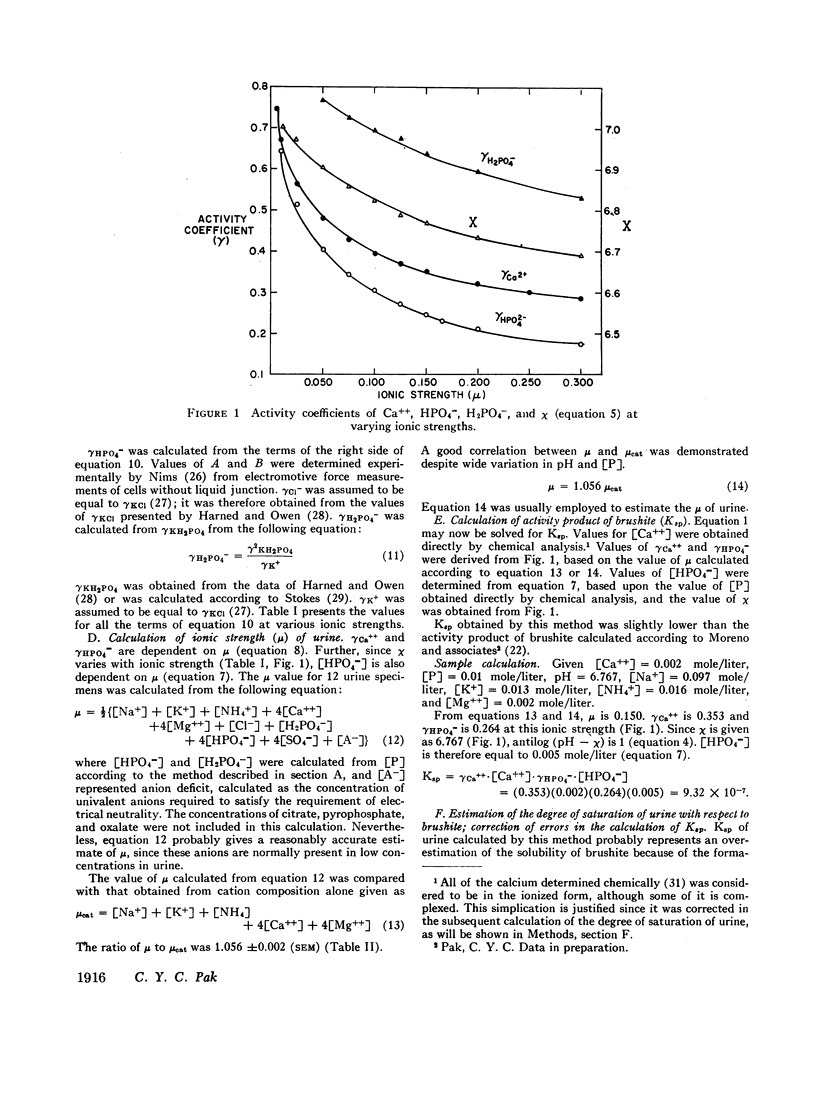
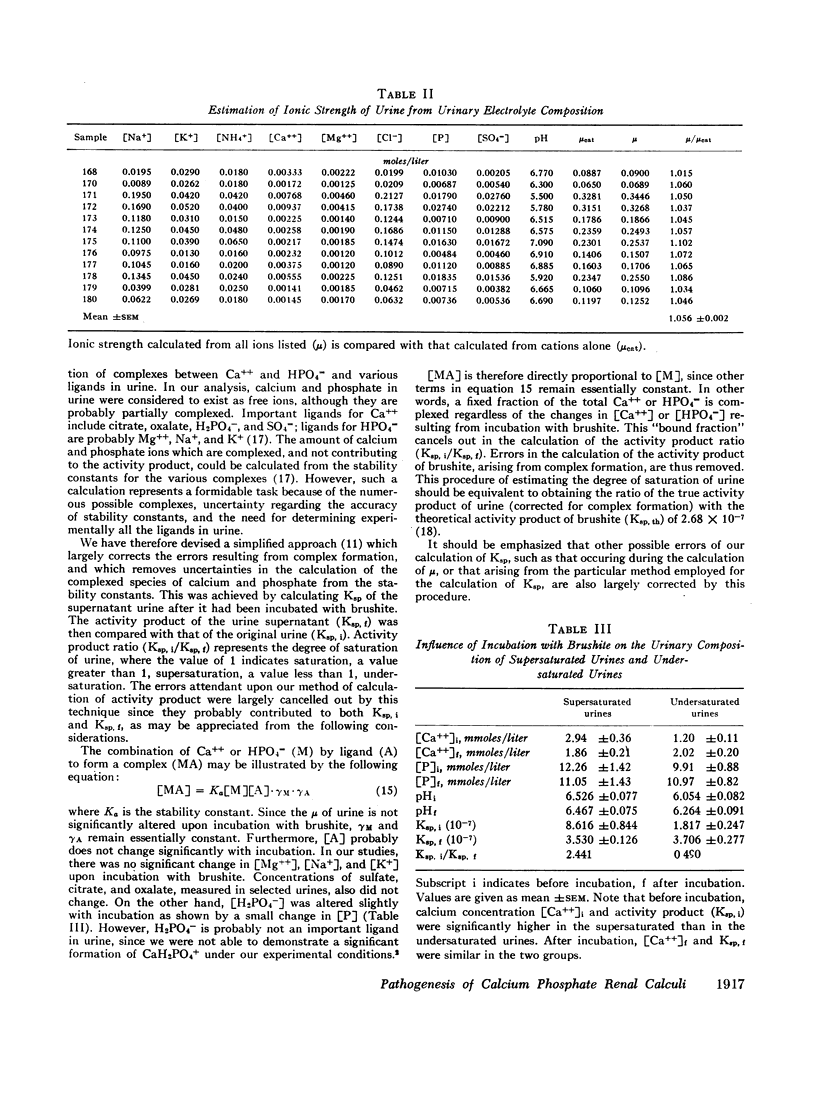
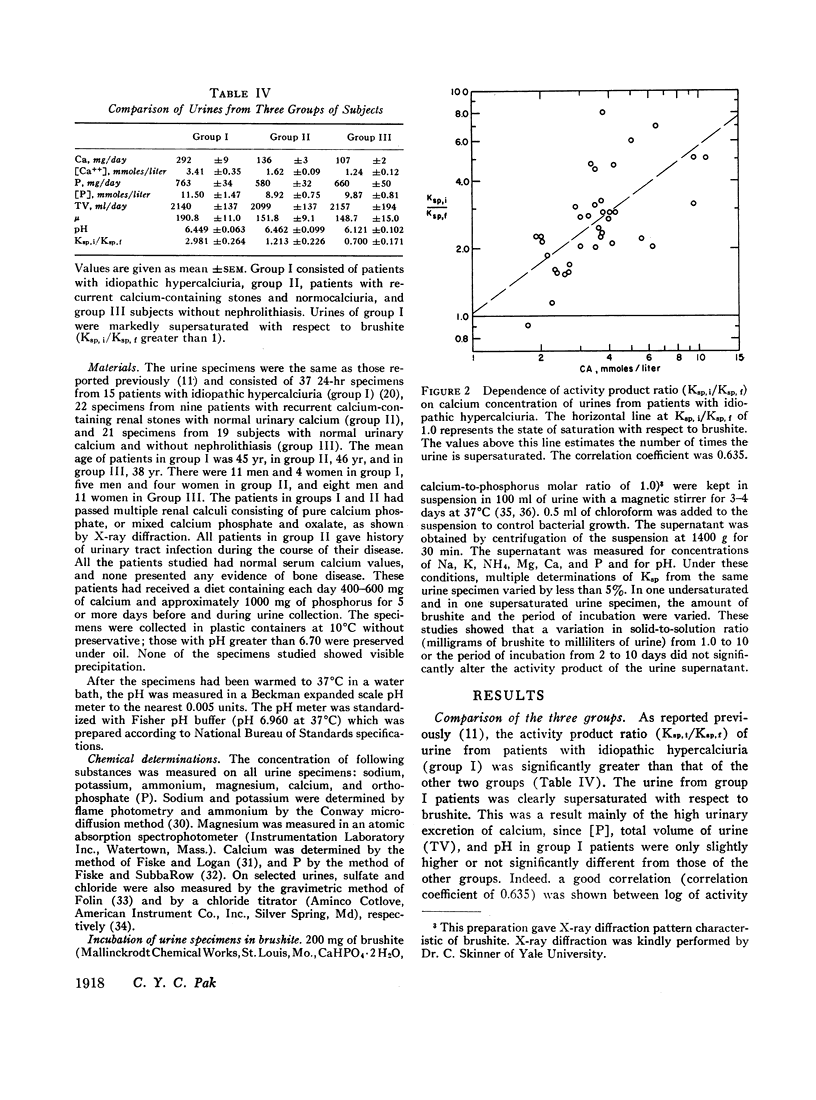
Abstract
Brushite (CaHPO4·2H2O) was considered to govern the formation of renal calculus of calcium phosphate origin. The degree of saturation of urine with respect to this phase was therefore calculated. This value was obtained from the ratio of the activity product of Ca++ and HPO4m (Ksp) before and after incubation of urine with brushite. The errors in the calculation of Ksp were largely eliminated by this procedure.
The urine of patients with idiopathic hypercalciuria and recurrent calcium-containing renal calculi was supersaturated with respect to brushit largely because of the high urinary concentration of Ca++. The urine of normocalciuric subjects was undersaturated except at high urinary pH. This technique of estimating the degree of saturation of urine should allow a quantitative assessment of the various therapeutic regimens recommended for patients with nephrolithiasis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS J. C., YARBRO C. L., GOLBY R. L., SELL I. T. Studies on the mechanism of formation of renal calculi. I. J Urol. 1955 Jun;73(6):930–937. doi: 10.1016/S0022-5347(17)67501-3. [DOI] [PubMed] [Google Scholar]
- BOYCE W. H., KING J. S., Jr Present concepts concerning the origin of matrix and stones. Ann N Y Acad Sci. 1963 Mar 5;104:563–578. doi: 10.1111/j.1749-6632.1963.tb17693.x. [DOI] [PubMed] [Google Scholar]
- Boyce W. H. Organic matrix of human urinary concretions. Am J Med. 1968 Nov;45(5):673–683. doi: 10.1016/0002-9343(68)90203-9. [DOI] [PubMed] [Google Scholar]
- COTLOVE E., TRANTHAM H. V., BOWMAN R. L. An instrument and method for automatic, rapid, accurate, and sensitive titration of chloride in biologic samples. J Lab Clin Med. 1958 Mar;51(3):461–468. [PubMed] [Google Scholar]
- ELLIOT J. S. Calcium phosphate solubility in urine. J Urol. 1957 Feb;77(2):269–274. doi: 10.1016/S0022-5347(17)66552-2. [DOI] [PubMed] [Google Scholar]
- Eanes E. D., Posner A. S. Intermediate phases in the basic solution preparation of alkaline earth phosphates. Calcif Tissue Res. 1968 Jul 15;2(1):38–48. doi: 10.1007/BF02279192. [DOI] [PubMed] [Google Scholar]
- Elliot J. S. Urinary calculus disease. Surg Clin North Am. 1965 Dec;45(6):1393–1404. doi: 10.1016/s0039-6109(16)37730-1. [DOI] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S., CARE A. D. EFFECT OF ORTHOPHOSPHATE ON URINARY PYROPHOSPHATE EXCRETION AND THE PREVENTION OF UROLITHIASIS. Lancet. 1964 May 16;1(7342):1065–1067. doi: 10.1016/s0140-6736(64)91267-x. [DOI] [PubMed] [Google Scholar]
- FLEISCH H., BISAZ S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am J Physiol. 1962 Oct;203:671–675. doi: 10.1152/ajplegacy.1962.203.4.671. [DOI] [PubMed] [Google Scholar]
- Fleisch H. Some new concepts on the pathogenesis and the treatment of urolithiasis. Urol Int. 1965;19(6):372–389. doi: 10.1159/000279353. [DOI] [PubMed] [Google Scholar]
- HENNEMAN P. H., BENEDICT P. H., FORBES A. P., DUDLEY H. R. Idiopathic hypercaicuria. N Engl J Med. 1958 Oct 23;259(17):802–807. doi: 10.1056/NEJM195810232591702. [DOI] [PubMed] [Google Scholar]
- Howard J. E., Thomas W. C., Jr, Barker L. M., Smith L. H., Wadkins C. L. The recognition and isolation from urine and serum of a peptide inhibitor to calcification. Johns Hopkins Med J. 1967 Mar;120(3):119–136. [PubMed] [Google Scholar]
- Howard J. E., Thomas W. C., Jr Control of crystallization in urine. Am J Med. 1968 Nov;45(5):693–699. doi: 10.1016/0002-9343(68)90205-2. [DOI] [PubMed] [Google Scholar]
- MacGregor J., Robertson W. G., Nordin B. E. Octocalcium phosphate: the phase governing the solubility equilibrium in apatitic calculi. Br J Urol. 1965 Oct;37(5):518–524. doi: 10.1111/j.1464-410x.1965.tb09640.x. [DOI] [PubMed] [Google Scholar]
- NEMAN W. F., TORIBARA T. Y., MULRYAN B. J. Synthetic hydroxyapatite crystals. 1. Sodium and potassium fixation. Arch Biochem Biophys. 1962 Sep;98:384–390. doi: 10.1016/0003-9861(62)90202-3. [DOI] [PubMed] [Google Scholar]
- Nordin B. E., Robertson W. G. Calcium phosphate and oxalate ion-products in normal and stone-forming urines. Br Med J. 1966 Feb 19;1(5485):450–453. doi: 10.1136/bmj.1.5485.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIEN E. L. Studies in urolithiasis. III. Physicochemical principles in stone formation and prevention. J Urol. 1955 Apr;73(4):627–652. doi: 10.1016/S0022-5347(17)67448-2. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Bartter F. C. Ionic interaction with bone mineral. I. Evidence for an isoionic calcium exchange with hydroxyapatite. Biochim Biophys Acta. 1967 Jul 25;141(2):401–409. doi: 10.1016/0304-4165(67)90115-8. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Diller E. C., Smith G. W., 2nd, Howe E. S. Renal stones of calcium phosphate: physicochemical basis for their formation. Proc Soc Exp Biol Med. 1969 Mar;130(3):753–757. doi: 10.3181/00379727-130-33648. [DOI] [PubMed] [Google Scholar]
- Pak C. Y., Skinner H. C. Ionic interaction with bone mineral. IV. Varying affinity of synthetic calcium phosphates for Ca2+. Biochim Biophys Acta. 1968 Sep 3;165(2):274–282. doi: 10.1016/0304-4165(68)90055-x. [DOI] [PubMed] [Google Scholar]
- Prien E. L., Prien E. L., Jr Composition and structure of urinary stone. Am J Med. 1968 Nov;45(5):654–672. doi: 10.1016/0002-9343(68)90202-7. [DOI] [PubMed] [Google Scholar]
- Robertson W. G., Peacock M., Nordin B. E. Activity products in stone-forming and non-stone-forming urine. Clin Sci. 1968 Jun;34(3):579–594. [PubMed] [Google Scholar]
- SCHWARTZ W. B., BANK N., CUTLER R. W. The influence of urinary ionic strength on phosphate pK2' and the determination of titratable acid. J Clin Invest. 1959 Feb;38(2):347–356. doi: 10.1172/JCI103808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERMEULEN C. W., LYON E. S., FRIED F. A. ON THE NATURE OF THE STONE-FORMING PROCESS. J Urol. 1965 Aug;94:176–186. doi: 10.1016/S0022-5347(17)63596-1. [DOI] [PubMed] [Google Scholar]
- VERMEULEN C. W., LYON E. S., GILL W. B. ARTIFICIAL URINARY CONCRETIONS. Invest Urol. 1964 Jan;1:370–386. [PubMed] [Google Scholar]
- VERMEULEN C. W., LYON E. S., MILLER G. H. Calcium phosphate solubility in urine as measured by a precipitation test; experimental urolithiasis. XIII. J Urol. 1958 Mar;79(3):596–606. doi: 10.1016/S0022-5347(17)66313-4. [DOI] [PubMed] [Google Scholar]
- Vermeulen C. W., Lyon E. S. Mechanisms of genesis and growth of calculi. Am J Med. 1968 Nov;45(5):684–692. doi: 10.1016/0002-9343(68)90204-0. [DOI] [PubMed] [Google Scholar]


