The amount of an active substance required for an assay on isolated plain muscle suspended in a bath depends upon the volume of the bath, which cannot conveniently be made much smaller than about 2 ml. A piece of intestine may be suspended in air and kept in good condition by a stream of a suitable solution running over its surface (Finkleman, 1930). This technique may be called superfusion, since the fluid runs over the tissue, by analogy with perfusion, in which the fluid runs through the tissue.
The effect of drugs on such a preparation may be tested by injecting them into the stream of fluid (Gaddum, Jang, and Kwiatkowski, 1939), and such preparations have been used for the assay of histamine (Kwiatkowski, 1941).
Greater sensitivity can be obtained by stopping the flow for a standard interval of time and applying the active solutions undiluted to the surface of the muscle. The object of the present paper is to describe an apparatus which has been developed during the last few years for this purpose and to present some results obtained with it.
Methods
The apparatus is shown in Fig. 1. The muscle is suspended in air in the middle of a wide glass tube which is immersed in warm water in a small copper bath. This copper bath is attached to a vertical rod and the lever is attached to the same rod. The warm water is mixed by means of a stream of air which is sufficiently violent to cause slight vibration of the whole apparatus and so diminish friction of the writing point on the drum. The vibration may be controlled by finding the appropriate height for the apparatus on the vertical bar. The appropriate salt solution is contained in a reservoir 20–40 cm. above the bath. It runs from this reservoir through a glass tap and a glass-wool filter, and is then warmed by passing through the water in the copper bath. The rate of flow is controlled by a resistance which consists of a capillary tube drawn out to a fine point and held inside a wider glass tube by passing the same rubber tube over both. The flow is adjusted to 1–5 ml./min. by altering the height of the reservoir. The superfusion fluid drips from a glass tube down the cotton which attaches the tissue to the lever. The end of this tube has been filed off at an acute angle in order to facilitate the administration of drugs. The height of the glass tube above the tissue must not be too small, or the solution may run down one side of the tissue and allow the other side to dry. If the height is sufficient (> 10 cm.), each drop is broken up on reaching the tissue, and wetting is even.
Fig. 1.

Solutions to be tested are contained in small test-tubes (12×65 mm.) which are warmed before use by placing them in a suitable holder attached to the copper bath. A small volume of this fluid is sucked into a teat pipette. The tap which controls the flow is then turned off for a standard time. The fine point of the teat pipette is placed in the filed-off end of the glass tube which forms the drop, and the teat is squeezed until a standard number of drops (generally 5 drops or about 0.2 ml.) has fallen; this takes 3–4 seconds. These drops are formed in a standard place in the angle between the thread and the glass tube, so that their size does not depend on the pipette, but their exact volume is not critical. The fluid is left in contact with the surface of the tissue for a standard time (10–60 sec.), and the tap is then opened and the normal flow restarted.
The effect of a drug depends on its concentration in the solutions tested, and it may be necessary to prepare a large number of different dilutions. This may be done quite rapidly by measuring out 0.2–1 ml. of a more concentrated solution into the small test-tubes with a 1-ml. measuring pipette and adding the appropriate volume of the superfusion fluid to make 1 ml. In this case the more concentrated solution must be very similar in pH and salt content to the superfusion fluid. Since fluids are applied direct to the tissue, small differences in their composition may affect the result. For example, superfusion fluid diluted with only 5% of water has been found to cause a contraction of guinea-pig's ileum.
In the experiments on guinea-pig's ileum the superfusion fluid was Tyrode's solution and the temperature of the bath was 30°–37° C. In the experiments on rat's uterus the fluid was de Jalon's solution (Gaddum, Peart, and Vogt, 1949), and the temperature was 25°–30° C. The magnification on the lever was 5—or sometimes more. The lever was adjusted to give a tension of 0.5–1 g. wt., which is partly counterbalanced by the weight of the muscle in air.
A modified form of the apparatus described above was used for experiments in which two tissues were suspended one above the other and the same fluid was superfused over them both in series (cf. Finkleman, 1930). In this way it was found that a substance was sometimes liberated from rat's small intestine which caused a contraction of rat's uterus. This observation led to experiments in which the rat's small intestine was perfused with Tyrode's solution at 37° C. through a polythene cannula tied in the superior mesenteric artery. All other vessels were tied except the portal vein, which was left open; both ends of the small intestine were tied. The tissue was suspended in a gauze hammock fixed in a funnel which was warmed in a bath; the outflow was collected from the funnel. The reservoir was at a height of about 50 cm. above the intestine and the rate of flow was kept constant (0.1–0.5 ml. per min.) by means of a capillary resistance.
Rats' uteri were generally prepared by the injection of stilboestrol (0.1 mg. per kg.) the day before the experiment, but this is not essential.
Crystalline preparations of trypsin and chymotrypsin containing less than 50% MgSO4.7H2O were obtained from Messrs. Armour. Oxytocin was used in the form of Pitocin (Parke, Davis). The preparation of substance P (P6) was made by Drs. A. H. Amin and T. B. B. Crawford by the method of Euler (1942). It was compared with a preparation obtained from Professor Euler and was estimated to contain 13.8 of his units per mg.
Results
Stoppage of the flow may itself cause contraction of superfused muscle which is probably due, sometimes at least, to the liberation of active substances which remain on the surface of the muscle. This effect is well shown by guinea-pig's ileum, and with this tissue it may be necessary to restrict the time of stoppage to 10–15 sec. This effect is partly due to choline and choline esters, and may be diminished by adding atropine to the fluid (Magnus, 1930), but some effect survives in atropine. Rat's uterus does not contract so readily when the flow is stopped.
Fluids are normally tested by applying 5 drops (ca. 0.2 ml.) in a short time (3–4 sec.). The fluid on the surface of the tissue is thus almost completely replaced, and this new fluid is now given a standard time to act. If more than 5 drops are used the effect may be slightly larger, but if less than 5 drops are used the effect may be much less, owing to incomplete replacement of the fluid on the surface of the tissue. Five drops has appeared to be a reasonable compromise between the conflicting claims of the accuracy which might be achieved with more drops and the economy which would be achieved with fewer.
When histamine is applied to guinea-pig's intestine the contraction of the muscle generally begins in less than 15 sec., but does not reach its maximum until about 20–30 sec. after the application of the drug when the muscle has already been superfused with drug-free fluid for some time. With slow-acting substances, such as trypsin and the substance R whose properties are discussed later in this paper, there may be no response until 30 sec. or more after the washing out of the drug has started, and the maximum may not be reached till a minute or two later (Fig. 3). These unconventional time-relations appear to be compatible with accurate assays. This preparation is, in fact, particularly suitable for the study of slow-acting drugs, since it is unnecessary to expose the tissue to the possibly deleterious effect of the drugs for long periods while slow effects are actually recorded on the drum. The muscle records its response some time after the drug has been washed away.
Fig. 3.

Rat's uterus superfused. Atropine 10−6. Slow responses to crystalline trypsin (20 mg./l.), and R2 (50 mg./l.) applied for 30 sec. at the signal.
The Assay of Histamine
The concentration of histamine which causes a contraction of superfused guinea-pig's ileum is similar to the final concentration in the bath when more conventional methods are used. The sensitivity of the method is thus similar to that which might be achieved with a bath-volume of 0.2 ml. Kwiatkowski (1941) has already emphasized the value of a method not unlike that used here, but his method is probably less sensitive because the histamine is more diluted and has less time to act.
In the assay shown in Fig. 2 the drugs were applied for 10 sec. at intervals of 2 min. Small concentrations of histamine (1–10 μg./l.) caused fairly regular effects. The experimental design used here gives a quick result. The unknown solutions alternate regularly with the standard solution which is itself used in two different doses alternately. The concentration of the unknown is chosen so as to cause effects intermediate between the effects of the two different doses of the standard, which precede and succeed it. The potency of the unknown is then calculated on the assumption that the effect is linearly related to log dose. The first three assays shown in Fig. 2 illustrate this design. In the fourth assay the sequence of doses was slightly different. Two estimates of the histamine concentration were made from each of these four assays, and the four duplicate results were 2.8, 2.6; 2.6, 2.2; 1.5, 1.6; and 1.7, 1.8 μg./l. These figures illustrate the fact that the superfused intestine behaves with reasonable consistency, but the two estimates in each pair are not independent, and the error of an assay is likely to be greater than that calculated directly from the differences between the duplicates.
Fig. 2.

Guinea-pig's ileum superfused. Approximate assays of four unknown solutions (A–D) in terms of histamine base (H–μg./l.).
Substance R
Tyrode's solution, or de Jalon's solution, which had been perfused through the rat's intestine by the method described above, caused a slow and delayed contraction, when applied to a superfused rat uterus in the presence of atropine (10−6). Samples collected in the first hour were not very active, but during the next 3–6 hours their activity was high and fairly constant. During this time they were generally still effective when diluted 20–50 times with the superfusion fluid. The substance responsible for this effect will be called substance R for convenience in this discussion.
It was soon found that substance R was precipitated by mixing the perfusion fluid with twenty volumes of cold acetone, and dry standard preparations were made in this way. These preparations (R1–R5) contained much inorganic salt, but were reasonably active and were used in most of the experiments. Later it was found that substance R could be precipitated quantitatively by adding only four volumes of cold acetone to the effluent and allowing the mixture to stand in the cold. A preparation made in this way was about five times more active than the earlier preparations (Fig. 4), but none of these preparations gave a clear solution in water.
Fig. 4.

Rat's uterus superfused. Atropine 10−6. Application 30 sec. (except 60 sec. at T*). The effect of crystalline trypsin (T) was irregular, but less than the effect of half the weight of R6. R2 = approx. 1/5 R6.
Fig. 3 shows the effect of the preparation R2 recorded on a fast drum to show the slow way in which the response develops. This figure also shows the response to a crystalline preparation of trypsin, which caused a similar slow response. This action of trypsin was described by Rocha e Silva (1939) in a comprehensive paper on the pharmacology of this substance. The response to substance R or trypsin may be obtained repeatedly, and in good preparations constant effects may be recorded at intervals of 5 min. Assays can be made by altering the concentrations, and these are most conveniently recorded on a slow drum.
The results shown in Fig. 4 prove that the preparation R6 was more active weight for weight than a crystalline preparation of trypsin. A crystalline preparation of chymotrypsin also caused a slow effect like that due to trypsin, but was about 10 times less active.
Substance R does not dialyse through cellophane. In one experiment more than 50% of the original activity was recovered when a solution had been dialysed against running tap water for 21 hr. In another experiment a solution was dialysed for 21 hr. against an equal volume of de Jalon's solution by shaking it in a dialyser like that used by Verney (1926). After this time no activity was detected in the de Jalon's solution and no loss in the original solution.
Neutral solutions of substance R appeared to be stable for at least several hours at room temperature, but were partly destroyed by boiling for 3–5 min. At pH 2 the activity disappeared quite rapidly at room temperature.
Substance R was not destroyed by trypsin or chymotrypsin. A solution containing R3 (5 mg./ml.) and crystalline trypsin (0.5 mg./ml.) was incubated for 1 hr. at 37° C. and then boiled for 3 min. to destroy the enzyme. A similar solution containing boiled trypsin was incubated at the same time and also boiled for 3 min. after the incubation. The amount of activity found in both of these solutions was between 30 and 40% of the original activity. The loss was presumably due to the boiling; the trypsin had no apparent action. Identical results were obtained with chymotrypsin. On the other hand, a preparation of substance P was completely inactivated by both these enzyme preparations in a few minutes (Amin, 1953). These results suffice to show that substance R is not readily destroyed by trypsin or chymotrypsin.
Substance R is not histamine, because histamine does not cause contraction of rat's uterus. It is distinguished from acetylcholine and 5-hydroxytryptamine (HT) by its failure to dialyse, and the slowness of its action, and by the fact that this action survives in the presence of atropine (10−6) or lysergic acid diethylamide (10−7). The latter substance has been found to anatagonize HT on rat's uterus (Gaddum, 1953). Fig. 5 shows that it abolished the effect of HT completely without affecting the response to the preparation R6.
Fig. 5.
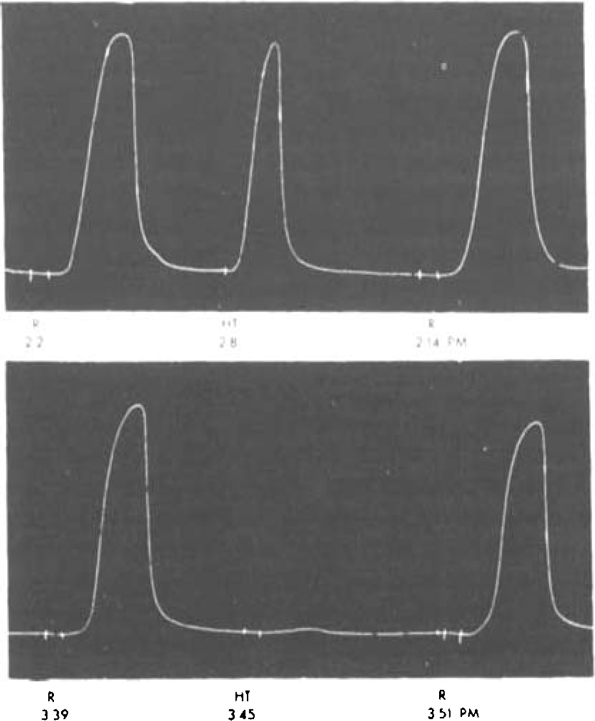
Rat's uterus superfused. Atropine 10−6. R—R6 (20 mg./l.); HT—5-hydroxytryptamine (100 μg./l.). Application for 30 sec. at times marked on record. Response to R relatively slow, From 3.26 p.m. Lysergic acid diethylamide (10−7); this abolished the response to HT, but not that to R6.
Bradykinin (Rocha e Silva, 1951) differs from substance R because it is dialysable and destroyed by chymotrypsin.
The possibility was considered that substance R might be identical with oxytocin, which is known to stimulate rat's uterus in low concentrations (Holton, 1948). This possibility is excluded by the results shown in Figs. 6 and 7. Fig. 6 shows that oxytocin had a good effect in a concentration of 100 μU./ml. (total dose about 25 μU.) and that this was similar to the effect of R2 in a concentration of 0.6 mg. per ml. Both these active solutions were then treated with thioglycollate by the method recommended by Ames, Moore, and van Dyke (1950) for the specific inactivation of posterior pituitary extracts. Oxytocin was almost completely inactivated in 38 min., and R2 survived for 56 min. with its activity at least as great as it had been originally.
Fig. 6.

Rat's uterus superfused. Atropine 10−6. Treatment with thioglycollate destroys oxytocin, but not substance R. R—Ra (60 mg./l.); RT same dose treated 56 min. OX—oxytocin (mU./ml.); OXT 0.2 mU./ml. treated 38 min.
Fig. 7.
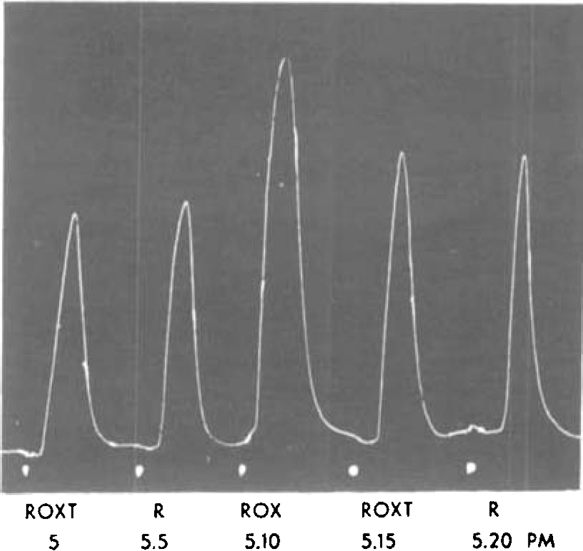
Rat's uterus superfused. Atropine 10−6. R—R2 (250 mg./l.): ROX—the same plus oxytocin (1 mU./ml.), ROXT—ROX treated with thioglycollate from 2.37 p.m. Thioglycollate was effective in destroying oxytocin in the presence of R2.
It might be suggested perhaps that R2 contained some substance which inhibited the action of thioglycollate. In the experiment shown in Fig. 7 oxytocin and R2 were therefore mixed in roughly equivalent amounts and the mixture was treated with thioglycollate. The results show that the mixture was partially inactivated and that the remaining activity was equivalent to that of the preparation R2 which had been used. This shows that thioglycollate was effective in the presence of R2, and is consistent with the view that it has no action on substance R.
Substance R is distinguished from substance P by the fact that it does not dialyse and is not destroyed by trypsin or chymotrypsin. It is also distinguished by the fact that it has comparatively little effect on the guinea-pig's ileum. The preparations R2 and P6 were compared with one another by their actions on guinea-pig's ileum in a bath in the presence of mepyramine. The comparison was difficult, since the effects were dissimilar, but, as judged by the immediate effect, the activity of R2 appeared to be about 0.3% of that of P6. This effect may have been due to a small amount of substance P in the preparation R2. These two preparations were then compared for their effects on superfused rat's uterus. When the maximum effects were similar, the latent period for R2 was longer than that for P6. An assay based on the maximum contraction led to the conclusion that the activity of R2 was now about 50% of that of P6—a result very definitely larger than that obtained on the guinea-pig's ileum (0.3%). This shows that the active substances in R2 and P6 are different.
In another experiment the whole of a rat's small intestine and contents were cut up in acetone, and the insoluble residue was extracted with acid. This extract was compared with the standard preparations P6 and R2 by its action on guinea-pig's ileum in a bath in the presence of mepyramine and atropine. It was also compared with these two standard preparations by its action on superfused rat uterus. The results are shown in Table I.
Table I.
Amount of Activity in the Whole Small Intestine of a Rat
| Using Assay | Guinea-pig Ileum | Rat Uterus | Ratio Ileum/Uterus |
|---|---|---|---|
| P6-equivalent, mg. | 3·3 | 85 | 1/26 |
| R2-equivalent, mg. | 1,150 | 170 | 6·8 |
| Ratio mg.R2/mg.P6 | 350 | 2 | 175 |
The discrepancy between the two estimates of the P6-equivalent is evidence that substance P was not the only substance present in the extract. The discrepancy between the two estimates of the R2-equivalent is evidence that substance R was not the only substance present. The results are compatible with the view that the experiment with guinea-pig's ileum gave a reasonably good estimate of the amount of substance P in the extract and that the experiment with the rat's uterus gave a reasonably good estimate of the amount of substance R. The consequences of this assumption can be calculated by considering the figures in the bottom line of Table I. These show, for example, that P6 was 350 times as active as R2 on the ileum. The P6 equivalent of the amount of substance R assumed to be present is thus 170/350 mg., or about 0.5 mg., which is almost negligible compared with 3.3 mg. This is a maximum estimate of the effect of substance R in this test, based on the assumption that the preparation R2 contained no substance P. If R2 did contain substance P, the figure 350 is too low and the figure 0.5 mg. is too high. It is thus unlikely that substance R seriously interferes with this test for the estimation of substance P except when the proportion of substance R present is even higher than in this experiment.
In the same way P6 was twice as active as R2 on rat's uterus. The R2 equivalent in this test of the amount of substance P which is assumed to be present is thus 3.3 × 2, or 6.6 mg., which is negligible compared with 170. Substance P is therefore unlikely to interfere with the use of the superfused rat's uterus for the estimation of substance R.
Estimates were made by this method of the amount of substance R in different parts of the rat's intestine. The small intestine was roughly divided into three fractions consisting of contents, mucous membrane, and muscle. The contents contained more than the mucous membrane, and the muscle contained little. Activity was also found in the contents of the colon, but not in the contents of the stomach. These data need amplification, but they suggest that substance R may be secreted by some gland into the small intestine.
Discussion
The technique described here is sensitive, and especially suitable for the study of slow-acting substances. The study of substance R by more conventional methods would be more troublesome. This substance cannot be histamine, acetylcholine, 5-hydroxytryptamine, oxytocin, or substance P. The only substances found to have an action as slow as that of substance R were trypsin and chymotrypsin. The fact that a simple acetone precipitate was actually more active than crystalline preparations of these enzymes suggests that substance R is not trypsin or chymotrypsin, but more evidence is required. The known properties of substance R are similar to those of kallikrein (Werle and Berek, 1950). The possibility that these two substances are the same has not yet been fully explored. However this may be, the fact remains that substance R may appear as a complication of experiments on the pharmacological activity of extracts of intestine, or of experiments designed to detect the release of active substances by the intestine under various experimental conditions.
Summary
The word superfusion is used to describe experiments in which suitable liquids run over tissues suspended in air. A new method of applying drugs to such tissues is described.
The value of superfused guinea-pig's ileum for the assay of histamine is confirmed.
Superfused rat's uterus may be used in assays involving oxytocin, the effective dose of which is about 0.02 milliunits.
Superfusion is particularly suitable for the study of slow-acting substances. This is illustrated by the analysis of the properties of “substance R,” which comes from rat's intestine and causes a slow contraction of rat's uterus. This substance does not dialyse through cellophane, and is insoluble in 80% cold acetone and rather unstable in watery solution. Its properties resemble those of some proteolytic enzymes, but its identity has still to be established.
References
- Ames RG, Moore DH, van Dyke HB. Endocrinol. 1950;46:215. [Google Scholar]
- Amin AH. 1953. Unpublished.
- Euler USv. Acta physiol. scand. 1942;4:373. [Google Scholar]
- Finkleman B. J. Physiol. 1930;70:145. doi: 10.1113/jphysiol.1930.sp002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH. J. Physiol. 1953;121:15P. [PubMed] [Google Scholar]
- Gaddum JH, Jang CS, Kwiatkowski H. J. Physiol. 1939;96:104. doi: 10.1113/jphysiol.1939.sp003760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Peart WS, Vogt M. J. Physiol. 1949;108:467. doi: 10.1113/jphysiol.1949.sp004350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton P. Brit. J. Pharmacol. 1948;3:328. doi: 10.1111/j.1476-5381.1948.tb00396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski H. J. Physiol. 1941;100:147. doi: 10.1113/jphysiol.1941.sp003931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus R. 1930. Lane Lecture, Stanford University, Pub. 2.
- Rocha e Silva M. Arquivos Inst. Biol. São Paulo. 1939;10:93. [Google Scholar]
- Rocha e Silva M. Bradicinina. 1951 São Paulo, 1951. [Google Scholar]
- Verney EB. J. Physiol. 1926;61:319. doi: 10.1113/jphysiol.1926.sp002296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werle E, Berek U. Biochem. Z. 1950;320:136. [Google Scholar]


