Abstract
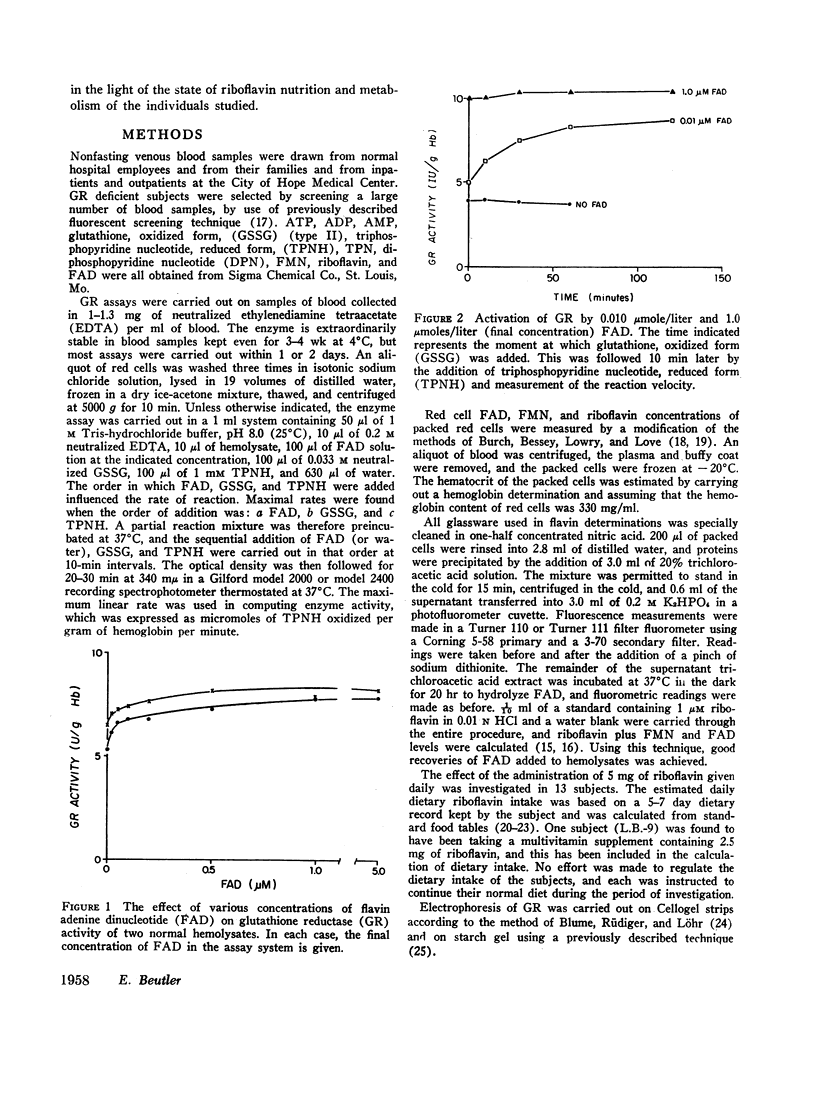
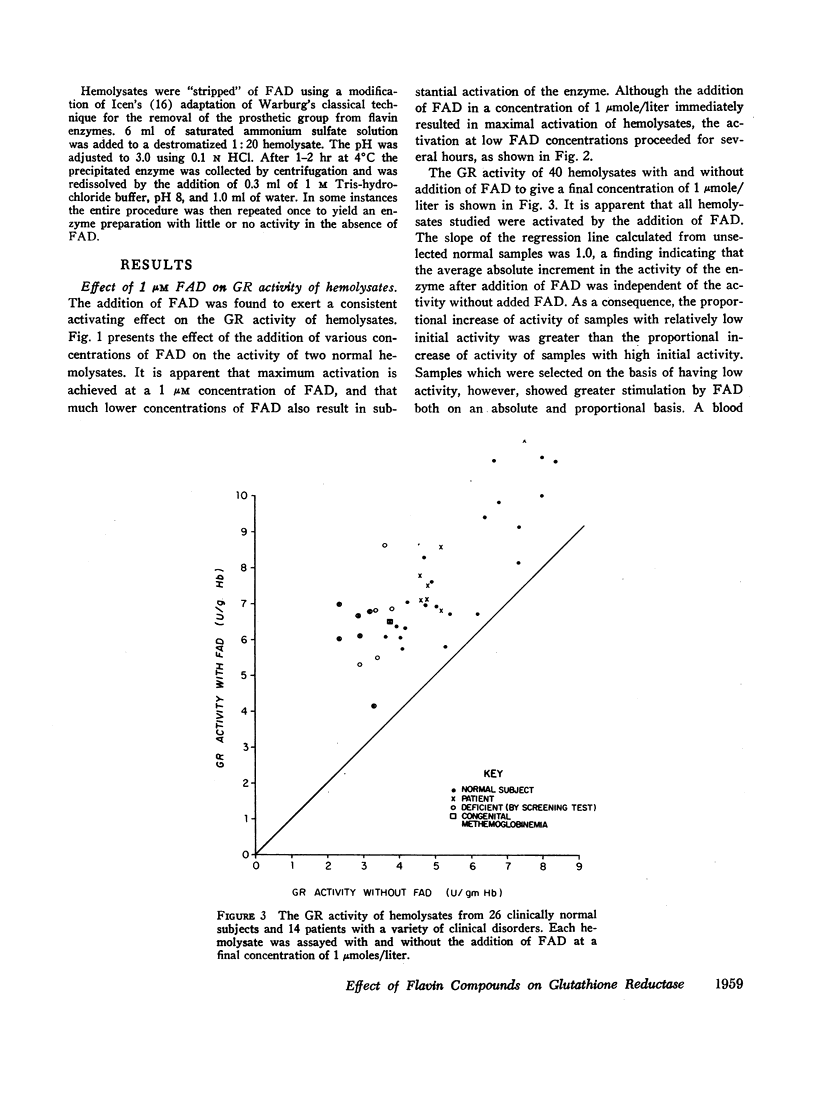
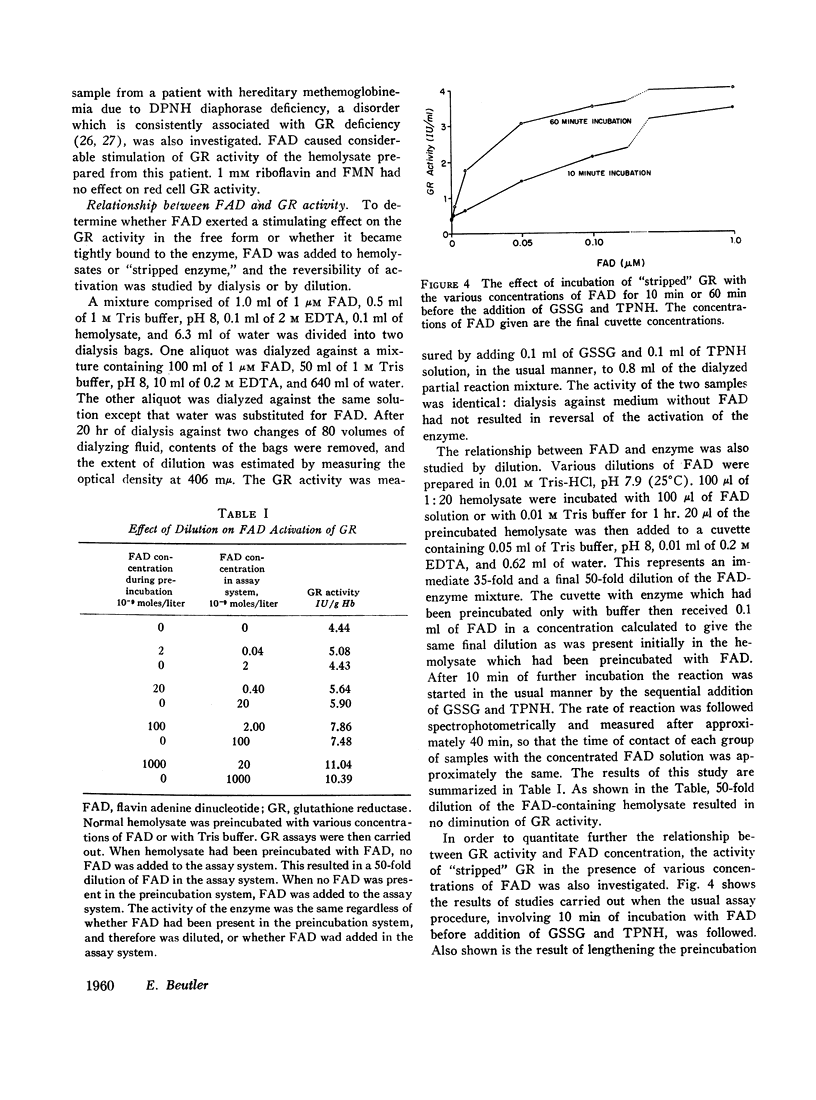
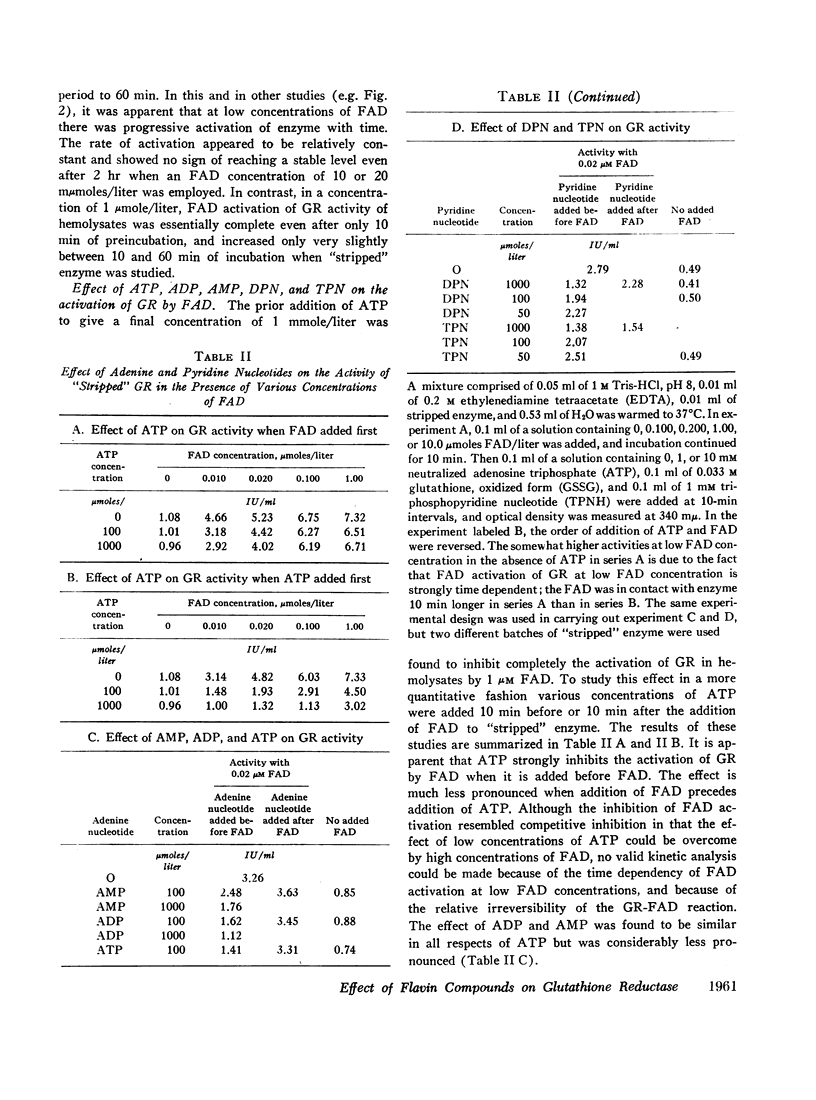
Increases or decreases of red cell glutathione reductase (GR) have been described in connection with many clinical abnormalities. We find that GR activity as measured in hemolysates represents only a portion of the available GR activity. The addition of small amounts of flavin adenine dinucleotide (FAD), but not of flavin mononucleotide or riboflavin, activates the GR of hemolysates. 1 μM FAD results in a maximal activation within 10 min; gradually increasing activation occurs at much lower, for example, 20 mμM FAD concentrations. Once FAD has activated GR, dilution or dialysis does not reverse activation of the enzyme. Activation of GR by FAD can be inhibited by adenosine triphosphate (ATP), and to a lesser extent by adenosine diphosphate (ADP) and adenosine monophosphate (AMP), if these adenine nucleotides are added before the addition of FAD, but only to a slight extent if FAD is added before the adenine nucleotides. The addition of FAD to GR does not alter its electrophoretic mobility but produces intensification of the bands.
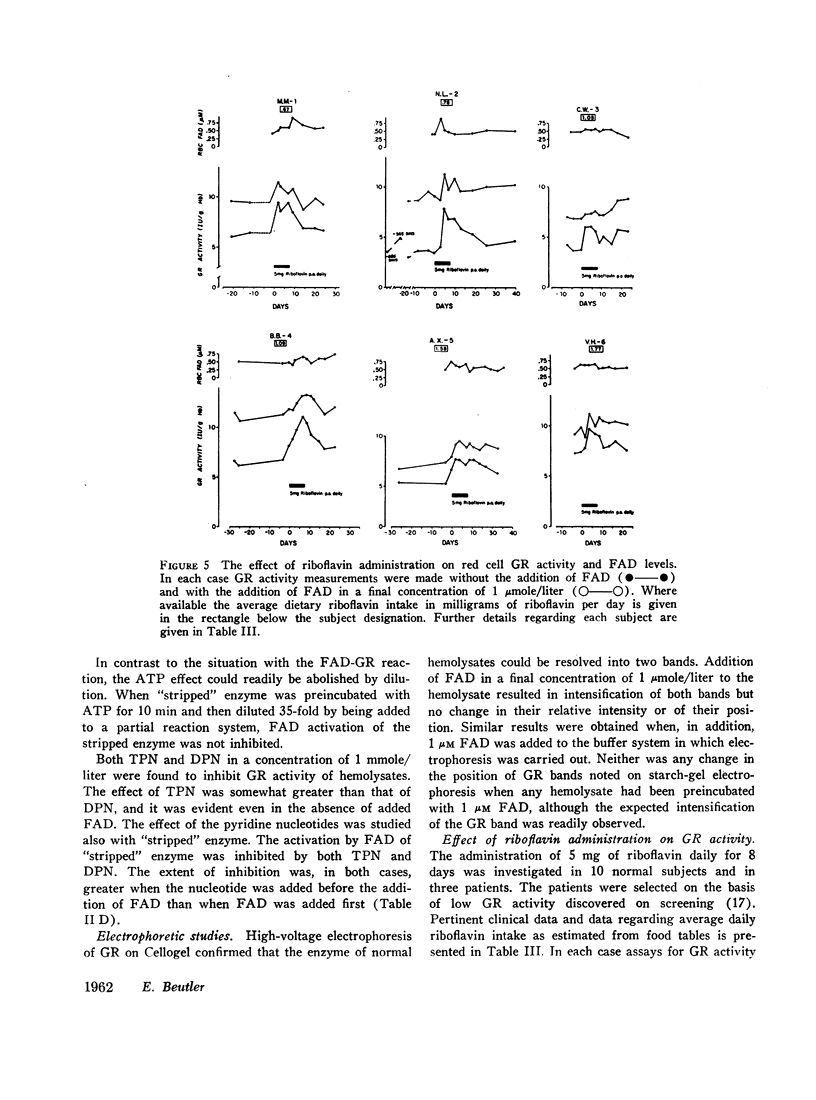
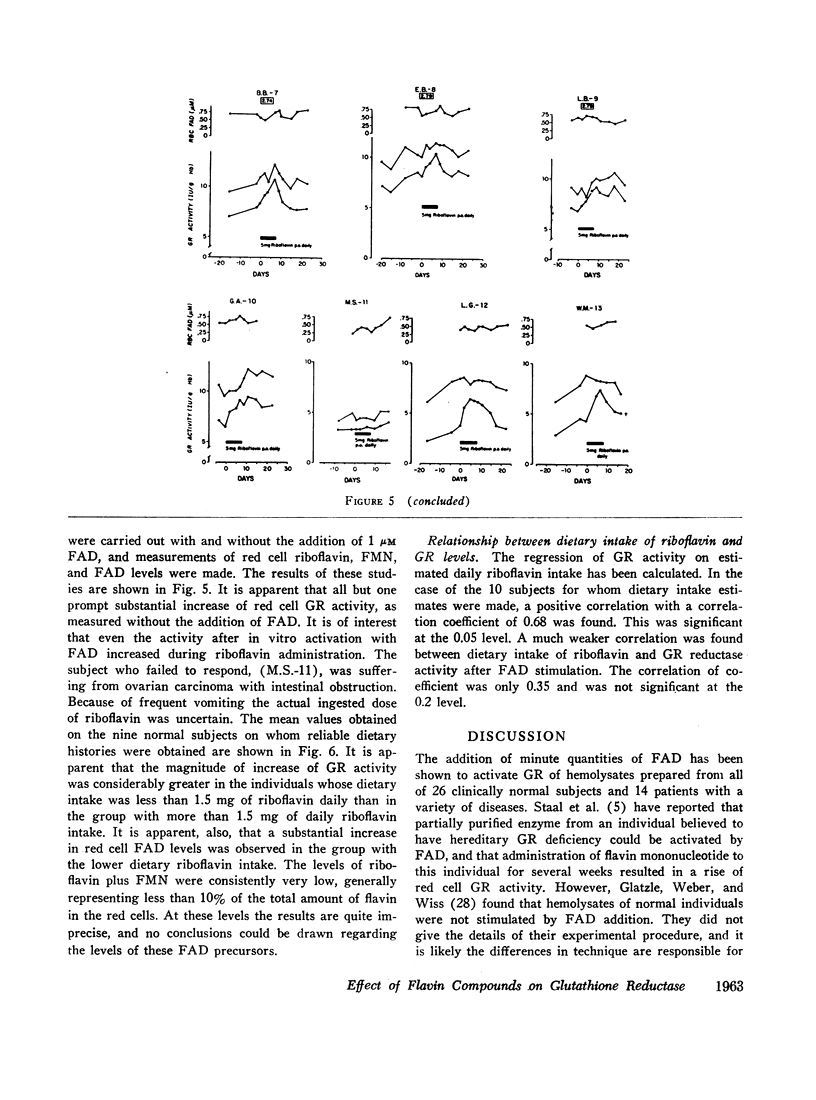
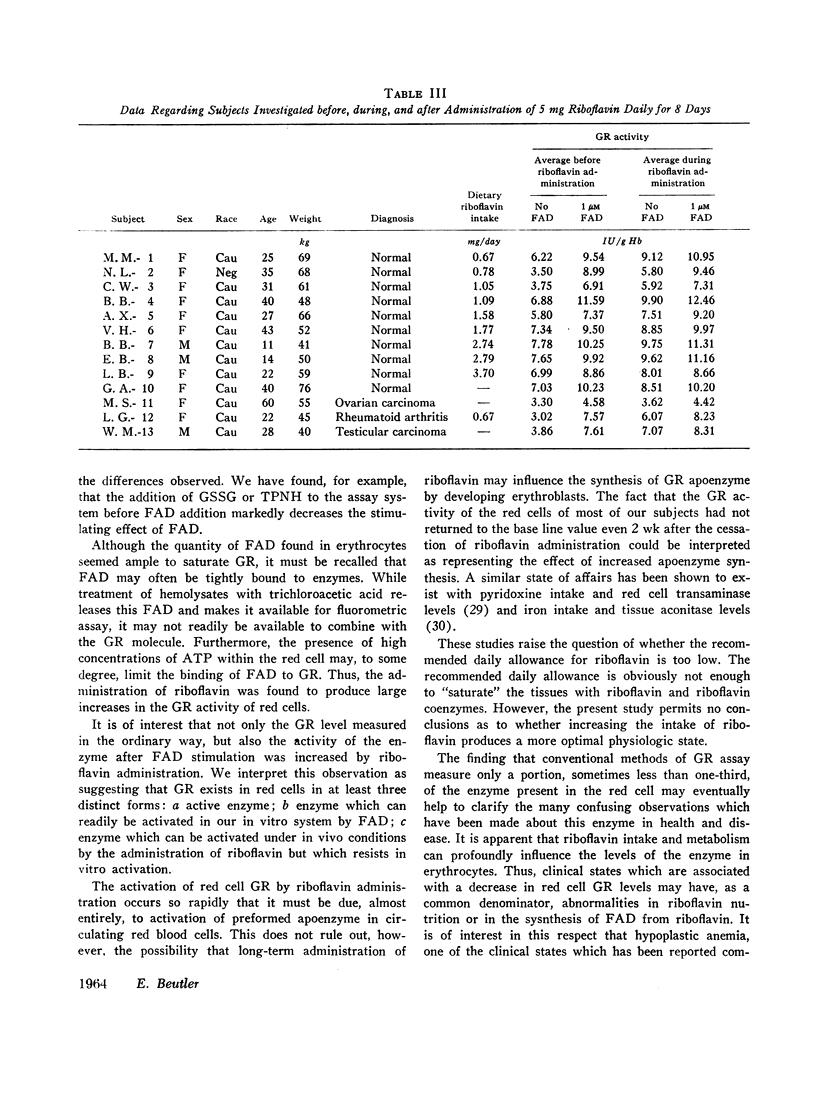
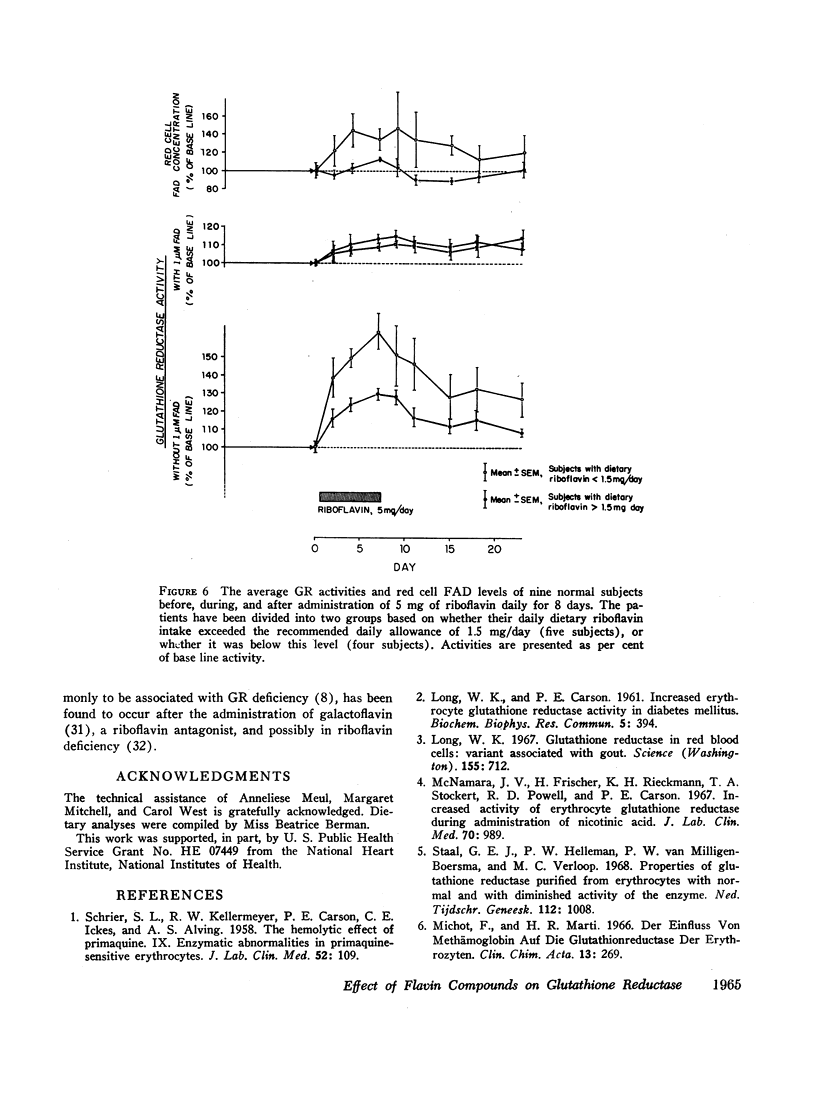
The administration of 5 mg of riboflavin daily produces marked stimulation of red cell GR activity within only 2 days. After cessation of riboflavin administration, the GR activity again begins to fall. The degree of stimulation of GR activity by riboflavin is inversely correlated with the level of dietary riboflavin intake. The base line GR activity of normal individuals is directly correlated with the level of dietary riboflavin intake. The previously unexplained variations of glutathione reductase in health and disease must be reevaluated in light of the state of riboflavin nutrition and metabolism of the subject.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEUTLER E. Iron enzymes in iron deficiency. VI. Aconitase activity and citrate metabolism. J Clin Invest. 1959 Sep;38:1605–1616. doi: 10.1172/JCI103939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUZARD J. A., KOPKO F. The flavin requirement and some inhibition characteristics of rat tissue glutathione reductase. J Biol Chem. 1963 Jan;238:464–468. [PubMed] [Google Scholar]
- Beutler E. A series of new screening procedures for pyruvate kinase deficiency, glucose-6-phosphate dehydrogenase deficiency, and glutathione reductase deficiency. Blood. 1966 Oct;28(4):553–562. [PubMed] [Google Scholar]
- Beutler E. Drug-induced hemolytic anemia. Pharmacol Rev. 1969 Mar;21(1):73–103. [PubMed] [Google Scholar]
- Blume K. G., Gottwik M., Löhr G. W., Rüdiger H. W. Familienuntersuchungen zum Glutathionreduktasemangel menschlicher Erythrocyten. Humangenetik. 1968;6(2):163–170. doi: 10.1007/BF00297725. [DOI] [PubMed] [Google Scholar]
- Blume K. G., Rüdiger H. W., Löhr G. W. Electrophoresis of glutathione reductase from human red blood cells. Biochim Biophys Acta. 1968 Mar 25;151(3):686–688. doi: 10.1016/0005-2744(68)90018-1. [DOI] [PubMed] [Google Scholar]
- FOY H., KONDI A. A case of true redcell aplastic anaemia successfully treated with riboflavin. J Pathol Bacteriol. 1953 Apr;65(2):559–564. doi: 10.1002/path.1700650228. [DOI] [PubMed] [Google Scholar]
- Glatzle D., Weber F., Wiss O. Enzymatic test for the detection of a riboflavin deficiency. NADPH-dependent glutathione reductase of red blood cells and its activation by FAD in vitro. Experientia. 1968 Nov 15;24(11):1122–1122. doi: 10.1007/BF02147797. [DOI] [PubMed] [Google Scholar]
- Hayduk K., Eggstein M., Kaufmann W., Waller H. D. Morbus Gaucher mit Glutathionreduktase-Mangel in den Blutzellen. Dtsch Med Wochenschr. 1968 May 24;93(21):1063–1066. doi: 10.1055/s-0028-1105191. [DOI] [PubMed] [Google Scholar]
- Icén A. Glutathione reductase of human erythrocytes. Purification and properties. Scand J Clin Lab Invest Suppl. 1967;96:1–67. [PubMed] [Google Scholar]
- Jacobs A., Cavill I. A., Hughes J. N. Erythrocyte transaminase activity. Effect of age, sex, and vitamin B6 supplementation. Am J Clin Nutr. 1968 May;21(5):502–507. doi: 10.1093/ajcn/21.5.502. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Beutler E. Electrophoretic study of glutathione reductase in human erythrocytes and leucocytes. Nature. 1968 Jan 20;217(5125):256–258. doi: 10.1038/217256a0. [DOI] [PubMed] [Google Scholar]
- LANE M., ALFREY C. P., Jr THE ANEMIA OF HUMAN RIBOFLAVIN DEFICIENCY. Blood. 1965 Apr;25:432–442. [PubMed] [Google Scholar]
- Long W. K. Glutathione reductase in red blood cells: variant associated with gout. Science. 1967 Feb 10;155(3763):712–713. doi: 10.1126/science.155.3763.712. [DOI] [PubMed] [Google Scholar]
- Marti H. R., Dorta T., Deubelbeiss K. A. Familiäre Methämoglobinämie durch Diaphorasemangel: eine dritte Schweizer Sippe. Schweiz Med Wochenschr. 1966 Mar 19;96(11):355–357. [PubMed] [Google Scholar]
- Michot F., Marti H. R. Der Einfluss von Methämoglobin auf die Glutathion-reductase der Erythrozyten. Clin Chim Acta. 1966 Mar;13(3):269–272. doi: 10.1016/0009-8981(66)90204-x. [DOI] [PubMed] [Google Scholar]
- SCHRIER S. L., KELLERMEYER R. W., CARSON P. E., ICKES C. E., ALVING A. S. The hemolytic effect of primaquine. IX. Enzymatic abnormalities in primaquine-sensitive erythrocytes. J Lab Clin Med. 1958 Jul;52(1):109–117. [PubMed] [Google Scholar]
- SCOTT E. M., DUNCAN I. W., EKSTRAND V. PURIFICATION AND PROPERTIES OF GLUTATHIONE REDUCTASE OF HUMAN ERYTHROCYTES. J Biol Chem. 1963 Dec;238:3928–3933. [PubMed] [Google Scholar]
- Staal G. E., Helleman P. W., van Milligen-Boersma L., Verloop M. C. Properties of glutathione reductase purified from erythrocytes with normal and with diminished activity of the enzyme. Ned Tijdschr Geneeskd. 1968 May 25;112(21):1008–1009. [PubMed] [Google Scholar]
- Waller H. D., Bremer J., Schönthal H., Dorow W. Hb C-Homozygotie mit Glutathionreductase-Mangel in den Blutzellen. Klin Wochenschr. 1967 Aug 15;45(16):824–826. doi: 10.1007/BF01745557. [DOI] [PubMed] [Google Scholar]
- Waller H. D., Löhr G. W., Zysno E., Gerok W., Voss D., Strauss G. Glutathionreduktasemangel mit hämatologischen und neurologischen Störungen. (Autosomal dominant verebliche Buldung eines pathologischen Enzyms) Klin Wochenschr. 1965 Apr 15;43(8):413–426. doi: 10.1007/BF01483846. [DOI] [PubMed] [Google Scholar]



