Abstract
After infection, most antigen-specific memory T cells reside in nonlymphoid tissues. Tissue-specific programming during priming leads to directed migration of T cells to the appropriate tissue, which promotes the development of tissue-resident memory in organs such as intestinal mucosa and skin. Mechanisms that regulate the retention of tissue-resident memory T cells include transforming growth factor-β (TGF-β)-mediated induction of the E-cadherin receptor CD103 and downregulation of the chemokine receptor CCR7. These pathways enhance protection in internal organs, such as the nervous system, and in the barrier tissues—the mucosa and skin. Memory T cells that reside at these surfaces provide a first line of defense against subsequent infection, and defining the factors that regulate their development is critical to understanding organ-based immunity.
The development and functional programming of the immune system is characterized by regionalization at multiple levels. For example, the generation of mature CD4+ and CD8+ T cells is compartmentalized in the thymus and follows a prescribed set of selection steps geared toward achieving a functionally responsive and minimally autoreactive peripheral repertoire1. Although certain stochastic events designate outcomes in this process, the system is essentially closed and under normal circumstances is not heavily influenced by extrathymic events. In contrast, mature T cells responding to antigens are considerably affected by the context in which antigen presentation occurs, which often represents a continuously changing environment. Thus, the immune response to infection is subjected to a dynamic process with active changes to cell types and their locations, concentrations of inflammatory and anti-inflammatory mediators, blood and lymph flow, and antigen concentrations. In the secondary lymphoid tissues, where T cell priming occurs, the sum of these alterations dictates the type of effector T cells generated and the nature of the memory populations produced.
Memory T cells are characterized by considerable heterogeneity at the phenotypic and functional levels. Early studies identified functionally distinct subsets of human peripheral blood effector and memory CD8+ T cell subsets on the basis of the expression of costimulatory and adhesion molecules2. Further analysis of human blood has linked the expression pattern of the lymph node-homing receptors CD62L and CCR7 to the functional status of memory CD4+ and CD8+ T cells3. That is, cells lacking these molecules have heightened constitutive effector functions (effector memory T cells (TEM cells)), whereas cells expressing these receptors are apparently in a resting state (central memory T cells (TCM cells)). These findings have led to the hypothesis that the two subsets are located in distinct tissues, with TCM cells in lymph node, spleen and blood, and TEM cells in spleen, blood and nonlymphoid tissues; indeed, this prediction has held true4,5. After immunization or infection, CD4+ and CD8+ memory T cells with the ability to rapidly produce cytokines, and with direct ex vivo cytotoxic activity in the case of CD8+ T cells, are present in essentially all nonlymphoid tissues, including the lung, liver and intestine. Both localized and systemic infections can lead to the generation of memory cells that accumulate in nonlymphoid tissues6. However, entry of memory T cells into nonlymphoid tissues and/or their residence there can lead to tissue-specific influences that affect the phenotype and function of the memory populations7-10. Moreover, in some tissues there seems to be one-way traffic of effector or memory T cells into the site with no means of exit. Thus, depending on the nonlymphoid tissue, long-term, resident, regional memory is established. Here we discuss the factors that regulate the regionalization of memory, including those that regulate T cell migration, retention and exit. Additionally, we discuss the influence of tissue location on the types of effector T cell functions that develop and consider the relevance of regional memory to immunoprotection.
Inductive and effector sites of peripheral tissues
The human body is separated from the outside world by barrier surfaces that carry out many functions to promote human health. Memory T cells reside in these tissues, which consist mainly of the intestine, lungs, skin and genital surfaces. These tissues have a vast surface area and therefore contain most the body’s memory T cells. Other tissues, such as the brain, bone marrow and liver, as well as essentially any tissue, may also contain memory T cells5,6. An appreciation of the structure and anatomy of any given tissue is essential to the understanding of T cell immunity at that site. Some of these sites, in particular the mucosal surfaces and the skin, share certain anatomical characteristics. In the case of the lungs, intestine and portions of the genitourinary tract11, the exterior environment is separated from the internal one by a single layer of epithelial cells covered by a mucus layer. The skin is more complex, with an outer epidermal layer largely devoid of lymphatics underlaid by a dermal layer composed mainly of connective tissue. Dendritic cells (DCs) underlying the epithelium in the mucosae or within the epidermal and dermal layers of the skin are poised to capture incoming antigens and subsequently migrate to secondary lymphoid tissues for antigen presentation to T cells.
In the tissues discussed above, the immune response is typically subdivided into events that occur at inductive sites, such as the Peyer’s patches and mesenteric lymph nodes (MLNs) for the intestine, and effector sites, such as the gut lamina propria and intraepithelial lymphocyte compartment; the latter is positioned above the basement membrane (Fig. 1). In the lung, antigen presentation initially occurs in the mediastinal lymph nodes, followed by the migration of activated T cells to the lung parenchyma and the bronchoalveolar space. Pathogens that invade the host through the intestinal mucosa typically first encounter antigen-presenting cells in either the Peyer’s patches or the lamina propria. Peyer’s patches are unique among T cell-inductive sites, other than the spleen, because they do not acquire antigens through lymphatics but instead acquire them via specialized epithelial cells called ‘M cells’ that are scattered over the dome of the Peyer’s patches and transfer antigen to DCs in the subepithelial dome. T cell activation can then proceed in the Peyer’s patches and/or in the MLNs, because DCs continuously traffic from the mucosa to the latter site12-14. For example, Shigella subspecies15, Salmonella subspecies16, reovirus, poliovirus and other enteric viruses17 gain access to the body mainly through the gastrointestinal tract by transit through M cells. Indeed, after oral infection with Salmonella typhimurium, antigen-specific CD4+ T cells are activated in the Peyer’s patches by local DCs18. M cells also provide an entry point through the lung epithelium for pathogens, including Mycobacterium tuberculosis19, and through the intestinal villous epithelium20. However, pathogens may also enter the body by direct transit through epithelial cells, as in humans infected with Listeria monocytogenes and in mice infected with recombinant L. monocytogenes expressing a modified internalin A protein that allows interaction with mouse E-cadherin21 or in L. monocytogenes–infected mice that transgenically express human E-cadherin22. Continual exposure of mucosal surfaces and the skin to potentially pathogenic microorganisms requires that the immune system handle diverse and often repeated insults. Thus, pathogen encounter or vaccination can generate lifelong immunologic memory, thereby providing protection against subsequent pathogen invasion.
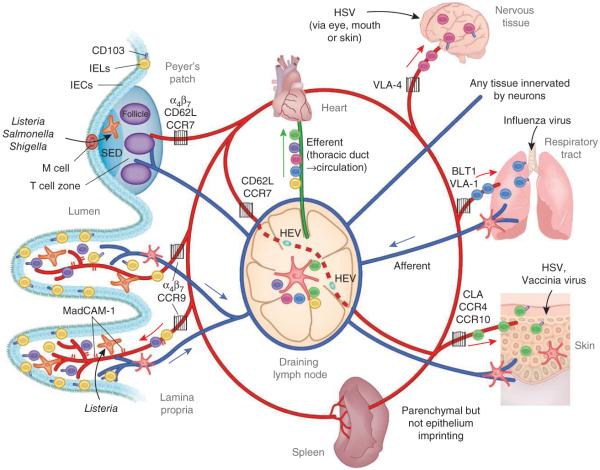
Figure 1.
The migration of effector and memory T cells to sites of localized infection. DC acquisition of antigen occurs at peripheral sites such as the lung, skin or gut after a breach in the epithelial layer or local infection. After capturing antigen, DCs access initial afferent lymphatics mediated by signals from integrins, chemokines and semaphorins to migrate to draining lymph nodes to activate naive or memory cells. For example, breach of the intestinal mucosa leads to T cell priming in the MLNs and/or Peyer’s patches. The spleen is poised to generate a substantial T cell response if pathogens are not compartmentally contained and gain access to the bloodstream. In either scenario, a robust T cell population expansion program follows with DC-mediated instruction for migration to the site of initial infection. Activated T cells and circulating memory cells exit the lymph node via the efferent lymphatics and return to circulation through the thoracic duct (colors indicate T cells instructed to home to specific tissues). As T cells migrate through the circulation, integrin and chemokine signals direct their emigration into tissues. In this manner, imprinted T cells have a specific key that allows access to restricted tissues (gates) under normal homeostatic conditions. IEL, intraepithelial lymphocyte; IEC, intestinal epithelial cell; VLA-4, integrin α4β1.
Memory T cell development and function
Determining how heterogeneous memory populations are generated and migrate to their appropriate locations has been a subject of much interest over the past decade. The lineage decision to generate memory occurs early after infection23-25. The fate of effector CD8+ T cells has been identified by expression of the receptors KLRG1 (killer cell lectin-like receptor subfamily G1) and CD127 (interleukin 7 (IL-7) receptor α-chain)26-29. Effector CD8+ T cells that express CD127 but not KLRG1 represent a true memory precursor effector cell (MPEC) population that generates long-lived CD8+ T cell memory and has stem cell-like renewal qualities (Fig. 2). Although CD127 is a marker for MPECs, IL-7-mediated signaling does not seem to select for the generation of memory T cells30-32. Conversely, CD8+ T cells expressing KLRG1 but not CD127 undergo apoptosis during contraction and are therefore called ‘short-lived effector cells’ (SLECs)28,29,31. Early effector CD8+ T cells express neither CD127 nor KLRG1, seem to be the earliest CD8+ T cell population generated after antigen encounter and give rise to both MPEC and SLEC subsets25,33,34 (Fig. 2). Cells expressing both CD127 and KLRG1 also appear in the effector and memory CD8+ T cell population, but it is unclear whether these cells represent an intermediary population or a distinct lineage of CD8+ T cells. The generation of these effector CD8+ T cell subsets varies greatly among different infection settings and after secondary infection; this is consistent with the regulation of their development by inflammatory mediators28,35. The composition of these subsets and how they might relate to memory development in nonlymphoid tissues has not yet been studied in detail, although some data are emerging. In the lung airways after influenza virus infection and in sensory ganglia after herpes simplex virus (HSV) infection, most antigen-specific effector CD8+ T cells are ‘early effector cells’, whereas MPECs increase in number over time36,37. Further experimentation is needed to determine the effects of tissue-specific factors on effector and memory T cell development in different tertiary tissues.
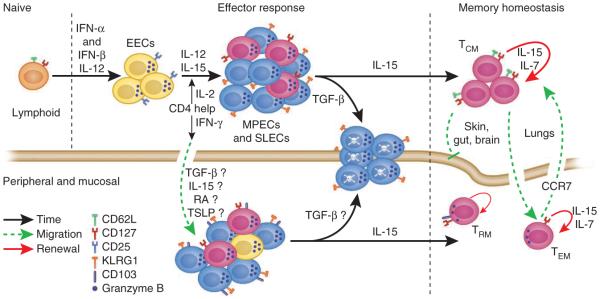
Figure 2.
The generation of a diverse and dynamic memory T cell population is a highly orchestrated process. Naive CD8+ T cells rapidly downregulate CD62L and CD127 after exposure to their cognate antigen in the proper contextual environment. IL-12, type I interferons (IFN-α and IFN-β) and IL-2 regulate the initial development of a heterogeneous effector T cell population in lymphoid tissues. CD4+ T cells are thought to be crucial to distinct aspects of this process. After certain infections, such as with l. monocytogenes, CD4+ T cell help is essential for the generation of a robust SLEC population and may also regulate the generation of TCM cells through IL-2 production25,34. However, IFN-γ produced by CD4+ T cells regulates recruitment of CD8+ T cells to the site of peripheral infection, such after infection of the genital tract with HSV109. Once T cells enter peripheral tissues, various molecules from the tissue microenvironment may further influence effector heterogeneity. For example, the cytokines TGF-β and TSLP can have tissue-specific effects on the differentiation of T cells in the intestinal mucosa71,83,110,111. Contraction of the T cell response is regulated in part by TGF-β and IL-15, which mediate opposing fates of the effector pool33,112. At least in lymphoid tissues, TGF-β seems to induce apoptosis of SLECs, whereas IL-15 promotes T cell survival and entry into the memory pool33,112. TCM cells reside mainly in lymphoid tissues and downregulate expression of granzyme B, whereas TEM and TRM cells reside in peripheral nonlymphoid tissues and maintain granzyme B expression and direct lytic activity. The migration of memory T cells into the skin, gut and brain seems to be restricted, whereas replenishment of liver and lung parenchyma (but not airway) memory T cells occurs through the circulation. Memory T cells located in peripheral tissues may also be regulated by CCR7-mediated emigration signals, which allows the potential for further modification of the TEM cell population102,103. EEC, early effector cell.
TCM and TEM cells arise as distinct lineages only in the MPEC population early in the immune response, and this lineage decision is influenced by T cell antigen receptor (TCR) signal strength, IL-2 and IL-15, among other factors24,25. The description of TCM and TEM cells has led to many studies attempting to delineate the factors that drive the differentiation of effector T cells and subsequent memory development. These efforts have provided insight into the physiological and anatomical differences between T cells that reside in lymphoid compartments and those that reside in nonlymphoid compartments. For example, TCM cells proliferate homeostatically faster than TEM cells do and gradually become the dominant memory population in lymphoid tissues38-40. Although some peripheral nonlymphoid tissues host a few TCM cells, these cells never predominate in the lung, liver or intestinal mucosa9,41. These results suggest that tertiary sites contain memory cells that are not derived from the recirculating pool or that cells undergo differentiation after infiltration of the tissue9.
The protective abilities of CD4+ and CD8+ TCM and TEM cells have also been explored in some detail41 and, depending on the infection, both have protective abilities42,43. Because TCM and TEM cells localize to different sites, on the basis of their expression of homing molecules, direct comparison of their protective abilities is often difficult and depends on the location of pathogen replication and therefore the site of T cell priming (for example, spleen versus draining lymph nodes)44. A distinction between ‘proliferative’ recall and ‘functional’ reactivation should also be considered in terms of regional memory. For example, soon after secondary oral infection, a CD8+ T EM intestinal intraepithelial lymphocyte may kill an infected intestinal epithelial cell through constitutive lytic activity but might not undergo secondary population expansion in situ. In contrast, TCM cells in the Peyer’s patches or MLNs encounter antigen later, proliferate extensively and migrate to the infected epithelium to provide additional protection and produce a new cadre of TEM cells. The particular cell type presenting antigen also influences the outcome of reactivation45. For example, influenza virus-specific effector CD8+ T cells are triggered to kill both CD45+ and CD45- target cells, whereas only costimulatory molecule-expressing CD45+ antigen-presenting cells induce the production of cytokines46. However, HSV reactivation in the dorsal root ganglia leads to DC-mediated re-expansion of resident memory cell populations47. Moreover, resident memory cells seem crucial for protection against HSV reactivation, as latently infected mice cannot avoid reactivation in the absence of CD8+ T cells or after a stress-induced compromise of CD8+ T cell function48. Given that HSV-specific CD8+ T cells residing in the latently infected trigeminal ganglia do not seem to be replenished by circulating cells49, loss of protection is probably due to loss of memory surveillance in the ganglia. Similarly, memory T cells residing in the skin provide protection against HSV infection50. In general, it is challenging to assess the protective abilities of tissue TEM cells in part because of difficulty in temporally and anatomically separating the events of memory T cell reactivation in secondary lymphoid tissue versus nonlymphoid tissue. For example, for direct testing of the protective ability of memory T cells residing in the intestinal epithelium, after challenge infection, the migration of new effectors into the intestine would need to be blocked without affecting the resident population. Thus, ongoing studies involve the development of systems to determine the intrinsic abilities of regional memory T cells.
Regulation of T cell entry into nonlymphoid tissues
Tissue-specific memory populations may be generated through a process called ‘imprinting.’ The best-characterized imprinting events are those that occur during T cell priming in the Peyer’s patches and MLNs. T cells responding to tissue-specific infection or immunization are primed in the draining lymph nodes, where they receive ‘instruction’ that directs their migration to the initial site of infection (Fig. 1). Imprinting for intestinal migration is mediated by specialized DC subsets that reside in the Peyer’s patches or that migrate from the lamina propria to the MLNs51,52. Similar events occur in skin-draining lymph nodes53,54. Through the action of retinoic acid, the integrin α4β7 and the chemokine receptor CCR9 are induced on intestinal mucosal T cells during priming55,56. The lack or blockade of either of these molecules prevents intestinal infiltration by effector T cells57-62. The addressin and α4β7 ligand MAdCAM-1 is constitutively expressed by a subset of vascular endothelial cells in the intestinal lamina propria and Peyer’s patches, and by high endothelial venules in the MLNs63-65 and the sacral lymph nodes that drain the genital tract66, whereas the ligand for CCR9 (CCL25) is constitutively expressed by intestinal epithelial cells in the small intestine52,62. The ability of DCs to provide gut-homing instruction to T cells is assigned to a subset of MLN- or Peyer’s patch-derived DCs that express the αE integrin (CD103) paired with the β7 chain. CD103+ DCs are also the main subset of migrating DCs that prime CD8+ T cells in the draining lymph node of the lung and the skin67-70 and ‘preferentially’ induce CD4+ regulatory T cells in the lamina propria and MLNs through a mechanism that depends on transforming growth factor-β (TGF-β) and retinoic acid 71,72. CD103 expression does not seem essential for gut-homing instruction, because a monocyte-derived inflammatory DC population in the intestine and MLNs that expresses E-cadherin and preferentially primes inflammatory responses of the TH17 subset of helper T cell responses can also home to the intestine73. In contrast with the response of CD103+ DCs, the generation of E-cadherin-positive DCs is impaired by TGF-β, which suggests that TGF-β may negatively regulate inflammatory responses but support inhibitory responses73 (Fig. 3).
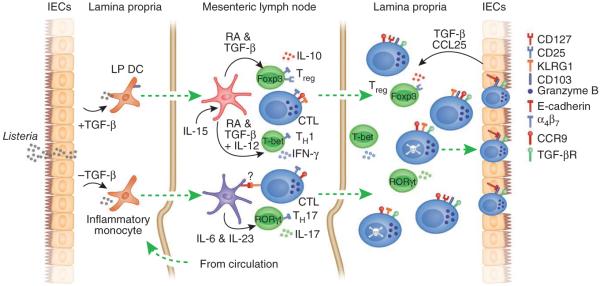
Figure 3.
Multifaceted roles of TGF-β in generating mucosal effector and memory T cell populations. TGF-β seems to regulate many aspects of CD8 memory T cell formation in mucosal and potentially other peripheral tissues. Initially, TGF-β derived from intestinal epithelial cells influences the antigen-presenting cells, which migrate to the draining lymph nodes to prime local T cell responses. The presence or absence of TGF-β regulates the expression of CD103 or E-cadherin on antigen-presenting cell subsets in the lamina propria (LP). CD103+ DCs, which also produce TGF-β, mediate imprinting of gut-homing T cells by a mechanism that depends on TGF-β and retinoic acid (RA) and are also responsible for the generation of regulatory T cells (Treg)71. In conjunction with IL-15, T helper type 1 (TH1) and TH17 cells are ‘preferentially’ generated, which skews the T cell response to a proinflammatory nature113. However, TGF-β also suppresses the generation of E-cadherin-positive DCs, which ‘preferentially’ drive a TH17 response in a colitis model73. It is unknown whether CD8+ T cells of the SLEC type, which express KLRG1, or other CD103+ CD8+ T cells can interact with E-cadherin expressed by these inflammatory DCs. Whatever holds true, CD4+ T cells primed by these DCs are fully able to migrate to the lamina propria. Once T cells emigrate into the lamina propria, α4β7 is rapidly downregulated and CD103 expression is upregulated in a TGF-β-dependent manner in a subset of lamina propria CD8+ T cells. CCL25 expression by intestinal epithelial cells mediates a chemotactic gradient to recruit effector CD8+ T cells into the epithelium. TGF-β signals also drive the apoptosis of CD8+ T cells of the SLEC type, at least in lymphoid tissues, but may also mediate distinct outcomes on the basis of the presence of other inflammatory or anti-inflammatory mediators33,113. In this system, TGF-β is probably produced by both the epithelium and lamina propria DCs, but the relative contributions of each source remain unclear. Foxp3, T-bet and RORγt are transcription factors; CTL, cytotoxic T lymphocyte; TGF-βR, TGF-β receptor.
Although mucosal lymphoid tissues preferentially prime CD8+ T cells to induce a gut-homing phenotype, this requirement is not absolute, as systemic infection induced by lymphocytic choriomenin-gitis virus, vesicular stomatitis virus or L. monocytogenes, as well as intraperitoneal administration of soluble antigen, also leads to intestinal infiltration by antigen-specific CD8+ T cells5,74,75. Indeed, splenic priming after intravenous infection with lymphocytic choriomeningitis virus induces a narrow window (4.5-7 days) of instruction for the intestinal migration of T cells75. However, although integrin α4β7 is upregulated early in the spleen of these mice, CCR9 is induced only in the MLNs, which suggests that infiltration of the lamina propria may have contributions from splenic priming whereas localization to the epithelium requires priming in the MLNs75. In addition, systemic infections probably prime T cell responses in the spleen and in various lymph nodes, leading to tissue-specific migration from multiple sources. DCs that acquire antigen from the skin induce a skin-homing phenotype on responding T cells characterized by expression of E-selectin and P-selectin ligands (such as CLA) and of CCR4 and/or CCR10 (refs. 53,54,76; Fig. 2). It is unclear whether lung-draining DCs impart a particular instruction pattern for migration into the lung parenchyma or the bronchoalveolar space, or whether T cell activation alone or inflammation alone is sufficient to drive infiltration8,77. Leukotriene B4, whose receptor BLT1 is expressed by activated CD8+ T cells, is one factor that controls the migration of effector cells to the lung and other tissues78,79. In addition, the α1β1 integrin VLA-1 is also involved in the migration or retention of effector and memory CD8+ T cells in the lung80,81. With the increasing heterogeneity of the CD4+ and CD8+ T cell response now being characterized, it is important to revisit these phenomena and determine whether distinct subsets of CD8 effectors are imprinted for mucosal migration. In such a manner, divergent effector CD8+ T cell populations may be imprinted differently for migration to nonlymphoid tissues.
Mechanisms of T cell retention in tertiary tissues
Once priming and imprinting has occurred, effector T cells enter the efferent lymphatics and subsequently return to the bloodstream through the thoracic duct (Fig. 1). The circulating cells may then interact with the appropriate adhesion ligands expressed by specialized endothelial cells to facilitate extravasation into the tissue. Recently emigrated cells may be further influenced by the local microenvironment of the tissue. For example, effector and memory CD8+ T cells migrating into the lamina propria and intraepithelial lymphocyte compartment upregulate CD103 and the activation marker CD69 (refs. 7,82,83). Indeed, although α4β7 expression is required for lymphocyte entry into the intestinal lamina propria and epithelium, once there, most effector and memory CD8+ T cells down-regulate α4β7 and upregulate CD103 (refs. 7,84). Thus, most intestinal CD8+ T cells have high expression of CD103, whereas most lymphoid CD8+ T cells lack or have low expression of CD103, which suggests a prominent role for this integrin in the mucosa. Because the ligand for CD103 is E-cadherin expressed by intestinal epithelial cells, researchers have hypothesized that CD103 is involved in retention of CD8+ T cells in the epithelium85. Parabiosis and tissue-grafting studies also suggest that most gut memory T cells do not emigrate from the gut and are only minimally replenished by circulating lymphocytes7,75,86. That idea is further supported by studies describing resident memory T cells (TRM cells) in other tissues47,50,87. TRM cells seem to be maintained long-term with less homeostatic turnover and at present are restricted to intestinal tissues7,75 and nervous tissues87-89 and the skin epidermal layer47,50. The idea that this strict compartmentalization of residence in the nervous tissue is complete in humans may be called into question by the finding that reactivation of JC polyo-mavirus occurs in some people treated with antibody to integrin α4; whether this is due to inhibition of T cell responses remains speculative90-92. In contrast, in the lung and liver parenchyma, memory CD8+ T cells seem to be repopulated with cells from the circulation7. However, although perfusion has been used for cell isolation in these studies, the extensive circulatory systems of these tissues could promote contamination by blood-borne lymphocytes. Further study is needed to test this possibility. As for the lung airways, after influenza virus infection, antigen-specific memory CD8+ T cells migrate to the airways only when residual cognate antigen is present8. Moreover, antigen-specific memory CD8+ T cells are preferentially retained in the mediastinal lymph nodes, whereas they freely migrate through other lymph nodes because of the presence of residual antigen in the mediastinal lymph nodes8. In contrast, Sendai virus-specific airway memory CD8+ T cells are continuously replenished from circulating cells77, although it is not known whether antigen is retained long term after this infection. Residual antigen is also involved in the induction and maintenance of CD103 and CD69 expression by influenza virus-specific CD8+ T cells93; these processes are involved in the migration and retention of these cells in the lung tissue10, thereby promoting protection.
The main similarity between memory CD8+ T cells resident in the gut, lung, skin and brain is their expression of CD103 and CD69 (refs. 7,50,82,87). CD103 binds E-cadherin, which is expressed mainly by epithelial cells and through homotypic interactions is essential for development and maintenance of tissue integrity94. KLRG1 also binds E-cadherin as well as N-cadherin, and the CD103-binding site is distinct from that of KLRG1 (ref. 95). Whether similar mechanisms exist for CD4+ T cells is not yet clear, although most CD4+ intestinal intraepithelial lymphocyte s and~20% of CD4+ lamina propria T cells also express CD103 (ref. 96). CD103 induction depends on TGF-β, which is produced by epithelial cells as well as by DC subsets (Fig. 3). In the absence of signaling through the TGF-β receptor, effector CD8+ T cells infiltrating the intestinal epithelium during graft-versus-host disease do not upregulate CD103 (ref. 83) and are less pathogenic. In the lungs, CD103-deficient CD8+ T cells are inefficiently retained after influenza viral infection10. CD103 may have other important roles in immune responses, such as facilitating movement through epithelial cell layers, as interaction with E-cadherin influences cellular shape and motility of lymphocytes within the skin epidermal layer97.
In addition, signal transduction via CD103 can augment T cell functions, including lytic activity and lytic granule polarization in tumor-infiltrating lymphocytes; these processes facilitate tumor clearance98. These abilities suggest that CD103 ligation provides critical signals for T cell function in tertiary tissues.
In addition to active retention mechanisms, the loss of molecules involved in signaling pathways that promote exit from tissues may also enhance tissue localization. For example, sphingosine 1-phosphate receptor type 1 (S1P1) is involved in thymocyte emigration and the exit of naive T cells from the lymph node, whereas CD69 may suppress S1P1 function through an intracellular interaction with this receptor99-101. In this way, CD69 expression decreases S1P1 function and prolongs the retention of activated T cells in the lymph node. CD69 promotes the migration or retention of CD8+ T cells in influenza virus-infected lungs10 and thus may have a general role in the tissue retention of effector and memory T cells. CCR7 is also involved in the exit of effector and memory CD8+ T cells from the lung and skin to the afferent lymphatics102,103 and may also operate in other tertiary tissues. Because most effector T cells downregulate CCR7 and TEM cells lack CCR7, this downregulation may be an additional mechanism by which exit from the tissues is regulated (Fig. 2). Thus, the theme of induction of specialized retention molecules and down-regulation of exit signals in several tissues suggests a fundamental mechanism by which effector and memory CD8+ T cells carry out surveillance functions in critical organ systems.
Implications and significance
Defining the processes that control the tissue-specific migration and retention of effector and memory T cells has major implications for disease pathogenesis and for the design of therapeutic interventions. Effective vaccination in humans induces long-lived antibody responses as well as T cell memory104. CD8+ T cell responses could be exploited for protection against infection with human immunodeficiency virus, whose major portals of entry are the genital and intestinal mucosa105,106. Similarly, influenza virus enters via the respiratory mucosa, and induction of memory CD8+ T cells specific for conserved viral epitopes could provide broad-based protection against multiple serotypes. Thus, designing vaccines that induce the proper homing and retention molecules on the responding T cells would probably substantially increase protective efficacy at the entry point of these and other pathogens. In contrast, tissue-specific effector and memory T cells may be involved in disease pathogenesis, such as in inflammatory bowel disease and multiple sclerosis. In these cases, inhibition, rather than promotion, of homing and retention might provide disease amelioration. Thus, treatment with antibodies to α4β7 and α4 is being tested to combat inflammatory bowel disease and multiple sclerosis, respectively, although such treatments have risks107,108. Defining the molecular and cellular events that surround the induction and maintenance of tissue-specific memory T cells may provide the foundation for the future development of new therapies.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu. Rev. Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Hamann D, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 4.Reinhardt RL, Khoruts A, Merica R, Zell T, Jenkins MK. Visualizing the generation of memory CD4 T cells in the whole body. Nature. 2001;410:101–105. doi: 10.1038/35065111. [DOI] [PubMed] [Google Scholar]
- 5.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 6.Masopust D, et al. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J. Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- 7.Klonowski KD, et al. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- 8.Zammit DJ, Turner DL, Klonowski KD, Lefrançois L, Cauley LS. Residual antigen presentation after influenza virus infection affects CD8 T cell activation and migration. Immunity. 2006;24:439–449. doi: 10.1016/j.immuni.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marzo AL, Yagita H, Lefrançois L. Cutting edge: migration to nonlymphoid tissues results in functional conversion of central to effector memory CD8 T cells. J. Immunol. 2007;179:36–40. doi: 10.4049/jimmunol.179.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y-T, et al. Environmental and antigen receptor-derived signals support sustained surveillance of the lungs by pathogen-specific cytotoxic T lymphocytes. J. Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat. Rev. Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu LM, MacPherson GG. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J. Exp. Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang F-P. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T cell areas of mesenteric lymph nodes. J. Exp. Med. 2000;191:435–444. doi: 10.1084/jem.191.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J. Immunol. 2006;176:4155–4162. doi: 10.4049/jimmunol.176.7.4155. [DOI] [PubMed] [Google Scholar]
- 15.Perdomo OJ, et al. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J. Exp. Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones BD, Ghori N, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer’s patches. J. Exp. Med. 1994;180:15–23. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neutra MR, Frey A, Kraehenbuhl JP. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 18.Salazar-Gonzalez RM, et al. CCR6-mediated dendritic cell activation of pathogen-specific T cells in Peyer’s patches. Immunity. 2006;24:623–632. doi: 10.1016/j.immuni.2006.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teitelbaum R, et al. The M cell as a portal of entry to the lung for the bacterial pathogen mycobacterium tuberculosis. Immunity. 1999;10:641–650. doi: 10.1016/s1074-7613(00)80063-1. [DOI] [PubMed] [Google Scholar]
- 20.Jang MH, et al. Intestinal villous M cells: an antigen entry site in the mucosal epithelium. Proc. Natl. Acad. Sci. USA. 2004;101:6110–6115. doi: 10.1073/pnas.0400969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schubert WD, et al. Structure of internalin, a major invasion protein of listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- 22.Disson O, et al. Modeling human listeriosis in natural and genetically engineered animals. Nat. Protoc. 2009;4:799–810. doi: 10.1038/nprot.2009.66. [DOI] [PubMed] [Google Scholar]
- 23.Lefrançois L, Obar JJ. Once a killer, always a killer: from cytotoxic T cell to memory cell. Immunol. Rev. 2010;235:206–218. doi: 10.1111/j.0105-2896.2010.00895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obar JJ, Lefrançois L. Early events governing memory CD8+ T-cell differentiation. Int. Immunol. 2010;22:619–625. doi: 10.1093/intimm/dxq053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Obar JJ, Lefrançois L. Early signals during CD8+ T cell priming regulate the generation of central memory cells. J. Immunol. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 27.Kaech SM, et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4:1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 28.Joshi NS, et al. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar S, et al. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 2008;205:625–640. doi: 10.1084/jem.20071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klonowski KD, Williams KJ, Marzo AL, Lefrançois L. Cutting edge: IL-7-independent regulation of IL-7 receptor α expression and memory CD8 T cell development. J. Immunol. 2006;177:4247–4251. doi: 10.4049/jimmunol.177.7.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hand TW, Morre M, Kaech SM. Expression of IL-7 receptor α is necessary but not sufficient for the formation of memory CD8 T cells during viral infection. Proc. Natl. Acad. Sci. USA. 2007;104:11730–11735. doi: 10.1073/pnas.0705007104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haring JS, et al. Constitutive expression of IL-7 receptor α does not support increased expansion or prevent contraction of antigen-specific CD4 or CD8 T cells following listeria monocytogenes infection. J. Immunol. 2008;180:2855–2862. doi: 10.4049/jimmunol.180.5.2855. [DOI] [PubMed] [Google Scholar]
- 33.Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-β and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Obar JJ, et al. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. Proc. Natl. Acad. Sci. USA. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui W, Joshi NS, Jiang A, Kaech SM. Effects of Signal 3 during CD8 T cell priming: bystander production of IL-12 enhances effector T cell expansion but promotes terminal differentiation. Vaccine. 2009;27:2177–2187. doi: 10.1016/j.vaccine.2009.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheridan BS, Cherpes TL, Urban J, Kalinski P, Hendricks RL. Reevaluating the CD8 T-cell response to herpes simplex virus type 1: involvement of CD8 T cells reactive to subdominant epitopes. J. Virol. 2009;83:2237–2245. doi: 10.1128/JVI.01699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Croom HA, et al. Memory precursor phenotype of CD8+ T cells reflects early antigenic experience rather than memory numbers in a model of localized acute influenza infection. Eur. J. Immunol. 2011;41:682–693. doi: 10.1002/eji.201040625. [DOI] [PubMed] [Google Scholar]
- 38.Tripp RA, Hou S, Doherty PC. Temporal loss of the activated L-selectin-low phenotype for virus-specific CD8+ memory T cells. J. Immunol. 1995;154:5870–5875. [PubMed] [Google Scholar]
- 39.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 40.Marzo AL, et al. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat. Immunol. 2005;6738:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodland DL, Kohlmeier JE. Migration, maintenance and recall of memory T cells in peripheral tissues. Nat. Rev. Immunol. 2009;9:153–161. doi: 10.1038/nri2496. [DOI] [PubMed] [Google Scholar]
- 42.Lefrançois L, Puddington L. Intestinal and pulmonary mucosal T cells: local heroes fight to maintain the status quo. Annu. Rev. Immunol. 2006;24:681–704. doi: 10.1146/annurev.immunol.24.021605.090650. [DOI] [PubMed] [Google Scholar]
- 43.Lefrançois L. Development, trafficking, and function of memory T-cell subsets. Immunol. Rev. 2006;211:93–103. doi: 10.1111/j.0105-2896.2006.00393.x. [DOI] [PubMed] [Google Scholar]
- 44.Klonowski KD, et al. CD8 T cell recall responses are regulated by the tissue tropism of the memory cell and pathogen. J. Immunol. 2006;177:6738–6746. doi: 10.4049/jimmunol.177.10.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Crowe SR, et al. Differential antigen presentation regulates the changing patterns of CD8+ T cell immunodominance in primary and secondary influenza virus infections. J. Exp. Med. 2003;198:399–410. doi: 10.1084/jem.20022151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hufford MM, Kim TS, Sun J, Braciale TJ. Antiviral CD8+ T cell effector activities in situ are regulated by target cell type. J. Exp. Med. 2011;208:167–180. doi: 10.1084/jem.20101850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 48.Freeman ML, Sheridan BS, Bonneau RH, Hendricks RL. Psychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infections. J. Immunol. 2007;179:322–328. doi: 10.4049/jimmunol.179.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Himmelein S, et al. Circulating herpes simplex type 1 (HSV-1)-specific CD8+ T cells do not access HSV-1 latently infected trigeminal ganglia. Herpesviridae. 2011;2:5. doi: 10.1186/2042-4280-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat. Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 51.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 52.Agace W. Generation of gut-homing T cells and their localization to the small intestinal mucosa. Immunol. Lett. 2010;128:21–23. doi: 10.1016/j.imlet.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 53.Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+ T cells activated in cutaneous or mucosal lymphoid tissues. J. Exp. Med. 2002;195:135–141. doi: 10.1084/jem.20011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mora JR, et al. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J. Exp. Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwata M, et al. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Svensson M, et al. Retinoic acid receptor signaling levels and antigen dose regulate gut homing receptor expression on CD8+ T cells. Mucosal Immunol. 2008;1:38–48. doi: 10.1038/mi.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lefrançois L, et al. The role of β7 integrins in CD8 T cell trafficking during an antiviral immune response. J. Exp. Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of a4-integrins in lymphocyte homing to mucosal tissues in vivo. J. Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 59.Svensson M, et al. CCL25 mediates the localization of recently activated CD8αβ+ lymphocytes to the small-intestinal mucosa. J. Clin. Invest. 2002;110:1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sandborn WJ, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N. Engl. J. Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 61.Feagan BG, et al. Treatment of ulcerative colitis with a humanized antibody to the α4β7 integrin. N. Engl. J. Med. 2005;352:2499–2507. doi: 10.1056/NEJMoa042982. [DOI] [PubMed] [Google Scholar]
- 62.Wurbel MA, Malissen M, Guy-Grand D, Malissen B, Campbell JJ. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J. Immunol. 2007;178:7598–7606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Streeter PR, Berg EL, Rouse BT, Bargatze RF, Butcher EC. A tissue-specific endothelial cell molecule involved in lymphocyte homing. Nature. 1988;331:41–46. doi: 10.1038/331041a0. [DOI] [PubMed] [Google Scholar]
- 64.Berg EL, et al. Homing receptors and vascular addressins: cell adhesion molecules that direct lymphocyte traffic. Immunol. Rev. 1989;108:5–18. doi: 10.1111/j.1600-065x.1989.tb00010.x. [DOI] [PubMed] [Google Scholar]
- 65.Briskin M, et al. Human mucosal addressin cell adhesion molecule-1 is preferentially expressed in intestinal tract and associated lymphoid tissue. Am. J. Pathol. 1997;151:97–110. [PMC free article] [PubMed] [Google Scholar]
- 66.Soderberg KA, Linehan MM, Ruddle NH, Iwasaki A. MAdCAM-1 expressing sacral lymph node in the lymphotoxin β-deficient mouse provides a site for immune generation following vaginal herpes simplex virus-2 infection. J. Immunol. 2004;173:1908–1913. doi: 10.4049/jimmunol.173.3.1908. [DOI] [PubMed] [Google Scholar]
- 67.Bedoui S, et al. Cross-presentation of viral and self antigens by skin-derived CD103+ dendritic cells. Nat. Immunol. 2009;10:488–495. doi: 10.1038/ni.1724. [DOI] [PubMed] [Google Scholar]
- 68.Kim TS, Braciale TJ. Respiratory dendritic cell subsets differ in their capacity to support the induction of virus-specific cytotoxic CD8+ T cell responses. Plos ONE. 2009;4:e4204. doi: 10.1371/journal.pone.0004204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ballesteros-Tato A, Leon B, Lund FE, Randall TD. Temporal changes in dendritic cell subsets, cross-priming and costimulation via CD70 control CD8+ T cell responses to influenza. Nat. Immunol. 2010;11:216–224. doi: 10.1038/ni.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.del Rio ML, Bernhardt G, Rodriguez-Barbosa JI, Forster R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 2010;234:268–281. doi: 10.1111/j.0105-2896.2009.00874.x. [DOI] [PubMed] [Google Scholar]
- 71.Coombes JL, et al. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-β and retinoic acid-dependent mechanism. J. Exp. Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun CM, et al. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Siddiqui KR, Laffont S, Powrie F. E-cadherin marks a subset of inflammatory dendritic cells that promote T cell-mediated colitis. Immunity. 2010;32:557–567. doi: 10.1016/j.immuni.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim SK, et al. Generation of mucosal cytotoxic T cells against soluble protein by tissue-specific environmental and costimulatory signals. Proc. Natl. Acad. Sci. USA. 1998;95:10814–10819. doi: 10.1073/pnas.95.18.10814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kunkel EJ, et al. Expression of the chemokine receptors CCR4, CCR5, and CXCR3 by human tissue-infiltrating lymphocytes. Am. J. Pathol. 2002;160:347–355. doi: 10.1016/S0002-9440(10)64378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ely KH, Cookenham T, Roberts AD, Woodland DL. Memory T cell populations in the lung airways are maintained by continual recruitment. J. Immunol. 2006;176:537–543. doi: 10.4049/jimmunol.176.1.537. [DOI] [PubMed] [Google Scholar]
- 78.Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH. Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat. Immunol. 2003;4:965–973. doi: 10.1038/ni972. [DOI] [PubMed] [Google Scholar]
- 79.Tager AM, et al. Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat. Immunol. 2003;4:982–990. doi: 10.1038/ni970. [DOI] [PubMed] [Google Scholar]
- 80.Richter M, et al. Collagen distribution and expression of collagen-binding α1β1 (VLA-1) and α2β1 (VLA-2) integrins on CD4 and CD8 T cells during influenza infection. J. Immunol. 2007;178:4506–4516. doi: 10.4049/jimmunol.178.7.4506. [DOI] [PubMed] [Google Scholar]
- 81.Ray SJ, et al. The collagen binding α1β1 integrin VLA-1 regulates CD8 T cell-mediated immune protection against heterologous influenza infection. Immunity. 2004;20:167–179. doi: 10.1016/s1074-7613(04)00021-4. [DOI] [PubMed] [Google Scholar]
- 82.Kim SK, Reed DS, Heath WR, Carbone F, Lefrançois L. Activation and migration of CD8 T cells in the intestinal mucosa. J. Immunol. 1997;159:4295–4306. [PubMed] [Google Scholar]
- 83.El-Asady R, et al. TGFβ-dependent CD103 expression by CD8+ T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J. Exp. Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim SK, Schluns KS, Lefrançois L. Induction and visualization of mucosal memory CD8 T cells following systemic virus infection. J. Immunol. 1999;163:4125–4132. [PubMed] [Google Scholar]
- 85.Schön MP, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin α E (CD103)-deficient mice. J. Immunol. 1999;162:6641–6649. [PubMed] [Google Scholar]
- 86.Poussier P, Edouard P, Lee C, Binnie M, Julius M. Thymus-independent development and negative selection of T cells expressing T cell receptor α/β in the intestinal epithelium: evidence for distinct circulation patterns of gut- and thymus-derived T lymphocytes. J. Exp. Med. 1992;176:187–199. doi: 10.1084/jem.176.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sheridan BS, Khanna KM, Frank GM, Hendricks RL. Latent virus influences the generation and maintenance of CD8+ T cell memory. J. Immunol. 2006;177:8356–8364. doi: 10.4049/jimmunol.177.12.8356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Assche G, et al. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn’s disease. N. Engl. J. Med. 2005;353:362–368. doi: 10.1056/NEJMoa051586. [DOI] [PubMed] [Google Scholar]
- 91.Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N. Engl. J. Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 92.Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon β-1a for multiple sclerosis. N. Engl. J. Med. 2005;353:369–374. doi: 10.1056/NEJMoa051782. [DOI] [PubMed] [Google Scholar]
- 93.Khanna KM, et al. In situ imaging reveals different responses by naive and memory CD8 T cells to late antigen presentation by lymph node DC after influenza virus infection. Eur. J. Immunol. 2008;38:3304–3315. doi: 10.1002/eji.200838602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb. Perspect. Biol. 2009;1:a003053. doi: 10.1101/cshperspect.a003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Y, et al. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lefrançois L, Barrett TA, Havran WL, Puddington L. Developmental expression of the αIELβ7 integrin on T cell receptor γδ and T cell receptor αβ T cells. Eur. J. Immunol. 1994;24:635–640. doi: 10.1002/eji.1830240322. [DOI] [PubMed] [Google Scholar]
- 97.Schlickum S, et al. Integrin α E(CD103)β 7 influences cellular shape and motility in a ligand-dependent fashion. Blood. 2008;112:619–625. doi: 10.1182/blood-2008-01-134833. [DOI] [PubMed] [Google Scholar]
- 98.Le Floc’h A. αEβ7 integrin interaction with E-cadherin promotes antitumor CTL activity by triggering lytic granule polarization and exocytosis. J. Exp. Med. 2007;204:559–570. doi: 10.1084/jem.20061524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Feng C, et al. A potential role for CD69 in thymocyte emigration. Int. Immunol. 2002;14:535–544. doi: 10.1093/intimm/dxf020. [DOI] [PubMed] [Google Scholar]
- 100.Shiow LR, et al. CD69 acts downstream of interferon-α/β to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- 101.Bankovich AJ, Shiow LR, Cyster JG. CD69 suppresses sphingosine 1-phosophate receptor-1 (S1P1) function through interaction with membrane helix 4. J. Biol. Chem. 2010;285:22328–22337. doi: 10.1074/jbc.M110.123299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Debes GF, et al. Chemokine receptor CCR7 required for T lymphocyte exit from peripheral tissues. Nat. Immunol. 2005;6:889–894. doi: 10.1038/ni1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bromley SK, Thomas SY, Luster AD. Chemokine receptor CCR7 guides T cell exit from peripheral tissues and entry into afferent lymphatics. Nat. Immunol. 2005;6:895–901. doi: 10.1038/ni1240. [DOI] [PubMed] [Google Scholar]
- 104.Amanna IJ, Slifka MK. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411:206–215. doi: 10.1016/j.virol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chakrabarti LA, Simon V. Immune mechanisms of HIV control. Curr. Opin. Immunol. 2010;22:488–496. doi: 10.1016/j.coi.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Streeck H, Nixon DF. T cell immunity in acute HIV-1 infection. J. Infect. Dis. 2010;202(Suppl 2):S302–S308. doi: 10.1086/655652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fontoura P. Monoclonal antibody therapy in multiple sclerosis: Paradigm shifts and emerging challenges. Mabs. 2010;2:670–681. doi: 10.4161/mabs.2.6.13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Reenaers C, Louis E, Belaiche J. Current directions of biologic therapies in inflammatory bowel disease. Therap. Adv. Gastroenterol. 2010;3:99–106. doi: 10.1177/1756283X09356872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol. Rev. 2005;206:132–148. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 111.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J. Exp. Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.DePaolo RW, et al. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]