Abstract
An important pathway by which plants detoxify heavy metals is through sequestration with heavy-metal-binding peptides called phytochelatins or their precursor, glutathione. To identify limiting factors for heavy-metal accumulation and tolerance, and to develop transgenic plants with an increased capacity to accumulate and/or tolerate heavy metals, the Escherichia coli gshII gene encoding glutathione synthetase (GS) was overexpressed in the cytosol of Indian mustard (Brassica juncea). The transgenic GS plants accumulated significantly more Cd than the wild type: shoot Cd concentrations were up to 25% higher and total Cd accumulation per shoot was up to 3-fold higher. Moreover, the GS plants showed enhanced tolerance to Cd at both the seedling and mature-plant stages. Cd accumulation and tolerance were correlated with the gshII expression level. Cd-treated GS plants had higher concentrations of glutathione, phytochelatin, thiol, S, and Ca than wild-type plants. We conclude that in the presence of Cd, the GS enzyme is rate limiting for the biosynthesis of glutathione and phytochelatins, and that overexpression of GS offers a promising strategy for the production of plants with superior heavy-metal phytoremediation capacity.
Heavy-metal pollution of soils and waters, mainly caused by mining and the burning of fossil fuels, is a major environmental problem. Heavy metals, unlike organic pollutants, cannot be chemically degraded or biodegraded by microorganisms. An alternative biological approach to deal with this problem is phytoremediation, i.e. the use of plants to clean up polluted waters and soils (Black, 1995; Salt et al., 1995a). Heavy metals or metalloids can be removed from polluted sites by phytoextraction, which is the accumulation of the pollutants in the plant biomass (Kumar et al., 1995). Compared with other remediation technologies, phytoremediation is less expensive (1000-fold less expensive than excavation and reburial of soil [Cunningham and Ow, 1996]) and is particularly suitable for treatment of large volumes of substrate with low concentrations of heavy metals. However, the presence of heavy metals inhibits plant growth, limiting the application of phytoremediation. Therefore, one trait that is of great significance to phytoremediation is the ability of plants to tolerate the toxic metals that are being extracted from the soil (Goldsbrough, 1998).
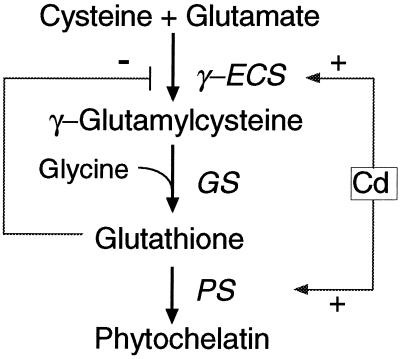
GSH plays several important roles in the defense of plants against environmental threats. Glutathione is not only a substrate for glutathione S-transferases, enabling neutralization of potentially toxic xenobiotics (Marrs, 1996), but is also a reductant of dehydroascorbate (Foyer and Haliwell, 1976). Moreover, GSH is the precursor for PCs, heavy-metal-binding peptides involved in heavy-metal tolerance and sequestration (Steffens, 1990). PCs constitute a family of peptides with the general structure (γ-Glu-Cys)n-Gly, where n = 2 to 11. PCs contain a high percentage of Cys sulfhydryl residues, which bind and sequester heavy-metal ions in stable complexes. PCs were induced by heavy metals such as Cd in all of the plants tested (Zenk, 1996). The roles of glutathione in heavy-metal tolerance and PC synthesis have been well illustrated in Cd-sensitive mutants of Arabidopsis. For example, the Cd-sensitive cad2 mutant was defective in glutathione biosynthesis (Howden et al., 1995). Glutathione is synthesized from its constituent amino acids in two sequential, ATP-dependent enzymatic reactions catalyzed by γ-ECS and GS, respectively (Fig. 1). PC synthase subsequently catalyzes the elongation of the (γ-Glu-Cys)n by transferring a γ-Glu-Cys group to glutathione or to PCs (Zenk, 1996; Chen et al., 1997).
Figure 1.
Regulation of GSH/PC biosynthesis in plants. PS, PC synthase. Cd enhances the transcription of ECS and activates the PC synthase enzyme, leading to the production of PCs and the depletion of GSH. γ-ECS is also subject to feedback inhibition by GSH.
Manipulating the expression of enzymes involved in glutathione and PC synthesis may be a good approach to enhancing heavy-metal tolerance in plants. The enzyme PC synthase does not appear to be a likely candidate to be rate limiting for PC synthesis, because it is constitutively expressed in plants (Steffens, 1990) and activated by the presence of heavy metals (Zenk, 1996). In any case, to our knowledge, no gene for PC synthase has yet been cloned. The genes encoding enzymes involved in GSH synthesis, on the other hand, may hold more promise. The rate-limiting step for glutathione synthesis in the absence of heavy metals is thought to be the reaction catalyzed by γ-ECS, because the activity of this enzyme is feedback regulated by glutathione and dependent on Cys availability (Fig. 1). This view was supported by the observation that overexpression of the Escherichia coli gshI gene, encoding γ-ECS, in poplar resulted in increased foliar glutathione levels (Arisi et al., 1997). Moreover, expression of tomato γ-ECS could restore some degree of heavy-metal tolerance to the cad2 Arabidopsis mutant. However, overexpression of this gene did not increase the Cd tolerance of wild-type Arabidopsis plants (Goldsbrough, 1998).
GS is not rate limiting for glutathione synthesis in the absence of heavy metals. Overexpression of the E. coli gshII gene, encoding GS, did not increase foliar glutathione levels in poplar (Foyer et al., 1995). However, under heavy-metal stress the regulation of glutathione biosynthesis undergoes a significant change. Heavy metals activate the PC synthase enzyme and thus induce the biosynthesis of PCs, resulting in a depletion of cellular glutathione levels (Fig. 1; Zenk, 1996). Consequently, the feedback inhibition of γ-ECS by glutathione is released. Furthermore, γ-ECS expression may be enhanced by heavy metals (Fig. 1). It was demonstrated that Cd enhances the transcription of the γ-ECS gene (Hatcher et al., 1995). In contrast, Cd may deactivate GS, because GS activity has previously been shown to be inhibited by Cd, whereas the same Cd treatment had no effect on γ-ECS activity (Schneider and Bergmann, 1995). Exposure of maize roots to Cd, in addition to causing a decline in GSH, caused an accumulation of γ-Glu-Cys, possibly because the activity of GS was reduced in vivo (Rauser et al., 1991). Therefore, under Cd stress the GS enzyme may become rate limiting for the biosynthesis of glutathione and PCs, and thus overexpression of gshII may alleviate the depletion of glutathione and enhance PC synthesis.
To test this hypothesis and to obtain plants with superior Cd accumulation and tolerance, we overexpressed the E. coli GS enzyme in the cytosol of Indian mustard (Brassica juncea), which is particularly suitable for phytoremediation because of its rapid biomass production and large trace-element accumulation capacity (Dushenkov et al., 1995). Indian mustard was also shown to produce a PC-Cd-sulfide complex (Speiser et al., 1992). The transgenic GS plants were compared with untransformed Indian mustard plants with respect to their Cd accumulation and tolerance, as well as their levels of heavy-metal-binding peptides.
MATERIALS AND METHODS
Materials
Indian mustard (Brassica juncea) seeds (accession no. 173874) were obtained from the North Central Regional Plant Introduction Station (Ames, IA). The gene construct used was described earlier by Strohm et al. (1995). It contains the Escherichia coli gshII gene, driven by the double-enhanced 35S cauliflower mosaic virus promoter, and the nptII gene, conferring kanamycin resistance.
Plant Transformation
All in vitro plant tissue cultures were grown at 25°C under continuous light. For transformation, Indian mustard hypocotyl segments were isolated from 3-d-old axenically grown seedlings (200–300 seedlings per transformation). The segments were immersed for 1 h in a suspension of the gshII-containing Agrobacterium tumefaciens strain C58pMP90 (A600 = 0.6) suspended in Murashige and Skoog medium; the bacteria were previously grown for 3 d at 28°C in liquid Luria-Bertani medium in the presence of 200 μm acetosyringone (3,5-dimethoxy-4-hydroxy-acetophenone; Fluka). After immersion in the bacterial suspension, the hypocotyls were blotted dry and transferred to medium containing Murashige and Skoog salts and vitamins (M5519, Sigma), 4 g L−1 agarose, 10 g L−1 Suc, Glc, and mannitol, 200 μm acetosyringone, 2 mg L−1 6-benzylaminopurine, and 0.1 mg L−1 naphthalene acetic acid. After 2 d of cultivation the hypocotyls were washed for 45 min in standard liquid Murashige and Skoog medium, blotted dry, and transferred to medium containing Murashige and Skoog salts and vitamins, 4 g L−1 agarose, 10 g L−1 Suc, Glc, and mannitol, 200 mg L−1 cefotaxime, 100 mg L−1 vancomycin, 20 mg L−1 kanamycin, 2 mg L−1 6-benzylaminopurine, 0.1 mg L−1 naphthalene acetic acid, and 30 μm AgNO3. After 11 d the hypocotyls were transferred to the same medium containing 10% coconut water. Established shoots were transferred to standard Murashige and Skoog medium containing 30 g L−1 Suc, 100 mg L−1 cefotaxime, and 1 mg L−1 indole-3-butyric acid to induce root formation.
Molecular Characterization of Transgenic Plants
PCR was used to identify GS transgenic lines among the kanamycin-resistant lines obtained. The PCR primers used were the following: the forward primer was directed against the 35S promoter with the sequence 5′ CCT TCG CAA GAC CCT TCC TC 3′, and the reverse primer was directed at the gshII gene with the sequence 5′ GGC TGG CAG GTA ATT TTG CGC 3′.
For western blotting, 7-d-old seedlings (shoots and roots separately) were ground in liquid nitrogen and extracted in 50 mm potassium phosphate buffer, pH 8.0, added at 1 mL g−1 fresh weight. After measurement of total protein concentration (Bradford, 1976), 10 μg of protein from each sample was separated by SDS-PAGE and blotted onto a Zeta-probe membrane (Bio-Rad) by electroblotting. We used the Bio-Rad Immun-lite kit for the immunodetection of separated proteins, and rabbit serum raised against purified E. coli glutathione synthetase as the first antibody (Arisi et al., 1997).
Plant Growth and Tolerance Experiments
Experiment I (Seedlings on Agar Medium)
T2 seeds from transgenic lines GS2, GS7, and GS10 and wild-type Indian mustard were sterilized by rinsing in 96% ethanol for 30 s, then in 1% hypochlorite solution for 30 min, and subsequently in sterile, deionized water for 50 min, all on a rocking platform. Fifty sterilized seeds were sown in a grid pattern in Magenta boxes (Sigma) on one-half-strength Murashige and Skoog medium containing 10 g L−1 Suc, 5 g L−1 Phytagar (Sigma), and different concentrations of CdSO4 (0, 0.15, 0.20, or 0.25 mm). These relatively high agar Cd concentrations were used because Cd toxicity in agar is usually much lower than in hydroponic conditions, possibly because of Cd absorption by agar. Also, the environmental conditions in agar experiments (rich nutrients, correct pH, and high moisture) are almost optimal for plant growth, which may make seedlings more tolerant to Cd. After 7 d at 25°C under continuous light, the individual seedlings were harvested, washed, and weighed, and the length of the longest root was measured.
Experiment II (Mature Plants in Hydroponic Solution)
Seeds of line GS7 and wild-type Indian mustard were sterilized and sown in Magenta boxes as described above. After 5 d on agar the seedlings were transferred to 4-inch pots containing coarse sand. The pots were maintained in a greenhouse with controlled temperature (24°C) and a short-day (9 h) photoperiod. The plants were watered twice a day, once with tap water and once with one-half-strength Hoagland solution (Hoagland and Arnon, 1938). After 6 weeks of growth under these conditions, the plants were gently washed in water to remove the sand adhering to the roots and transferred to a nutrient film technique setup (Zayed, 1987). The plants were placed in channels, and one-fourth-strength Hoagland nutrient solution amended with 0.1 mm Cd (as CdSO4) was circulated along the plant roots. Plant growth and Cd accumulation were measured. The nutrient film technique setup was maintained under the same greenhouse conditions described above. Plants were harvested after 10 d and thoroughly washed under running deionized water to remove any trace elements adhering to the tissue. Total fresh weights of the plants were measured before and after the experiment to determine the effect of different concentrations of Cd on growth. Shoot and root tissues were separated and dried at 70°C for 3 d. The dried tissues were weighed and then ground in a Wiley mill (Thomas Scientific).
Experiment III (Mature Plants in Hydroponic Solution)
GS7, GS10, and wild-type Indian mustard plants were grown in the nutrient film technique setup and treated as described in “Experiment II,” except that the Cd treatment was started when the plants were 5 weeks old, and the duration of the Cd treatment was 14 d.
GSH, PC2, and Total Thiol Analysis
Nonprotein thiol, GSH, and PC were measured from the plants used in experiment II. Extracts were prepared according to the method described by Galli et al. (1996) by adding 300 μL of a solution containing 1 m NaOH and 1 mg L−1 NaBH4 to 100 mg of a homogenized plant sample. The homogenate was centrifuged for 3 min at 13,000g at 4°C. Three-hundred microliters of the supernatant was acidified by adding 50 μL of 37% HCl. Nonprotein thiol contents were measured photospectrometrically by adding 20 μL of this solution to 1 mL of 5,5′-dithiobis(2-nitrobenzoic acid) (Ellman's reagent [Ellman, 1959]), followed by the measurement of A412. GSH and PCs were analyzed by HPLC using a system similar to that described by Grill et al. (1987). Low-Mr thiols were separated on a C18 reverse-phase column (Waters/Millipore) using a 0% to 20% acetonitrile gradient in 0.05% (v/v) phosphoric acid for 20 min. On-line postcolumn derivatization with Ellman's reagent was used to specifically monitor sulfhydryl-containing compounds. The reagent consisted of 0.1 m potassium-phosphate buffer (pH 7.5) and 75 mm Ellman's reagent; the absorption was measured at 412 nm. The flow rate for the solvent and reagent was 1.0 mL min−1. The amounts of GSH and PC (PC2) were calculated from standard curves using GSH and PC2 (provided by Dr. M.H. Zenk, Ludwing-Maximilans-Universität, Munich, Germany) as the markers.
Elemental Analysis
Elemental analysis was carried out after acid digestion of dried and ground tissue samples according to the method of Zarcinas et al. (1987). The concentrations of trace elements in the acid digest were measured by inductively coupled plasma emission spectroscopy (Fassel, 1978). Standards (National Institute of Standards and Technology) and blanks were run with all samples for quality control. As a negative control, plants that had not been supplied with trace elements were also analyzed for trace-element concentrations.
Statistical analyses were performed using the JMP IN statistical package (SAS Institute, Cary, NC).
RESULTS
Production and Characterization of Transgenic GS Plants
Seven kanamycin-resistant Indian mustard lines were obtained after transformation with the gshII construct, and were designated GS1, GS2, GS4, GS6, GS7, GS10, and GS13. All seven plant lines showed a PCR product when PCR was conducted using primers directed against the 35S promoter and the gshII gene (not shown). Homozygous T2 lines from individual T1 plants of lines GS7, GS10, and GS2, showing different gshII expression levels (see below), were used for subsequent experiments. These transgenic T1 and T2 plants did not shown any phenotypic differences from the untransformed Indian mustard plants.
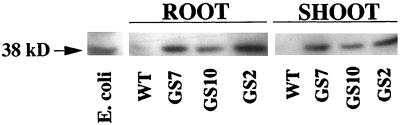
Antiserum raised against E. coli GS was used to analyze the GS expression in the transgenic lines at the protein level. On western blots both shoot and root tissues from all of the GS lines contained a protein with the same molecular mass as that from E. coli GS (38 kD), which reacted with the antiserum (Fig. 2); no band was detected in the wild-type extract. The expression levels of the E. coli GS protein were similar in roots and shoots; line GS10 showed lower E. coli GS expression levels than lines GS7 and GS2 (Fig. 2). Similar differences in gshII expression between the plant lines were observed at the mRNA level (results not shown).
Figure 2.
Western blot of seedling extracts from the wild-type (WT) and GS-overexpressing (GS2, GS7, and GS10) transgenic Indian mustard seedlings (digitized image). Equal amounts (10 μg) of total protein were loaded onto each lane, separated on a 10% SDS-PAGE gel, blotted, and treated with antiserum raised against purified E. coli GS protein. Samples were pooled from 25 seedlings each and grown on one-half-strength Murashige and Skoog medium. E. coli extract was included as a positive control.
GS Plants Show Improved Cd Accumulation and Tolerance
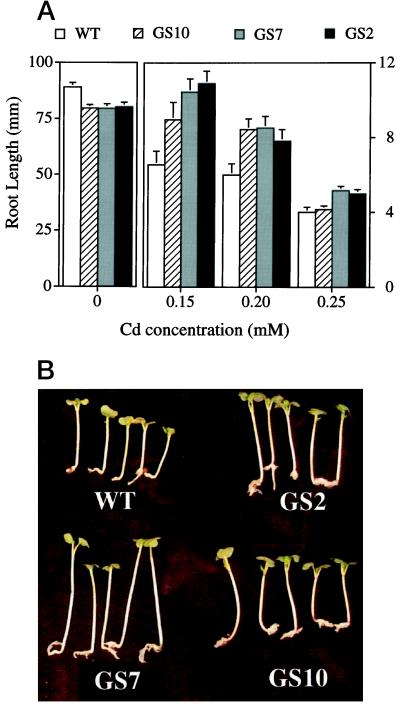
Two types of experiments using seedlings or mature plants were conducted to test Cd tolerance. For the seedling experiment, seeds of lines GS7, GS10, and GS2 and from wild-type Indian mustard were sown on agar medium containing 0, 0.15, 0.20, or 0.25 mm CdSO4, and root length was measured after 7 d. Root length is considered to be a reliable parameter for trace-element tolerance (Murphy and Taiz, 1995). At all three Cd concentrations, the GS seedlings had significantly longer roots than the wild-type seedlings (Fig. 3A). For example, at 0.15 mm Cd the roots of line GS7 seedlings were 60% longer (P < 0.001) than those of wild-type seedlings. The transgenic seedlings were also taller than wild-type seedlings under Cd treatment (Fig. 3B). Under control conditions the GS seedlings had slightly, but significantly, shorter roots than the wild type (Fig. 3A; P < 0.05).
Figure 3.
Effect of Cd on the growth of wild-type (WT) and GS-overexpressing (GS2, GS7, and GS10) Indian mustard seedlings grown for 7 d on agar medium containing different concentrations of CdSO4. A, Root length (the averages and se values of 50 seedlings). B, Five representative seedlings from each line, grown on 0.20 mm CdSO4 (digitized image). Using analysis of variance, each of the transgenic lines was shown to be significantly different (P < 0.05) from the wild type (WT) at each Cd concentration, with the exception of line GS10 at 0.25 mm Cd.
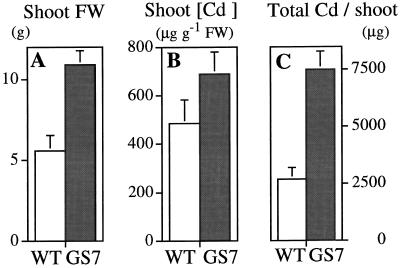
The first Cd-tolerance experiment with mature plants was performed with plants from transgenic line GS7 and the wild type. After 10 d of growth on 0.10 mm CdSO4 the GS7 plants had attained a 2-fold higher fresh weight than the wild type (P < 0.01; Fig. 4A). There were no significant differences between the plant lines with respect to initial fresh weight (data not shown) or between the final fresh weight of untreated plants (113.2 ± 13.1 and 122.3 ± 12.3 g for the wild type and line GS7, respectively). The Cd concentrations were approximately 40% higher in shoots of GS7 plants compared with the wild type (P = 0.13; Fig. 4B); no significant differences were found with respect to root Cd concentration. As a result of the increased tolerance to Cd and the increased shoot Cd accumulation, the total amount of harvestable Cd per plant shoot was about 3-fold higher for GS7 plants than for the wild type (P < 0.01; Fig. 4C).
Figure 4.
Growth (A), shoot Cd concentration (B), and shoot Cd accumulation (C) by wild-type (WT) and GS-overexpressing (GS7) Indian mustard plants treated with 0.1 mm CdSO4. In A, the final fresh weight (FW) was used as a parameter of growth, because the initial fresh weights of the wild-type and GS plants were not significantly different. Values shown are the average and se of eight replicates for individual plants. P < 0.01 (A); P = 0.13 (B); and P < 0.01 (C).
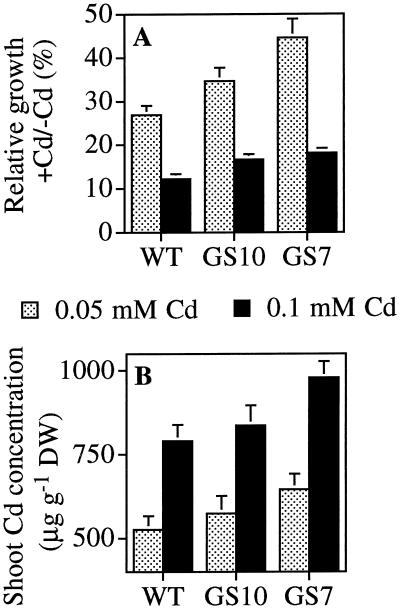
In the second experiment using mature plants, 6-week-old GS7, GS10, and wild-type plants were exposed to 0.05 or 0.10 mm CdSO4 for 14 d. Again, the GS7 plants showed superior Cd tolerance: they suffered less growth inhibition by Cd than the wild type (P < 0.05; Fig. 5A). For instance, in nutrient solution amended with 0.05 mm Cd, the relative growth of GS7 plants was 45% of that of untreated GS7 controls, whereas the relative growth of wild-type plants was only 27%. The growth of GS10 plants was intermediate between GS7 and the wild type, in accordance with the gshII expression levels, which were lower in GS10 than in GS7 plants (Fig. 2). The shoot Cd concentrations in GS7 plants were 19% to 22% higher than in wild-type plants (P < 0.05; Fig. 5B). Similar to Cd tolerance, line GS10 showed intermediate Cd accumulation between GS7 and the wild type (Fig. 5B), in accordance with its lower gshII expression level. No significant differences were found with respect to root Cd concentrations.
Figure 5.
Growth inhibition (A) and shoot Cd concentrations (B) of wild-type (WT) and GS-overexpressing (GS7 and GS10) Indian mustard plants treated with 0.05 or 0.1 mm CdSO4. In A, the parameter used for growth is the relative increase in fresh weight of Cd-treated plants as the percentage of untreated plants of the same line; this parameter was chosen to normalize the different initial fresh weights for plants from different lines. Values shown are the average and se of 12 replicates (A), or of 6 pooled samples from 2 plants each (B). P < 0.05 for GS7 versus the wild type, and not significant for GS10 versus the wild type (A and B). DW, Dry weight.
GS Plants Have Higher Levels of GSH, PC2, Thiols, S, and Ca
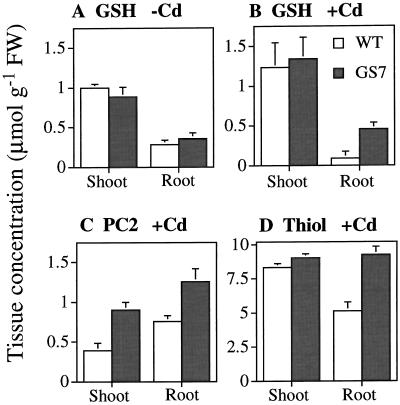
To investigate the effect of GS overexpression on the production of heavy-metal-binding compounds, the levels of GSH, PC (PC2), and total thiol were determined in leaf and root samples collected from GS7 and wild-type plants used in the first mature-plant experiment described above, which were treated with 0.1 mm Cd.
The GSH levels were 5-fold higher in roots of Cd-treated GS7 plants than in wild-type roots (P < 0.05; Fig. 6B). There were no significant differences between the plant lines with respect to GSH contents in shoot tissues of Cd-treated plants (Fig. 6B) or in roots or shoots of untreated plants (Fig. 6A). It is interesting that the GSH levels in roots from Cd-treated wild-type plants were significantly lower than those in untreated wild-type plants, whereas the GS7 plants showed similar GSH levels under both conditions (Fig. 6, A and B).
Figure 6.
Tissue concentrations of glutathione (A and B), PC (PC2, C), and total thiol (D) in wild-type (WT) and GS-overexpressing (GS7) Indian mustard plants grown in the absence (A) or presence (B–D) of 0.1 mm CdSO4. Note that tissue PC2 levels in the absence of Cd were below the detection limit in all of the samples. Values shown are the average and se of three replicate samples from four plants each. A, No significant differences between line GS7 and the wild type. B, P < 0.05 for roots, not significant for shoots. C, P < 0.05 for roots, P < 0.01 for shoots. D, P < 0.01 for roots, not significant for shoots. FW, Fresh weight.
In Cd-treated plants the levels of the smallest PC molecule, PC2, were 1.7-fold higher in roots of GS7 plants compared with the wild type (P < 0.05), and 2.3-fold higher in shoots of GS7 than in the wild type (P < 0.01; Fig. 6C). In untreated plants no PC2 was detectable in either plant line.
The thiol levels (including Cys, γ-Glu-Cys, GSH, and PC2) in roots of Cd-treated GS7 plants were approximately 2-fold higher than in the wild type (P < 0.01; Fig. 6D). There were no differences between the plant lines with respect to shoot thiol content in Cd-treated or untreated plants. In both GS7 and wild-type plants the total thiol levels were 3-fold higher in shoots and 10-fold higher in roots of Cd-treated plants compared with untreated plants of the same line.
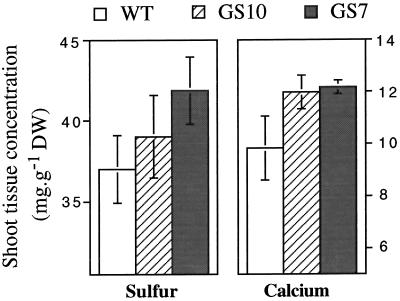
The GS7, GS10, and wild-type plants used in the second mature-plant experiment (0 or 0.1 mm Cd treatment) were analyzed for shoot and root levels of Ca, Cu, Fe, Mg, Mn, S, and Zn. S and Ca were present at higher levels in shoots of Cd-treated GS7 plants compared with wild-type shoots (Fig. 7; P < 0.10); GS10 plants showed intermediate values between the wild type and line GS7. There were no differences among the plant lines with respect to root Ca or S concentrations in Cd-treated or untreated plants (data not shown). The Ca levels in roots and shoots of Cd-treated plants were significantly lower than those in untreated plants in both GS and the wild type (P < 0.001); S levels were not significantly affected by Cd in either plant line. No differences were found between GS and wild-type plants with respect to the levels of Mg, Zn, Cu, Mn, and Fe (data not shown).
Figure 7.
Shoot tissue concentrations of S and Ca in wild-type (WT) and GS-overexpressing (GS7 and GS10) Indian mustard plants treated with 0.1 mm CdSO4. Values shown are the average and se of six pooled samples from two plants each. S, P < 0.10 for GS7 versus the wild type, not significant for GS10 versus the wild type; Ca, P < 0.10 for GS7 versus the wild type and for GS10 versus the wild type. S and Ca concentrations in roots were not significantly different between the wild-type and transgenic plants, either under control conditions or after Cd treatment. DW, Dry weight.
DISCUSSION
Our results show that overexpression of the E. coli gshII gene in Indian mustard resulted in enhanced production of GSH and PCs and improved Cd accumulation and tolerance. The physiological and biochemical effects observed were correlated with the ghsII expression levels. These results suggest that in the presence of Cd, the GS enzyme is rate limiting for the biosynthesis of glutathione and PCs, and that the levels of glutathione and/or PCs determine the plant's capacity to accumulate and tolerate Cd.
Under unstressed conditions GS does not appear to be rate limiting for glutathione synthesis, because the glutathione levels did not differ significantly in unstressed GS7 and wild-type plants (Fig. 6A), and there was no detectable PC in either plant line. These results agree with those of Foyer et al. (1995), who found that overexpression of the same gene did not affect poplar glutathione levels. Under Cd stress, however, the GS enzyme appears to become rate limiting for the biosynthesis of glutathione and PCs. In roots of Cd-treated wild-type plants, the GSH levels were 3-fold lower (caused by depletion attributable to PC synthesis) than in unstressed wild-type plants. In the tissues of GS plants, the rate limitation was overcome and GSH levels were the same in Cd-treated and untreated plants (Fig. 6, A and B). Furthermore, the PC2 levels in roots and shoots of the GS plants were about 2-fold higher than in wild-type plants (Fig. 6C).
The roots appear to be the major site of PC synthesis. First, the PC2 levels were higher in roots than in shoots of Cd-treated wild-type and transgenic plants (Fig. 6C). Second, the depletion in glutathione was observed only in the roots of wild-type plants, but not in the shoots (Fig. 6, A and B). Third, the Cd-induced increase in total thiol (a substantial fraction of which represents PCs of various lengths) was 10-fold in roots and only 3-fold in shoots. The notion that PCs are produced mainly in the root was also suggested by Salt et al. (1995b).
The most likely explanation for the increased Cd tolerance of the GS plants is that these plants produced more PCs. The 2-fold increase in PC levels in the shoots and roots of the GS transgenic plants is expected to lead to a greater capacity to detoxify and sequester Cd, because PCs bind heavy metals, followed by sequestration of the Cd-PC complex in the vacuole (Zenk, 1996). The Cd-PC complex is further complexed in the vacuole with sulfide. It has been suggested that metal tolerance by plants may be limited by the availability of reduced S for Cys and sulfide synthesis (Goldsbrough, 1998). It is interesting that the levels of total S were higher in the shoots of the GS plants compared with the wild type (Fig. 7A). Furthermore, because GSH has an important role in plant-stress tolerance, the higher GSH levels in the roots of the GS plants may have contributed to their increased Cd tolerance.
Cd significantly reduced the tissue Ca concentration in both wild-type and GS plants, but overexpression of GS diminished this decrease in Ca in shoots (Fig. 7B). The Ca concentration in roots was not significantly different between the wild-type and transgenic plants (data not shown). Two recent studies have focused on the interaction of Cd2+ and Ca2+ (Ibekwe et al., 1996; Beyersmann and Hechtenberg, 1997). Cd2+ is a Ca2+ channel blocker, and Cd2+ interferes with the Ca2+ messenger system by binding calmodulin, a Ca2+-binding protein that regulates a variety of enzymes and cell processes (Cheung, 1984). The increased levels of Cd-binding peptides in the GS plants may contribute to reducing the effect of Cd on the Ca-calmodulin interaction.
The GS plants accumulated more Cd (at higher concentrations) than wild-type plants in their shoots, but not in their roots. The translocation of Cd from the root to the shoot through the xylem is thought to be driven by transpiration (Salt et al., 1995b). Because more Cd was bound by PCs and stored in the vacuole in the GS plants, vital biochemical and physiological processes were less damaged than in wild-type plants. This led to increased total leaf surface area in GS plants and, therefore, to the accumulation of more Cd (as a result of greater transpiration per plant). Furthermore, GS plants may have absorbed more Cd because they sustained less damage by Cd to the root surface. Root water uptake was shown to be one of the primary mechanisms affected by Cd stress in plants (Marchiol et al., 1996). The higher PC levels in the roots of the GS plants may have alleviated this negative effect of Cd on root water uptake.
In conclusion, this study provides insight into the regulation of GSH and PC biosynthesis and heavy-metal sequestration. In addition, we have successfully developed transgenic plants that have an increased capacity for Cd accumulation and tolerance. These GS plants offer great promise for enhancing the efficiency of Cd phytoextraction from polluted soils and wastewater. These plants may also show increased tolerance to, and accumulation of, other heavy metals, because PCs are thought to play a role in tolerance of a range of heavy metals, especially nonessential heavy metals such as mercury and lead (Goldsbrough, 1998).
ACKNOWLEDGMENTS
We thank Dr. M.H. Zenk for generously providing PC2 standard references, Dr. A.C.M. Arisi for providing GS antibodies, and X.L. Du and Dr. G. Noctor for help with biochemical analyses. We also thank Dr. M. de Souza for reviewing the manuscript.
Abbreviations:
- γ-EC
γ-glutamyl-Cys
- γ-ECS
γ-glutamyl-Cys synthetase
- GS
glutathione synthetase
- PC
phytochelatin
- PC2
(γ-Glu-Cys)2-Gly
Footnotes
This work was supported by a grant from the Electric Power Research Institute (no. W04163 to N.T.) and by a TALENT stipend from the Dutch Organization for Scientific Research to E.A.H.P.-S.
LITERATURE CITED
- Arisi ACM, Noctor G, Foyer CH, Jouanin L. Modification of thiol contents in poplar (Populus tremula × P. alba) overexpressing enzymes involved in glutathione synthesis. Planta. 1997;203:362–372. doi: 10.1007/s004250050202. [DOI] [PubMed] [Google Scholar]
- Beyersmann D, Hechtenberg S. Cadmium, gene regulation, and cellular signalling in mammalian cells. Toxicol Appl Pharmacol. 1997;144:247–261. doi: 10.1006/taap.1997.8125. [DOI] [PubMed] [Google Scholar]
- Black H. Absorbing possibilities: phytoremediation. Environ Health Perspect. 1995;103:1106–1108. doi: 10.1289/ehp.951031106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou J, Goldsbrough PB. Characterization of phytochelatin synthase from tomato. Physiol Plant. 1997;101:165–172. [Google Scholar]
- Cheung WY. Calmodulin: its potential role in cell proliferation and heavy metal toxicity. Fed Proc. 1984;43:2995–2999. [PubMed] [Google Scholar]
- Cunningham SD, Ow D. Promises and prospects of phytoremediation. Plant Physiol. 1996;110:715–719. doi: 10.1104/pp.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushenkov SPBA, Kumar N, Motto H, Raskin I. Rhizofiltration: the use of plants to remove heavy metals from aqueous streams. Environ Sci Technol. 1995;29:1239–1245. doi: 10.1021/es00005a015. [DOI] [PubMed] [Google Scholar]
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fassel VA. Quantitative elemental analyses by plasma emission spectroscopy. Science. 1978;202:183–191. doi: 10.1126/science.202.4364.183. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Haliwell B. The presence of glutathione and glutathione reductase in chloroplasts: a proposed role in ascorbic acid metabolism. Planta. 1976;133:21–25. doi: 10.1007/BF00386001. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Souriau N, Perret S, Lelandais M, Kunert KJ, Pruvost C, Jouanin L. Over-expression of glutathione reductase but not glutathione synthetase leads to increases in antioxidant capacity and resistance to photoinhibition in poplar trees. Plant Physiol. 1995;109:1047–1057. doi: 10.1104/pp.109.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli U, Schuepp H, Brunold C. Thiols in cadmium- and copper-treated maize (Zea mays L.) Planta. 1996;198:139–143. [Google Scholar]
- Goldsbrough PB (1998) Metal tolerance in plants: the role of phytochelatins and metallothioneins. In N Terry, GS Banuelos, eds, Phytoremediation of Trace Elements. Ann Arbor Press, Ann Arbor, MI (in press)
- Grill E, Winnacker EL, Zenk MH. Phytochelatins, a class of heavy-metal-binding peptides from plants, are functionally analogous to metallothioneins. Proc Natl Acad Sci USA. 1987;84:439–443. doi: 10.1073/pnas.84.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatcher EL, Chen Y, Kang YJ. Cadmium resistance in A549 cells correlates with elevated glutathione content but not antioxidant enzymatic activities. Free Radical Biol Med. 1995;19:805–812. doi: 10.1016/0891-5849(95)00099-j. [DOI] [PubMed] [Google Scholar]
- Hoagland D, Arnon DI (1938) The water culture method for growing plants without soil. Bull Calif Agric Stat, p 346
- Howden R, Anderson CR, Goldsbrough PB, Cobbett CS. A cadmium-sensitive, glutathione-deficient mutant of Arabidopsis thaliana. Plant Physiol. 1995;107:1067–1073. doi: 10.1104/pp.107.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibekwe AM, Angle JS, Chaney RL, van Berkum P. Zinc and cadmium toxicity to alfalfa and its microsymbiont. J Environ Qual. 1996;25:1032–1040. [Google Scholar]
- Kumar PBAN, Dushenkov V, Motto H, Raskin I. Phytoextraction: the use of plants to remove heavy metals from soils. Environ Sci Technol. 1995;29:1234–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- Marchiol L, Leita L, Martin M, Peressotti A, Zerbi G. Physiological responses of two soybean cultivars to cadmium. J Environ Qual. 1996;25:562–566. [Google Scholar]
- Marrs K. The functions and regulation of glutathione S-transferases in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:127–158. doi: 10.1146/annurev.arplant.47.1.127. [DOI] [PubMed] [Google Scholar]
- Murphy A, Taiz L. A new vertical mesh transfer technique for metal-tolerance studies in Arabidopsis. Ecotypic variation and copper-sensitive mutants. Plant Physiol. 1995;108:29–38. doi: 10.1104/pp.108.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauser WE, Schupp R, Rennenberg H. Cysteine, γ-glutamylcysteine and glutathione levels in maize seedlings. Distribution and translocation in normal and cadmium-exposed plants. Plant Physiol. 1991;97:128–138. doi: 10.1104/pp.97.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salt DE, Blaylock M, Kumar NPBA, Dushenkov V, Ensley BD, Chet I, Raskin I. Phytoremediation: a novel strategy for the removal of toxic metals from the environment using plants. Biotechnology. 1995a;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- Salt DE, Price RC, Pickering IJ, Raskin I. Mechanisms of cadmium mobility and accumulation in Indian mustard. Plant Physiol. 1995b;109:1427–1433. doi: 10.1104/pp.109.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Bergmann L. Regulation of glutathione synthesis in suspension cultures of parsley and tobacco. Bot Acta. 1995;108:34–40. [Google Scholar]
- Speiser D, Abrahamson SL, Banuelos G, Ow D. B. juncea produces a phytochelatin-cadmium-sulfide complex. Plant Physiol. 1992;99:817–821. doi: 10.1104/pp.99.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens JC. The heavy metal-binding peptides of plants. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:553–575. [Google Scholar]
- Strohm M, Jouanin L, Kunert KJ, Pruvost C, Polle A, Foyer CH, Rennenberg H. Regulation of glutathione synthetase in leaves of transgenic poplar (Populus tremula × P. alba) overexpressing glutathione synthase. Plant J. 1995;7:141–145. [Google Scholar]
- Zarcinas BA, Cartwright B, Spouncer LR. Nitric acid digestion and multi-element analysis of plant material by inductively coupled plasma spectrometry. Commun Soil Sci Plant Anal. 1987;18:131–146. [Google Scholar]
- Zayed AM (1987) Influence of sodium chloride on ion uptake and yield of tomatoes and lettuce grown by hydroponics. PhD thesis. Wye College, University of London
- Zenk MH. Heavy metal detoxification in higher plants: a review. Gene. 1996;179:21–30. doi: 10.1016/s0378-1119(96)00422-2. [DOI] [PubMed] [Google Scholar]