Abstract
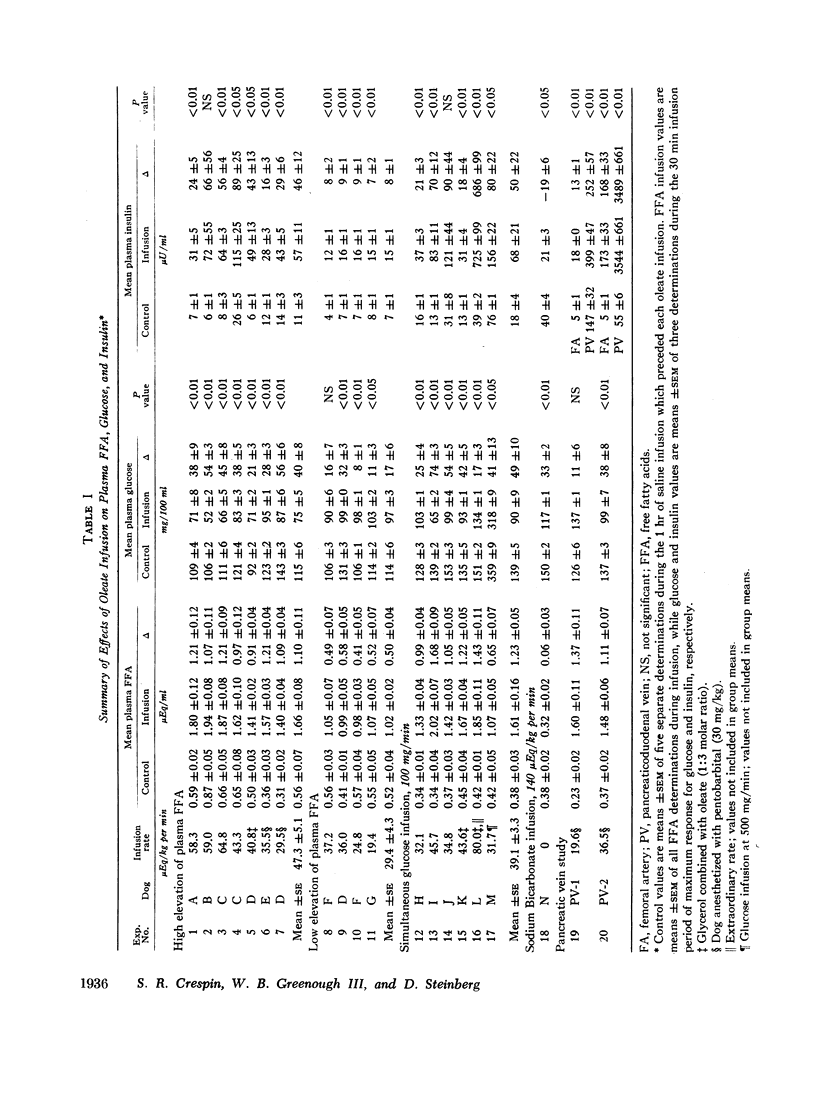
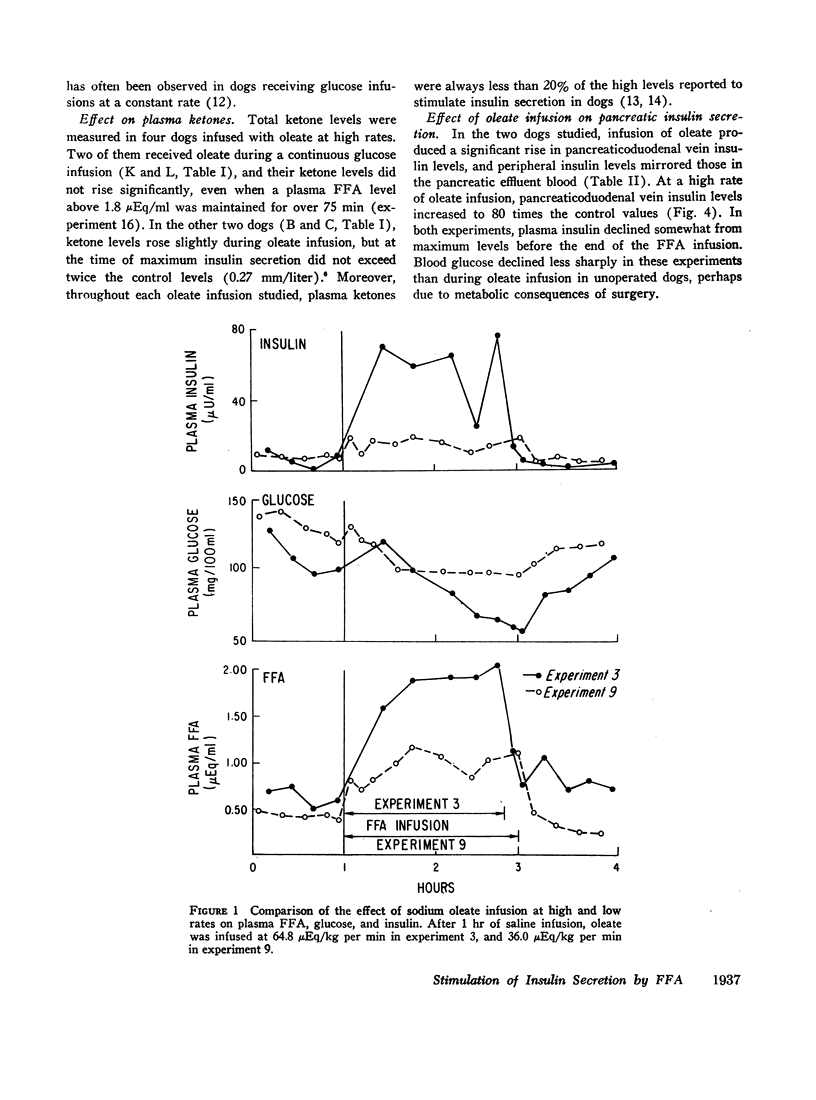
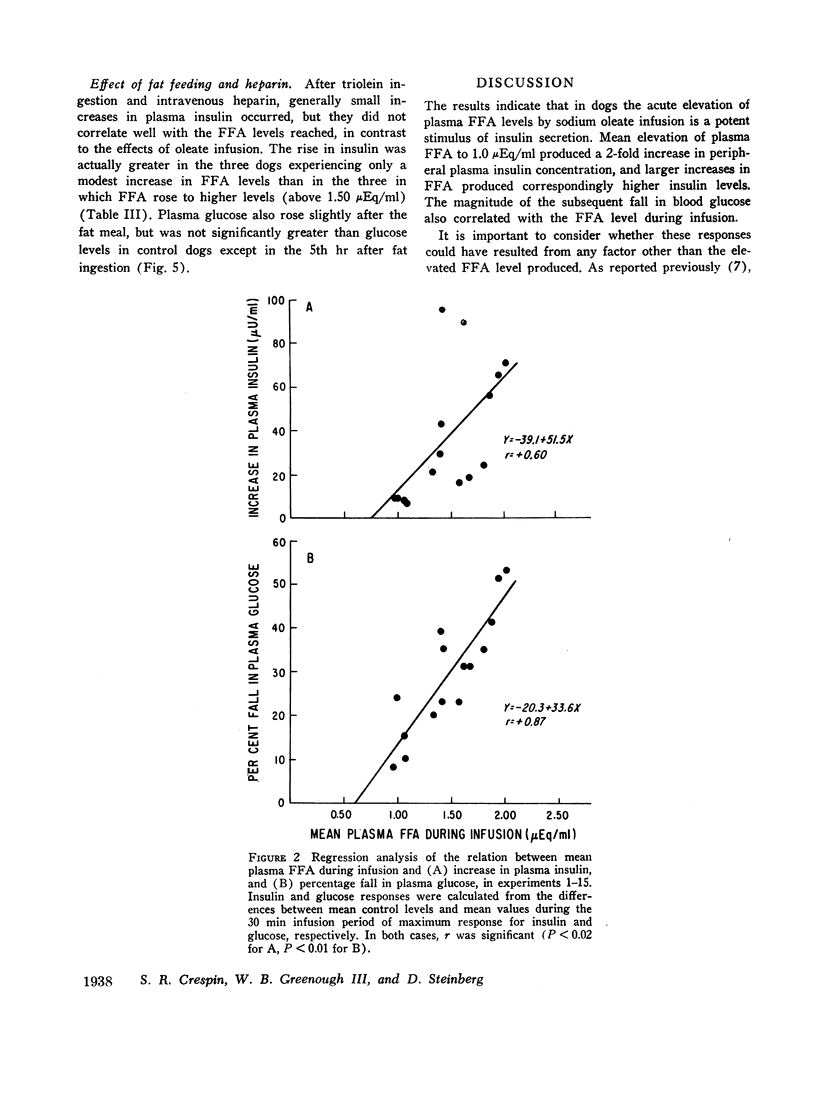
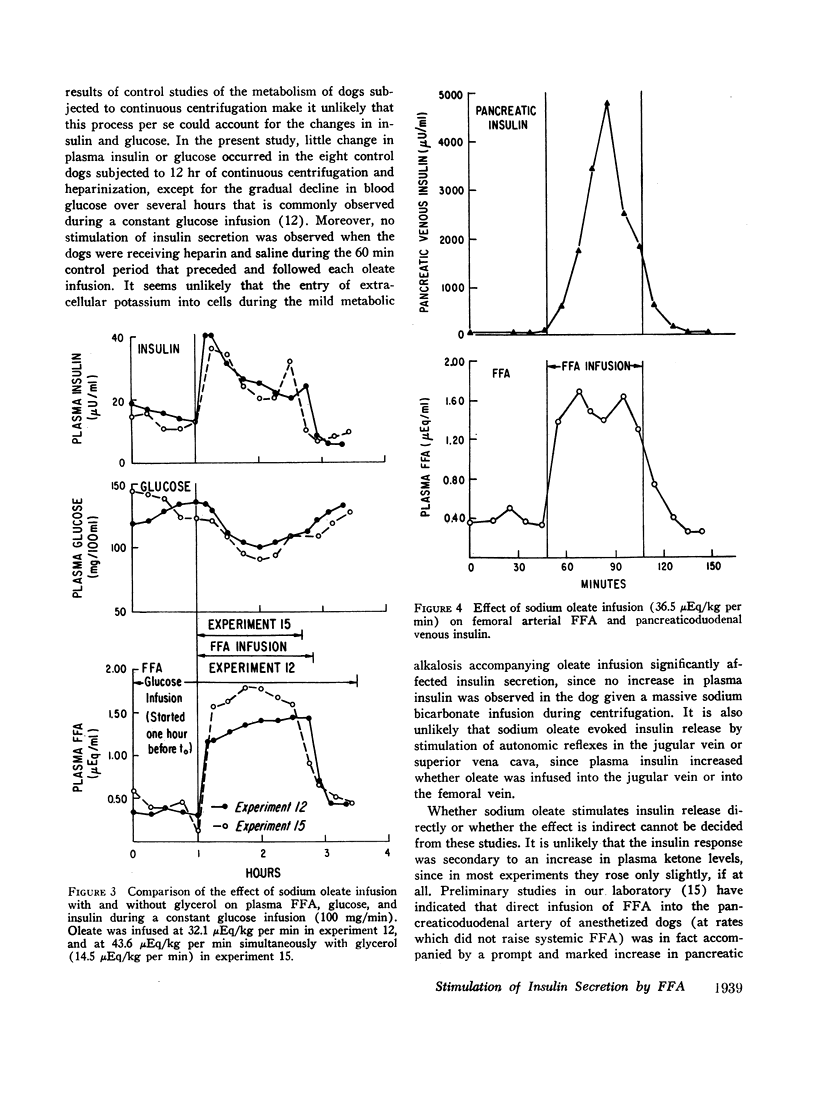
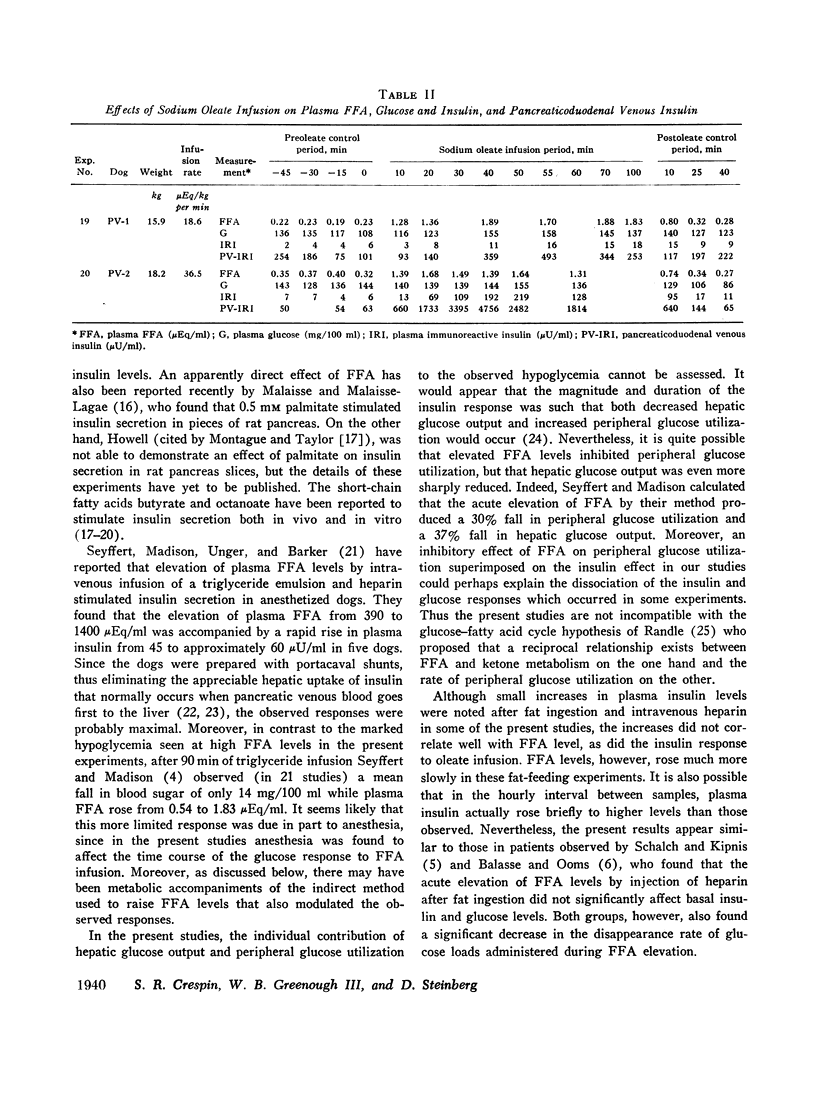
The acute elevation of plasma free fatty acid (FFA) levels by direct infusion of sodium oleate into the plasma of conscious dogs was accompanied by the rapid onset of a 2- to 12-fold increase in plasma immunoreactive insulin, and, subsequently, a marked fall in plasma glucose, even in dogs receiving intravenous glucose throughout the infusion. The magnitude of both the insulin and glucose responses correlated with the mean FFA level during infusion. A large increase in plasma insulin and fall in glucose also occurred when glycerol was infused with oleate in order to simulate endogenous lipolysis more closely. Insulin levels in pancreaticoduodenal vein blood rose markedly during oleate infusion, while plasma ketone levels rose only slightly.
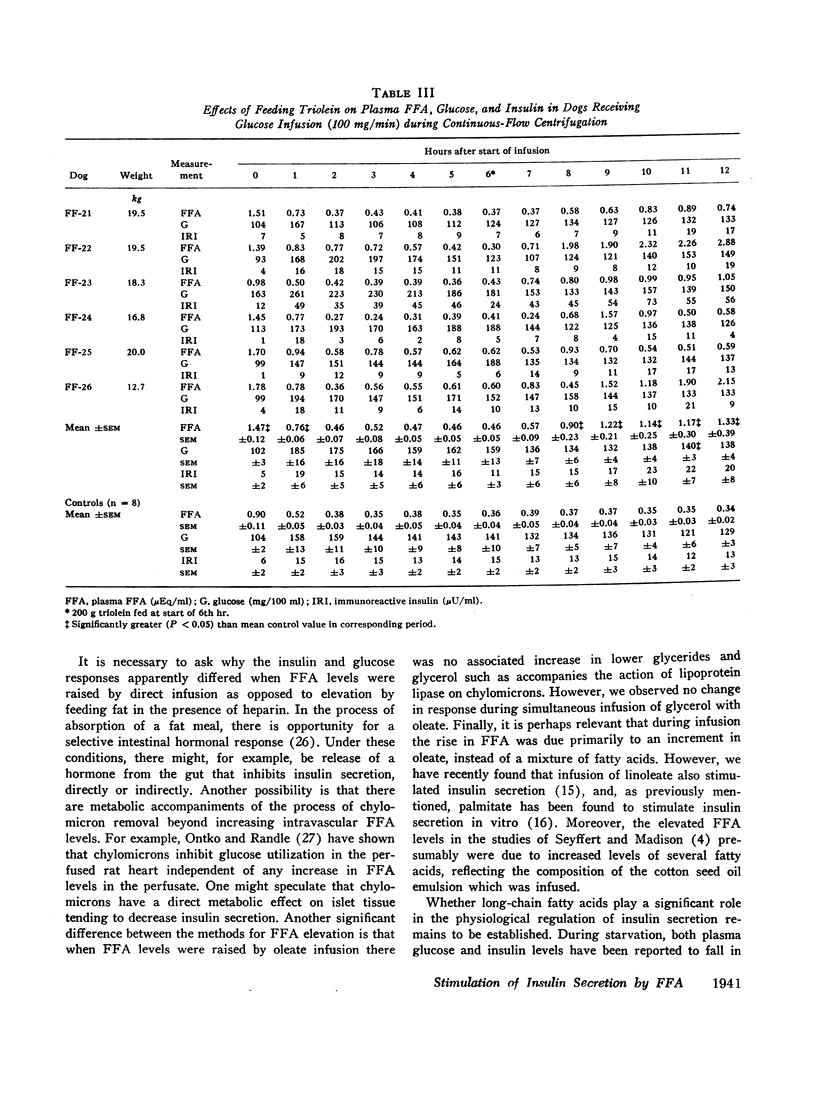
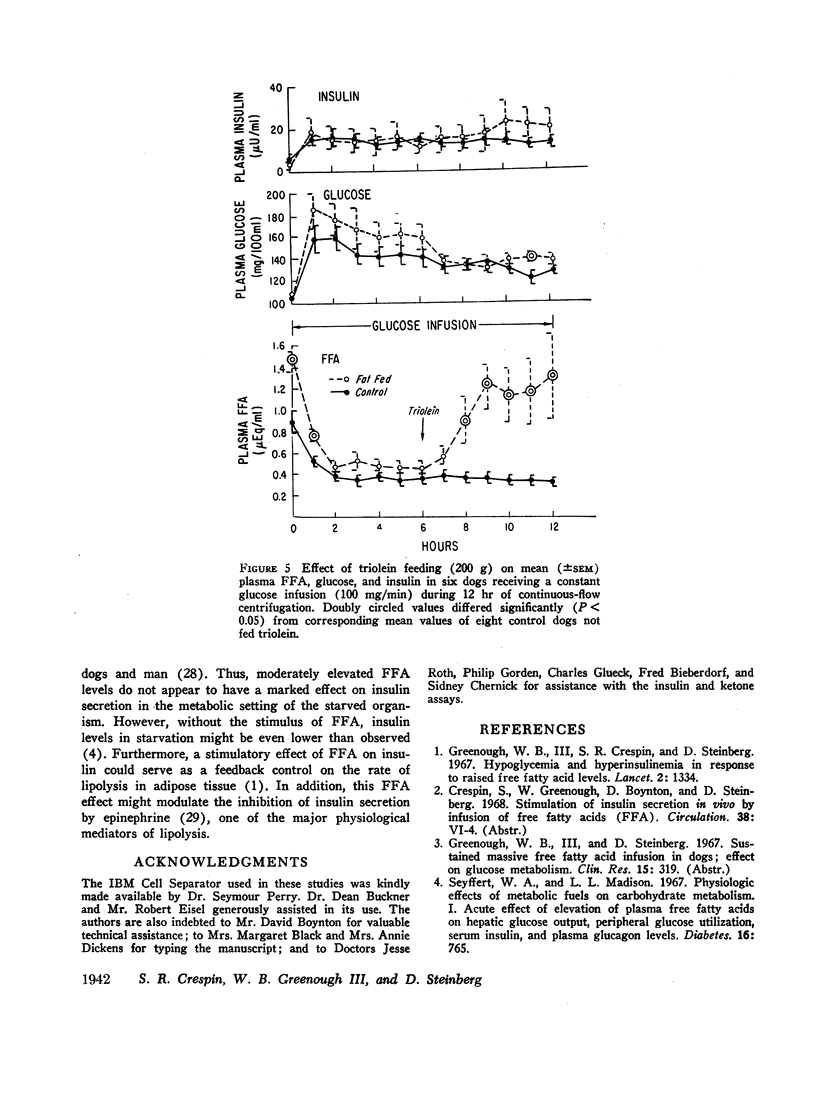
In contrast to the effects of oleate infusion, elevation of plasma FFA to correspondingly high levels by triolein ingestion and intravenous heparin produced only small increases in plasma insulin, which did not correlate well with the FFA level reached, and small increases in plasma glucose.
The results indicate that under certain conditions elevated FFA levels may be a potent stimulus of insulin secretion. This response is modified under other conditions such as during chylomicron removal under the influence of heparin. This effect may play a role in the regulation of lipolysis and ketone formation, but determination of the exact mechanism of FFA stimulation of the pancreas and its physiological significance will require further investigation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balasse E., Ooms H. A. Effet d'une élévation aiguë du taux des acides gras libres (nefa) sur la tolérance glucidique et la réponse insulinique a l'hyperglycémie chez l'homme normal. Rev Fr Etud Clin Biol. 1968 Jan;13(1):62–67. [PubMed] [Google Scholar]
- FELTS P. W., CROFFORD O. B., PARK C. R. EFFECT OF INFUSED KETONE BODIES ON GLUCOSE UTILIZATION IN THE DOG. J Clin Invest. 1964 Apr;43:638–646. doi: 10.1172/JCI104949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freinkel N., Metzger B. E. Oral glucose tolerance curve and hypoglycemias in the fed state. N Engl J Med. 1969 Apr 10;280(15):820–828. doi: 10.1056/NEJM196904102801508. [DOI] [PubMed] [Google Scholar]
- Greenough W. B., 3rd, Crespin S. R., Steinberg D. Hypoglycaemia and hyperinsulinaemia in response to raised free-fatty-acid levels. Lancet. 1967 Dec 23;2(7530):1334–1336. doi: 10.1016/s0140-6736(67)90917-8. [DOI] [PubMed] [Google Scholar]
- Greenough W. B., 3rd, Crespin S. R., Steinberg D. Infusion of long-chain fatty acid anions by continuous-flow centrifugation. J Clin Invest. 1969 Oct;48(10):1923–1933. doi: 10.1172/JCI106159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADISON L. L., COMBES B., ADAMS R., STRICKLAND W. The physiological significance of the secretion of endogenous insulin into the portal circulation. III. Evidence for a direct immediate effect of insulin on the balance of glucose across the liver. J Clin Invest. 1960 Mar;39:507–522. doi: 10.1172/JCI104065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADISON L. L., MEBANE D., UNGER R. H., LOCHNER A. THE HYPOGLYCEMIC ACTION OF KETONES. II. EVIDENCE FOR A STIMULATORY FEEDBACK OF KETONES ON THE PANCREATIC BETA CELLS. J Clin Invest. 1964 Mar;43:408–415. doi: 10.1172/JCI104925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEBANE D., MADISON L. L. HYPOGLYCEMIC ACTION OF KETONES. I. EFFECTS OF KETONES ON HEPATIC GLUCOSE OUTPUT AND PERIPHERAL GLUCOSE UTILIZATION. J Lab Clin Med. 1964 Feb;63:177–192. [PubMed] [Google Scholar]
- Madison L. L., Seyffert W. A., Jr, Unger R. H., Barker B. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism. 1968 Apr;17(4):301–304. doi: 10.1016/0026-0495(68)90097-8. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F. Stimulation of insulin secretion by noncarbohydrate metabolites. J Lab Clin Med. 1968 Sep;72(3):438–448. [PubMed] [Google Scholar]
- Manns J. G., Boda J. M. Insulin release by acetate, propionate, butyrate, and glucose in lambs and adult sheep. Am J Physiol. 1967 Apr;212(4):747–755. doi: 10.1152/ajplegacy.1967.212.4.747. [DOI] [PubMed] [Google Scholar]
- Montague W., Taylor K. W. Regulation of insulin secretion by short chain fatty acids. Nature. 1968 Mar 2;217(5131):853–853. doi: 10.1038/217853a0. [DOI] [PubMed] [Google Scholar]
- Porte D., Jr, Graber A. L., Kuzuya T., Williams R. H. The effect of epinephrine on immunoreactive insulin levels in man. J Clin Invest. 1966 Feb;45(2):228–236. doi: 10.1172/JCI105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RANDLE P. J., GARLAND P. B., HALES C. N., NEWSHOLME E. A. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963 Apr 13;1(7285):785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Sanbar S. S., Evans J. R., Lin B., Hetenyi G., Jr Further studies on the effect of octanoate on glucose metabolism in dogs. Can J Physiol Pharmacol. 1967 Jan;45(1):29–38. doi: 10.1139/y67-003. [DOI] [PubMed] [Google Scholar]
- Sanbar S. S., Martin J. M. Stimulation by octanoate of insulin release from isolated rat pancreas. Metabolism. 1967 May;16(5):482–484. doi: 10.1016/0026-0495(67)90140-0. [DOI] [PubMed] [Google Scholar]
- Schalch D. S., Kipnis D. M. Abnormalities in carbohydrate tolerance associated with elevated plasma nonesterified fatty acids. J Clin Invest. 1965 Dec;44(12):2010–2020. doi: 10.1172/JCI105308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyffert W. A., Jr, Madison L. L. Physiologic effects of metabolic fuels on carbohydrate metabolism. I. Acute effect of elevation of plasma free fatty acids on hepatic glucose output, peripheral glucose utilization, serum insulin, and plasma glucagon levels. Diabetes. 1967 Nov;16(11):765–776. doi: 10.2337/diab.16.11.765. [DOI] [PubMed] [Google Scholar]
- Soeldner J. S., Slone D. Critical variables in the radioimmunoassay of serum insulin using the double antibody technic. Diabetes. 1965 Dec;14(12):771–779. doi: 10.2337/diab.14.12.771. [DOI] [PubMed] [Google Scholar]
- Vance J. E., Buchanan K. D., Williams R. H. Effect of starvation and refeeding on serum immunoreactive glucagon and insulin levels. J Lab Clin Med. 1968 Aug;72(2):290–297. [PubMed] [Google Scholar]



