.21875. [DOI] [PubMed] [Google Scholar]
- 98.Vreugdenhil AC, Rousseau CH, Hartung T, Greve JW, van ‘t Veer C, Buurman WA. Lipopolysaccharide (LPS)-binding protein mediates LPS detoxification by chylomicrons. J Immunol. 2003;170(3):1399–1405. doi: 10.4049/jimmunol.170.3.1399. [DOI] [PubMed] [Google Scholar]
- 99.Velagapudi VR, Hezaveh R, Reigstad CS, Gopalacharyulu P, Yetukuri L, Islam S, et al. The gut microbiota modulates host energy and lipid metabolism in mice. J Lipid Res. 2010;51(5):1101–1112. doi: 10.1194/jlr.M002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gustafsson BE, Einarsson K, Gustafsson J. Influence of cholesterol feeding on liver microsomal metabolism of steroids and bile acids in conventional and germ-free rats. J Biol Chem. 1975;250(21):8496–8502. [PubMed] [Google Scholar]
- 101.Gerard P, Lepercq P, Leclerc M, Gavini F, Raibaud P, Juste C. Bacteroides sp. strain D8, the first cholesterol-reducing bacterium isolated from human feces. Appl Environ Microbiol. 2007;73:5742–5749. doi: 10.1128/AEM.02806-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gustafsson B, Swenander Lanke L (1960) Bilirubin and urobilins in germfree, ex-germfree, and conventional rats. J Exp Med 112:975–981 [DOI] [PMC free article] [PubMed]
- 103.Oresic M, Seppanen-Laakso T, Yetukuri L, Backhed F, Hanninen V. Gut microbiota affects lens and retinal lipid composition. Exp Eye Res. 2009;89(5):604–607. doi: 10.1016/j.exer.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Caesar R, Fak F, Backhed F. Effects of gut microbiota on obesity and atherosclerosis via modulation of inflammation and lipid metabolism. J Intern Med. 2010;268(4):320–328. doi: 10.1111/j.1365-2796.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 105.Epstein S, Zhu J, Burnett M, Zhou Y, Vercellotti G, Hajjar D. Infectionand atherosclerosis: potential roles of pathogen burden and molecular mimicry. Arterioscler Thromb Vasc Biol. 2000;20:1417–1420. doi: 10.1161/01.ATV.20.6.1417. [DOI] [PubMed] [Google Scholar]
- 106.Ott S, El Mokhtari N, Musfeldt M. Detection of diverse bacterial signatures in atherosclerotic lesions of patients with coronary heart disease. Circulation. 2006;113:929–937. doi: 10.1161/CIRCULATIONAHA.105.579979. [DOI] [PubMed] [Google Scholar]
- 107.Koren O, Spor A, Felin J, Fak F, Stombaugh J, Tremaroli V, et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4592–4598. doi: 10.1073/pnas.1011383107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maziere C, Conte MA, Dantin F, Maziere JC. Lipopolysaccharide enhances oxidative modification of low density lipoprotein by copper ions, endothelial and smooth muscle cells. Atherosclerosis. 1999;143(1):75–80. doi: 10.1016/S0021-9150(98)00277-9. [DOI] [PubMed] [Google Scholar]
- 109.Wiedermann CJ, Kiechl S, Dunzendorfer S, Schratzberger P, Egger G, Oberhollenzer F, et al. Association of endotoxemia with carotid atherosclerosis and cardiovascular disease: prospective results from the bruneck study. J Am Coll Cardiol. 1999;34(7):1975–1981. doi: 10.1016/S0735-1097(99)00448-9. [DOI] [PubMed] [Google Scholar]
- 110.Amar J, Ruidavets JB, Bal Dit Sollier C, Bongard V, Boccalon H, Chamontin B, et al. Soluble CD14 and aortic stiffness in a population-based study. J Hypertens. 2003;21(10):1869–1877. doi: 10.1097/00004872-200310000-00014. [DOI] [PubMed] [Google Scholar]
- 111.Andraws R, Berger JS, Brown DL. Effects of antibiotic therapy on outcomes of patients with coronary artery disease: ameta-analysis of randomized controlled trials. JAMA. 2005;293:2641–2647. doi: 10.1001/jama.293.21.2641. [DOI] [PubMed] [Google Scholar]
- 112.Kocaman SA, Sahinarslan A, Akyel A, Timurkaynak T, Boyaci B, Cengel A. The association of circulating monocyte count with coronary collateral growth in patients with diabetes mellitus. Acta Diabetol. 2010;47(1):49–54. doi: 10.1007/s00592-009-0097-4. [DOI] [PubMed] [Google Scholar]
- 113.Dalmasso G, Nguyen HT, Yan Y, Laroui H, Charania MA, Ayyadurai S, et al. Microbiota modulate host gene expression via microRNAs. PLoS One. 2011;6(4):e19293. doi: 10.1371/journal.pone.0019293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120(15):1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 115.Ferre P, Foufelle F (2010) Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab Suppl 2:83–92 [DOI] [PubMed]
- 116.Postic C, Girard J. The role of the lipogenic pathway in the development of hepatic steatosis. Diabetes Metab. 2008;34(6 Pt 2):643–648. doi: 10.1016/S1262-3636(08)74599-3. [DOI] [PubMed] [Google Scholar]
- 117.Day CP. Genetic and environmental susceptibility to non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):255–260. doi: 10.1159/000282098. [DOI] [PubMed] [Google Scholar]
- 118.Han DW. Intestinal endotoxemia as a pathogenetic mechanism in liver failure. World J Gastroenterol. 2002;8(6):961–965. doi: 10.3748/wjg.v8.i6.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang HY, de Han W, Su AR, Zhang LT, Zhao ZF, Ji JQ, et al. Intestinal endotoxemia plays a central role in development of hepatopulmonary syndrome in a cirrhotic rat model induced by multiple pathogenic factors. World J Gastroenterol. 2007;13(47):6385–6395. doi: 10.3748/wjg.13.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology. 2003;37(5):1043–1055. doi: 10.1053/jhep.2003.50182. [DOI] [PubMed] [Google Scholar]
- 121.Eisenbarth G. Type I diabetes mellitus: a chronic auto-immune disease? N Engl J Med. 1986;314:1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- 122.Assan R, Feutren G, Sirmai J, Laborie C, Boitard C, Vexiau P, et al. Plasma C-peptide levels and clinical remissions in recent-onset type I diabetic patients treated with cyclosporin A and insulin. Diabetes. 1990;39:768–774. doi: 10.2337/diabetes.39.7.768. [DOI] [PubMed] [Google Scholar]
- 123.Burcelin RG, Eddouks M, Beylot M, Normand S, Boitard C, Feutren G, et al. Hypersensitivity to insulin during remissions in cyclosporin-treated IDDM patients. Diabetes Care. 1993;16(6):881–888. doi: 10.2337/diacare.16.6.881. [DOI] [PubMed] [Google Scholar]
- 124.King C, Sarvetnick N. The incidence of type-1 diabetes in NOD mice is modulated by restricted flora not germ-free conditions. PLoS One. 2011;6(2):e17049. doi: 10.1371/journal.pone.0017049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pozzilli P, Signore A, Williams AJ, Beales PE. NOD mouse colonies around the world—recent facts and figures. Immunol Today. 1993;14(5):193–196. doi: 10.1016/0167-5699(93)90160-M. [DOI] [PubMed] [Google Scholar]
- 126.McInerney MF, Pek SB, Thomas DW. Prevention of insulitis and diabetes onset by treatment with complete Freund’s adjuvant in NOD mice. Diabetes. 1991;40(6):715–725. doi: 10.2337/diabetes.40.6.715. [DOI] [PubMed] [Google Scholar]
- 127.Sadelain MW, Qin HY, Lauzon J, Singh B. Prevention of type I diabetes in NOD mice by adjuvant immunotherapy. Diabetes. 1990;39(5):583–589. doi: 10.2337/diabetes.39.5.583. [DOI] [PubMed] [Google Scholar]
- 128.Karvonen M, Tuomilehto J, Libman I, LaPorte R (1993) A review of the recent epidemiological data on the worldwide incidence of type 1 (insulin-dependent) diabetes mellitus. World Health Organization DIAMOND Project Group. Diabetologia 36(10):883–892 [DOI] [PubMed]
- 129.Patterson CC, Dahlquist G, Soltesz G, Green A. Is childhood-onset type I diabetes a wealth-related disease? An ecological analysis of European incidence rates. Diabetologia. 2001;44(Suppl 3):B9–B16. doi: 10.1007/PL00002961. [DOI] [PubMed] [Google Scholar]
- 130.Levy-Marchal C, Patterson CC, Green A. Geographical variation of presentation at diagnosis of type I diabetes in children: the EURODIAB study. European and dibetes. Diabetologia. 2001;44(Suppl 3):B75–B80. doi: 10.1007/PL00002958. [DOI] [PubMed] [Google Scholar]
- 131.Green A, Patterson CC. Trends in the incidence of childhood-onset diabetes in Europe 1989–1998. Diabetologia. 2001;44(Suppl 3):B3–B8. doi: 10.1007/PL00002950. [DOI] [PubMed] [Google Scholar]
- 132.Holt PG, Somerville C, Baron-Hay MJ, Holt BJ, Sly PD. Functional assessment of CD2, CD3 and CD28 on the surface of peripheral blood T-cells from infants at low versus high genetic risk for atopy. Pediatr Allergy Immunol. 1995;6(2):80–84. doi: 10.1111/j.1399-3038.1995.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 133.Holt PG. Postnatal maturation of immune competence during infancy and childhood. Pediatr Allergy Immunol. 1995;6(2):59–70. doi: 10.1111/j.1399-3038.1995.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 134.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 135.Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, et al. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One. 2010;5(5):e10507. doi: 10.1371/journal.pone.0010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brugman S, Klatter FA, Visser JT, Wildeboer-Veloo AC, Harmsen HJ, Rozing J, et al. Antibiotic treatment partially protects against type 1 diabetes in the bio-breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia. 2006;49(9):2105–2108. doi: 10.1007/s00125-006-0334-0. [DOI] [PubMed] [Google Scholar]
- 137.Pischon T, Hu F, Rexrode K, Girman C, Manson J, Rimm E. Inflammation, the metabolic syndrome, and risk of coronary heart disease in women and men. Atherosclerosis. 2008;197:392–399. doi: 10.1016/j.atherosclerosis.2007.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Firatli E. The relationship between clinical periodontal status and insulin-dependent diabetes mellitus. Results after 5 years. J Periodontol. 1997;68:136–140. doi: 10.1902/jop.1997.68.2.136. [DOI] [PubMed] [Google Scholar]
- 139.D’Aiuto F, Sabbah W, Netuveli G, Donos N, Hingorani A, Deanfield J. Association of the metabolic syndrome with severe periodontitis in a large US population-based survey. J Clin Endocrinol Metab. 2008;93:3989–3994. doi: 10.1210/jc.2007-2522. [DOI] [PubMed] [Google Scholar]
- 140.Vergnes JN, Kaminski M, Lelong N, Musset AM, Sixou M, Nabet C (2011) Maternal dental caries and pre-term birth: results from the EPIPAP study. Acta Odontol Scand 69(4):248–256 [DOI] [PubMed]
- 141.Löe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–334. [PubMed] [Google Scholar]
- 142.Soell M, Hassan M, Miliauskaite A, Haïkel Y, Selimovic D. The oral cavity of elderly patients in diabetes. Diabetes Metab. 2007;33(Suppl 1):S10–S18. doi: 10.1016/S1262-3636(07)80053-X. [DOI] [PubMed] [Google Scholar]
- 143.Slots J. Rapid identification of important periodontal microorganisms by cultivation. Oral Microbiol Immunol. 1986;1:48–57. doi: 10.1111/j.1399-302X.1986.tb00318.x. [DOI] [PubMed] [Google Scholar]
- 144.Doan N, Contreras A, Flynn J, Morrison J, Slots J. Proficiencies of three anaerobic culture systems for recovering periodontal pathogenic bacteria. J Clin Microbiol. 1999;37:171–174. doi: 10.1128/jcm.37.1.171-174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saito T, Shimazaki Y. Metabolic disorders related to obesity and periodontal disease. Periodontology. 2007;43:254–266. doi: 10.1111/j.1600-0757.2006.00186.x. [DOI] [PubMed] [Google Scholar]
- 146.Hettne K, Weeber M, Laine M, Ten Cate H, Boyer S, Kors J. Automatic mining of the literature to generate new hypotheses for the possible link between periodontitis and atherosclerosis: lipopolysaccharide as a case study. J Clin Periodontol. 2007;34:1016–1024. doi: 10.1111/j.1600-051X.2007.01152.x. [DOI] [PubMed] [Google Scholar]
- 147.Darré L, Vergnes J-N, Gourdy P, Sixou M. Efficacy of periodontal treatment on glycaemic control in diabetic patients: a meta-analysis of interventional studies. Diabetes Metab. 2008;34:497–506. doi: 10.1016/j.diabet.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 148.Grossi S. Treatment of periodontal disease and control of diabetes: an assessment of the evidence and need for future research. Ann Periodontol. 2001;6:138–145. doi: 10.1902/annals.2001.6.1.138. [DOI] [PubMed] [Google Scholar]
- 149.Miller L, Manwell M, Newbold D, Reding M, Rasheed A, Blodgett J. The relationship between reduction in periodontal inflammation and diabetes control: a report of 9 cases. J Periodontol. 1992;63:843–848. doi: 10.1902/jop.1992.63.10.843. [DOI] [PubMed] [Google Scholar]
- 150.Roberfroid M. Prebiotics: the concept revisited. J Nutr. 2007;137(3 Suppl 2):830S–837S. doi: 10.1093/jn/137.3.830S. [DOI] [PubMed] [Google Scholar]
- 151.Roberfroid MB. Inulin-type fructans: functional food ingredients. J Nutr. 2007;137(11 Suppl):2493S–2502S. doi: 10.1093/jn/137.11.2493S. [DOI] [PubMed] [Google Scholar]
- 152.Gibson GR. Dietary modulation of the human gut microflora using prebiotics. Br J Nutr. 1998;80(4):S209–S212. [PubMed] [Google Scholar]
- 153.Guarner F. Studies with inulin-type fructans on intestinal infections, permeability, and inflammation. J Nutr. 2007;137:2568S–2571S. doi: 10.1093/jn/137.11.2568S. [DOI] [PubMed] [Google Scholar]
- 154.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: involvement of glucagon-like peptide-1. Obes Res. 2005;13(6):1000–1007. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 155.Knauf C, Cani PD, Kim DH, Iglesias MA, Chabo C, Waget A, et al. Role of central nervous system glucagon-like peptide-1 receptors in enteric glucose sensing. Diabetes. 2008;57(10):2603–2612. doi: 10.2337/db07-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23(6):679–692. doi: 10.1097/MOG.0b013e3282f0cffc. [DOI] [PubMed] [Google Scholar]
- 157.Collado MC, Meriluoto J, Salminen S. Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol. 2007;45(4):454–460. doi: 10.1111/j.1472-765X.2007.02212.x. [DOI] [PubMed] [Google Scholar]
- 158.Fukushima Y, Kawata Y, Mizumachi K, Kurisaki J, Mitsuoka T. Effect of bifidobacteria feeding on fecal flora and production of immunoglobulins in lactating mouse. Int J Food Microbiol. 1999;46(3):193–197. doi: 10.1016/S0168-1605(98)00183-4. [DOI] [PubMed] [Google Scholar]
- 159.McCarthy J, O’Mahony L, O’Callaghan L, Sheil B, Vaughan EE, Fitzsimons N, et al. Double blind, placebo controlled trial of two probiotic strains in interleukin 10 knockout mice and mechanistic link with cytokine balance. Gut. 2003;52(7):975–980. doi: 10.1136/gut.52.7.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Menard S, Candalh C, Bambou JC, Terpend K, Cerf-Bensussan N, Heyman M. Lactic acid bacteria secrete metabolites retaining anti-inflammatory properties after intestinal transport. Gut. 2004;53(6):821–828. doi: 10.1136/gut.2003.026252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Osman N, Adawi D, Molin G, Ahrne S, Berggren A, Jeppsson B. Bifidobacterium infantis strains with and without a combination of oligofructose and inulin (OFI) attenuate inflammation in DSS-induced colitis in rats. BMC Gastroenterol. 2006;6:31. doi: 10.1186/1471-230X-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Trevisi P, De Filippi S, Minieri L, Mazzoni M, Modesto M, Biavati B et al (2008) Effect of fructo-oligosaccharides and different doses of Bifidobacterium animalis in a weaning diet on bacterial translocation and toll-like receptor gene expression in pigs. Nutrition 24(10):1023–1029 [DOI] [PubMed]
- 163.Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543–547. doi: 10.1038/nature09646. [DOI] [PubMed] [Google Scholar]
- 164.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 165.Kondo S, Xiao J, Satoh T, Odamaki T, Takahashi S, Sugahara H, et al. Antiobesity effects of bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74(8):1656–1661. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 166.Velayudham A, Dolganiuc A, Ellis M, Petrasek J, Kodys K, Mandrekar P, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–997. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Medina J, Fernandez-salazar L, Garcia-Buey L, Moreno-Otero R. Approach to the pathogenesis and treatment of nonalcoholic steatohepatitis. Diabetes Care. 2004;27:2057–2066. doi: 10.2337/diacare.27.8.2057. [DOI] [PubMed] [Google Scholar]
- 168.Esposito E, Iacono A, Bianco G, Autore G, Cuzzocrea S, Vajro P, et al. Probiotics reduce the inflammatory response induced by a high-fat diet in the liver of young rats. J Nutr. 2009;139:905–911. doi: 10.3945/jn.108.101808. [DOI] [PubMed] [Google Scholar]
- 169.Andreasen A, Larsen N, Pedersen-Skovsgaard M, Møller M, Svendsen K, Jakobsen M et al (2010) Effects of Lactobacillus acidophilus NCFM on insulin sensitivity and the systemic inflammatory response in human subjects. Br J Nutr 104(12):1831–1838 [DOI] [PubMed]
- 170.Laitinen K, Poussa T, Isolauri E, Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group (2009) Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br J Nutr 101:1679–1687 [DOI] [PubMed]


