The specific antagonism of some actions of histamine by low concentrations of antihistamine drugs characterizes one type of histamine receptor for which we suggest the symbol H1. Such receptors occur in guinea-pig ileum and bronchi (Arunlakshana & Schild, 1959). Several other actions of histamine, for example, stimulation of gastric acid secretion, inhibition of rat uterus and stimulation of isolated atria, cannot be specifically antagonized (Loew, 1947; Ashford, Heller & Smart. 1949; Dutta, 1949; Trendelenberg, 1960). These actions are likely to be mediated by other histamine receptors.
The effects of many compounds related to histamine have previously been examined on the isolated ileum and uterus of the guinea-pig and the blood pressure of the cat (Dale & Dudley, 1921; Vartiainen, 1935; Walter, Hunt & Fosbinder, 1941; Hunt & Fosbinder, 1942; Niemann & Hayes, 1942; Huebner, Turner & Scholz, 1949; Lee & Jones, 1949; Craver, Barrett, Cameron & Herrold, 1951; Ingle & Taylor, 1963) and on gastric acid secretion (Koch, Luckhardt & Keeton, 1920; Burgess & Ivy, 1932; Sacks, Ivy, Burgess & Vandolah, 1932; Schnedorf & Ivy, 1935; Grossman, Robertson & Rosiere, 1952; Ivy & Liepins, 1958; Lin, Alphin, Henderson & Chen, 1961; Lin, Benslay & Henderson, 1961; Lin, Henderson, Chen & Benslay, 1961), but the effects on smooth muscle and acid secretion have not been directly and quantitatively compared.
In the present work we have compared the characteristics of a typical antihistamine-sensitive receptor in guinea-pig ileum with the histamine receptors in rat uterus and stomach, using histamine analogues selected on the basis of availability and structural relevance. Only a limited number of compounds have been examined, but their relative activities support the differentiation of histamine receptors into at least two types. The results also suggest some correlation between the stimulant activity of histamine analogues on rat stomach and their inhibitory action on rat uterus. However, classification of other histamine receptors must await the discovery of specific antagonists.
The receptors of isoprenaline in rat uterus were differentiated from those for histamine by means of the β-blocking agents dichloroisoprenaline (DCI) and pronethalol.
METHODS
Gastric acid secretion
The method of Rosenoer & Schild (1962) for the continuous recording of gastric acid secretion in the rat was used. This involves perfusion of the stomach of the rat, anaesthetized with urethane, with a phosphate-citrate buffer, and passage of the effluent over a microflow electrode (E.I.L. Type SMF 23) connected to a pH meter (E.I.L. model 23A) and pen recorder (Fielden Servograph Type R.L.I). The meter was connected through a potential dividing resistance network, incorporating a backing-off battery, which enabled an expanded scale to be used. Full scale deflection of the recorder was equivalent to 2.5 pH units. Lister hooded female rats were used weighing 180 to 200 g. Drugs were injected intravenously.
Smooth muscle preparations in vitro
Longitudinal contractions of isolated muscle were recorded isotonically with a frontal writing lever on a smoked drum. An automatic assay apparatus (Boura, Mongar & Schild, 1954) was used with organ baths of 2 to 6 ml. capacity.
Rat uterus
Segments of uterus from rats weighing 180 to 220 g were suspended in oxygenated de Jalon solution at 30° C. Contractions, approximately 80% maximal, were induced by injecting equal doses of carbachol. A dose cycle of 3 to 4 min and contact time of 1 to 1½ min were used with a carbachol concentration of 10−7 to 2 × 10−8. Assays were begun when 10 or more equal carbachol responses had been obtained. Inhibitory drugs were injected 1 min before each third dose of carbachol, and the subsequent reduced response compared with the preceding carbachol control. This procedure is similar to the assay of sympathomimetic amines described by Gaddum & Lembeck (1949) but in their experiments the amines were removed before contraction occurred. Where there was little variation in the controls, alternate carbachol contractions were inhibited and the reductions calculated from the average height of control responses for each assay block. In some experiments acetylcholine 10−6 to 2 × 10−6 was used as the stimulant in place of carbachol.
Guinea-pig ileum
Terminal ileum, from guinea-pigs weighing 250 to 500 g, was suspended in oxygenated Tyrode solution at 37° C. pA2 values were determined by the method of Schild (1947).
Materials
The following compounds were used: histamine acid phosphate (British Drug Houses), carbachol (Aldrich Chemical Co.), N-methylhistamine dihydrobromide (Ely Lilly & Co.), N-benzylhistamine dihydrochloride* (Ward, Blenkinsop & Co.), NN-diethylhistamine dihydrochloride (May & Baker), 4-β-chloroethylimidazole (Ciba), 3,5-β-pyrazolethylamine dihydrochloride (I.C.I.), 2-β-pyridylethylamine and 4-β-pyridylethylamine (Midland Tar Distillers), β-phenylethylamine hydrochloride (British Drug Houses), dichloroisoprenaline (Burroughs Wellcome & Co.), pronethalol (I.C.I.), diphenhydramine (Parke, Davis & Co.) and mepyramine maleatae and promethazine (May & Baker).
Bases were dissolved in an equivalent of hydrochloric acid for administration. All doses are expressed as concentration of base.
RESULTS
Assays of histamine analogues as stimulants of gastric acid secretion in the rat
Most experiments were planned as 2 + 2 assays in which as least two doses of the analogues and two of histamine were administered in random order in the same rat. As the sensitivity of the rat stomach increases during the first two or three responses (Rosenoer & Schild, 1962), before commencing each assay a response to carbachol (1 to 2 μg) was elicited followed by one or two responses to histamine (100 to 200 μg). This allowed the preparation to stabilize and enabled its sensitivity to be judged. In the present experiments the concentration of phosphate-citrate buffer was half that used by Rosenoer & Schild (1962) for carbachol-stimulated secretion. Histamine in doses of 50 to 300 μg then gave adequate pH. changes with flow rates of 1 ± 0.2 ml./min. Typical responses to histamine and some histamine analogues are illustrated in Fig. 1. The analogues, like histamine, gave graded responses, each lasting approximately 45 min.
Fig. 1.
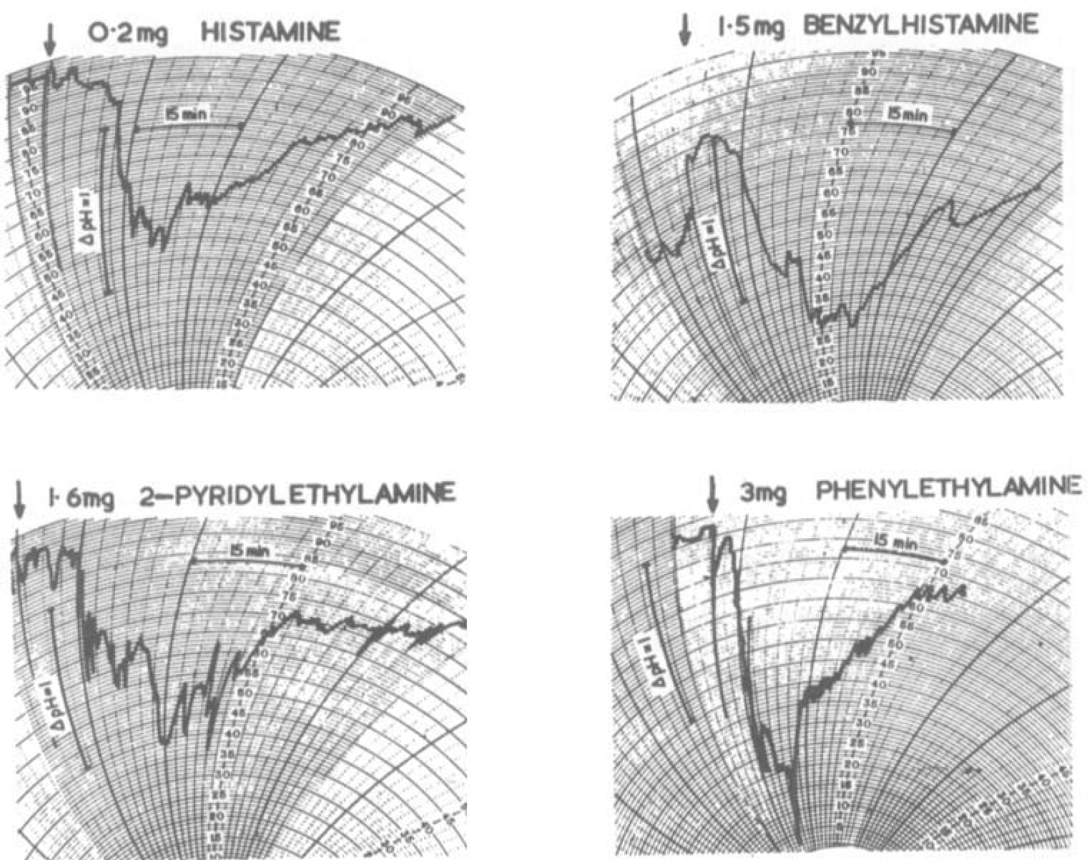
Responses of different perfused rat stomach preparations; change of pH of effluent following intravenous injections of stimulants.
The secretory activities, compared with histamine, of some related compounds are summarised in Table 1. The effect of NN-diethylhistamine (IV) was also examined with the buffer-perfused cat stomach. The activity relative to histamine agreed closely with that obtained using the rat. Appreciable variations in sensitivity may nevertheless occur between animals (Table 1) but as each animal served as its own control the validity of the assays was only affected by the extent to which sensitivity varied differentially between histamine and each analogue. This variability was most marked with the pyrazolethylamine (Table 1, VI). The pyridylethylamines (VII and VIII) appeared to potentiate subsequent secretory responses. They were therefore assayed on a 2 + 1 basis by first establishing a histamine dose/response relationship and then giving a single injection of the pyridylethylamine.
Table 1.
Inhibition of Rat Gastric Acid Secretion and Isolated Rat Uterus by Compounds Related to Histamine
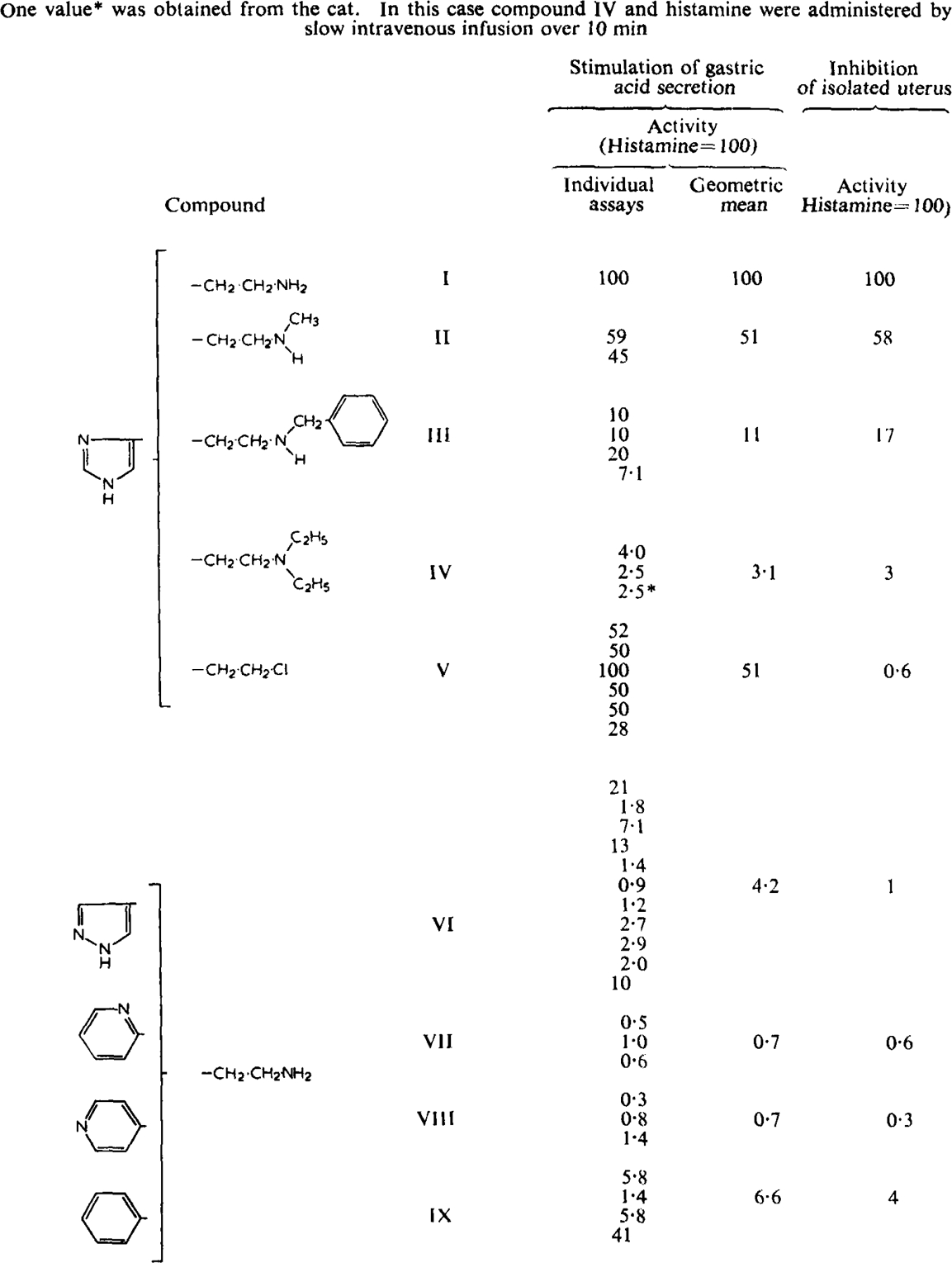 |
β-Phenylethylamine (IX) is included in Table 1 since it stimulated gastric acid secretion in the rat. In the cat, however, it was inactive when administered by slow intravenous infusion in doses up to ten times that effective with histamine.
β-Chloroethylimidazole (V) was examined in more detail. Although it was one of the most powerful stimulants examined, comparable to histamine itself, its secretory action on rat stomach was completely blocked by vagotomy and by atropine (1 mg/kg i.v.) in contrast to that of histamine, which was only slightly reduced by atropine. In the corresponding guinea-pig and cat preparations it was inactive. β-Chlorethylimidazole, lowered the blood pressure of the cat and the rat but its effects were different from those of histamine. They were of very short duration and were associated with reductions in heart rate. The depressor effect of histamine in the cat could be further distinguished from that of β-chloroethylimidazole by promethazine (0.5 mg/kg i.v.) which partially antagonized the effect of histamine but not that of β-chloroethylimidazole.
Another histamine analogue without a basic side chain, 4-β-hydroxyethylimidazole, and imidazole itself were usually inactive but occasionally small erratic increases in rat gastric acid secretion were produced.
2-Mercaptohistamine, when given in appropriate concentration, antagonized the effect of histamine on gastric acid secretion in the rat, but the compound was a partial agonist. It stimulated secretion of gastric acid in the rat at dose levels which varied between animals, precluding its use as a tool for quantitative measurements of antagonism.
Inhibition of isolated rat uterus
Differentiation of the inhibitory actions of isoprenaline from those of histamine by means of β-blocking drugs: The principle of these experiments was to inhibit carbachol contractions with isoprenaline and histamine and determine whether or not both inhibitory effects were equally antagonized by β-blocking agents. Concentrations of histamine and isoprenaline which gave suitably graded inhibition of the carbachol (10−7 to 2 × 10−6) responses were selected by preliminary test and an assay block obtained containing twenty-four responses. Each third injection of carbachol was preceded by a dose of one or other inhibitor varying in random order. The reservoir for the organ bath was then changed to one containing the β-blocking agent in de Jalon's solution. After allowing about 20 min for the tissue to become equilibrated, during which time regular stimulation by carbachol was maintained, a second similar comparative assay of histamine and isoprenaline was performed with the uterus in continued contact with the β-blocking agent. In the presence of dichloroisoprenaline (DCI) (10−8 to 10−9) the inhibitory action of isoprenaline was reduced. In contrast the inhibitions produced by histamine were increased (Fig. 2). Lower concentrations of DCI gave equivocal results and at higher concentrations DCI itself produced too great an inhibition of the carbachol response. A similar differentiation of receptors for histamine and sympathomimetic amines was demonstrated with pronethalol. Neither histamine nor isoprenaline was antagonized by mepyramine (10−9) in the bathing fluid. The histamine inhibitions were slightly enhanced.
Fig. 2.
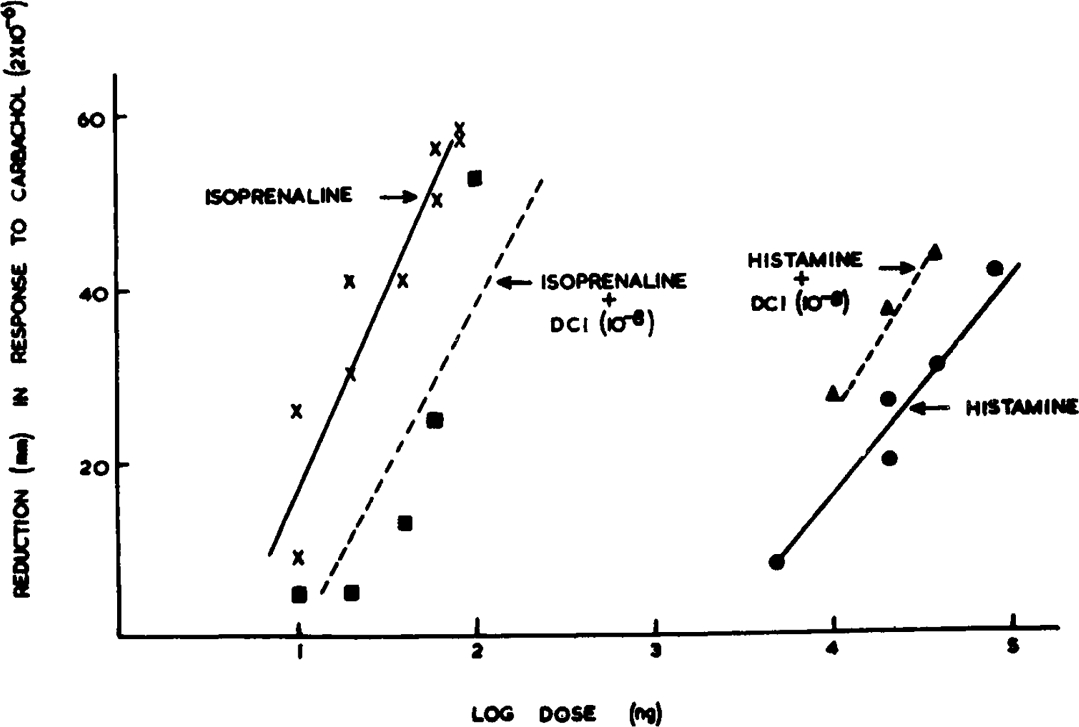
Inhibition of 2 × 10−6 carbachol-induced contractions of rat uterus by isoprenaline and histamine in the presence and absence of dichloroisoprenaline (DCI).
Inhibitory action of histamine analogues: Part of a typical assay with rat uterus is illustrated in Fig. 3. The activities of the analogues in inhibiting carbachol or acetylcholine contractions are given in Table 1. When the activity ratios with histamine are expressed on a logarithmic scale (Fig. 4) the correlation coefficient between the activities of the analogues on rat uterus and rat stomach is r = 0.95 (df = 5, P = 0.001). Chloroethylimidazole (V) which, as already discussed, acts by a different mechanism, was omitted from the calculation.
Fig. 3.
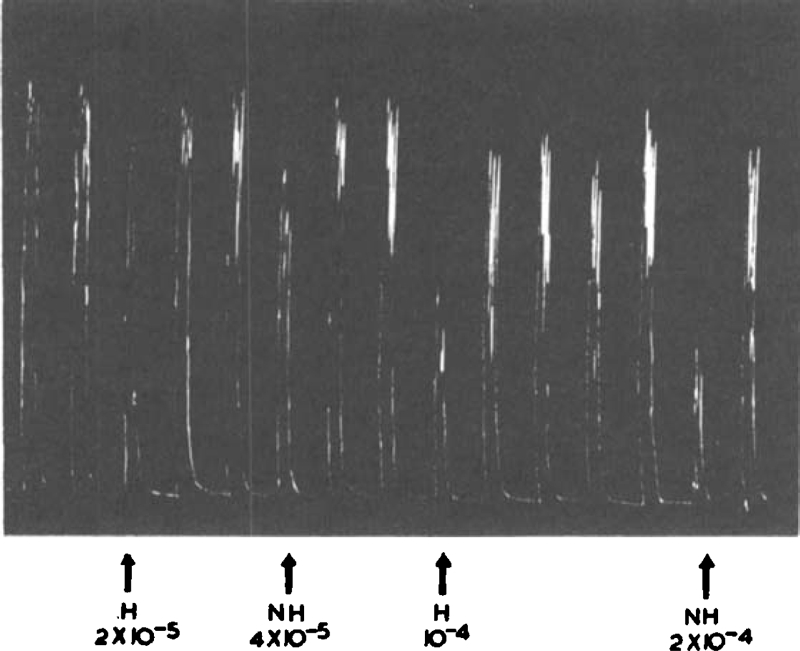
Rat uterus stimulated intermittently by acetylcholine (10−6). Histamine and N-methylhistamine were injected into the bath 1 min before the stimulant; de Jalon solution.
Fig. 4.
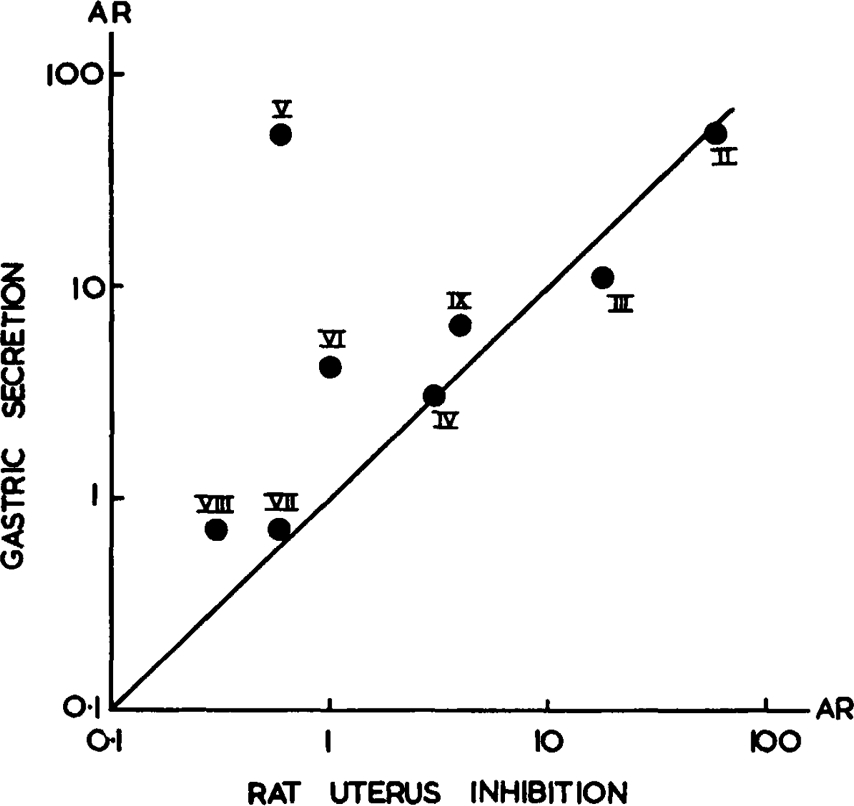
Correlation between activity ratios (A.R.) relative to histamine = 100 by acid secretion and by uterus inhibition assays; log scale; r = 0.95 (omitting V). Line through origin represents equal activity ratios.
The possibility that 4-pyridylethylamine (VIII) and phenylethylamine (IX), which are less closely related to histamine, might be acting on sympathetic receptors was ruled out by their behaviour with DCI. In contrast to isoprenaline neither amine was antagonized by DCI. One of the experiments is illustrated in Fig. 5.
Fig. 5.
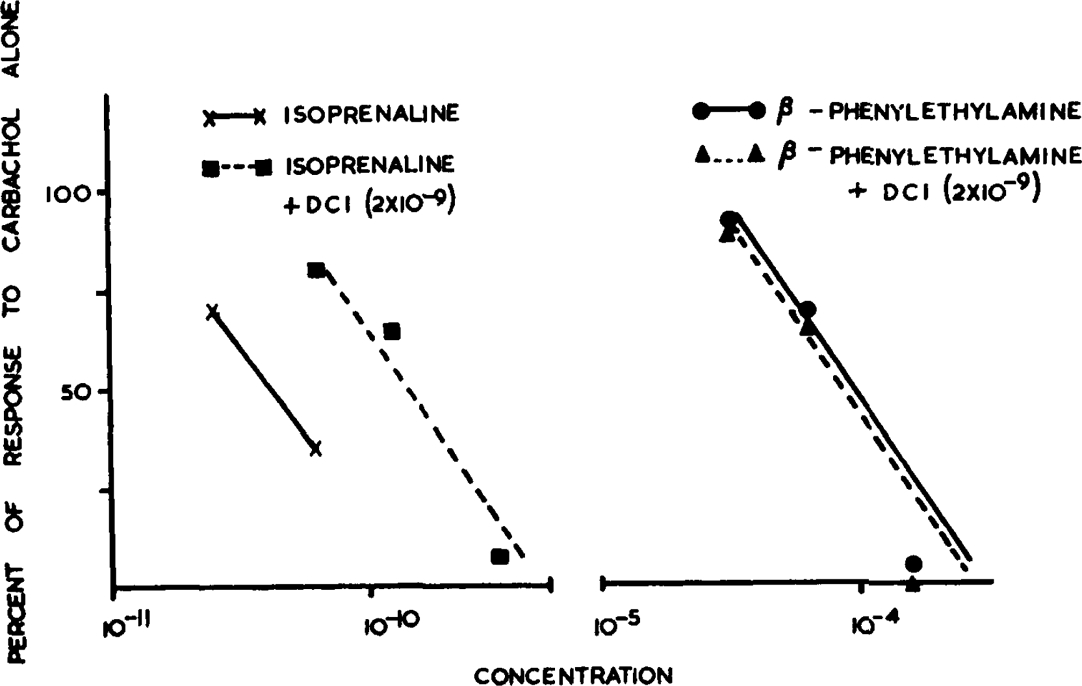
Contractions of rat uterus induced by carbachol (1.3 × 10−6) inhibited by isoprenaline and β-phenylethylamine. Antagonism of isoprenaline by 2 × 10−9 dichloroisoprenaline (DCI).
Stimulation of isolated guinea-pig ileum
Table 2 summarizes the activities of the histamine analogues in stimulating guinea-pig ileum, and the pA2 values of mepyramine against the effective compounds. The structural specificity for high levels of stimulant activity is clearly evident. Substitution of the terminal amino group by methyl had little effect on activity but substitution by larger groups, or modification of the ring structure, markedly diminished activity. The pA2 values varied slightly with the sensitivity of the preparation but in any one comparison they were very similar. The pA2 of mepyramine against 4-β-pyridylethylamine (VIII) was remarkably close to the other values in view of the marginal stimulant activity of this compound. Fig. 6 shows in a more direct way that another antihistamine drug, diphenhydramine, has the same antagonistic activity against histamine and 2- and 4-β-pyridylethylamine.
Table 2.
Stimulation of Isolated Ileum of the Guinea-Pig by Analogues of Histamine and Antagonism by Mepyramine
Each pA2 value obtained with an analogue of histamine is accompanied by a pA2 value obtained with histamine on the same piece of tissue
| Compound No. | Activity (A1) (Histamine = 100) | pA2 Mepyramine against analogue | pA2 Mepyramine against histamine |
|---|---|---|---|
| III | 10 | 9·3 | 9·3 |
| 9·2 | 9·2 | ||
| IV | 1 | 9·3 | 9·3 |
| V | <0·01 | — | — |
| VI | 0·06 | 9·8 | 9·7 |
| VII | 3 | 9·7 | 9·7 |
| VIII | 0·01 | 9·8 | 9·8 |
| 9·7 | |||
| IX | <0·01 | — | — |
Fig. 6.
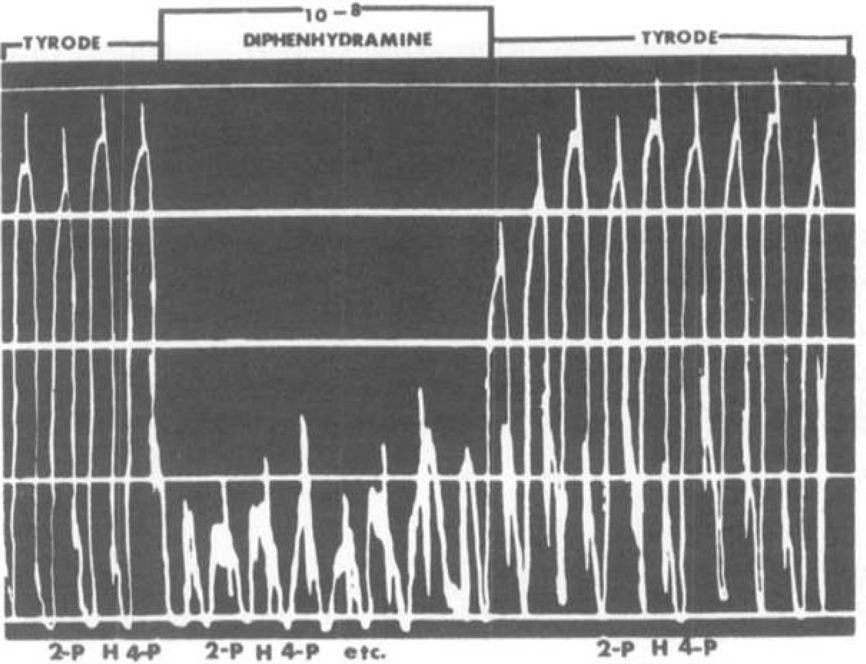
Guinea-pig ileum. Equal antagonism of histamine (H) 0.03 μg/ml., 2-pyridylethylamine (2-P) 0.6 μg/ml. and 4-pyridylethylamine (4-P) 150 μg/ml. by diphenhydramine 10−8. Tyrode solution.
DISCUSSION
Acid gastric secretion
The most satisfactory way of defining receptors is by means of antagonists, but, at present, no specific antagonist is known for the secretory stimulant action of histamine on the stomach. A search for this property in the compounds described, and in other histamine derivatives, has been unproductive. It was therefore only possible to make an examination of gastic receptor characteristics by comparing a series of agonists. Because of their structural relation to histamine, they have been tentatively assumed to act in a similar manner. This assumption may well not be valid for analogues which lack one or more of the reactive groups of histamine. Furthermore, the possibility has not been ruled out that the agonists act indirectly. 3-β-Aminoethyl-1,2,4-triazole (betazole), for example, has recently been shown to release histamine from the glandular stomach of the rat (Haverback, Stubrin, & Dyce, 1965).
A basic side chain is probably required to stimulate the gastric histamine receptor. Interaction of this part of the molecule with the receptor is indicated by the inactivity of β-hydroxyethylimidazole and the effect of substituting the terminal amino group; activity decreased with substitution in the order N-methylhistamine>N-benzylhistamine>NN-diethylhistamine. Although β-chloroethylimidazole had considerable gastric stimulatory activity in the rat this effect was qualitatively quite different from that of histamine. Abolition by atropine and vagotomy suggested that the activity was mediated centrally rather than through the gastric histamine receptor.
The imidazole ring also has a major role in the secretory action of histamine. 3,5-β-Pyrazolethylamine, which stimulates secretion in the dog stomach (Grossman et al., 1952), was active in the rat but much less so than histamine. The lower basicity or reactivity of the pyrazole ring compared with imidazole (Albert, Goldacre & Phillips, 1948) may account for the quantitative difference. Although modification of the imidazole ring results in a marked fall in activity, the spatial relationship of the basic groups in the ring and side chain do not appear to be critical for the gastric receptor. 2- and 4-β-Pyridylethylamines (VII & VIII), for example, are approximately equiactive on rat stomach. It is more surprising that β-phenylethylamine (IX), without a reactive group in the ring, has gastric stimulatory activity in the rat. The gastric effects of this compound, which is inactive in the cat, have not been further investigated. It may produce an increase in gastric acid secretion by a mechanism independent of the histamine receptor; possibly an indirect vascular effect.
Inhibition of rat uterus
Before examining the effects of histamine analogues on isolated rat uterus, the problem had to be solved whether histamine acts on the same receptor as isoprenaline. which also relaxes the preparation. To test this possibility the β-blocking agents dichloroisoprenaline and pronethalol were used. In each case the difficulty arose that the β-blocking agents themselves inhibited the rat uterus, but, within a limited range of concentrations, it was possible to obtain drug antagonism by β-blocking agents without much inhibition of the tissue. Both dichloroisoprenaline and pronethalol reduced the inhibitory effect of isoprenaline in concentrations which either enhanced, or did not modify, the inhibitory effects of histamine. It was therefore concluded that histamine does not act on the β-adrenergic receptor.
The correlation between the activity ratios of the β-substituted ethylamines on the rat stomach and uterus was surprising in view of the contrast between the two preparations. Examination of a larger number of compounds would test whether the simpler in vitro preparation could be used as a preliminary guide to secretory action. It already appears that neither preparation has very exacting requirements, with regard to the structure of the aromatic nucleus, at least for low levels of activity.
The H1 Receptor
For H1 histamine receptors in the guinea-pig ileum, the effects of modifying the side chain of histamine are rather similar to those obtained with rat uterus and stomach. In contrast, modification of the aromatic ring has a greater effect on H1 activity. Of particular interest is the fact that the activities of 2- and 4-β-pyridylethylamines (VII & VIII) on guinea-pig ileum differ by more than 100-fold. This is presumably due to the fact that only the 2-isomer possesses the structure
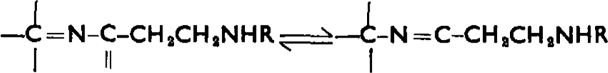 |
(R = alkyl or aralkyl) which is required for considerable H1 activity. Other minimal structural units have previously been suggested (Walter et al., 1941, Niemann and Hayes, 1942, Lee and Jones, 1949). The similarity of pA2 values nevertheless demonstrates that both isomers have an affinity for H1 receptors. Possibly a wide variety of histamine analogues have such an affinity, but only those with rather special structural features have strong intrinsic activity. It appears that if the H1 receptor is defined in terms of antagonists it can be affected by a wider spectrum of compounds than was formerly thought.
It is not yet possible to define structural characteristics of the histamine receptors. Rocha e Silva (1961) has suggested an interesting possible model for histamine receptors of the type found in guinea-pig ileum, but the scheme of interaction does not include the basic ring nitrogen, which is important for H1 histamine activity. Lee & Jones (1949) suggested that this nitrogen might be involved in intramolecular hydrogen bonding with the side chain. The nature of the structurally less-specific rat uterus and gastric histamine receptors is even more obscure. It is possible that the formation of histamineadenine-dinucleotide (HAD) from nicotinamide-adenine-nucleoside (NAD) is a link in at least one type of histamine receptor activation. Alivisatos (1965) has presented evidence for the formation of HAD in vivo and has shown that the relatively inactive histamine derivatives, histidine and imidazole acetic acid, do not displace nicotinamide from NAD. Alivisatos (1960) earlier suggested that this type of reaction, fission of an onium structure by a suitable imidazole, could be the mechanism of hydrogen ion production by the parietal cells. Recent evidence for the physiological participation of histamine as well as gastrin in the secretion of gastric acid (Kahlson Rosengren Svahn & Thunberg, 1964, Code, 1965, Haverback, Stubrin & Dyce, 1965, Levine, 1965, Shore, 1965) may encourage further study of the gastric histamine receptor and a wider search for specific antagonists.
SUMMARY
The characteristics of some histamine receptors were investigated by comparing the relative activities of several histamine analogues. Three preparations were used (i) the perfused rat stomach for gastric secretory action (ii) the isolated rat uterus, stimulated with carbachol, for smooth muscle inhibitory action and (iii) the isolated guinea-pig ileum for smooth muscle stimulatory action.
N-Methylhistamine, N-benzylhistamine, NN-diethylhistamine, β-chloroethylimidazole, 3,5-β-pyrazolethylamine, 2- and 4-β-pyridylethylamine and β-phenlyethylamine stimulated rat gastric acid secretion and inhibited rat uterus. The mode of action of β-chloroethylimidazole was shown to differ from that of histamine. There was a marked correlation between the relative activities of the other compounds on the two preparations.
The receptors for isoprenaline in rat uterus were differentiated from those for histamine by means of the β-blocking drugs dichloroisoprenaline and pronethalol.
On guinea-pig ileum, substitution of the terminal amino group of histamine by methyl had little effect on activity but substitution by larger groups, or modification of the ring, markedly diminished activity.
pA2 values showed that 4-β-pyridylethylamine, as well as compounds more closely related to histamine, has an affinity for histamine receptors in guinea-pig ileum.
The symbol H1 is suggested for receptors which are specifically antagonized by low concentrations of antihistamine drugs.
No specific antagonists were found for the actions of histamine on rat uterus and stomach. These actions are therefore unlikely to be mediated by H1 receptors.
The relative activities of several histamine analogues support the differentiation of histamine receptors into at least two classes.
Acknowledgments
The authors wish to thank Miss Sheliah Tong for her valuable technical assistance. We are grateful to Imperial Chemical Industries, May & Baker Ltd., Midland Tar Distillers and Ward Blenkinsopp & Co. for gifts of compounds, and to Dr Henry Adam for letting us have a rare sample of N-methylhistamine.
Footnotes
For simplicity trivial rather than systematic nomenclature has been used for these compounds. Substituents are attached to the terminal nitrogen atom on the side chain.
REFERENCES
- Albert A, Goldacre R, Phillips J. The strength of heterocyclic bases. J. chem. Soc. 1948:2240–2249. [Google Scholar]
- Alivisatos SGA. Mechanism of the gastric secretion of hydrogen ions. Nature, Lond. 1960;186:368–370. doi: 10.1038/186368a0. [DOI] [PubMed] [Google Scholar]
- Alivisatos SGA. Enzymatic interactions of histamine with pyridine coenzymes. Fedn Proc. 1965;24:769–773. [PubMed] [Google Scholar]
- Arunlakshana O, Mongar JL, Schild HO. Potentiation of pharmacological effects of histamine by histaminase inhibitors. J. Physiol., Lond. 1954;123:32–54. doi: 10.1113/jphysiol.1954.sp005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br. J. Pharmac. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashford CA, Heller H, Smart GA. The effects of antihistamine substances on gastric secretion. Br. J. Pharmac. Chemother. 1949;4:153–161. doi: 10.1111/j.1476-5381.1949.tb00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boura A, Mongar JL, Schild HO. Improved automatic apparatus for pharmacological assays on isolated preparations. Br. J. Pharmac. Chemother. 1954;9:24–30. doi: 10.1111/j.1476-5381.1954.tb00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess JP, Ivy AC. Effect of some imidazoles on gastric secretion. Proc. Soc. exp. Biol. Med. 1930;28:115–116. [Google Scholar]
- Code CF. Histamine and gastric secretion: a later look, 1955–1965. Fedn Proc. 1965;24:1311–1321. [PubMed] [Google Scholar]
- Craver BN, Barrett W, Cameron A, Herrold E. Pharmacological actions of 35 derivatives of 4-methyl, 5-ethyl or 2-substituted imidazoles. Archs int. Pharmacodyn. Ther. 1951;87:33–48. [PubMed] [Google Scholar]
- Dale HH, Dudley HW. The physiological action of β-iminazolylethylamines. J. Pharmac. exp. Ther. 1921;18:103–110. [Google Scholar]
- Dutta NK. Some pharmacological properties common to antihistamine compounds. Br. J. Pharmac. Chemother. 1949;4:281–289. doi: 10.1111/j.1476-5381.1949.tb00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddum JH, Lembeck F. The assay of substances from the adrenal medulla. Br. J. Pharmac. Chemother. 1949;4:401–407. doi: 10.1111/j.1476-5381.1949.tb00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman MI, Robertson C, Rosiere CE. The effect of some compounds related to histamine on gastric acid secretion. J. Pharmac. exp. Ther. 1952;104:277–283. [PubMed] [Google Scholar]
- Haverback BJ, Stubrin MI, Dyce BJ. Relationship of histamine to gastrin and other secretagogues. Fedn Proc. 1965;24:1326–1333. [PubMed] [Google Scholar]
- Huebner CF, Turner RA, Scholz CR. Studies of imidazole compounds. IV. Derivatives of 4-ethylimidazole. J. Am. chem. Soc. 1949;71:3942–3944. [Google Scholar]
- Hunt WH, Fosbinder RJ. A study of some β-2- and β-4-pyridylalkylamines. J. Pharmac. exp. Ther. 1942;75:299–307. [Google Scholar]
- Ingle PHB, Taylor H. Synthesis of some histamine derivatives having potential histamine-like or antihistamine activity. J. Pharm. Pharmac. 1963;15:620–623. doi: 10.1111/j.2042-7158.1963.tb12847.x. [DOI] [PubMed] [Google Scholar]
- Ivy AC, Liepins KW. Effects of derivatives and inhibitors of histamine metabolism on gastric secretion. Am. J. Physiol. 1958;195:521–524. doi: 10.1152/ajplegacy.1958.195.2.521. [DOI] [PubMed] [Google Scholar]
- Kahlson G, Rosengren E, Svahn D, Thunberg R. Mobilization and formation of histamine in the gastric mucosa as related to acid secretion. J. Physiol., Lond. 1964;174:400–416. doi: 10.1113/jphysiol.1964.sp007494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch FC, Luckhardt AB, Keeton R. Gastrin studies. V. Chemical studies on gastrin bodies. Am. J. Physiol. 1920;52:508–520. [Google Scholar]
- Lee HM, Jones RG. The histamine activity of some β-aminoethyl heterocyclic nitrogen compounds. J. Pharmac. exp. Ther. 1949;95:71–78. [PubMed] [Google Scholar]
- Levine RJ. Effect of histidine decarboxylase inhibition on gastric acid secretion in the rat. Fedn Proc. 1965;24:1331–1333. [PubMed] [Google Scholar]
- Lin TM, Alphin RS, Henderson FG, Chen KK. 3-β-Aminoethyl-1,2,4-triazole, a potent stimulant of gastric secretion. J. Pharmac. exp. Ther. 1961;134:88–94. [PubMed] [Google Scholar]
- Lin TM, Benslay DN, Henderson FG. Comparative study of histamine and histamine metabolites on gastric secretion. Fedn. Proc. 1961;20:251. [Google Scholar]
- Lin TM, Henderson FG, Chen KK, Benslay DN. Structure-activity and enzyme substrate relationships of histamine, histamine metabolites and analogues in stimulation of gastric secretion. Int. pharmac. Meet. 1. Stockholm. 1961;7:351–356. [Google Scholar]
- Loew ER. Pharmacology of antihistamine compounds. Physiol. Rev. 1947;27:542–573. doi: 10.1152/physrev.1947.27.4.542. [DOI] [PubMed] [Google Scholar]
- Niemann C, Hayes JT. The relation between structure and histamine-like activity. J. Am. chem. Soc. 1942;64:2288–2289. [Google Scholar]
- Rocha e Silva M. On the nature of the receptors for histamine. Chemotherapia. 1961;3:544–559. doi: 10.1159/000219570. [DOI] [PubMed] [Google Scholar]
- Rosenoer VM, Schild HO. The assay of urogastrone. J. Physiol., Lond. 1962;162:155–162. doi: 10.1113/jphysiol.1962.sp006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks J, Ivy AC, Burgess JP, Vandolah JE. Histamine as the hormone for gastric secretion. Am. J. Physiol. 1932;101:331–338. [Google Scholar]
- Schild HO. pA, a new scale for the measurement of drug antagonism. Brit. J. Pharmac. Chemother. 1947;2:189–206. doi: 10.1111/j.1476-5381.1947.tb00336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnedorf JG, Ivy AC. Effects of methylhistamine and hydroxyethylglyoxaline on gastric secretion and blood pressure in the dog. Proc. Soc. exp. Biol. Med. 1935;32:777–778. [Google Scholar]
- Shore PA. Release of histamine from stomach by vagus-stimulating drugs: association with gastric acid secretion. Fedn Proc. 1965;24:1322–1325. [PubMed] [Google Scholar]
- Trendelenberg U. The action of histamine and 5-hydroxytryptamine on isolated mammalian atria. J. Pharmac. exp. Ther. 1960;130:450–460. [PubMed] [Google Scholar]
- Vartiainen A. Action of certain new histamine derivatives. J. Pharmac. exp. Ther. 1935;54:265–282. [Google Scholar]
- Walter LA, Hunt WH, Fosbinder RJ. β-(2- and 4-Pyridylalkyl)amines. J. Am. chem. Soc. 1941;63:2771–2773. [Google Scholar]


