The work which led up to the investigation described in this paper was concerned with the influence of adrenaline on the action of acetylcholine or on tissue functions normally elicited by acetylcholine. Previously experiments were carried out on animals with a normal circulation or on perfused organs, and any adrenaline effects observed were accompanied by vasoconstriction, so that it was conceivable that its action was due to vascular changes.
The improvement in the transmission of impulses along the motor nerve (Bülbring and Burn, 1939) or the lowering of threshold to submaximal stimuli applied to the sciatic nerve (Bülbring and Whitteridge, 1941) by injecting adrenaline into the circulation might have been due to changes in the distribution of blood in the vascular bed of the nerve trunk leading to an alteration of electrical resistance. However, there was a discrepancy in time relations, the increased nervous excitability lagging behind and long outlasting the vascular effect of adrenaline.
In fatigued skeletal muscle adrenaline—and other vasoconstrictor substances—augment muscle contractions (Bülbring and Burn, 1940), but Maibach (1928) and Corkill and Tiegs (1933) obtained the same result on frog muscle suspended in a bath, showing thereby that the action of adrenaline on the fatigued nerve muscle preparation can be observed without its vascular action. Presumably, in fatigue, neuromuscular transmission gradually fails and is restored by adrenaline. When, for instance, Bülbring and Whitteridge (1941) recorded muscle tension and nerve action potentials, a striking absence of parallelism between the effect of adrenaline on muscle and nerve was observed. At a time when no effect of adrenaline on the nerve response to maximal shocks at 1 per sec. could be seen, 100 per cent increase was seen in the fatigued muscle.
In non-fatigued skeletal muscle stimulated indirectly with maximal shocks, it was found that adrenaline augmented the effect of prostigmine. The results were taken to indicate that adrenaline facilitates neuromuscular transmission and increases the action of acetylcholine on the muscle (Bülbring and Burn, 1942). Though the dose of adrenaline used was always the same, producing a similar degree of vasoconstriction each time, its effect on the muscle was dependent not only on the presence of prostigmine and on the amount of prostigmine given, but also and chiefly on the rate of stimulation, i.e. on the rate of accumulation of acetylcholine produced at the motor ending. Nevertheless, vascular changes along the nerve might have occurred and it seemed desirable to repeat the experiments on an isolated mammalian nerve-muscle preparation in which the vascular action of adrenaline is without effect.
Dale and Gaddum (1930) described experiments on the denervated kitten's diaphragm suspended in a bath. Therefore an attempt was made to dissect a slip of kitten's diaphragm with the phrenic nerve attached, to suspend it in an isolated organ bath and record the contractions to nerve stimulation. But even with single shocks at a slow rate (5 per min.) the muscle twitches slowly declined, the oxygenation presumably being insufficient. The thinner muscle of a rat's diaphragm was, however, found to work satisfactorily and was used for all the experiments to be described.
Method
Adult rats were used. The rat was killed and bled out. After removing the skin over the chest, the thorax was opened along the right side of the sternum and the frontal part of the right thoracic wall was removed. The mediastinum behind the sternum was severed and a cut was made just above the frontal insertion of the diaphragm, taking care not to damage the phrenic nerve, which sometimes attaches itself to the ribs. The frontal part of the left thoracic wall was then removed and the phrenic nerve was seen quite distinctly. Both left lobes of the lung were removed. The left abdominal muscles were cut along the costal margin and the last rib was held with a pair of forceps. A strip of diaphragm was now cut out. Two converging cuts were made through the ribs towards the tendinous part of the diaphragm parallel to its muscle fibres, 3 mm. to the right and 3 mm. to the left of the point where the phrenic nerve enters the diaphragm. The strip was cut out beyond the tendinous part with about 2.5 cm. of phrenic nerve attached to it. The preparation had a fan-like shape, being 3 mm. wide at the tendinous end, while at the costal margin it was about 12 mm. wide. The preparation was fixed by a glass rod with a pin pushed through an intercostal space holding it at the bottom of the organ bath. A thread tied around the tendinous end was attached to a light isotonic heart lever writing on a smoked drum. The nerve was stimulated by a pair of electrodes of the following design (see Fig. 1). Two platinum plates were enclosed in a small block of perspex with wires running up in a perspex handle 6 cm. long. The platinum plates were 1 mm. thick and 3 mm. square, they were lying parallel in the perspex block, 5 mm. square and 6 mm. thick, in such a way that they were separated by 2 mm. perspex. A hole about 1 mm. wide was drilled centrally through this block containing the plates to take the phrenic nerve which thus passed through a tube of perspex with two platinum rings. This arrangement ensured a good contact for the electrical stimulation and also provided a moist chamber for the nerve, preventing it from drying up. The electrodes were fixed vertically just touching the surface of the solution inside the bath. After the phrenic nerve had been pulled up through the hole a moist piece of cotton wool was placed on top. Single shocks were applied to the nerve from a neon lamp circuit at rates varying from 5 to 50 per min. Constant muscle contractions could be obtained for many hours in response to single shocks at rates not exceeding 12 per min. With faster rates fatigue set in if they were applied for prolonged periods. A tetanus was not maintained by this isolated preparation.
Fig. 1.
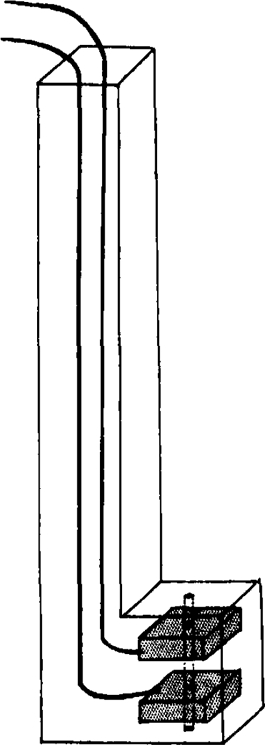
Perspex electrodes.
Some experiments were performed using fluid electrodes (Collison, 1933) in which the nerve was stimulated in a chamber sealed off with wax at the bottom so that the fluid surrounding the nerve at the point of stimulation was not in contact with the fluid surrounding the muscle.
Tyrode's solution containing double the usual amount of dextrose was used. A sintered glass gas-distribution-tube was fixed at the bottom of the bath providing vigorous oxygenation with a mixture of 95 per cent O2 + 5 per cent CO2. The capacity of the bath was 100 c.c. and the temperature was kept between 36° and 37° C.
Results
1. The Action of Adrenaline
When the phrenic nerve was stimulated with single maximal shocks a series of muscle contractions of equal height was obtained. The tension developed in different preparations varied from 10–25 g. It remained constant for several hours if the rate of stimulation did not exceed 12 per min. With faster rates the muscle slowly became fatigued. While the muscle contracted maximally and showed no sign of fatigue, the addition of adrenaline to the bath had no effect on the size of contractions. However, when the phrenic nerve was stimulated with submaximal shocks, the muscle contractions were slightly increased by the addition of 10 μg.–50 μg. adrenaline to the bath. This increase never exceeded 20 per cent. In Fig. 2a is shown a record of maximal contractions of the diaphragm; their size was not affected by adrenaline. In Fig. 2b the same muscle was stimulated with submaximal shocks; 50 μg. adrenaline now caused a bigger muscle response. When this experiment was repeated on another preparation using fluid electrodes (Collison, 1933) the same result was obtained (Fig. 2c). The chamber in which the nerve was enclosed for stimulation was sealed off with wax at the bottom. Thus the adrenaline which was added to the bath had no direct access to the nerve at the site of stimulation and therefore presumably exerted its action nearer to the muscle. In Fig. 3 is shown another experiment in which fluid electrodes were used. The action of adrenaline on maximal stimuli at a slow rate of 6 per min. (a), was compared with that on submaximal stimuli at the same rate (b). A similar adrenaline effect was observed on submaximal stimuli at a faster rate of 18 per min. in (c). Maximal stimuli were then applied (d) and after prolonged stimulation fatigue reduced the muscle contractions to the same size as they had been initially in response to the submaximal stimulation in (c). The adrenaline effect was very similar both in (c) and (d), suggesting an improvement of transmission in both, which was maintained in (c) but not in the fatigued muscle (d).
Fig. 2.
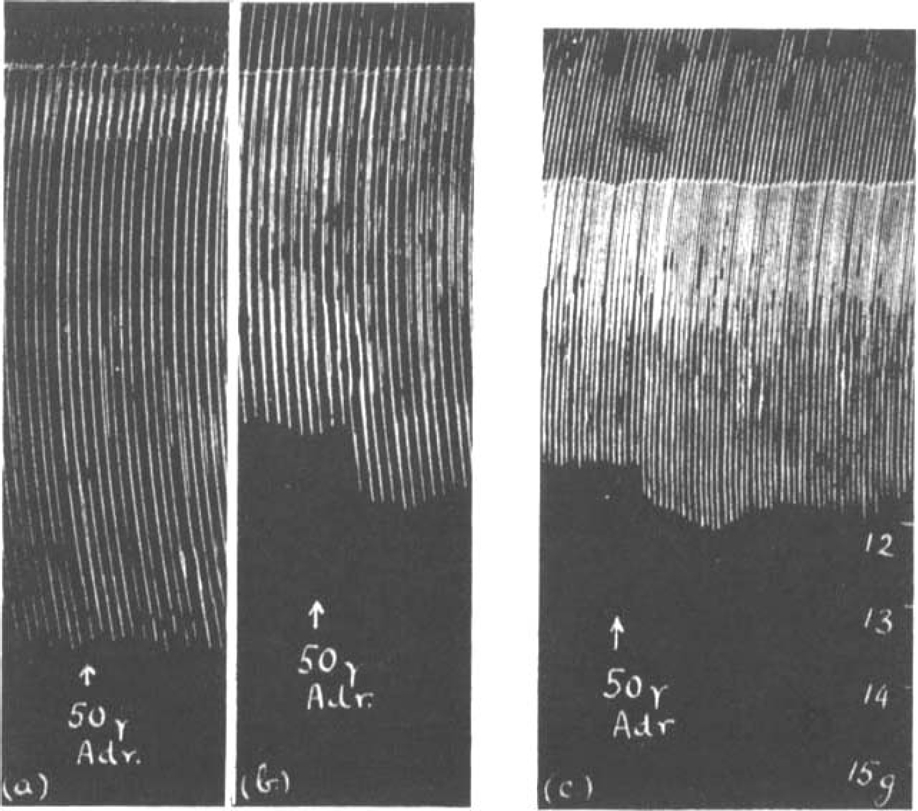
Contractions of rat's diaphragm. (a) and (b) stimulation of phrenic nerve with open electrodes, (c) with fluid electrodes. The effect of 50 μg. adrenaline on the muscle response to maximal (a) and submaximal single shocks (b) and (c).
Fig. 3.
Rat's phrenic nerve-diaphragm preparation. Comparison of the adrenaline effect at different rates of stimulation: (a) maximal, 6 per min.; (b) submaximal, 6 per min.; (c) submaximal 18 per min.; (d) muscle fatigued by maximal stimulation 18 per min.
2. The Action of Prostigmine and Eserine
As adrenaline had no effect by itself upon maximal muscle twitches the modification of the action of prostigmine and eserine by additional adrenaline was studied with maximal stimuli only. The effect of prostigmine was found to be dependent on the dose and on the rate of stimulation. With slow stimulation (5 per min.) the threshold dose producing increased tension was about 0.1 μg. prostigmine or a concentration of 1 in 1,000 million. The accompanying table was compiled from observations in 20 different experiments.
It can be seen from Table I that small doses of 0.1 μg.–0.2 μg. prostigmine caused an increase of muscle contractions at slow and at fast stimulation rates up to 24 per min. The effect of ten times that amount of prostigmine was uncertain, sometimes producing a temporary augmentation followed by depression.
Table I.
The Effect of Prostigmine on the Size of Muscle Contractions Elicited by Maximal Single Shocks
| Rate of stimulation per min. | ||||
|---|---|---|---|---|
| Dose of Prostigmine | 5–8 | 9–12 | 14–16 | 18–24 |
| 0·1 μg.–0·2 μg. | no effect or increase | increase | increase | increase |
| 0·25 μg.–0·3 μg. | increase | increase | uncertain | — |
| 0·4 μg.–0·5 μg. | increase | uncertain | increase followed by depression | — |
| 1–2 μg. | uncertain | increase followed by depression | depression | depression |
| 10 μg. | depression | — | — | — |
At a slow rate of stimulation, as is seen from Table I, a small dose of prostigmine might either have no effect or cause a rise in muscle contractions. If now adrenaline was added to the bath a further augmentation was sometimes observed. But mostly the adrenaline had no apparent effect unless the prostigmine was washed out before the adrenaline was added. After doses of prostigmine exceeding 0.3 μg. or with faster stimulation, adrenaline usually caused depression of the muscle contractions, but again an augmentation was almost always seen if the prostigmine was first washed out.
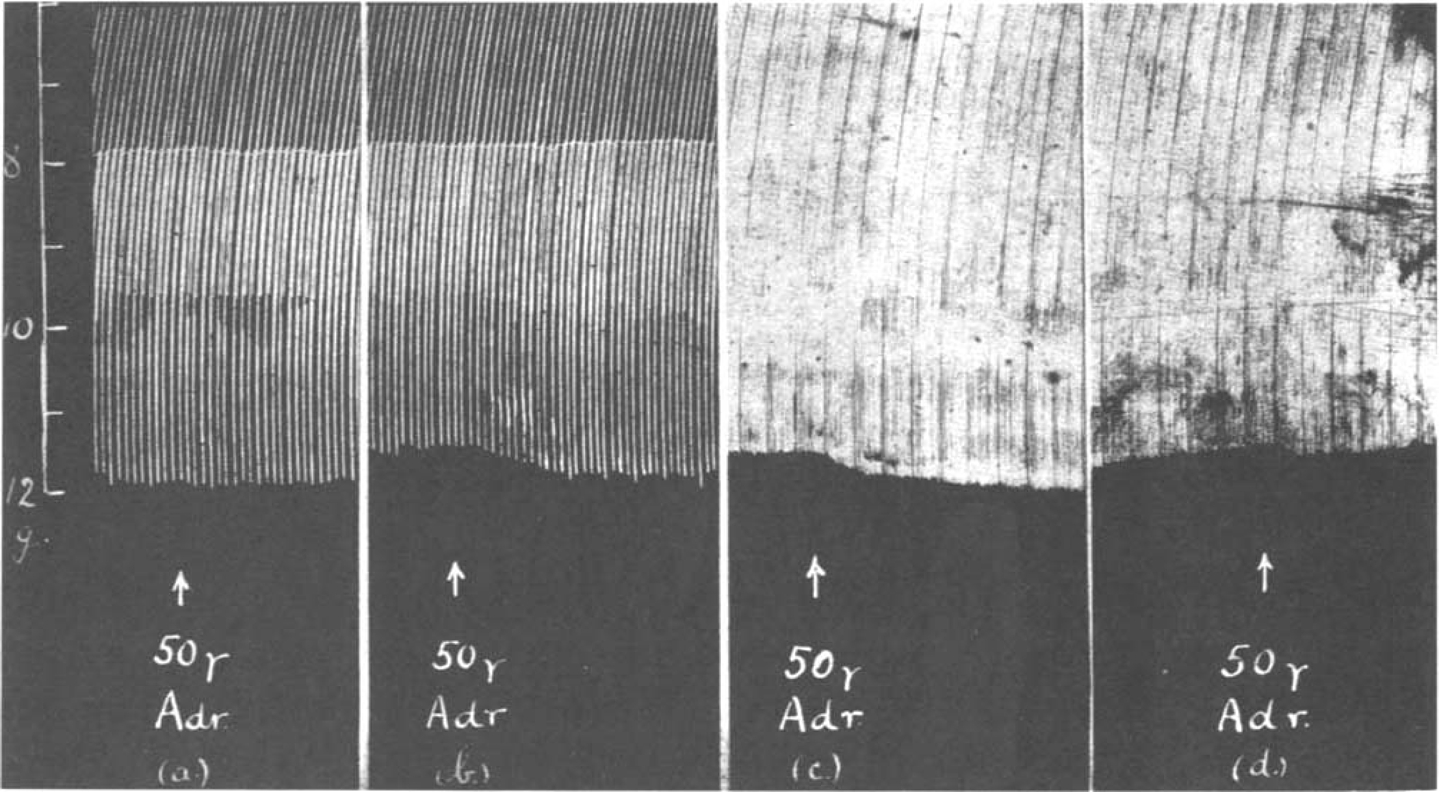
Fig. 4 shows the contractions of the diaphragm in response to maximal shocks at 9 per min. A dose of 0.25 μg. prostigmine added to the bath increased the size of contractions. After washing out, 20 μg. adrenaline caused a further increase. If the prostigmine was not washed out, the opposite effect was often observed. In Fig. 5 the rate of stimulation was faster, 18 per min. After 0.1 μg. prostigmine, which by itself scarcely affected the size of contractions, had been washed out, 10 μg. adrenaline caused an augmentation (Fig. 5a); but when the same small dose of 0.1 μg. prostigmine had been repeated, again producing no immediate effect of its own, and was followed 2 min. later by 10 μg. adrenaline, a depression resulted as shown in Fig. 5b.
Fig. 4.
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 9 per min. Prostigmine causes an increase of muscle contractions, and after a wash out (W) adrenaline causes a further augmentation.
Fig. 5.
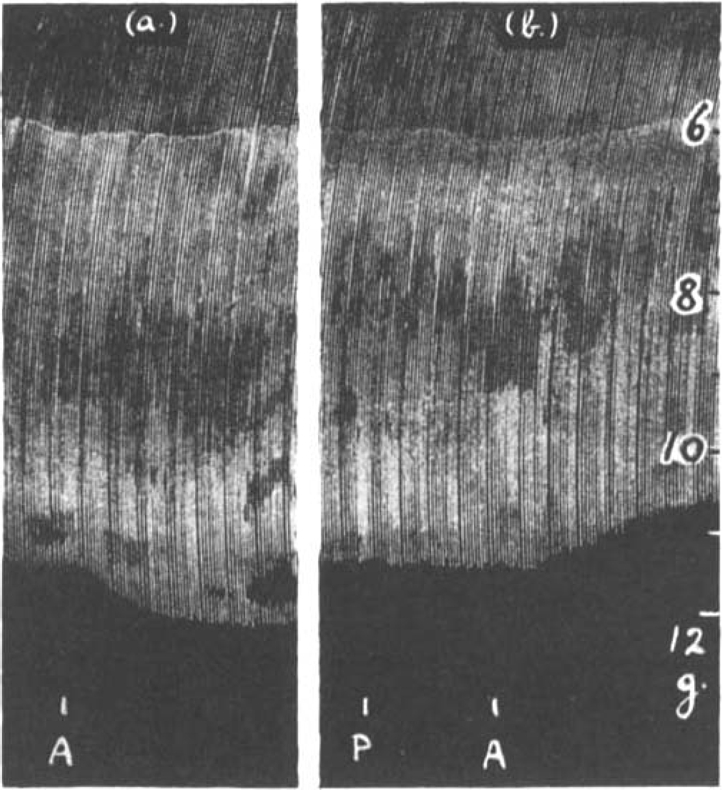
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 18 per min. 0.1 μg. prostigmine had been given beforehand and was washed out; in (a) 10 μg. adrenaline (A) caused a rise in muscle contractions. In (b) when 0.1 μg. prostigmine (P) was not washed out 10 μg. adrenaline (A) caused a fall in muscle contractions.
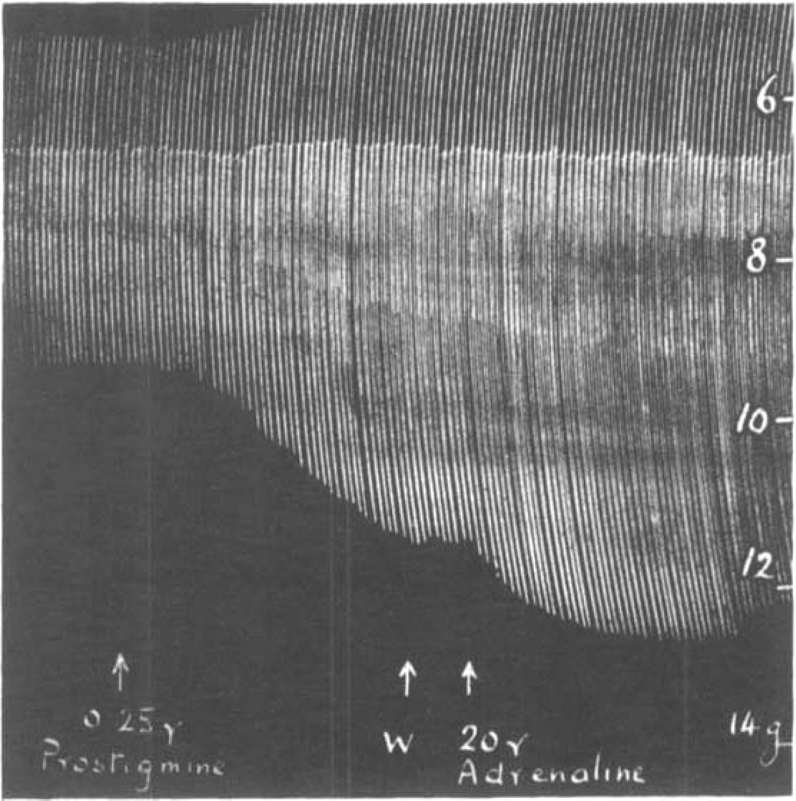
It may be assumed that the isolated muscle, without a circulation to remove added prostigmine, is progressively affected by the drug, however small the dose, as long as it is left in the bath. Thus more and more acetylcholine is prevented from destruction by cholinesterase. If adrenaline is now added as well, it can only increase muscle contractions before the stage of acetylcholine excess is reached. The augmentation should therefore be shown best after the prostigmine has been removed from the bath. This is demonstrated in the experiment shown in Fig. 6. The rate of stimulation was 7 per min.; 0.1 μg. prostigmine was added to the bath. It increased muscle contractions and was allowed to act for 3 min., when it was washed out. Two minutes later adrenaline was added causing a further increase. After the washing out, the muscle contractions slowly returned to their initial level. The dose of 0.1 μg. prostigmine was now repeated and allowed to act for 5 min. causing an augmentation which was, however, less than that produced by the addition of adrenaline before. When now adrenaline was added no effect was seen, the size of muscle twitches slowly declining until the solution was changed. The muscle contractions, instead of returning to their initial size, now gradually increased as though the full adrenaline effect only developed at this stage. Similar observations were made repeatedly and an example is shown in Fig. 7. The rate of stimulation was 14 per min. in this experiment. A dose of 0.1 μg. prostigmine produced three times an almost identical augmentation (P). The first dose was washed out, whereupon adrenaline caused its typical further augmentation (A1). After the solution had been changed, the muscle contractions returned to normal. The second dose of prostigmine was not washed out, whereupon adrenaline (A2) caused a slight depression. After another wash out, the muscle contractions increased and a further rise was obtained with adrenaline (A3). When this was washed out, contractions once more returned to normal. The third dose of prostigmine was washed out but no adrenaline was added until 10 min. later, when it produced its typical rise (A1).
Fig. 6.
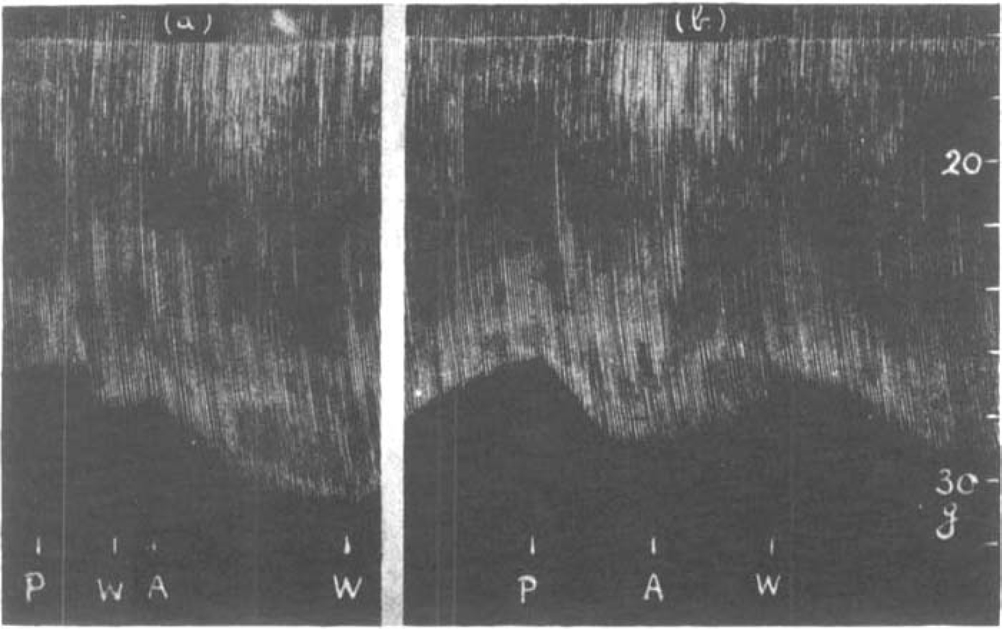
Rat's phrenic nerve-diaphragm preparation. Effect of adrenaline (a) after and (b) before washing out a previous dose of prostigmine. Maximal single shocks 7 per min. P = 0.1 μg. prostigmine; W = wash, A = 10 μg. adrenaline
Fig. 7.
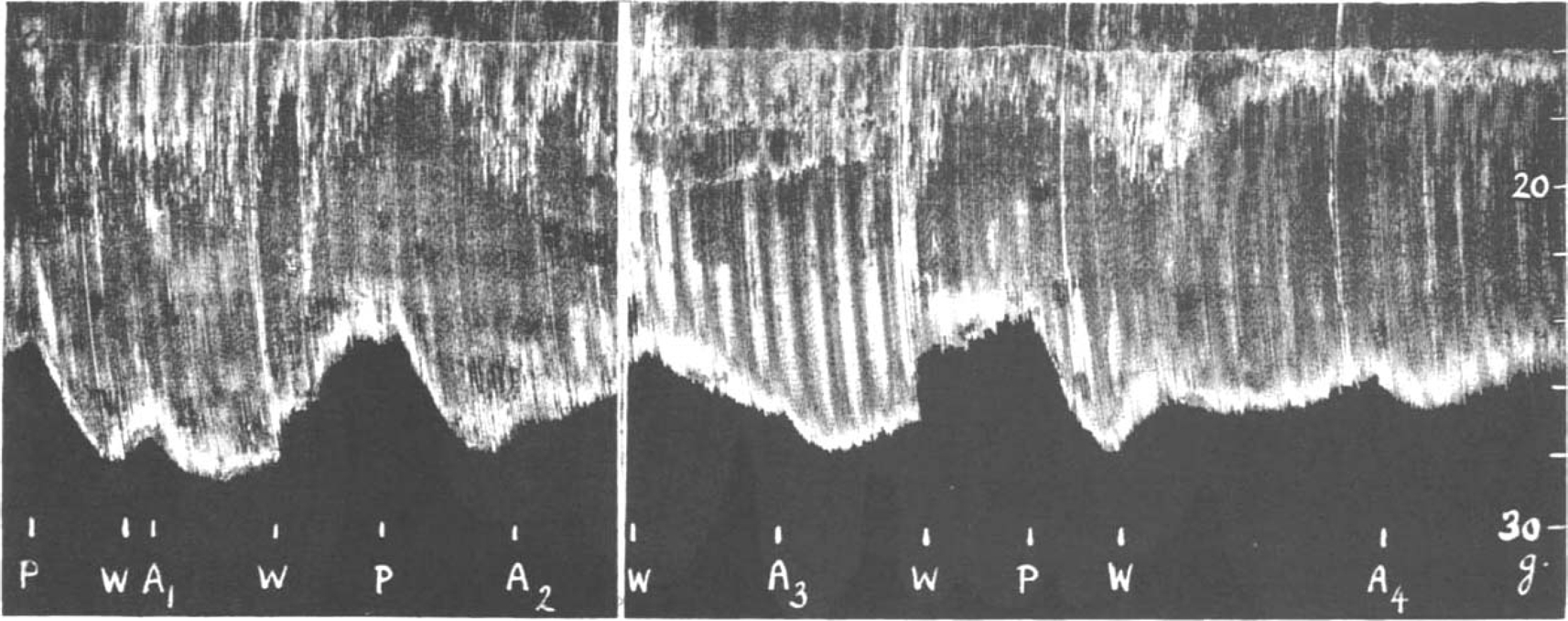
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 14 per min. P = 0.1 μg. prostigmine, W = wash, A = 20 μg. adrenaline. For description see text.
There is no qualitative difference between the action of prostigmine and that of eserine, but they differ quantitatively. The threshold dose of eserine was found to be as low as 0.01 μg. or 1 in 10,000 million, which produced a rise in muscle contractions during slow stimulation at 5 per min. In a fresh preparation as little as 0.1 μg. eserine was seen to cause depression (Fig. 8a). This depression had not passed off 35 min. later though the solution had been changed twice. When adrenaline was added a further depression was observed (Fig. 8b). The preparation was now left for 4 hours, but in spite of washing, the height of contractions did not return to normal. At this stage, however, 0.2 μg. eserine produced a big rise in tension and, after it had been washed out, adrenaline caused a further rise (Fig. 8c). After the solution had been changed, the contractions were allowed to return to their former level and 0.2 μg. eserine was once more added (Fig. 8d) followed—(without wash)—by adrenaline which caused a depression similar to that of 4 hours previously in Fig. 8b. The two opposite effects of adrenaline shown in Fig. 8c and d in the presence of eserine correspond precisely to those shown in Fig. 5a and b, in the presence of prostigmine.
Fig. 8.
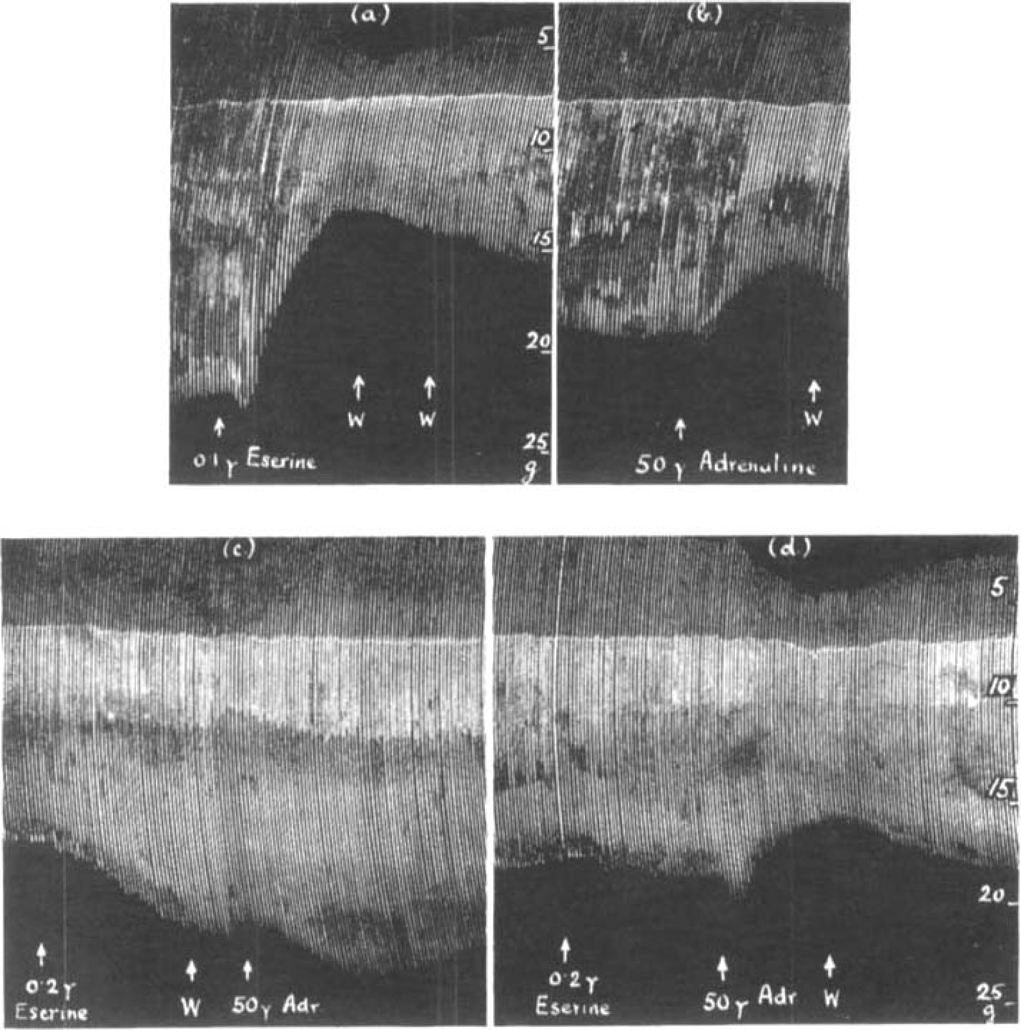
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 5 per min. W. = wash. (a) shows the depression caused by 0.1 μg. eserine on a fresh preparation, (b) the effect of 50 μg. adrenaline 35 min. later, (c) shows the augmentation caused by 0.2 μg. eserine in the same preparation 3 hours later, and the further increase due to 50 μg. adrenaline after eserine had bean washed out. In (d) 50μg. adrenaline was added without removing the eserine; note the similarity between (d) and (b).
There was no doubt that if the rat's diaphragm had been prepared for several hours and was stimulated continuously at a slow rate, its sensitiveness to the action of prostigmine and eserine gradually declined. With stimulation at 8 per min. in a fresh preparation, 2 μg. prostigmine caused a short increase of muscle contractions followed by a depression. In another preparation, which had been working for several hours, 20 μg. prostigmine still produced a big increase which then gave way to depression. Similarly adrenaline is more likely to produce a muscular depression in the beginning of an experiment than at a later stage. In Fig. 9a adrenaline following 0.2 μg. eserine caused a big depression, but 3 hours later as shown in Fig. 9b, the same dose produced a big increase.
Fig. 9.
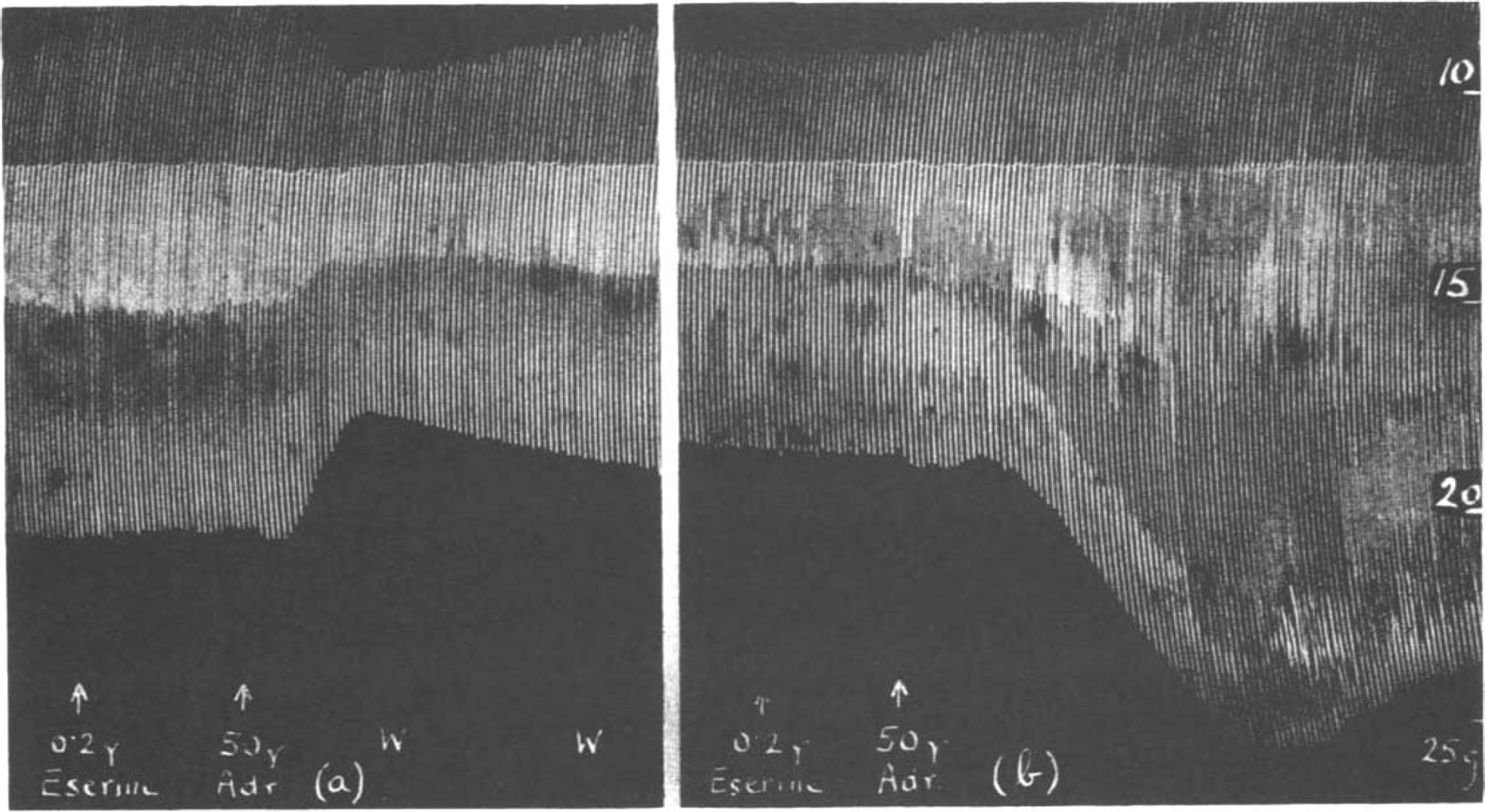
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 6 per min. In a fresh preparation (a) adrenaline caused a depression in the presence of eserine; 3 hours later (b) it caused augmentation.
3. The Action of Atropine, Curarine and Procaine
The action of atropine has been studied both before and after prostigmine or eserine. In big doses atropine shows its well-known “curare-like” action. The contractions of the isolated rat's diaphragm were diminished by doses of 4–5 mg. atropine, a concentration of 1 in 20,000 to 1 in 25,000. Sometimes, however, a very slight transitory increase was seen before the gradual decline. With smaller doses of atropine up to 0.5 mg. no effect on normal muscle contractions could be seen; but after the addition of 1–2 mg. to the bath, making a concentration of 1 in 50,000 to 1 in 100,000, the curious observation was made that the muscle responses to maximal single shocks were increased as is shown in Fig. 10a and 11a. There was never a sudden increase, but the effect was always gradual, at the most 20 per cent and more often less. This increase of normal muscle contractions was only obtained in fresh preparations.
Fig. 10.
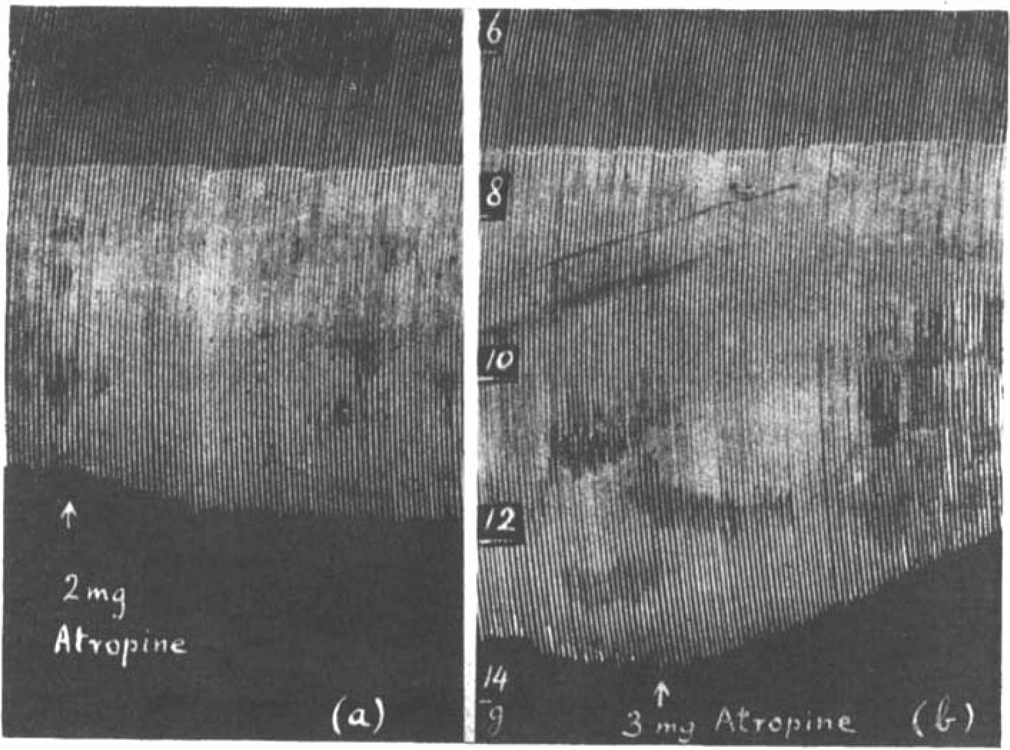
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 8 per min. The effect of atropine on (a) the contractions of a fresh muscle, (b) on the contractions increased by 0.2 μg. eserine.
The observation that eserine, 1 in 1,000 million, on a fresh preparation might cause a depression of muscle tension produced by maximal shocks (see Fig. 8a) raised the question whether a fresh preparation was in a condition of mild acetylcholine paralysis? Dale and Gaddum (1930) observed that atropine antagonized the action of acetylcholine on the isolated denervated kitten's diaphragm. Brown (1937) showed that in the frog's gastrocnemius the injection of 0.1 c.c. atropine sulphate, 1 in 1,000, abolished the muscle response to motor nerve stimulation and depressed, but did not abolish, the muscle response to a close intra-arterial injection of acetylcholine. On the other hand, Abdon (1940) found that those doses of atropine which abolished the muscle twitch caused by acetylcholine injected intra-arterially, did not have any influence on the muscle contractions provoked by motor nerve stimulation. In his experiments on frogs the muscle was immersed in atropine sulphate, 1 in 10,000, while in rabbits 10 mg./kg. atropine sulphate was injected intravenously. Abdon suggested that authors who failed to abolish the effect of intra-arterially injected acetylcholine seemed to have used too small amounts of atropine. But Brown used 10 times as much as Abdon and the discrepancy of their findings cannot therefore be explained in this way.
In the isolated rat's diaphragm atropine caused a depression of muscle contractions in response to maximal nerve stimuli when they were increased by eserine or prostigmine. This is shown in Fig. 10b. While the size of muscle contractions was still increasing, owing to the addition of 0.2 μg. eserine to the bath, 3 mg. atropine reduced them to their original size.
On the other hand, when the muscle contractions were depressed by an overdose of eserine, atropine augmented them. In Fig. 11 is shown an experiment in which atropine, at the beginning of the experiment (Fig. 11a), caused some increase of muscle contractions. The solution was changed several times and the preparation was stimulated continuously for 3 hours. After this period 20 μg. eserine was given and first increased the size of contractions, then depressed them below their original size. When now 2 mg. atropine was added an augmentation was seen (Fig. 11b). There is a striking similarity between the effects in Fig. 10a and b, which suggests that in both instances atropine removes a depression which is due to an excess of acetylcholine.
Fig. 11.
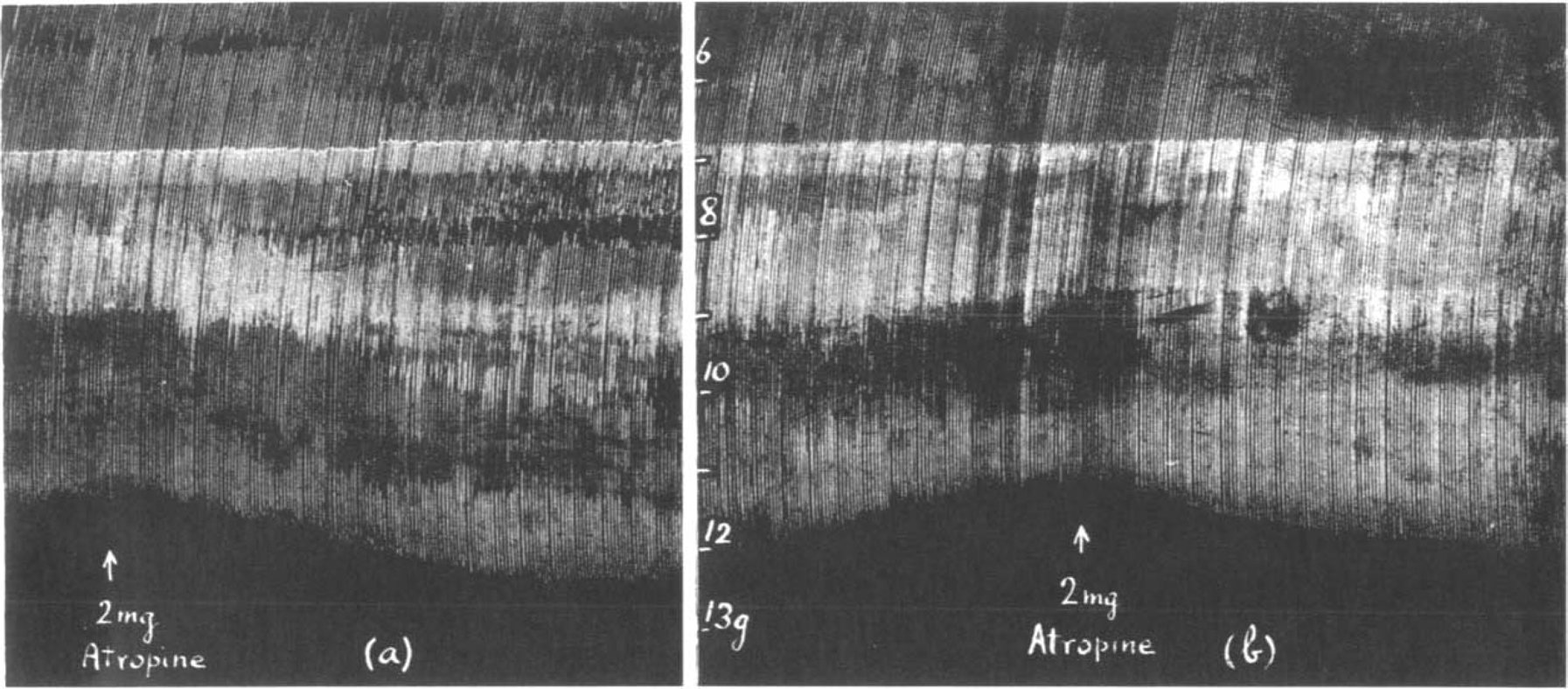
Similar to Fig. 10. Maximal single shocks 9 per min. The effect of atropine on (a) the contractions of a fresh muscle, (b) on the contractions depressed by 20 μg. eserine.
Briscoe (1936) has shown that subparalytic doses of curarine restore muscle contractions previously depressed by prostigmine, and it seemed interesting to compare the action of atropine with that of curarine. The dependence of the action of prostigmine and of eserine on the frequency of stimulation was studied by Briscoe (1936) and by Bacq and Brown (1937). In Fig. 12a is shown the effect of periods of fast stimulation on the normal muscle before and after 0.5 μg. eserine. Though at a rate of 5 per min. the size of contractions was increased in the presence of eserine, faster stimulation (18 and 50 per min.) caused a rapid decline, the steepness of which was directly proportional to the rate of stimulation. After the administration of larger doses of eserine (2 μg. in Fig. 12b) while the nerve was stimulated at 17 per min. muscle contractions declined rapidly below normal. The addition of 1 mg. atropine stopped this decline and started a slow increase. Further 2 mg. atropine had no more effect than to increase the overthrow of the lever; (this is due to the muscle relaxing more quickly). When 50 μg. curarine was added to the bath a sudden increase of contractions took place and now, in the presence of curarine, it was found that the relation between the size of muscle contractions and the rate of stimulation was reversed. At a slow rate the height of contractions declined, at a fast rate it increased. It is generally accepted that curarine raises the threshold of the muscle for acetylcholine. Therefore, when the rate of stimulation is slow and, in the presence of eserine, only small amounts of acetylcholine accumulate, the muscle contractions may decline below their maximal size, but with fast stimulation, when more acetylcholine accumulates, a normal height of contractions may be attained.
Fig. 12.
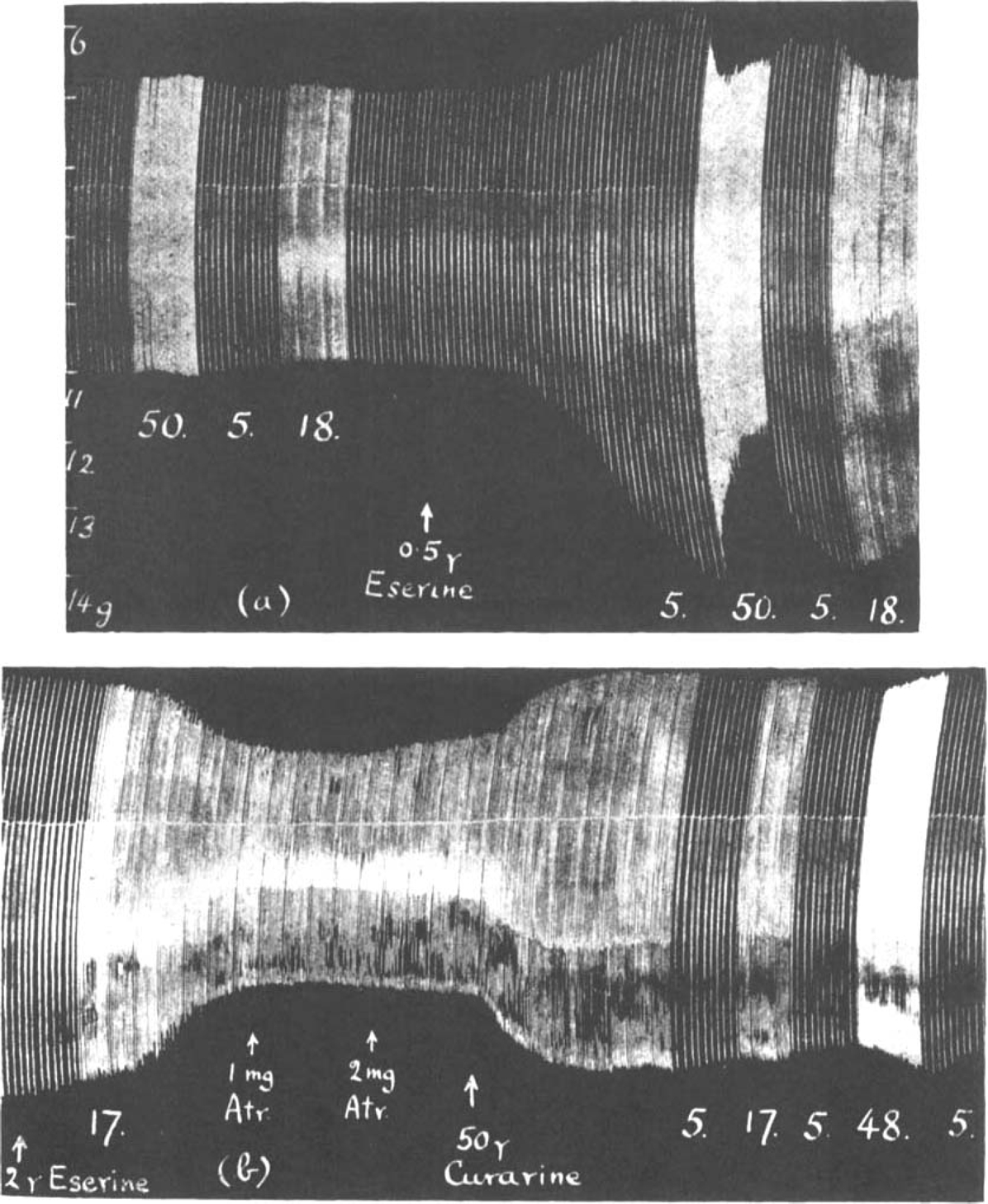
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks at rates per min. indicated by figures. Note that after eserine (a) contractions decline during fast and recover during slow rates, while after curarine (b) contractions decline during slow and increase during rapid rates.
If one assumes that atropine has only a very slight threshold-raising, curarelike action, this would explain why it was found to be more effective in antagonizing an eserine depression at slow rates of stimulation than at faster rates (compare Figs. 11b and 12b). Fig. 13 shows the muscle contractions of a preparation fully eserinized (100 μg.) and atropinized (4 mg.). At a rate of 5 per min. contractions were bigger than at 18 or 50 per min. But even at this fastest rate no severe depression was seen. If atropine exerted its action only by raising the threshold of the muscle, like curarine but to a lesser degree, then 50 stimuli per min. in the presence of an enormous dose of eserine should have depressed muscle contractions much further. One may therefore attempt to explain the action of atropine by assuming that atropine reduces the amount of acetylcholine formed. This possibility was first considered by Brown (1937).
Fig. 13.
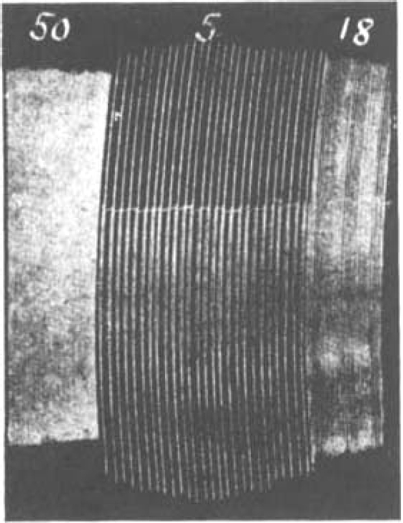
Maximal single shocks at 50, 5 and 18 per min. in a fully eserinized and atropinized phrenic nerve-diaphragm preparation.
Harvey (1939) showed that procaine suppressed the output of acetylcholine from the superior cervical ganglion during preganglionic stimulation. He suggested that in skeletal muscle procaine, besides acting like curare, also diminished the liberation of acetylcholine from the motor nerve endings. This view was supported by Jaco and Wood (1944). Fig. 14 shows an experiment in which the effect of procaine was observed (a) during a prostigmine-augmentation, (b) during a depression after an overdose of prostigmine. The increased muscle contractions in (a) were reduced by procaine to their normal height. The diminished muscle contractions in (b) were also at first slightly reduced but then steadily increased although they did not reach their original size. Nevertheless, the gradual onset of this effect is similar to the records obtained with atropine.
Fig. 14.
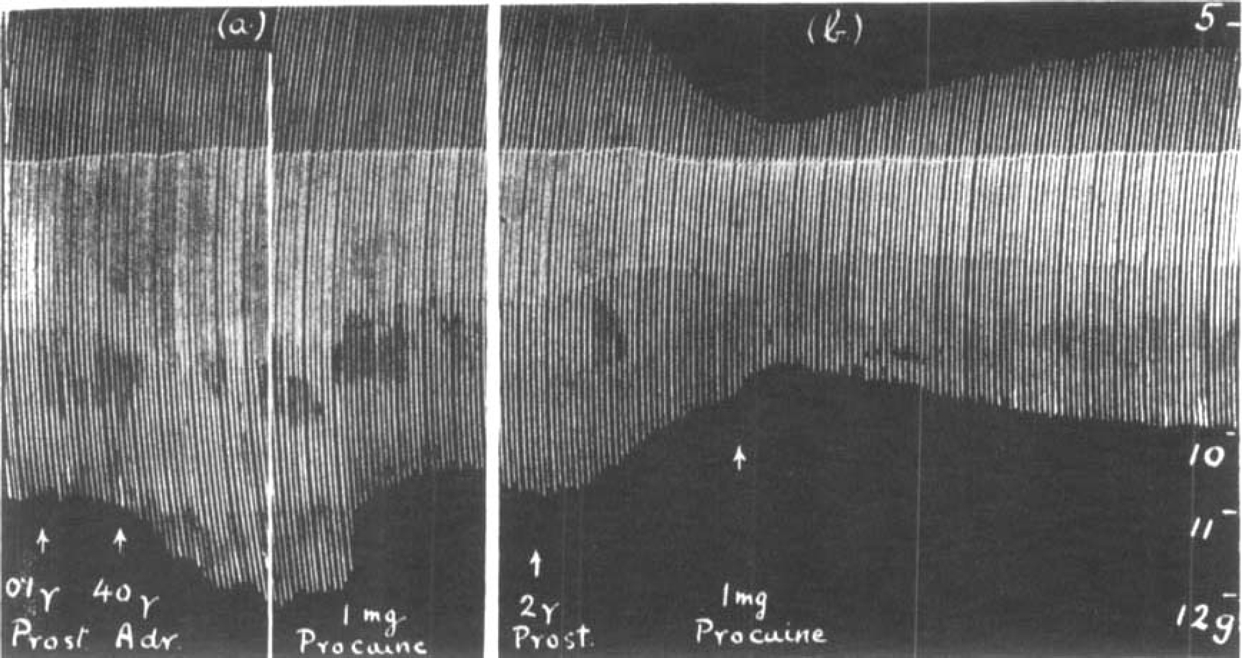
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 8 per min. The effect of 1 mg. procaine on the size of muscle contractions (a) when increased. (b) when depressed by prostigmine.
It is possible to observe the effect of excess acetylcholine on the muscle contractions by increasing the rate of nerve stimulation in the presence of an anticholinesterase and also by adding acetylcholine to the bath. If atropine and procaine act like curarine by raising the threshold of the muscle to acetylcholine then the muscle response to acetylcholine released from the nerve and to acetylcholine introduced from outside should be affected in the same way. If, however the activity of the nerve is affected by atropine and procaine, then in their presence the response of the muscle to the application of fast stimulation should differ from the response to the addition of acetylcholine to the bath.
In Fig. 15 (a) is shown the effect of fast stimulation on a muscle treated with prostigmine. The size of muscle contractions declined and a further depression was produced by the addition of 0.5 mg. acetylcholine to the bath. In (b) after 35 μg. curarine the muscle contractions slowly increased during the period of faster stimulation and the addition of 0.5 mg. acetylcholine did not interrupt this slow augmentation. As curarine raises the threshold of the muscle to acetylcholine neither the rapid stimulation nor the addition of 0.5 mg. acetylcholine to the bath caused a depression. We know that in the presence of curarine the nerve liberates acetylcholine as before (Dale, Feldberg and Vogt, 1936) and the amount introduced into the bath remained the same. But the muscle, being less sensitive, was not paralysed.
Fig. 15.
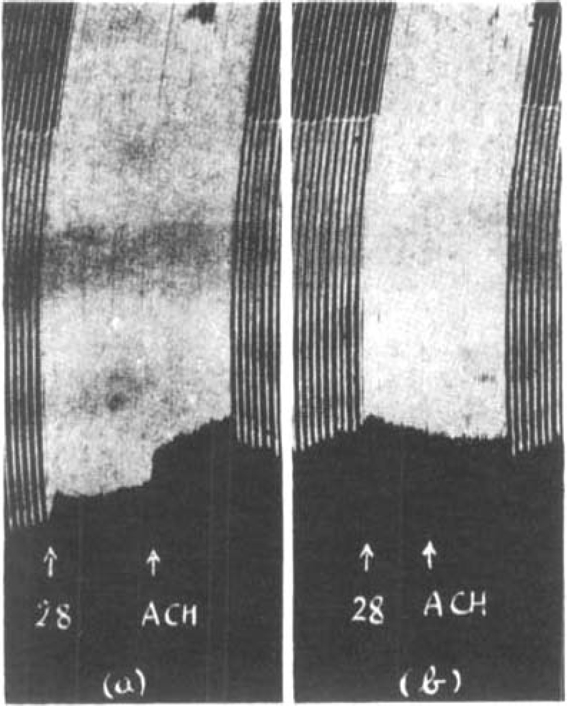
Rat's phrenic nerve-diaphragm preparation. Maximal single shocks 5 per min: 2 μg. prostigmine present throughout. The effect of increasing the rate of stimulation to 28 per min. and of 0.5 mg. acetylcholine (a) before and (b) after 35 μg. curarine.
In Fig. 16 is shown a comparison between the action of atropine and curarine. Throughout 2 μg. prostigmine was added to the bath to ensure the accumulation of acetylcholine. In (a) a depression was produced by fast stimulation and a further depression by additional acetylcholine. In (b) 2 mg. atropine was added. The muscle contractions were at first slightly depressed during fast stimulation but steadily regained their original height; the addition of 0.5 mg. acetylcholine caused a marked depression. After the preparation had been washed out several times during an interval of 20 min., 35 μg. curarine was added to the bath (c); this abolished both the depression due to rapid nerve stimulation and that due to added acetylcholine. After another interval in which the curarine was washed out the sequence was once more repeated in the presence of atropine (d).
Fig. 16.
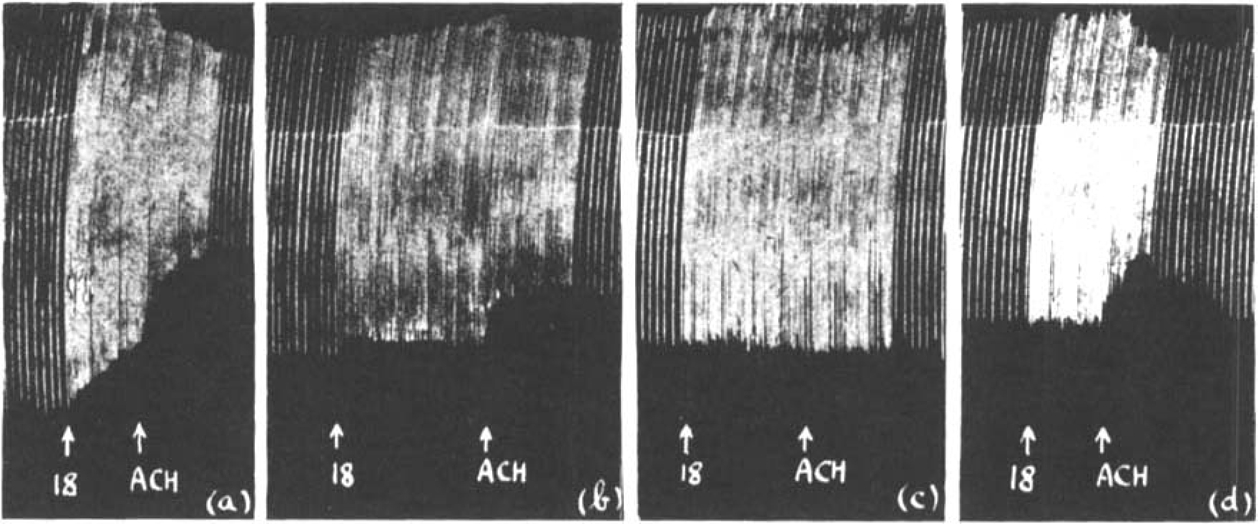
Similar to Fig. 15. Maximal single shocks 6 per min.; 2 μg. prostigmine present throughout. The effect of increasing the rate of stimulation to 18 per min., and of 0.5 mg. acetylcholine (a) before and (b) after 2 mg. atropine; (c) after 35 mg. curarine: (d) after 2 mg. atropine.
The action of procaine was indistinguishable from that of atropine. This is shown in Fig. 17. Throughout 2 μg. prostigmine was present in the bath and a depression was first produced by fast stimulation and secondly by additional acetylcholine. In the presence of 2 μg. prostigmine only (a) both effects were marked. After 2 mg. procaine (b) and also after 2 mg. atropine (d), muscle contractions at first declined with increased rate of stimulation but soon remained steady, whereas the addition of 0.5 mg. acetylcholine caused a progressive depression. When the bath was changed, both after procaine (c) and after atropine (e), rapid stimulation caused an initial depression followed by a gradual increase of contractions, but added acetylcholine caused a profound decline. Finally, in the presence of 35 μg. curarine (f), both effects were abolished.
Fig. 17.
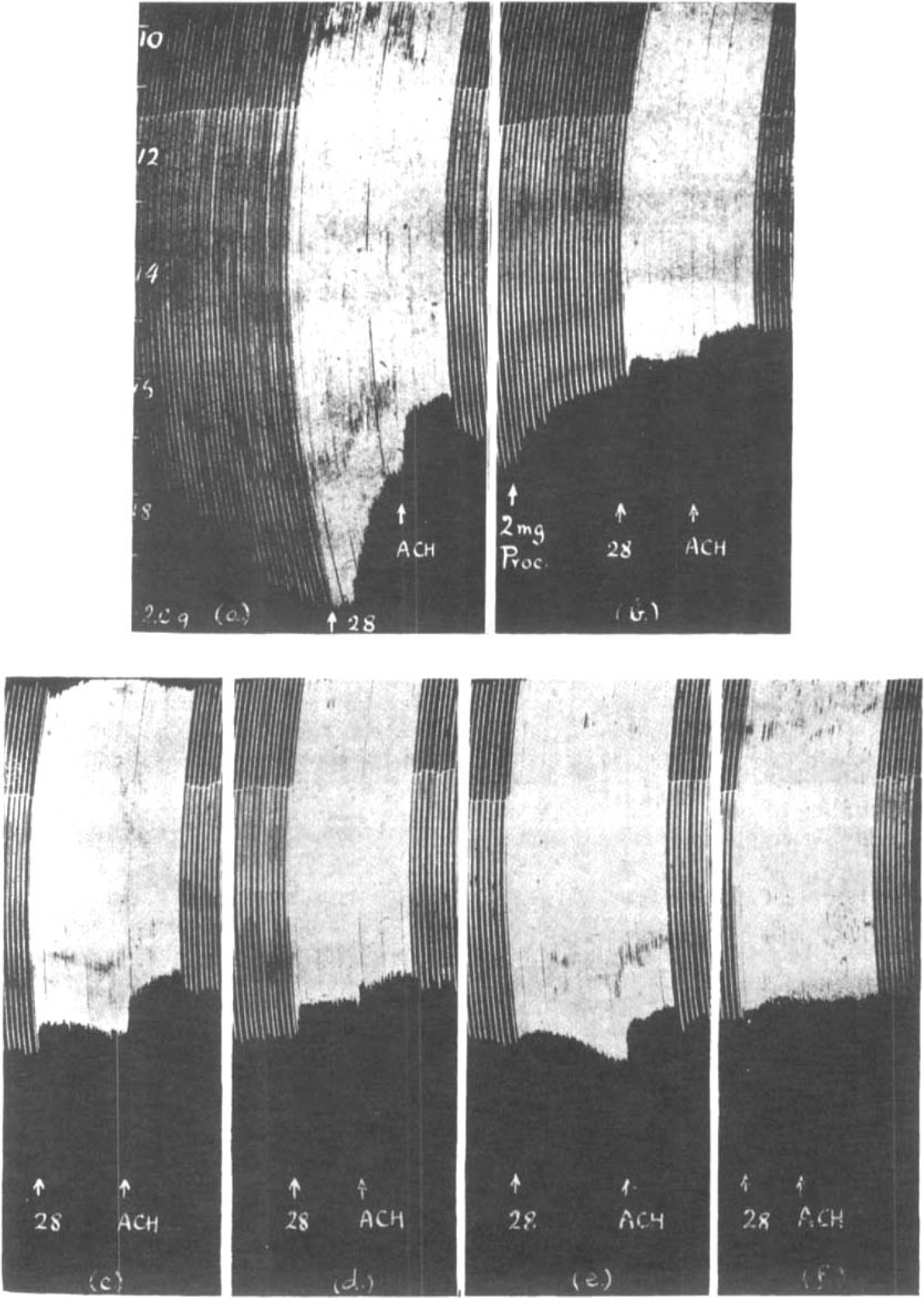
Similar to Fig. 15. Maximal single shocks 6 per min.; 2 μg. prostigmine present throughout. The effect of increasing the rate of stimulation to 28 per min., and of 0.5 mg. acetylcholine (a) before and (b) after 2 mg. procaine; (c) after washing out; (d) after 2 mg. atropine, and (e) after washing out; (f) after 35μg. curarine.
Discussion
The action of adrenaline was studied on an isolated mammalian nerve muscle preparation in order to exclude vascular effects. The results described in this paper confirm the conclusions drawn from previous experiments in which adrenaline was injected into the bloodstream. They leave no doubt that adrenaline has an action on the muscular response to nerve stimulation which is independent of its vasoconstrictor action.
It was found that muscle contractions in response to submaximal nerve stimuli are increased if adrenaline is added to the fluid in the bath in which the muscle is suspended. This increased muscle response is seen not only when the nerve is stimulated with open electrodes, in which the adrenaline may reach the site of nerve stimulation, but also when the nerve is stimulated with fluid electrodes, where the adrenaline cannot reach the point of stimulation. Adrenaline has no effect on the muscle response to maximal motor nerve stimuli. It causes an augmentation in the size of maximal contractions only when fatigue has occurred after prolonged maximal stimulation. This phenomenon is generally attributed to an improvement of neuromuscular transmission.
It is difficult to explain why in the non-fatigued preparation adrenaline increases the muscle response to submaximal stimuli whereas it does not affect maximal contractions. The results so far obtained with submaximal stimulation suggest that adrenaline acts by improving neuromuscular transmission; but further investigation is in progress. This explanation would be in agreement with observations on the perfused superior cervical ganglion (Bülbring, 1944) in which adrenaline improved synaptic transmission and thus increased the response of the nictitating membrane to submaximal stimulation of the preganglionic nerve fibres. The effective concentration of adrenaline in the perfusion fluid of the ganglion was, however, 1 in 100 to 1 in 200 million, whereas in the fluid surrounding the isolated muscle it was 1 in 2 to 1 in 10 million. The discrepancy may be due to the different way in which adrenaline reaches the tissue; much less may be required if it is carried in the circulation than if it acts by diffusion from outside. Dale and Gaddum (1930) used an even stronger concentration of adrenaline, 1 in 75,000, which increased the action of acetylcholine on denervated muscle.
Another action on skeletal muscle was described by Bülbring and Burn (1942), who found that adrenaline augmented the action of eserine or prostigmine. It was possible that this was due to a vascular effect. But the results were interpreted as indicating that adrenaline lowered the threshold of the muscle, thereby potentiating the action of acetylcholine which accumulated in the presence of the anticholinesterase. This view is supported by the results obtained on the isolated preparation. If the phrenic nerve is stimulated with maximal shocks at slow rates the contractions of the diaphragm are increased by prostigmine or eserine and a further increase is produced by the addition of adrenaline to the bath. If the nerve is stimulated at more rapid rates or if the anticholinesterase is allowed to exert a more prolonged action, then adrenaline causes a depression of muscle contractions. Both observations indicate that adrenaline in the absence of vascular changes intensifies the effect on the muscle of an accumulation of acetylcholine.
In the isolated muscle, especially at the beginning of an experiment, the effects of excess acetylcholine are more readily observed than in a muscle with its normal circulation. This fact is reminiscent of conditions in the perfused superior cervical ganglion described by Brown and Feldberg (1936). They showed that in the perfused ganglion during continued maximal preganglionic stimulation the output of acetylcholine started at a high level and fell rapidly during the first 20–60 min. after which it continued at a steady low level. Consequently, when the perfusion fluid contained eserine, the effect of excess acetylcholine on the contraction maintained by the nictitating membrane during continued preganglionic stimulation differed in the initial stage from the effect later on. An increase in strength of stimulation, as well as the injection of acetylcholine into the perfusion fluid, at first caused a depression, but 44 min. later both produced an augmentation. The results obtained on the isolated striated muscle preparation suggest that similar conditions prevail. In the beginning of an experiment motor nerve stimulation seems to produce such a large amount of acetylcholine that the addition even of a small dose of an anticholinesterase may cause an accumulation sufficiently high to produce paralysis. The same dose of eserine, given later, may cause an increase of muscle contractions. Similarly, in a fresh preparation, after a dose of eserine which by itself may exert no action, adrenaline causes a depression, whereas several hours later the same dose of adrenaline causes augmentation.
It seems possible that when this isolated muscle is freshly prepared, acetylcholine accumulates in it even in the absence of an anticholinesterase. This hypothesis is supported by the observation that, in a fresh muscle, contractions were slightly augmented by the addition of atropine to the bath. This observation was puzzling until it was shown that atropine antagonized any effects of increased amounts of acetylcholine accumulated by nerve stimulation in the same way as it antagonizes the action of acetylcholine, eserine and prostigmine in the spinal cord (Bülbring and Burn, 1941). Thus, when muscle contractions were increased by eserine or prostigmine, the addition of atropine reduced them to normal. On the other hand, when muscle contractions were decreased by an overdose of an anticholinesterase or by a faster rate of stimulation atropine counteracted the depression. In both instances atropine acts like curarine. A difference was, however, observed when excess of acetylcholine was not only produced by rapid nerve stimulation in the presence of eserine or prostigmine but also by addition of acetylcholine from outside. Curarine in the presence of an anticholinesterase prevented the muscular depression caused by fast stimulation and that caused by a dose of acetylcholine alike. But atropine did not prevent the depressant effect of a dose of acetylcholine added to the bath. The conclusion was therefore drawn that atropine interfered with the liberation of acetylcholine from the motor nerve ending rather than that atropine affected the muscle in the same way as curarine. Brown (1937) came to the same conclusion when he found that in frog's muscle atropine rendered nerve stimulation ineffective, leaving, however, a large part of the acetylcholine response still present.
In the phrenic nerve-diaphragm preparation the effects caused by atropine were very similar to those obtained with procaine. As procaine depresses the activity of sensory nerves a similar action on motor nerves is not surprising. It is well known that atropine possesses a weak local anaesthetic action. But while Harvey (1939) showed that procaine diminished the output of acetylcholine from the perfused superior cervical ganglion during preganglionic stimulation, Brücke (1937) showed that atropine increased the output of acetylcholine in the frog's heart during vagus stimulation. The amount of acetylcholine escaping into the perfusion fluid may not be a true measure of the amount liberated from the nerve, but may be modified by the amount which is capable of or prevented from combining with the receptive substance. Recently Dawes (1946) has shown that atropine and procaine in large dosage exert a similar action on heart muscle, i.e. both of them prolong the refractory period in the same way as quinidine.
Summary
A strip of the rat's diaphragm with the phrenic nerve can be used as an isolated mammalian nerve muscle preparation.
If single submaximal shocks are applied to the phrenic nerve the contractions of the diaphragm muscle are increased by the addition of adrenaline to the bath. This effect is observed whether the adrenaline reaches the site of nerve stimulation or not. Unless the muscle is fatigued, adrenaline has no effect on the muscle response to maximal nerve stimuli.
The muscle contractions elicited by maximal nerve stimuli are increased by small doses of eserine or prostigmine at slow rates of stimulation. A depression is produced by prolonged action or by an overdose of the anticholinesterase or by increasing the stimulation rate in the presence of the anticholinesterase.
Adrenaline augments the action of eserine and prostigmine. This may be observed as an increase or as a decrease in the size of muscle contractions according to various conditions. Muscle contractions are increased when the amount of the anticholinesterase is small and when the rate of stimulation is slow. The size of contractions is depressed by adrenaline after an overdose of the anticholinesterase or with faster rates of stimulation.
The depressant effect of eserine or prostigmine and of subsequent adrenaline is more readily observed in a fresh preparation than in one which has been stimulated for several hours. The possibility that acetylcholine may accumulate during the initial stages of motor nerve stimulation in this isolated preparation is discussed.
Atropine increases slightly the size of muscle contractions elicited by maximal nerve stimuli in a fresh preparation.
If muscle contractions are increased by eserine or prostigmine, atropine reduces them to their normal size. If muscle contractions are depressed by an overdose of an anticholinesterase or by a faster rate of stimulation then atropine counteracts the depression.
In the presence of an anticholinesterase curarine abolishes the depressant effects of excess acetylcholine, whether this be produced by rapid nerve stimulation or by adding acetylcholine to the bath. Atropine differs from curarine. It abolishes the depressant effect of rapid motor nerve stimulation but it does not abolish the depression caused by the addition of acetylcholine to the bath.
The action of atropine was found to be very similar to that of procaine. The conclusion was therefore drawn that atropine acts not only by raising the threshold of the muscle, as curarine does, but also by interfering with nervous activity.
References
- Abdon NA. Acta Physiol. Scand. 1940;1:153. [Google Scholar]
- Bacq ZM, Brown GL. J. Physiol. 1937;89:45. doi: 10.1113/jphysiol.1937.sp003461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL. J. Physiol. 1937;89:438. doi: 10.1113/jphysiol.1937.sp003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GL, Feldberg W. J. Physiol. 1936;88:265. doi: 10.1113/jphysiol.1936.sp003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscoe G. Lancet. 1936;1:469. [Google Scholar]
- Brücke FThv. J. Physiol. 1937;90:81 P. [Google Scholar]
- Bülbring E. J. Physiol. 1944;103:55. doi: 10.1113/jphysiol.1944.sp004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Burn JH. J. Physiol. 1939;97:250. doi: 10.1113/jphysiol.1939.sp003804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Burn JH. J. Pharmacol. 1940;68:150. [Google Scholar]
- Bülbring E, Burn JH. J. Physiol. 1941;100:377. doi: 10.1113/jphysiol.1941.sp003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Burn JH. J. Physiol. 1942;101:224. doi: 10.1113/jphysiol.1942.sp003977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E, Whitteridge D. J. Physiol. 1941;99:201. doi: 10.1113/jphysiol.1941.sp003893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collison LW. J. Physiol. 1933;80:14 P. [Google Scholar]
- Corkill SB, Tiegs OW. J. Physiol. 1933;78:161. doi: 10.1113/jphysiol.1933.sp002995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH, Feldberg W, Vogt M. J. Physiol. 1936;86:353. doi: 10.1113/jphysiol.1936.sp003371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale HH, Gaddum JH. J. Physiol. 1930;70:109. doi: 10.1113/jphysiol.1930.sp002682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes GS. 1946. in the press.
- Harvey AM. Bull. Johns Hopkins Hosp. 1939;65:223. [Google Scholar]
- Jaco NT, Wood DR. J. Pharmacol. 1944;82:63. [Google Scholar]
- Maibach S. Z. Biol. 1928;88:207. [Google Scholar]


