Abstract
Stimulation of the vagal non-adrenergic inhibitory innervation caused the release of adenosine and inosine into vascular perfusates from the stomachs of guinea-pigs and toads.
Stimulation of portions of Auerbach's plexus isolated from turkey gizzard caused the release of adenosine triphosphate (ATP), adenosine diphosphate (ADP) and adenosine monophosphate (AMP).
ATP, added to solutions perfused through the toad stomach vasculature, was broken down to adenosine, inosine and adenine.
Of a series of purine and pyrimidine derivatives tested for inhibitory activity on the guinea-pig isolated taenia coli, ATP and ADP were the most potent.
ATP caused inhibition of twelve other gut preparations previously shown to contain non-adrenergic inhibitory nerves. The inhibitory action of ATP was not prevented by tetrodotoxin.
Quinidine antagonized relaxations of the guinea-pig taenia coli caused by catecholamines or adrenergic nerve stimulation. Higher concentrations of quinidine antagonized relaxations caused by ATP or non-adrenergic inhibitory nerve stimulation.
When tachyphylaxis to ATP had been produced in the rabbit ileum, there was a consistent depression of the responses to non-adrenergic inhibitory nerve stimulation but not of responses to adrenergic nerve stimulation.
It is suggested that ATP or a related nucleotide is the transmitter substance released by the non-adrenergic inhibitory innervation of the gut.
Introduction
For many years it was assumed that the only inhibitory control of mammalian gastrointestinal smooth muscle is exerted by adrenergic sympathetic nerve fibres. It was only recently realized that a second type of inhibitory neurone of parasympathetic or intrinsic origin innervates the gut (see, for example, Burnstock, Campbell, Bennett & Holman, 1964; Holman & Hughes, 1965; Martinson, 1965; Bennett, Burnstock & Holman, 1966; Burnstock, Campbell & Rand, 1966). This type of nerve fibre is not cholinergic, nor is it adrenergic, as is shown by the combined results of pharmacological, electrophysiological and histochemical studies (Campbell, 1970). However, as yet, no evidence has been presented to show what transmitter substance is released by these inhibitory nerves. Experiments designed to determine the nature of the inhibitory transmitter substance are reported in this paper. The approach used is that which Loewi (1921) applied to the cardiac vagus, namely stimulation of the nerve supply to a perfused organ and examination of the perfusate for substances which mimic the nerve action. A brief report of part of the study has already been made (Satchell, Burnstock & Campbell, 1969).
Methods
Nutrient media
Isolated preparations from toads were perfused with McKenzie's solution (Campbell, Burnstock & Wood, 1964) kept at room temperature. Mammalian preparations were perfused with or mounted in a modified Krebs solution (Bülbring, 1953) kept at 35° C. Preparations of Auerbach's plexus isolated from turkey gizzards were placed in the medium described by Ginsborg (1960) kept at 38° C. All media were bubbled with a mixture of 95% oxygen and 5% carbon dioxide.
Preparations
For tests of the biological activity of perfusates or of known purine and pyrimidine derivatives the following isolated preparations of mammalian gut were suspended as strips or as Magnus preparations: guinea-pig stomach, ileum, taenia coli and colon; rabbit stomach, ileum and colon; rat stomach, duodenum and colon; mouse ileum and colon. To test the effects of stimulation of extrinsic sympathetic or intramural nerves, preparations of guinea-pig taenia coli and rabbit ileum were set up as described by Burnstock et al. (1966) and Holman & Hughes (1965) respectively. In all these experiments, the Krebs solution contained hyoscine (10−7 to 10−6 g/ml). Muscle activity was recorded either with an isotonic lever writing on a smoked drum or with Grass FT 0·03 tension transducers recording on a Gilson polygraph. Grass S5 stimulators were used to deliver rectangular pulses for nerve stimulation, as described in the text.
Isolated preparations of guinea-pig stomach—vagus nerves and of toad stomach—vagosympathetic nerves were perfused via the coeliac artery for 20 min to remove traces of blood. The preparations were then placed in small chambers and 2·5 ml of nutrient medium was recycled through each preparation for 30 min, using the apparatus shown in Fig. 1. Electrical stimuli were applied to the nerves via platinum ring electrodes shielded in plastic. Some preparations of toad stomach were dissected to allow stimulation of either the intracranial portion of the vagus nerves or the cervical sympathetic contribution to the vagosympathetic trunks. Effects of ATP on the perfused toad stomach were recorded with an intraluminal catheter connected to a water manometer, recording on a smoked drum.
Fig. 1.
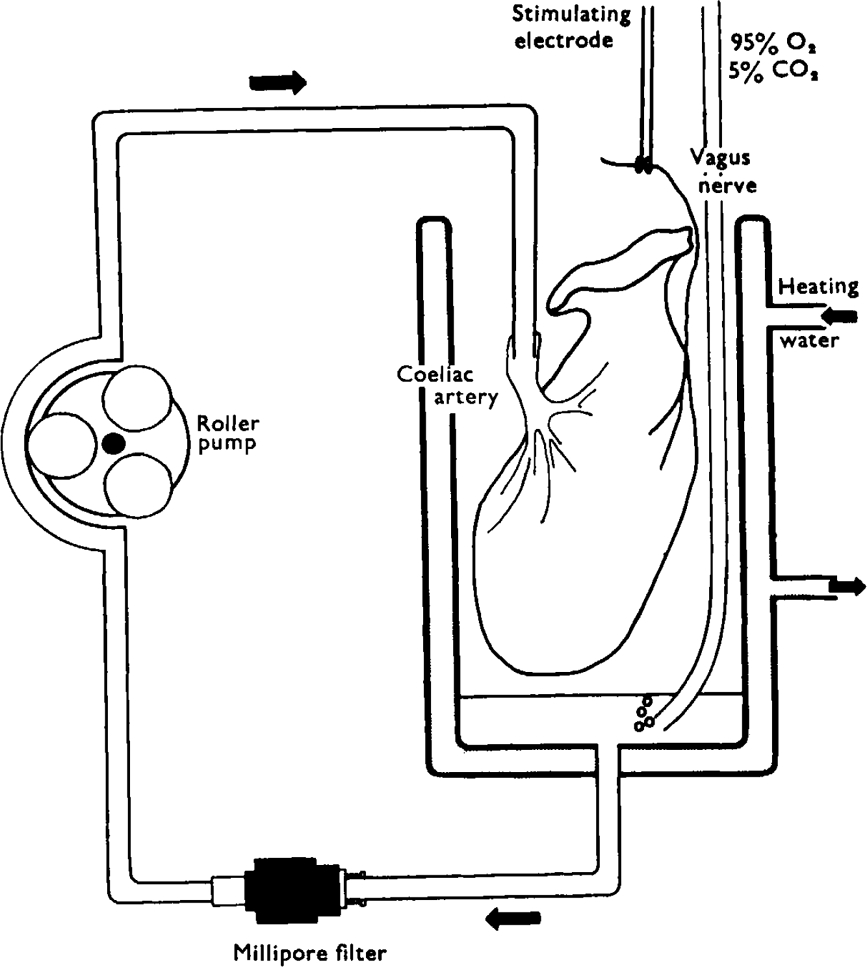
Diagram of apparatus used for closed-circuit perfusion of stomach preparations. A small volume (2·5 ml) of nutrient solution is pumped by the roller pump through a cannula into the coeliac artery. Fluid draining from the gastric veins collects in the bottom of the constant temperature organ bath where it is bubbled with 95% oxygen and 5% carbon dioxide. The fluid is then drawn through a Millipore filter to be recycled. Shielded platinum wire ring-electrodes are placed around the vagus nerves for stimulation
Gizzards were obtained from freshly killed turkeys and approximately 200 mg of Auerbach's plexus was dissected from the margins of four large gizzards. The tissue was weighed into two 100 mg portions. Each portion was placed in a small bath containing 0·6 ml of oxygenated nutrient medium at 38° C. The test bath contained platinum wire electrodes which were used to apply stimuli to the preparations.
Purification of perfusates
In the initial stages of the study, gastric perfusates were concentrated by freezedrying and subjected to paper chromatography. Developed chromatograms were divided into areas which were eluted by shaking with water; the eluates were concentrated and tested for pharmacological activity on guinea-pig taenia coli preparations. Following the location of an inhibitory fraction and its identification as a purine nucleoside, optimum yields of the compound were obtained by making the perfusates 3% with respect to perchloric acid and shaking them with activated charcoal (05 mg/ml extract) for 1 hour. The charcoal was then centrifuged down and washed once with distilled water; the purines were recovered by suspending the charcoal in 10% aqueous pyridine solution at 37° C for 3 hours. Solutions were concentrated by freeze-drying, applied to Whatman No. 1 chromatography paper and run for 12 h in ascending solvents, either (1) n-butanol, acetic acid, water (120:30:50) or (2) iso-butyric acid, water, 0·880 ammonia, 0·1 m versene (100:55·8:4·2:1·6).
Drugs
Drugs used were adenine, adenosine, adenosine-5′-monophosphate disodium salt (AMP), adenosine-5′-diphosphate trisodium salt (ADP), adenosine-5′-triphosphate disodium salt (ATP), adrenaline bitartrate, caffeine, inosine, inosine-5′-monophosphate disodium salt (IMP), guanosine-5′-monophosphate disodium salt (GMP), hyoscine hydrobromide, noradrenaline bitartrate, quinidine sulphate, quinine sulphate, tetrodotoxin, uridine, uridine-5′-monophosphate disodium salt (UMP), 2, 6-xylenol.
All concentrations are expressed in terms of the forms given above.
Results
Release of inhibitory substances on nerve stimulation
The best established approach to determining the nature of a transmitter substance released by a particular set of nerves is that used initially by Loewi (1921) in his discovery of “Vagusstoff”. The nerve supply to a perfused organ is stimulated and the perfusate is examined for the presence of biologically active material which mimics the effects of nerve stimulation. This approach was applied to the non-adrenergic inhibitory innervation of the stomach.
Release of inhibitory material from the stomach. Non-adrenergic inhibitory nerves are present in the vagal supply to the stomach of both guinea-pigs (Campbell, 1966b) and toads (Campbell, 1969), the organs which were chosen for study. Initial experiments were carried out on perfused toad stomachs; the vagosympathetic nerve trunks were stimulated for 45 s in every 2 min with pulses of 2 ms duration at 30 Hz. Over the 30 min collection period the stimulus voltage was raised progressively from 5 to 40 V. As controls, preparations taken from animals of similar weight were perfused for 30 min without nerve stimulation. The biological activity of perfusates from stimulated and non-stimulated stomachs was tested on atropinized preparations of guinea-pig taenia coli; the perfusate from stimulated stomachs caused relaxation whereas the perfusate from non-stimulated preparations was not effective.
Inhibitory activity was found in only one area of the paper chromatogram with an Rf value of 0·48 (solvent 1). A second area at Rf 0·72 (solvent 1) was found to contain a substance which caused contraction of the taenia coli, but no further studies of this substance have been made.
A series of tests were made with a variety of reagents to give a preliminary identification of the active substance in the inhibitory area of the chromatograms. Use of the silver nitrate-bromophenol blue reagent (Wood, 1955) caused the appearance of two blue spots in the area of inhibitory activity, indicating the presence of purine compounds. At this stage further purification was achieved by adsorption of nucleotides and nucleosides on activated charcoal and it was possible to identify the purine materials as adenosine and inosine. The presence of these compounds was confirmed by estimation of the specific absorption maxima in ultraviolet light, which are 260 and 248·5 nm respectively, and by the comparison of their Rf values with those of authentic adenosine and inosine chromatographed in a variety of solvent systems. Adenosine, but not inosine, caused relaxation of the taenia coli (sec below) and would explain the biological activity of the inhibitory area.
Using extracts purified on activated charcoal, a chromatographic comparison was made between the amounts of purine compounds released from stimulated and non-stimulated stomachs. Figure 2 is an ultraviolet photoprint of such a chromatogram. showing that adenosine is present in trace amounts in the perfusate from the non-stimulated stomach but in appreciably larger amounts in the perfusate from the stimulated preparation. Inosine is also present in the control perfusate and appears in greater amounts in perfusate from the stimulated stomach. This experiment was carried out four times on toad stomachs with similar results. In addition, two comparisons were made between perfusates from guinea-pig stomachs stimulated via the vagus nerves or not stimulated. Vagal stimulation caused an increase in the release of adenosine and inosine, comparable to that seen in the toad stomach.
Fig. 2.
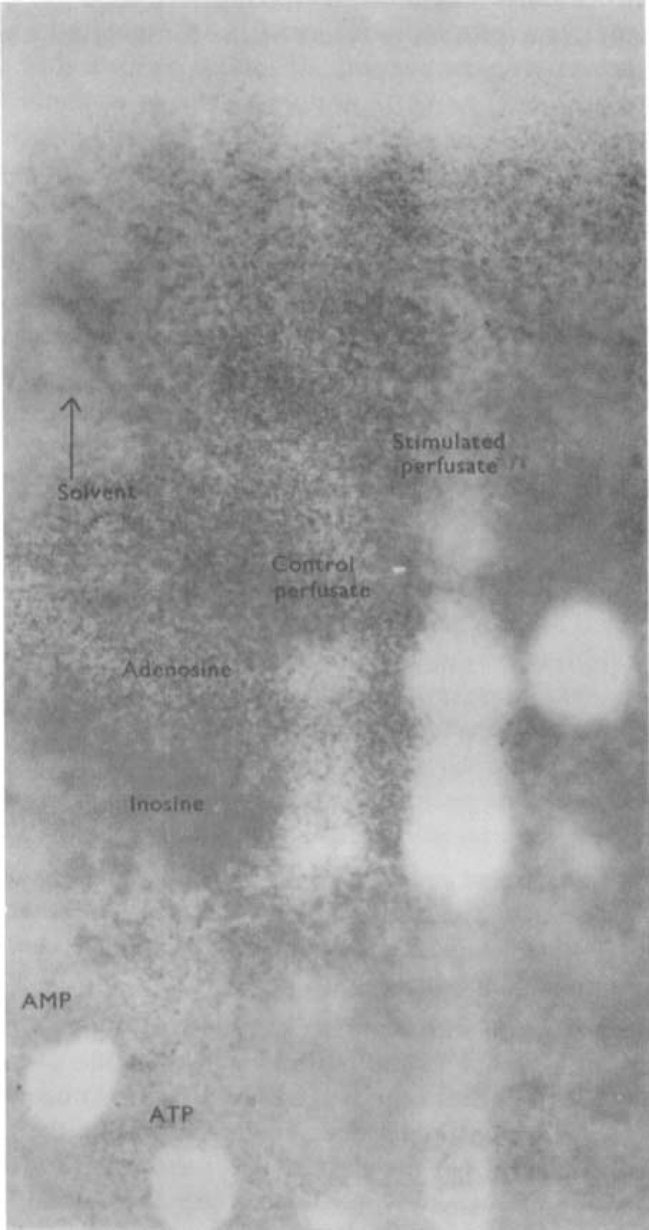
Chromatographic evidence for purine release from toad stomach stimulated via the vagosympathetic nerve. The figure shows an ultraviolet photoprint of a chromatogram spotted with (from the left) AMP, ATP, perfusate from unstimulated stomach, perfusate from stimulated stomach, adenosine. Chromatogram run for 12 h ascending in solvent 1 (see text). Vagosympathetic nerve trunks were stimulated for 45 s in every 2 min for a total of 30 min with pulses of 2 ms duration, at 30 Hz; the voltage was progressively increased from 5 to 40 V. Note that stimulation increases the amounts of adenosine and inosine released from the stomach.
Source of the nucleoside efflux on stimulation. Both the vagosympathetic trunk of the toad and the guinea-pig vagus nerve are mixed nerves, containing both non-adrenergic inhibitory and cholinergic excitatory nerve fibres. It was not possible in the above experiments to determine which type of nerve fibre was causing the release of nucleosides. However, the toad vagus nerve proper, accessible as the intracranial vagal roots, provides only the non-adrenergic inhibitory innervation of the stomach; the cholinergic innervation may be stimulated separately in the cervical sympathetic contribution to the vagosympathetic trunk (Campbell, 1969). To solve the problem of which type of nerve fibre mediates the nucleoside release, a further four chromatographic comparisons were carried out. In two experiments, perfusate from non-stimulated preparations was compared with perfusate from stomachs stimulated via the vagus nerve roots of one side. In the other two experiments, the cervical sympathetic trunk was stimulated. Stimulation was for 45 s every 2 min at 10 Hz and 10 V throughout the period of collection. Stimulation of the intracranial vagal roots caused a marked increase in the amounts of adenosine and inosine released. In contrast, stimulation of the cervical sympathetic chain did not increase the release of nucleosides; in fact, it appeared that slightly less nucleoside was released during sympathetic stimulation, an observation which might be explained by changes in regional perfusion. It is clear that the nucleoside release is a specific result of stimulating the non-adrenergic inhibitory nerves.
Nucleotide release from isolated Auerbach's plexus. The results described above, while indicating that activity in non-adrenergic inhibitory nerves causes an increased nucleoside efflux, do not indicate the source of the nucleoside. It was possible that the nucleosides were released from the gastric muscle as part of the response to the inhibitory nerves, or that stimulation had opened new perfusion channels and freed entrapped blood cells containing nucleosides. To clarify this problem, the effect of electrical stimulation on the release of purine compounds from isolated portions of Auerbach's plexus was determined.
Auerbach's plexus in the mammalian gut is too fine and too firmly buried in the muscle layers for isolation. However, the plexus in the gizzard of birds is concentrated in a thick layer on the margins lying just beneath the serosa (Malinovsky, 1963). This plexus must contain a considerable number of non-adrenergic inhibitory neurons to provide the dense innervation of the gizzard musculature by these nerves, demonstrated by Bennett (1969). To study the release of purine compounds from stimulated inhibitory nerves in the absence of muscle and vascular sources of ATP. pieces of thick lateral enteric plexus were dissected from the gizzard of turkeys, as described under Methods. Purines were isolated from the nutrient media by adsorption on activated charcoal and chromatography as described for gastric perfusate. The resting isolated plexus did not release detectable amounts of any purine nucleotide or nucleoside into the medium. However, when the isolated plexus was stimulated with pulses of 2 ms at 50 Hz for 45 s every 2 min. the medium collected after 30 min contained an appreciable amount of AMP and also traces of ATP and ADP (Fig. 3). Both the resting and the stimulated nerve preparations, extracted with perchloric acid after the experimental period, were found to contain considerable quantities of ATP, ADP and AMP.
Fig. 3.
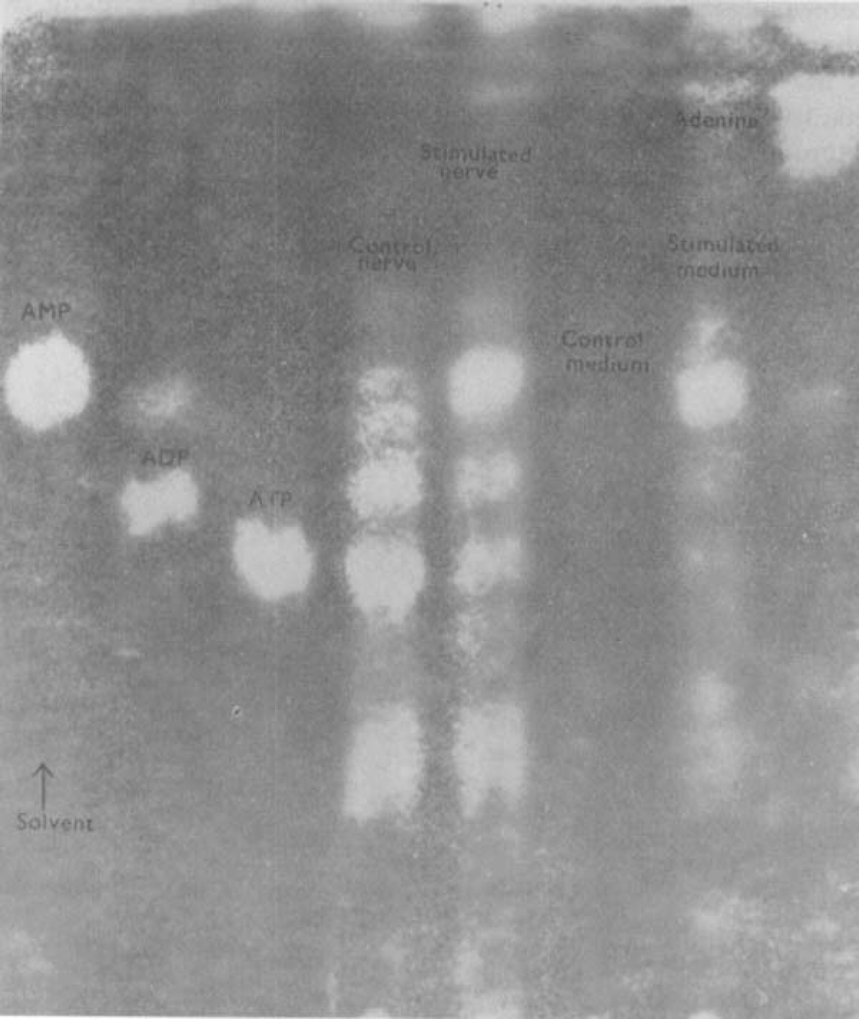
Release of nucleotides from isolated Auerbach's plexus of turkey gizzard. The figure shows an ultraviolet photoprint of a chromatogram spotted with, from the left, AMP, ADP, ATP, extracted unstimulated plexus, extracted stimulated plexus, medium of unstimulated plexus, medium of stimulated plexus (pulses of 2 ms at 50 Hz, trains of 45 s every 2 min for 30 min, voltage progressively increasing from 5 to 50 V), adenine. Note that stimulation causes the release of AMP and traces of ADP and ATP into the medium. Comparable levels of nucleotides were found in extracts of stimulated and unstimulated plexuses. Chromatogram run for 12 h ascending in solvent 2 (see text)
Breakdown of ATP in stomach preparations
As shown above, adenosine and inosine were released from stimulated stomachs of toad or guinea-pig whereas the main purine compound released on stimulation from isolated Auerbach's plexus of the avian gizzard was AMP. These results might indicate that the inhibitory nerves act by releasing AMP, which is broken down by gastric tissues to adenosine and inosine. However, the possibility arises that the AMP coming from the plexus represents a release of ATP which is broken down either by the conditions of incubation or by ATPase in the plexus. To determine whether such a breakdown of ATP does occur in the stomach, ATP was added to 2·5 ml of nutrient solution which was recycled through the toad stomach vasculature for 30 min. Figure 4 shows an ultraviolet photoprint of the chromatographed perfusate. It can be seen that the ATP added to the perfusate was converted to adenosine, inosine and a trace of adenine. This result implies that, if the inhibitory transmitter substance were ATP, it would be degraded to the nucleosides which are found in perfusates of stimulated preparations. However, the result cannot be taken as evidence against the role of AMP as the transmitter substance.
Fig. 4.
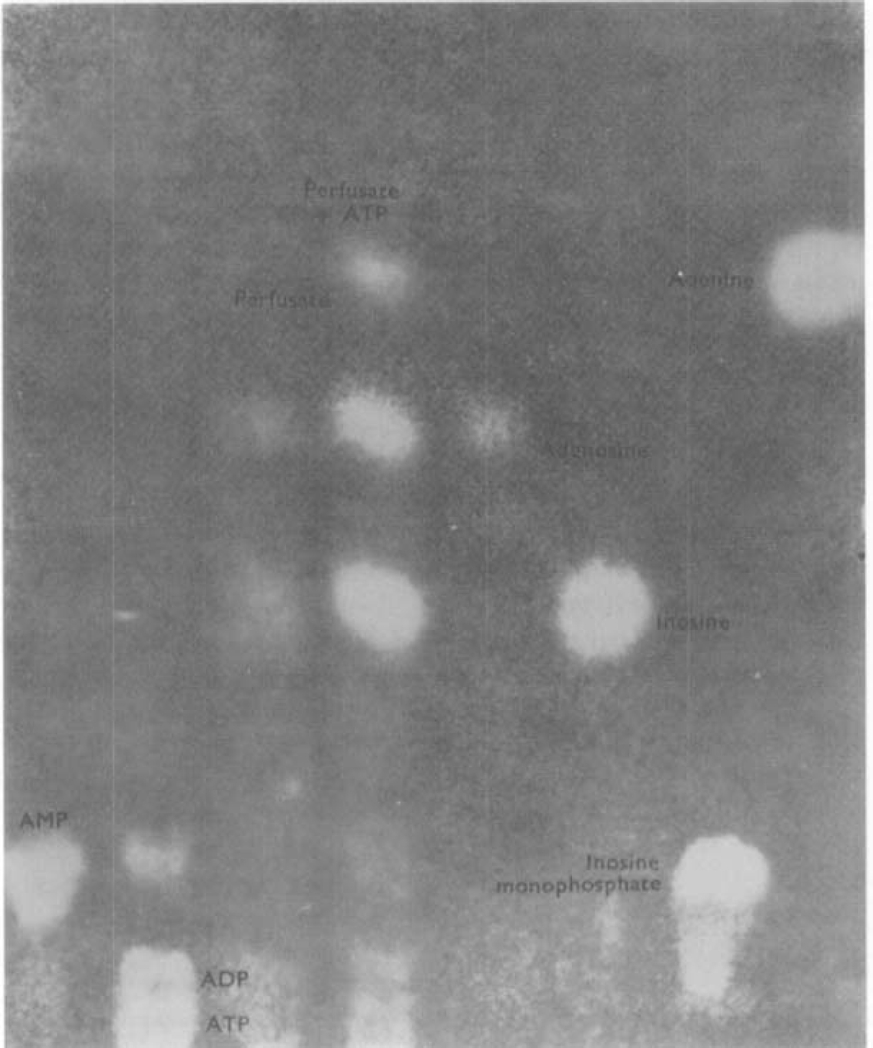
Breakdown of ATP perfused through the toad stomach vascular bed. The figure shows an ultraviolet photoprint of a chromatogram spotted with (from the left) AMP, ATP + ADP control perfusate, perfusate with ATP added before recycling, adenosine, inosine, IMP, adenine. Chromatogram run for 12 h ascending in solvent 1. Note that the ATP added to the medium was broken down to adenosine and inosine
Actions of applied purine and pyrimidine derivatives
In the previous section it was shown that stimulation of the non-adrenergic inhibitory nerves is associated with the release of purine derivatives. To determine whether any of these compounds could act as an inhibitory transmitter substance, a study was made of the actions of purine and pyrimidine derivatives on gut muscle.
Relative inhibitory potencies. The inhibitory action of the purine nucleoside adenosine on mammalian gut muscle was first shown by Drury & Szent-Györgi (1929). Barsoum & Gaddum (1935) reported that adenosine had inhibitory actions on the gut of a variety of mammals, birds and amphibians. Some other nucleosides and nucleotides were also found to relax the gut (Drury, 1936). If any compound of this group were the non-adrenergic inhibitory transmitter substance, it would probably have a relatively strong inhibitory potency. Accordingly, the potencies of a range of naturally-occurring purine and pyrimidine compounds in causing relaxation of the guinea-pig isolated atropinized taenia coli were examined.
The results of the study are closely comparable with those reported for the same tissue by Axelsson & Holmberg (1969). The most potent inhibitory compounds tested were ATP and ADP; these compounds were about equipotent and the threshold concentration for causing relaxation was about 10−7m (Fig. 5). Typically, the relaxation caused by ATP or ADP reached its maximum more rapidly than did relaxations induced by catecholamines (Fig. 6). In a comparable manner, the taenia often contracted back to normal tone more rapidly after ATP or ADP was washed from the bath than after catecholamine responses. In these respects the nucleotide effects are reminiscent of the response of the taenia to non-adrenergic inhibitory nerve stimulation, which has been shown previously to be more rapid in onset and offset than responses to adrenergic nerve stimulation (Burnstock et al., 1966). Both AMP and adenosine were about 100 times less potent than ATP in causing relaxation of the taenia, acting above a threshold concentration of about 10−5m. The relaxations caused by these compounds were rather slow in onset. The purine base adenine, the deaminated nucleoside inosine and its mononucleotide IMP, had no effect on the taenia in concentrations as high as 10−3m. However, the related mononucleotide GMP caused relaxation when applied in concentrations greater than about 3 × 10−5m (compare Axelsson & Holmberg, 1969). The pyrimidine nucleoside uridine and its mononucleotide UMP were without effect in concentrations up to 10−3m. It can be seen that, of this group of compounds, ATP and ADP are most likely to be able to act as a transmitter substance in view of their high inhibitory potencies.
Fig. 5.
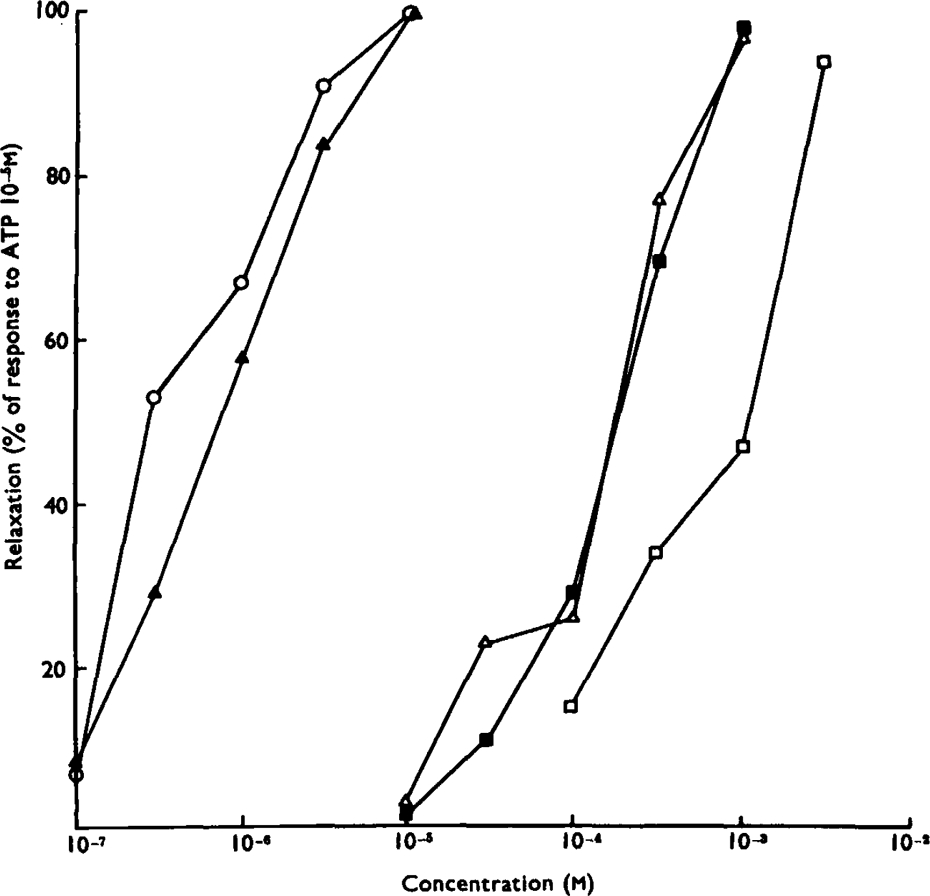
Relative potencies of purine compounds in causing relaxation of the isolated guinea-pig taenia coli. Ordinate: amplitude of relaxation as a percentage of the response to ATP (10−5m). Abscissa: molar concentration (log scale). Dose-response curves are shown for ATP (▴), ADP (○), AMP (δ), adenosine (▪) and GMP (□). The tissues were exposed to the agonists for 30 s. Each point is the mean of values obtained on three preparations
Fig. 6.
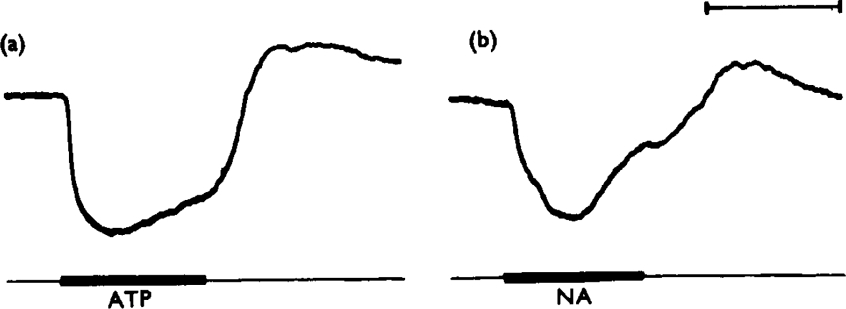
Relative rapidity of gut responses to ATP. Consecutive, approximately equal sub-maximal relaxations of a guinea-pig isolated taenia coli, (a) caused by ATP (10−5 g/ml) and (b) by noradrenaline (NA, 10−7 g/ml). Drugs were applied for 1 mm, as shown by the bars. Note that the ATP response reaches its maximum after 19 s whereas the response to noradrenaline takes 28 s to become maximal. Time marker, 1 minute
Site of action of ATP. Nicotinic stimulant drugs appear to cause relaxation of gut muscle by stimulating the non-adrenergic inhibitory neurones within the gut wall (Burnstock et al., 1966). The possibility arises that ATP might also cause relaxation of the gut indirectly, acting via inhibitory nerves. To test this, the effects of tetrodotoxin on ATP responses were studied. It was found that tetrodotoxin (2 × 10−7 g/ml) abolished the inhibitory responses of the atropinized taenia coli to stimulation of either perivascular or of intramural nerves without affecting the relaxation produced by ATP (Fig. 7). This result shows that the inhibitory effect of ATP is not caused by the initiation of action potentials in inhibitory nerves, although the possibility still remains that ATP has a “tyramine-like” action—that is, it releases an inhibitory transmitter substance by displacement from transmitter stores without initiating neuronal action potentials (Bell, 1968).
Fig. 7.
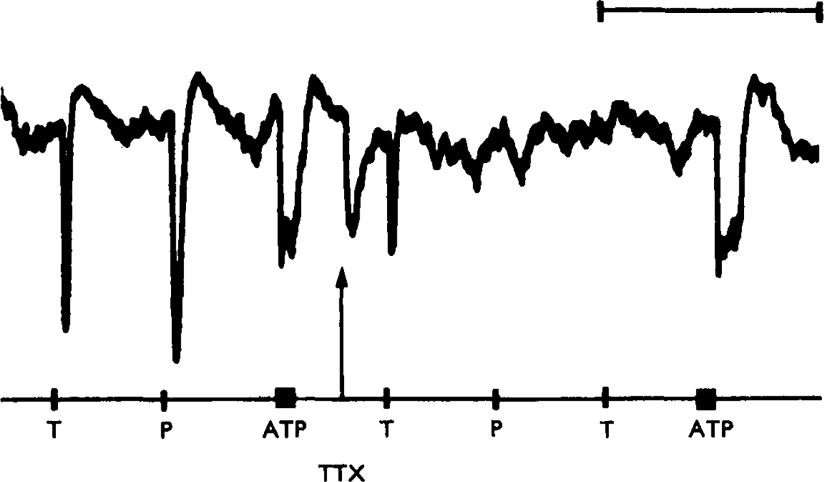
Isolated guinea-pig taenia coli treated with hyoscine (4× 10−7 g/ml). Control responses to transmural stimulation (T, 10 Hz for 10 s), perivascular nerve stimulation (P, 10 Hz for 10 s) and ATP (bar, 2× 10−6 g/ml) are shown at the left of the trace. At the arrow, tetrodotoxin (TTX, 1·5 ×10−7 g/ml) was added to the organ bath. Responses to both transmural and perivascular nerve stimulation were rapidly abolished, but the response to ATP was not reduced. Time marker, 10 minutes
Effects of A TP on selected gut segments. If the inhibitory transmitter substance is a nucleoside or a nucleotide, the compound must be able to induce relaxation of all gut segments innervated by non-adrenergic inhibitory nerves. A study was therefore made of the effects of ATP, as one of the most potent compounds in this group, on the following tissues known to contain non-adrenergic inhibitory nerves: guinea-pig stomach circular muscle (Campbell, 1966b), ileum (Holman & Hughes, 1965) and descending colon (Holman & Hughes, 1965; Furness, 1969); rabbit stomach circular muscle (Paton & Vane, 1963), ileum (Holman & Hughes, 1965; Day & Warren, 1968) and descending colon (Holman & Hughes, 1965; Furness, 1969); rat gastric fundus (Heazell, 1969), ileum and colon (Holman & Hughes, 1965); mouse duodenum and colon (Holman & Hughes, 1965); toad (Bufo marinus) stomach (Campbell, 1969). The gut segments were mounted either as strips with the mucosa dissected off or as Magnus preparations. Contractions of the saline-perfused isolated toad stomach were recorded by means of an intraluminal catheter. All media contained hyoscine (10−6 g/ml).
ATP (10−6 to 10−4 g/ml) caused relaxation of all twelve gut segments tested (Table 1). The inhibitory responses to ATP were complicated by excitatory actions which were of two types. Preparations indicated as having only inhibitory responses to ATP often showed a contraction or a period of increased spontaneous activity after the ATP had been washed from the organ bath. Similar, but generally smaller, contractions occurred after inhibitory concentrations of noradrenaline had been washed out (Fig. 8a) and it is thought that the contraction is a ‘rebound’ phenomenon, as proposed for nerve-mediated responses of the taenia coli (Bennett, 1966; Campbell, 1966a), and not a direct response to ATP.
Table 1.
Effects of ATP on segments of gastrointestinal tract
| Animal | Gut segment | ATP (g/ml) | Response |
|---|---|---|---|
| Guinea-pig | stomach | 10−5–10−4 | — |
| ileum | 10−6–10−4 | —(low conc.)+(high conc.) | |
| colon | 5 × 10−6–2 × 10−5 | — | |
| Rabbit | stomach | 10−5-10−4 | —(low conc.)+(high conc.) |
| ileum | 10−6-10−4 | — | |
| colon | 10−5-10−4 | — | |
| Rat | stomach | 2 × 10−6–2 × 10−5 | — followed by + |
| duodenum | 2 × 10−6–10−5 | — | |
| colon | 2 × 10−5 | — | |
| Mouse | ileum | 5 × 10−6–2 × 10−5 | — |
| colon | 5 × 10−6–5 × 10−5 | —(low conc.)+(high conc.) | |
| Toad | stomach | 5 × 10−7–5 × 10−4 | —, +, or + followed by − |
+ = contraction during application of ATP.
− = relaxation or inhibition of spontaneous movements.
Fig. 8.
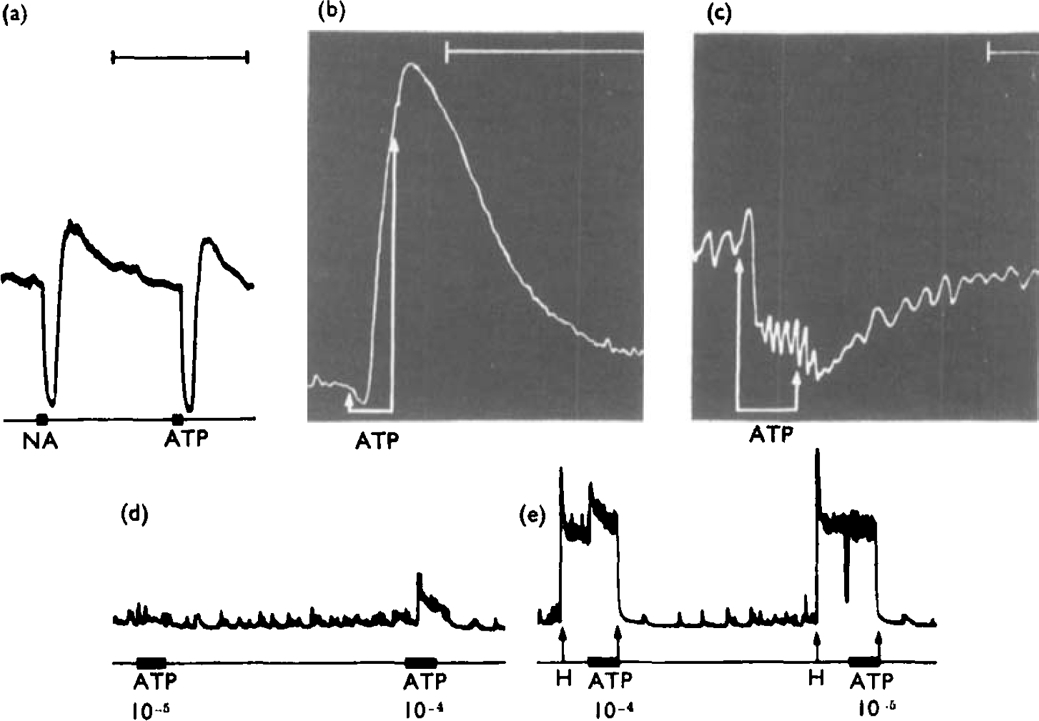
Responses of selected gut segments to ATP. Preparations treated with hyoscine (10−6 g/ml). (a), Guinea-pig isolated taenia coli. Both noradrenaline (NA, 2 × 10−7 g/ml) and ATP (ATP, 10−5 g/ml), applied for 15 s (bars) caused relaxation, followed by a secondary “rebound” contraction when the drugs were washed from the organ bath. (b), Isolated rat gastric fundus strip. ATP (10−6 g/ml), applied for 1 min between arrows, caused an initial slight relaxation followed by a contraction while the drug was still in the organ bath. (c). Isolated, Ringer-perfused toad stomach. ATP (2·5 × 10−7 g/ml in perfusate), applied for 6 min between the arrows, caused a slight initial rise followed by a marked fall in intragastric pressure. (d), (e), Guinea-pig isolated ileum. In a low-tone preparation (d) ATP (10−5 g ml) applied for 60 s (bar) was without effect. A higher concentration (10−4 g/ml) caused contraction. (e), When the preparation was contracted with histamine (H. 6·5 × 10−8 g/ml, applied between arrows), ATP 10−4 g/ml still caused contraction but 10−3 g/ml caused a brief relaxation. Time markers, 5 minutes. The time marker for (a) also refers to (d) and (c)
The second type of excitatory action of ATP occurred while the nucleotide was still present in the organ bath and is regarded as a direct excitatory effect. In the rat gastric fundus strip preparation, all effective concentrations of ATP caused a relaxation followed by a contraction before the organ bath was washed out (Fig. 8b). The perfused toad stomach responded to intravascular ATP with either contraction or relaxation in different preparations; occasionally a brief contraction preceded the relaxation (Fig. 8c). There was no obvious relationship between the concentration of ATP infused and the type of response seen. In the remaining three preparations showing an excitatory response to ATP, the guinea-pig ileum, the rabbit stomach and the mouse colon, contractions tended to occur only when high concentrations of ATP were applied. The preparations of guinea-pig ileum and rabbit stomach commonly had low tone. In these preparations low concentrations of ATP had no effect and high concentrations caused contraction; in preparations with raised tone, the lower concentrations were seen to cause relaxation (Fig. 8d, e). It should be noted that similar dual effects of ATP on gut segments have been reported previously. For instance, adenine compounds have been reported to contract (Brecht & Epple, 1952) or relax (Malchikova & Poskonova, 1969) the amphibian stomach, and to excite (Buchthal & Kahlson, 1944; Naess & Schanche, 1957) or inhibit (Barsoum Gaddum, 1935; Ewing, Schlenk & Emerson, 1949; Moulton, Spector & Willough- by, 1957; Ambache & Freeman, 1968) the guinea-pig ileum. These observations raise the possibility that there are at least two types of receptor for ATP, comparable to α- and β-adrenoceptors.
Blockade of the inhibitory action of ATP
If the non-adrenergic inhibitory nerves act by releasing ATP, any procedure which prevents responses to ATP should also inhibit transmission from the inhibitory nerves to smooth muscle.
Substances which prevent A TP actions. A variety of compounds have been reported to antagonize the actions of adenosine or ATP on gut muscle and other tissues. We have tested the actions of some of these compounds on the guinea-pig atropinized taenia coli. Caffeine has been reported to antagonize ATP-induced inhibition of the rabbit small intestine (Nichols & Walaszek, 1963). However, caffeine, in concentrations as high as 5 × 10−5 g/ml, did not affect relaxations of the taenia coli induced by ATP (2 × 10−6 g/ml) or by perivascular or transmural stimulation of adrenergic or non-adrenergic nerves respectively. At a concentration of 10−4 g/ml, caffeine lowered the tone of the muscle considerably but did not have any selective blocking action on the responses.
Quinine was reported to antagonize the relaxing effects of adenosine on the fowl rectal caecum (Madinaveitia & Raventos, 1949) but in concentrations up to 10−5 g/ml quinine had no effect on responses of the taenia coli to ATP or to stimulation of adrenergic or non-adrenergic inhibitory nerves. Similarly, 2,6-xylenol (2 × 10−5 g/ml), a phenol reported to antagonize ATP-induced pulmonary vasoconstriction (Lunde, Waaler & Walløe, 1968), was without effect on the inhibitory responses of the taenia coli.
More encouraging results were obtained with quinidine, a drug shown to block the inhibitory effects of ATP on the rabbit ileum (Bowman & Hall, 1970). Quinidine (1–5 ×10−5 g/ml) reduced or abolished the effects of adrenaline or noradrenaline (10−8 to 10−7 g/ml) and of perivascular adrenergic nerve stimulation on the taenia coli, usually without reducing responses to ATP (10−6–2 × 10−5 g/ml) or to transmural stimulation of the non-adrenergic inhibitory nerves (Fig. 9a, b). When the concentration of quinidine was raised to 2 × 10−4 g/ml, the responses to both ATP and stimulation of non-adrenergic inhibitory nerves were consistently reduced and occasionally they were abolished (Fig. 9c). At a concentration of 3 × 10−4 g/ml, quinidine impaired the activity and tone of the taenia.
Fig. 9.
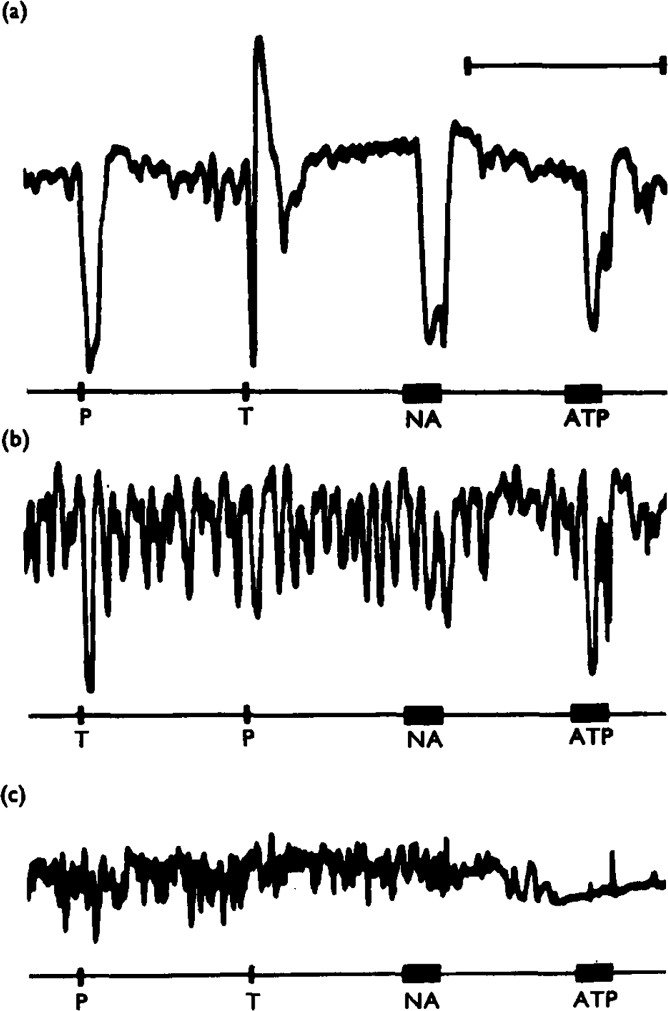
Effects of quinidine on inhibitory responses of the guinea-pig taenia coli, treated with hyoscine (10−6 g/ml). Panel (a) shows control responses to perivascular nerve stimulation (P, 20 Hz for 10 s), transmural stimulation (T. 10 Hz for 10 s), noradrenaline (NA. 5 × 10−8 g/ml for 40 s) and ATP (ATP. 4 × 10−5 g/ml for 40 s). In (b). the preparation had been treated with quinidine (5 × 10−5 g/ml) for 65 minutes. The responses to perivascular nerve stimulation and to noradrenaline were virtually abolished, whereas the responses to transmural stimulation and to ATP were not reduced in amplitude. In (c), the preparation had been treated with quinidine (2 × 10−1 g/ml) for a further 20 minutes. The responses to transmural stimulation and to ATP were now also abolished. Time marker, 5 minutes
Tachyphylaxis to ATP. It has been reported that the inhibitory action of ATP on the rabbit ileum shows marked tachyphylaxis (Mihich, Clarke & Philips, 1954; Kim, Shulman & Levine, 1968). Attempts were made to produce tachyphylaxis to ATP in the guinea-pig taenia coli but no desensitization was ever seen. However, ATP tachyphylaxis could readily be achieved in segments of rabbit ileum. Rabbit ileum desensitized to high concentrations of ATP showed very low tone and irregular spontaneous activity, and these effects were partially counteracted by increasing the Ca2+ concentration of the medium 2–4 fold (Burn & Gibbons, 1964).
Almost complete desensitization of the rabbit ileum to any particular dose of ATP could be achieved by repeatedly adding the dose to the organ bath at 3–5 min intervals without washing out the bath. Six Finkleman preparations of ileum were desensitized to ATP (10−5 g/ml, four preparations; 10−4 g/ml. two preparations) without any reduction of the inhibitory response to perivascular adrenergic nerve stimulation (Fig. 10c, d). In one further preparation, the perivascular nerve effects were abolished after the muscle had been desensitized to ATP (10−5 g/ml) and the response was partially restored when the stimulating voltage was increased. This result suggested that ATP might act as a local anaesthetic drug. To test this, the perivascular adrenergic innervation of two preparations was stimulated with alternate bursts of pulses of submaximal and supramaximal voltage; in neither preparation did desensitization to ATP (10−5 g/ml) cause an appreciable reduction of the responses.
Fig. 10.
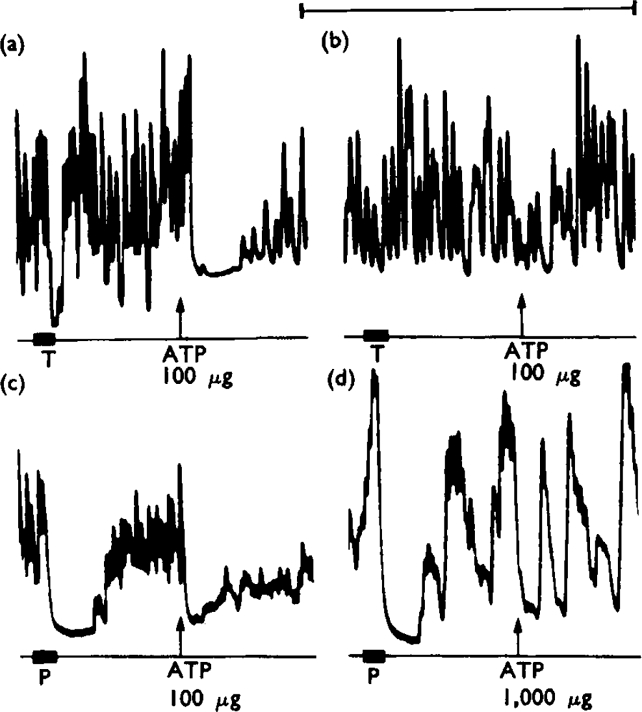
Effects of desensitization of rabbit isolated ileum to ATP. Preparations treated with hyoscine (10−6 g/ml). Panels (a) and (b) show responses of a preparation subjected to degenerative section of the perivascular adrenergic innervation 6 days previously. In (a), transmural stimulation (T, 10 Hz for 20 s) caused inhibition. At the arrow, ATP (100 μg to make 10−5 g/ml) added to the organ bath and left in contact with the tissue caused a transient inhibition. Panel (b) starts 15 min after (a). In the interval, three further applications of ATP (100 μg) had been made. The responses to both transmural stimulation and ATP (100 μg) are virtually abolished. Calcium concentration of medium four times normal. Panels (c) and (d) show responses of a preparation with normal perivascular adrenergic innervation. In (c), perivascular nerve stimulation (P, 10 Hz for 20 s) and ATP (100 μg to make 10−5 g/ml, added at arrow and left in) caused inhibition. Panel (d) starts 40 min after (c), after five 100 μg and two 1,000 μg applications of ATP. ATP (1,000 μg applied at the arrow) was ineffective but the response to perivascular nerve stimulation was still present. Calcium concentration of medium twice normal. Time marker, 5 minutes
The effect of ATP desensitization on the non-adrenergic inhibitory innervation was tested on transmurally stimulated preparations in which adrenergic transmission had been prevented by treatment with guanethidine (5 × 10−6 g/ml, four preparations) or by degenerative section of the perivascular innervation 6 days previously (one preparation). When desensitization to ATP (10−5 g/ml) had been achieved, the inhibitory response to transmural stimulation was abolished in three of the preparations (Fig. 10a, b) and was reduced in the remaining two. Increasing the desensitizing dose of ATP to 10−4 g/ml in the latter two preparations caused an abolition of the response to stimulation in one case, although it did not reduce the response any further in the other. It seems clear that when the rabbit ileum was desensitized to ATP, there was a selective reduction of the inhibitory effects of the non-adrenergic innervation.
Discussion
In this paper the nature of the transmitter substance released by the non-adrenergic inhibitory nerves supplying gastro-intestinal muscle has been examined. The results suggest that either ATP or some closely related compound is involved. The following criteria, based on the summary given by Eccles (1964) would need to be satisfied before ATP could be established as the non-adrenergic inhibitory transmitter substance: ATP and the enzymes necessary for its formation must be present in the inhibitory nerves; ATP must be released from the nerves when they are activated; ATP and the transmitter substance released on nerve stimulation must have the same effect on gastro-intestinal muscle; an enzyme which inactivates ATP should occur in the tissue; drugs which are found to alter the normal nerve-muscle transmission should similarly alter the effects of applied ATP. We shall examine the extent to which these criteria have been satisfied.
Formation and storage of ATP. With regard to the formation and presence of ATP within the non-adrenergic inhibitory nerves, little need be said. Both ATP and enzymic systems which can produce ATP have been found in all cellular tissues examined. It would, therefore, be surprising to find that the inhibitory nerve fibres were unable to produce and retain ATP. The critical question is whether ATP is stored in the nerve terminals in such a way that it can be released during nerve activity. The most obvious approach to this question would be to try to isolate an ATP-storing granular fraction, comparable with ‘synaptic vesicles’, from tissues containing the non-adrenergic inhibitory nerves.
Evidence has been presented recently that large granular vesicles found predominantly in some axon profiles in the toad lung are associated with the non-adrenergic inhibitory transmitter substance (Robinson, McLean & Burnstock, 1970). Comparable vesicle composition has been described for axon profiles in the gut of both toad (Rogers & Burnstock, 1966) and mammals (Bennett & Rogers, 1967). An attempt to isolate and identify this vesicular fraction is being carried out.
Release of ATP. The second criterion for ATP, or a related compound, as the transmitter substance has been satisfied, since it has been shown that stimulation of the vagal non-adrenergic inhibitory nerves of the stomachs of both toads and guinea-pigs causes an increase in the venous efflux of adenosine and inosine from these organs. As is discussed below, the adenosine and inosine released are likely to be products of the breakdown of adenosine nucleotides. The efflux of nucleosides from the guinea-pig stomach could conceivably have resulted from stimulation not of the non-adrenergic inhibitory innervation but of the cholinergic excitatory fibres which run in the vagus (Greeff & Holtz, 1956). However, this problem does not arise in the studies on the toad stomach. The vagal innervation of the toad gastric muscle is solely of the non-adrenergic inhibitory type (Campbell, 1969) and stimulation of the vagal roots, like stimulation of the mixed vago-sympathetic trunk, caused increased nucleoside efflux. In contrast, stimulation of the cholinergic gastric fibres in the cervical sympathetic contribution to the vagosympthetic trunk does not increase the resting efflux of nucleosides. The stimulated release of nucleosides is therefore a specific concomitant of activity in the non-adrenergic inhibitory fibres.
Several explanations of the increased efflux of nucleosides from the stomach, other than that ATP is the inhibitory transmitter substance, should be considered. First, the original ATP might have been released not as a transmitter substance but as a direct result of the process of axon membrane activation, in the manner suggested by Abood, Koketsu & Miyamoto (1962). Second, the nucleotide might have been released from the muscle cells as part of the specific response of the muscle fibres to stimulation of the non-adrenergic inhibitory innervation. Third, the ATP might have been released from trapped red blood cells in vascular backwaters which become perfused only when the vagus nerves are stimulated. Fourth, the ATP might have been released not as the transmitter substance proper, but as a component of the vesicular storage system for the true transmitter, as occurs during release of catecholamines from the adrenal medulla (Douglas, 1968). Finally the nucleotide might have been released not from the non-adrenergic inhibitory nerves, but from antidromically-stimulated sensory nerve fibres in the vagus nerve (Holton & Holton, 1954).
Two lines of argument indicate that the increased nucleoside efflux is not due simply to the release of ATP from nerve membranes, an event which is claimed to occur as any excitable membrane undergoes the permeability changes responsible for the action current (Abood et al., 1962). First, the efflux does not increase when the cervical sympathetic supply to the toad stomach is stimulated, but only when the vagal fibres are active. Second, the amount of nucleotide released from the stomach appears to be much greater than can be explained by membrane release alone. We have not yet attempted to give quantitative values for the amounts of nucleoside recovered in the gastric perfusate, although a quantitative study is now in progress. However, an examination of chromatographic spot size and density has been made, showing that the adenosine output from the toad stomach increases from about 20 nmol in resting preparations to about 50 nmol in preparations stimulated via the vagosympathetic nerve. During the 30 min collection period, the preparations were stimulated with 20,250 pulses which elicited an increase of about 30 nmol in adenosine output. These figures give a value for stimulated adenosine release of about 1·5 pmol/pulse. In contrast, Abood et al. (1962) found that the output of ATP from stimulated frog ventral nerve roots exceeded the unstimulated output by about (1 nmol/g)/h. Their preparations were stimulated at 50 Hz, giving an ATP release of 0·006 (pmol/g)/pulse. To obtain the release per pulse observed in the toad stomach, one would have to stimulate 250 g of nerve releasing at the rate observed in the sciatic roots. In a toad stomach, weighing a few grams, the weight of nervous tissue must be more like 0·25 g.
There is no direct evidence to disprove that the nucleoside release is coming from the muscle cells or from entrapped red blood cells, because there are no drugs which are known to prevent specifically the action of the inhibitory transmitter substance. The best evidence against these possibilities is that stimulation of isolated portions of Auerbach's plexus from turkey gizzards, dissected away from the underlying muscle layers, causes a greatly increased efflux of purine compounds. Since the plexus, which contains non-adrenergic inhibitory neurones supplying the gizzard musculature (Bennett, 1969), releases nucleotide, there is no reason at this stage to propose a further release of purine compounds from any cell type other than the nerves.
There is little reason to think that ATP is released from the non-adrenergic inhibitory nerves as part of the ejected intracellular storage site for another transmitter substance. First, although ATP does have such a binding function in the catecholamine-containing vesicles of adrenergic neurones, Stjärne (1969) has been unable to find evidence of ATP release from the spleen when the adrenergic nerves are stimulated. He expressed the belief that catecholamine release from neuronal stores is qualitatively different from release of adrenal medullary amines (Douglas, 1968). Second, if the release of nucleoside reported here represents the storage-ATP for the true inhibitory transmitter substance, it might be expected that the substance would be found in the perfusates after nerve stimulation. In fact, inhibitory activity was found in only one region of chromatograms of the gastric perfusates, and this activity could be attributed to the nucleosides found in the region.
It is not likely that the nucleoside efflux from the stomach is derived from ATP released from antidromically stimulated afferent neurones in the vagus nerve. Holton (1959) showed that ATP is released from mammalian afferent dendritic terminals in concentrations sufficient to cause regional vasodilatation. If the gastric adenosine were derived from this source, it would be expected that some sign of the pharmacological action of the released ATP on the stomath should appear. In fact, the fibres in the vagal trunk mediating gastric inhibition are preganglionic, and therefore efferent, in both guinea-pigs (Bülbring & Gershon, 1967; Ohga, Nakazato & Saito, 1969) and toads (Campbell, 1969), and no effects on motility attributable to the antidromic stimulation of vagus sensory fibres have been observed.
Effects of ATP on gastro-intestinal muscle. It has been shown that ATP and the transmitter substance released from the non-adrenergic inhibitory nerves have similar actions on gut muscle. In each of twelve different gut segments, all of which have previously been shown to contain non-adrenergic inhibitory nerves, taken from guinea-pigs, rabbits, rats, mice and toads, ATP was found to have inhibitory actions. By using tetrodotoxin, it was established that ATP does not exert its inhibitory action indirectly, either by stimulating the non-adrenergic or the adrenergic inhibitory innervation. The story is complicated by the fact that excitatory actions of ATP on some of the tissues were also found, but this is no argument against the hypothesis that ATP is the inhibitory transmitter substance. For example, noradrenaline, acting on α-adrenoceptors, is well established as the transmitter substance released by sympathetic vasoconstrictor nerves, in spite of the fact that β-adrenoceptors mediating vasodilatation occur in the same tissues.
The inhibitory non-adrenergic nerves in the gut have been shown to cause hyper-polarization of the smooth muscle cell membranes in the taenia coli (Bennett, et al., 1966). It has already been shown that ATP causes hyperpolarization of taenia coli cells (Imai & Takeda, 1967; Axelsson & Holmberg, 1969), so again there is a parallelism between the actions of ATP and the nerve released transmitter substance. Not much weight can be placed on the similarities until it is possible to determine the muscle membrane reversal potential for the action of both ATP and the endogenous transmitter substance. However, smooth muscle cells are elecrically coupled to a variable degree and part or even all of the post-synaptic membrane potential change recorded in any one cell with intracellular microelectrodes during transmission may in fact be electrotonically propagated from other cells (Bennett & Merrillees, 1966; Bennett, 1967). Under these conditions, it is not possible to make an accurate estimation of the equlibrium potential for the membrane under the influence of the transmitter substance.
Inactivation of ATP. If the non-adrenergic inhibitory nerves act on the gut by releasing ATP, the action of the ATP must be terminated by recapture of ATP into the nerve fibres or by breakdown of ATP into compounds with greatly reduced inhibitory potencies. No evidence relating to nervous uptake of ATP is available, but there is clear evidence that ATP can be broken down in the tissue. When ATP is added to a perfusion fluid recycled through the vasculature of the toad stomach, very little unaltered ATP is recovered. Instead the perfusate contains increased amounts of ADP, AMP and especially adenosine and inosine. Dephosphorylation of ATP to form AMP or adenosine would decrease inhibitory potency 100-times, judging from the potency ratios observed on the guinea-pig taenia coli. Further breakdown by deamination to form inosine would decrease potency by more than 10,000 times. No evidence has been presented that the observed breakdown of ATP is due to enzymic activity, but the gut is known to contain both 5′-nucleotidase and adenosine deaminase (White, Handler & Smith, 1968). It can therefore be concluded that this criterion for considering ATP as the inhibitory transmitter substance has been fulfilled.
The extensive breakdown of ATP could explain the fact that ATP itself does not form part of the increased efflux of purine compounds from stimulated organs. For instance, the adenosine and inosine recovered from perfused stomachs could well be breakdown products of ATP or some other purine nucleotide released from the nerves. The fact that AMP is released on stimulation of isolated Auerbach's plexus might lead one to believe that the transmitter substance is AMP itself, but it should be remembered that ATP is labile in solution and, if released, could well have broken down to AMP during the experiment.
Blockade of ATP. One line of experiments has apparently been successful in demonstrating that when the action of ATP is prevented, transmission from the non-adrenergic inhibitory nerves is also antagonized. The rabbit ileum shows tachyphylaxis to ATP and the induction of tachyphylaxis causes a consistent reduction of responses to stimulation of the non-adrenergic, but not of the adrenergic, inhibitory innervation. This evidence suggests strongly that the non-adrenergic inhibitory nerves act by releasing ATP. Evidence consistent with this hypothesis was obtained from experiments with quinidine. Quinidine, in moderate concentrations, prevents the effects of applied noradrenaline. In high concentrations, quinidine antagonizes both the action of ATP and the response to non-adrenergic inhibitory nerve stimulation. Because of the lack of specificity, the effects of quinidine must be interpreted with caution. However, the fact remains that quinidine antagonized the nerve action as it would have to do if the nerves acted by releasing ATP.
It can be seen that, in broad outline, all five criteria for ATP as the transmitter substance released by the non-adrenergic nerves have been fulfilled. In the above discussion, for convenience, we have based the argument on the hypothesis that ATP is the non-adrenergic inhibitory substance. In fact the evidence presented would hold equally well for AMP, ADP and probably other purine nucleotides. There is no valid reason for choosing between these nucleotides, but we favour ATP and ADP in view of their high inhibitory potency.
Acknowledgments
This work was supported by the Australian Research Grants Committee and by the National Heart Foundation of Australia.
REFERENCES
- Abood LG, Koketsu K, Miyamoto S. Outflux of various phosphates during membrane depolarization of excitable tissue. Am. J. Physiol. 1962;202:469–474. doi: 10.1152/ajplegacy.1962.202.3.469. [DOI] [PubMed] [Google Scholar]
- Ambache N, Freeman MA. Atropine-resistant longitudinal muscle spasms due to excitation of non-cholinergic neurones in Auerbach's plexus. J. Physiol., Lond. 1968;199:705–728. doi: 10.1113/jphysiol.1968.sp008674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson J, Holmberg B. The effects of extracellularly applied ATP and related compounds on electrical and mechanical activity of the smooth muscle taenia coli from the guinea-pig. Acta physiol. scand. 1969;75:149–156. doi: 10.1111/j.1748-1716.1969.tb04366.x. [DOI] [PubMed] [Google Scholar]
- Barsoum GS, Gaddum JH. The pharmacological estimation of adenosine and histamine in blood. J. Physiol., Lond. 1935;85:1–14. doi: 10.1113/jphysiol.1935.sp003298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C. Differential effects of tetrodotoxin on sympathomimetic actions of nicotine and tyramine. Br. J. Pharmac. Chemother. 1968;32:96–103. doi: 10.1111/j.1476-5381.1968.tb00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR. Rebound excitation of the smooth muscle cells of the guinea-pig taenia coli after stimulation of intramural inhibitory nerves. J. Physiol., Lond. 1966;185:124–131. doi: 10.1113/jphysiol.1966.sp007975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR. The effect of intracellular current pulses in smooth muscle cells of the guinea-pig vas deferens at rest and during transmission. J. gen. Physiol. 1967;50:2459–2476. doi: 10.1085/jgp.50.10.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Burnstock G, Holman ME. Transmission from intramural inhibitory nerves to the smooth muscle of the guinea-pig taenia coli. J. Physiol., Lond. 1966;182:541–558. doi: 10.1113/jphysiol.1966.sp007836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Merrillees NCR. An analysis of the transmission of excitation from autonomic nerves to smooth muscle. J. Physiol., Lond. 1966;185:520–535. doi: 10.1113/jphysiol.1966.sp008000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MR, Rogers DC. A study of the innervation of the taenia coli. J. cell Biol. 1967;33:573–596. doi: 10.1083/jcb.33.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett T. Nerve-mediated excitation and inhibition of the smooth muscle cells of the avian gizzard. J. Physiol., Lond. 1969;204:669–686. doi: 10.1113/jphysiol.1969.sp008938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman WC, Hall MT. Inhibition of rabbit intestine mediated by α- and β-adrenoceptors. Br. J. Pharmac. 1970;38:399–415. doi: 10.1111/j.1476-5381.1970.tb08528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht K, Epple O. Uber die Wirkung von Acetylcholin und Adenosintriphosphat auf isolierte lebende und mit Wasser-Glycerin extrahiertel quergestreifte und glatte Muskeln des Frosches. Pflügers Arch. ges. Physiol. 1952;255:315–326. doi: 10.1007/BF00370176. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Kahlson G. The motor effect of adenosine triphosphate and allied phosphorous compounds on smooth mammalian muscle. Acta physiol. scand. 1944;8:325–334. [Google Scholar]
- Bulbring E. Measurements of oxygen consumption in smooth muscle. J. Physiol., Lond. 1953;122:111–134. doi: 10.1113/jphysiol.1953.sp004983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulbring E, Gershon MD. 5-hydroxytryptamine participation in the vagal inhibitory innervation of the stomach. J. Physiol., Lond. 1967;192:823–846. doi: 10.1113/jphysiol.1967.sp008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn JH, Gibbons WR. The part played by calcium in determining the response to stimulation of sympathetic postganglionic fibres. Br. J. Pharmac. Chemother. 1964;22:520–548. doi: 10.1111/j.1476-5381.1964.tb01708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Bennett M, Holman ME. Innervation of the guinea-pig taenia coli: Are there intrinsic inhibitory nerves which are distinct from sympathetic nerves. Int. J. Neuropharmac. 1964;31:163–166. doi: 10.1016/0028-3908(64)90003-6. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Campbell G, Rand MJ. The inhibitory innervation of the taenia of the guinea-pig caecum. J. Physiol., Lond. 1966;182:504–526. doi: 10.1113/jphysiol.1966.sp007834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. Nerve-mediated excitation of the taenia of the guinea-pig caecum. J. Physiol., Lond. 1966a;185:148–159. doi: 10.1113/jphysiol.1966.sp007977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. The inhibitory nerve fibres in the vagal supply to the guinea-pig stomach. J. Physiol., Lond. 1966b;185:600–612. doi: 10.1113/jphysiol.1966.sp008004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell G. The autonomic innervation of the stomach of a toad (Bufo marinus) Comp. Biochem. Physiol. 1969;3:693–706. doi: 10.1016/0010-406x(69)92069-6. [DOI] [PubMed] [Google Scholar]
- Campbell G. Autonomic nervous supply to effector tissues. In: Bulbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle. London: Arnold; 1970. [Google Scholar]
- Campbell G, Burnstock G, Wood M. A method for distinguishing between adrenergic and cholinergic excitatory innervation of smooth muscle. Q. Jl exp. Physiol. 1964;49:268–276. doi: 10.1113/expphysiol.1964.sp001731. [DOI] [PubMed] [Google Scholar]
- Day MD, Warren PR. A pharmacological analysis of the responses to transmural stimulation in isolated intestinal preparations. Br. J. Pharmac. Chemother. 1968;32:227–240. doi: 10.1111/j.1476-5381.1968.tb00967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas WW. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br. J. Pharmac. 1968;34:451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury AN. The physiological activity of nucleic acid and its derivatives. Physiol. Rev. 1936;16:292–325. [Google Scholar]
- Drury AN, Szent-Gyorgi A. The physiological activity of adenine compounds with especial reference to their acion upon the mammalian heart. J. Physiol., Lond. 1929;68:213–237. doi: 10.1113/jphysiol.1929.sp002608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eccles JC. The Physiology of Synapses. Berlin: Springer; 1964. [Google Scholar]
- Ewing PL, Schlenk F, Emerson GA. Comparison of smooth muscle effects of crotono-side (isoguanosine) and adenosine. J. Pharmac. exp. Ther. 1949;97:379–383. [PubMed] [Google Scholar]
- Furness JB. An electrophysiological study of the innervation of the smooth muscle of the colon. J. Physiol., Lond. 1969;205:549–562. doi: 10.1113/jphysiol.1969.sp008982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsborg BL. Spontaneous activity in muscle fibres of the chick. J. Physiol., Lond. 1960;150:707–717. doi: 10.1113/jphysiol.1960.sp006413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greeff K, Holtz P. Untersuchungen am isolierten Vagus-Magcn Präparat. Arch. exp. Path. Pharmak. 1956;227:424–435. [PubMed] [Google Scholar]
- Heazell MA. A non-adrenergic component to the inhibitory innervation of the fundus of the rat stomach. Br. J. Pharmac. 1969;36:186–187P. [PMC free article] [PubMed] [Google Scholar]
- Holman ME, Hughes JR. Inhibition of intestinal smooth muscle. Aust. J. exp. Biol. med. Sci. 1968;43:277–290. doi: 10.1038/icb.1965.27. [DOI] [PubMed] [Google Scholar]
- Holton FA, Holton P. The capillary dilator substance in dry powders of spinal roots; a possible role of adenosine triphosphate in chemical transmission from nerve endings. J. Physiol., Lond. 1954;126:124–140. doi: 10.1113/jphysiol.1954.sp005198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton P. The liberation of adenosine triphosphate on antidromic stimulation of sensory nerves. J. Physiol., Lond. 1959;145:494–504. doi: 10.1113/jphysiol.1959.sp006157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Takeda K. Effects of vasodilators upon the isolated taenia coli of the guinea-pig. J. Pharmac. exp. Ther. 1967;156:557–564. [PubMed] [Google Scholar]
- Kim TS, Shulman J, Levine RA. Relaxant effect of cyclic adenosine-3′, 5′-monophosphate on the isolated rabbit ileum. J. Pharmac. exp. Ther. 1968;163:36–42. [PubMed] [Google Scholar]
- Loewi O. Uber humorale Uberträgbarkeit der Herznervenwirkung. Pflügers Arch. ges. Physiol. 1921;189:239–242. [Google Scholar]
- Lunde PKM, Waaler BA, Walløe L. The inhibitory effect of various phenols upon ATP-induced vasoconstriction in sol ated perfused rabbit lungs. Acta physiol. scand. 1968;72:331–337. doi: 10.1111/j.1748-1716.1968.tb03855.x. [DOI] [PubMed] [Google Scholar]
- Madinaveitia J, Raventos J. Antimalarial compounds as antagonists of adenosine. Br. J. Pharmac. Chemother. 1949;4:81–92. doi: 10.1111/j.1476-5381.1949.tb00518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchikova LS, Poskonova MA. Adrenaline and adenil nucleotide effects on spontaneous activity and acetylcholine contraction of smooth muscles. Fiziol. Zh. SSSR. 1969;55:1378–1384. [PubMed] [Google Scholar]
- Malinovsky L. The nerve supply of the stomach in the domestic pigeon (Columba domestica) Cesk. Morfol. 1963;11:16–27. [PubMed] [Google Scholar]
- Martinson J. Vagal relaxation of the stomach. Experimental reinvestigation of the concept of the transmission mechanism. Acta physiol. scand. 1965;64:453–462. doi: 10.1111/j.1748-1716.1965.tb04204.x. [DOI] [PubMed] [Google Scholar]
- Mihich E, Clarke DA, Philips FS. Effect of adenosine analogs on isolated intestine and uterus. J. Pharmac. exp. Ther. 1954;111:335–342. [PubMed] [Google Scholar]
- Moulton R, Spector WG, Willoughby DA. Histamine release and pain production by xanthosine and related compounds. Br. J. Pharmac. Chemother. 1957;12:365–370. doi: 10.1111/j.1476-5381.1957.tb00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naess K, Schanche S. The action of barbiturates on the intestine. Acta pharmac. tox. 1957;13:90–95. doi: 10.1111/j.1600-0773.1957.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Nichols RE, Walaszek ES. Antagonism of the vaso-depressor effect of ATP by caffeine. Fedn Proc. 1963;22:308. [Google Scholar]
- Ohga A, Nakazato Y, Saito K. An analysis of the vago-vagal reflex relaxation of the stomach. J. physiol. Soc. Japan. 1969;31:92–93. [PubMed] [Google Scholar]
- Paton WDM, Vane JR. An analysis of the responses of the isolated stomach to electrical stimulation and to drugs. J. Physiol., Lond. 1963;165:10–46. doi: 10.1113/jphysiol.1963.sp007040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson PM, McLean JR, Burnstock G. A study of large granular vesicles: Ultrastructural identification of non-adrenergic inhibitory nerve fibres in toad lung. J. Pharmac. exp. Ther. 1970 submitted. [PubMed] [Google Scholar]
- Rogers D, Burnstock G. “Multi-axonal” autonomic junctions in intestinal smooth muscle of the toad (Bufo marinus) J. comp. Neurol. 1966;126:625–652. [PubMed] [Google Scholar]
- Satchell DG, Burnstock G, Campbell G. Evidence for a purine compound as the transmitter in non-adrenergic inhibitory neurones in the gut. Aust. J. exp. Biol. med. Sci. 1969;47:24. [Google Scholar]
- Stjärne L. Release of catecholamines from adrenal medulla as compared to that from sympathetic nerves. Acta physiol. scand. 1969;77(Suppl. 330):27. [Google Scholar]
- White A, Handler P, Smith EL. Principles of Biochemistry. 4th ed. New York: McGraw-Hill; 1968. pp. 619–644. [Google Scholar]
- Wood T. A reagent for the detection of chloride and of certain purines and pyrimidines on paper chromatograms. Nature, Lond. 1955;176:175–176. doi: 10.1038/176175a0. [DOI] [PubMed] [Google Scholar]


