Abstract
In this perspective, we review published data which support the concept that many or most chronic and progressive lung diseases also involve the lung vessels and that microvascular abnormalities and endothelial cell death contribute to the pathobiology of emphysema. Lung vessel maintenance depends on Vascular Endothelial Growth Factor signaling and both are compromised in the emphysematous lung tissue. Although hypoxic pulmonary vasoconstriction has been considered as an important factor contributing to the vascular remodeling in chronic obstructive pulmonary disease (COPD) (COPD/emphysema, it is now clear that inhaled cigarette smoke can damage the lung vessels independent of the lung vascular tone. We propose that a “sick lung circulation” rather than the right heart afterload may better explain the cardiac abnormalities in COPD patients which are usually summarized with the term “cor pulmonale.” The mechanisms and causes of pulmonary hypertension are likely complex and include vessel loss, in situ thrombosis, and endothelial cell dysfunction. Assessment of the functional importance of pulmonary hypertension in COPD requires hemodynamic measurements during exercise.
Keywords: cor pulmonale, exercise, lung endothelial cells, pulmonary hypertension, pulmonary vascular remodeling
INTRODUCTION
We owe the first description of emphysema to Laenec[1] and the first thorough description of the lung vessel pathology in chronic obstructive pulmonary disease (COPD) to Liebow,[2] and we find the 1963 statement of G.W. Wright[3] that the injury in emphysema was of a “vasculonecrotic nature” remarkable. From the vantage point of the first decade of the 21st Century the concept that lung vessels are involved in most, if not all, chronic and progressive lung diseases is still perhaps more intuitive than accepted knowledge. The topic of the vascular involvement and pulmonary hypertension (PH) in emphysema/COPD has been previously reviewed and appears and disappears periodically on the radar screen of investigators interested in new treatment strategies for patients with COPD/emphysema. Whereas, understandably, the overwhelmingly large segment of COPD publications addresses issues of airway mechanics and airway pathology, the fact that the improved survival of COPD patients shown in the long-term oxygen treatment trial was associated with a small reduction in the pulmonary artery pressure[4] gives clinicians pause and repeatedly raises the question whether the pulmonary hypertension in COPD patients should be treated—and if so, with what drugs? The topic of pulmonary vascular involvement and pulmonary hypertension has many fascinating aspects and has been recently reviewed,[5–7] yet many questions remain unresolved. More recently systemic disease components in COPD are being discussed; however, earlier studies have already begun to address issues of hypercoagulability and deep venous thrombosis in patients with COPD.[8,9] Here we review recent data and concepts of the pulmonary vascular pathophysiology and pathobiology, introduce the more general concept of a “sick lung circulation” in integrated systems biology of COPD and propose new mechanistic concepts for the condition that we call cor pulmonale.
EMPHYSEMA
Emphysema is defined as “an anatomic alteration of the lung characterized by an abnormal airspace enlargement distal to the terminal bronchioles accompanied by destructive changes of the alveolar walls”[10] and is a variable component of the syndrome COPD which is now understood to have also extrapulmonary systemic manifestations.[11] Laennec, who described emphysema in 1838, entertained the hypothesis that emphysema was the result of chronic bronchitis, which he called “catarrh.” The modern view that chronic airway inflammation causes emphysematous airspace destruction and that cigarette smoking drives and maintains airway inflammation is dominant and complemented by the “chronic infection” hypothesis.[12,13] One unanswered question is: How does airway inflammation (bronchiolitis) cause the disappearance of the surrounding alveoli? One hypothesis is that there is a gradient of proteases which is released from neutrophils and macrophages. This protease gradient is responsible for the “digestion” of alveolar septae surrounding the small airways. A second question is why there are patients (never smokers) who develop severe emphysema without significant airflow limitation, and a third is why individuals with the genetic α1-antitrypsin deficiency usually do not develop emphysema-unless they smoke cigarettes. The definition of “destructive changes of the alveolar walls” includes the loss of lung capillaries, but not the loss of precapillary arterioles which is apparent on pulmonary angiography of patients with emphysema.
Of interest, a mutation in a gene encoding a copper transporter protein is responsible for the congenital emphysema in Menkes disease[14] which finds it correlate in the emphysema of the “blotchy mouse”,[15] and emphysema has been described in patients with coeliac disease,[16] pointing towards malnutrition or autoimmunity, or both, as etiologies of emphysema in these non-smoking patients. An animal model of autoimmune emphysema has recently been described.[12] This model is based on antibodies and T lymphycytes which cause lung endothelial cell apoptosis.
COPD and pulmonary hypertension
Vascular pathology
The topic of pulmonary hypertension in patients with COPD, with and without cor pulmonale has been reviewed previously.[17–22] New clinical imaging technology to study COPD is being developed ,[23,24] however, a detailed study of the lung vessels still requires invasive angiography. Histological and morphometric studies of the lung vessels require lung tissue samples which become available only after lung cancer surgery, lung volume reduction or lung transplantation. The Lung Division of the NIH HLB Institute has established a COPD lung tissue repository; here lung tissue can be requested for cellular and molecular studies.[25]
Pulmonary vascular remodeling: The role of cigarette smoke and chronic hypoxia
Our concepts of pulmonary vascular remodeling in COPD/ emphysema have evolved to a large extent because of the work of the group of Joan Albert Barbera in Barcelona. Their histological examination of lung tissue resected from patients with cancer and nearly normal lung function revealed lung arteriolar abnormalities of muscularization and significant intima fibrosis that had to be attributed to cigarette smoking and not to hypoxia; the patients were not hypoxemic and Barcelona is at sea level. Thus, these findings have challenged the previous mechanistic explanation that hypoxia vasoconstriction was the root cause of the pulmonary vascular changes observed in the lungs from COPD patients. Indeed animal experiments of chronic cigarette smoke exposure have reproduced some of these pulmonary vascular changes.[26] In severe, end-stage COPD in patients that undergo lung transplantation most certainly, large regions of the lung tissue are hypoxic and we can propose that hypoxia will contribute to the lung vascular remodeling in these patients. Taken together, it is likely that early in the COPD syndrome development there are direct toxic effects of cigarette smoke on the lung vessels, and perhaps in later stages of COPD, there is hypoxia-induced lung vessel remodeling. Many investigators, even today, find it difficult to understand how inhaled cigarette smoke can injure lung vessels. They believe that the lung airway compartment is sufficiently separated from the vascular compartment and ask, “How does the cigarette smoke get to the lung vessels?” Without a doubt particles of the cigarette smoke reach the alveoli and volatile components can diffuse from the terminal respiratory bronchioles into the surrounding tissue areas, but we also know that stable volatile components of the cigarette smoke, for example the very aggressive aldehyde acrolein, reach the systemic circulation and their endothelial cells. With this information in mind, we understand that lung vessels are exposed to cigarette smoke components both from the outside and inside (see below).
Pulmonary resistance vessels, the arterioles, become muscularized after a period of chronic hypoxia and we can interpret this phenomenon as a “meaningful,” adaptive response of the lung vessels in order to match lung blood flow to ventilation, to adjust to high-altitude living, and to protect the lung capillaries against flooding due to increased precapillary pulmonary arterial pressure. This hypoxia-induced pulmonary vessel muscularization can be explained by vascular smooth muscle cell hypertrophy and hyperplasia but it is probably more complex. The muscularization can also be in part explained, at least in rodent studies—by the participation of endothelial cells and cells arriving from the systemic circulation via the vasa vasorum.[27,28]
(Fig. 1) distinguishes lung vascular remodeling as an adaptive response to wall stress, i.e., muscularization from the response to endothelial cell (EC) injury and apoptosis and the loss of the EC monolayer integrity. The latter response can be extremely complex and involve a local immune response (perivascular accumulation of inflammatory cells) recruitment of bone marrow-derived precursor cells, phenotypic alterations of EC and VSMC via endothelial cell to mesenchymal cell transition (EMT)[29] and the deposition of an altered matrix which is produced by the phenotypically altered vascular cells. In this schematic we attempt a synopsis of the most relevant mechanisms of lung vessel remodeling
Figure 1.

Comparison of adaptive pulmonary vascular remodeling with the complex cellular changes which can be a consequence of the damage of the endothelial cell (EC) monolayer
As mentioned, we owe the original description of the vascular pathology in emphysematous lungs to A. Liebow.[2] The most frequently listed vascular abnormalities, some of their underlying mechanisms and their potential consequences, are shown in (Fig. 2).
Figure 2.
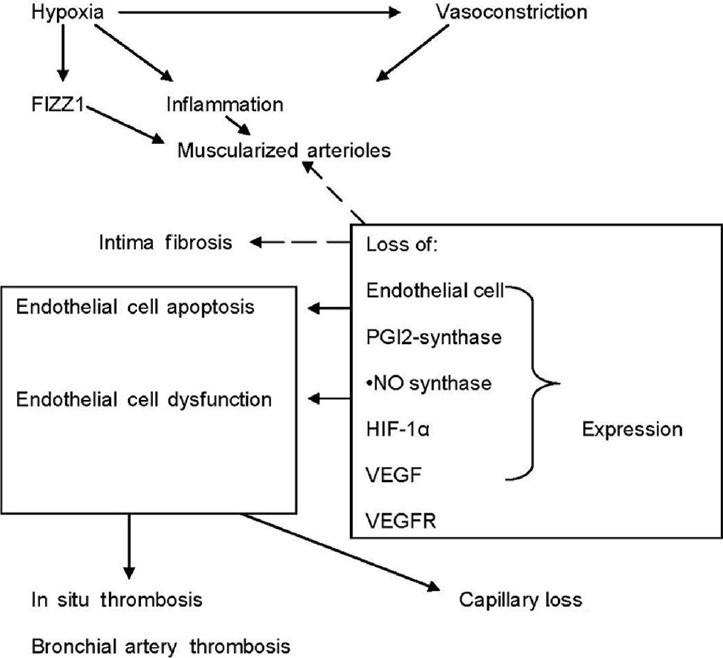
This diagram highlights the components of pulmonary vascular alterations observed in COPD/emphysema lungs and some of their proposed mechanisms. Hypoxia and vasoconstriction likely independently affect the lung vessels and promote remodeling. Remodeling affects the three layers of the vessels: intima, media and adventitia. Endothelial cell apoptosis may be critically important and lead to both intima fibrosis and muscularization. The large box symbolizes an endothelial cell and illustrates alterations in gene expression which affects endothelial cell function.
The modern view of pulmonary vascular remodeling is that in addition to the fluid-mechanical effects of hypoxic vasoconstriction—and thus increased shear stress of the resistance vessels, during hypoxia—there is a role for HIF-1α-dependent mechanisms. Here it is important to remember that hypoxia is linked via HIF-1α to “inflammation” and the innate immune response.[30,31] To summarize: pulmonary arteriolar muscularization, once understood as a consequence of smooth muscle contraction, has now become the result of complicated actions and interactions of transcription factors[32,33] and genes encoding growth factor proteins and proteins encoding multiple enzymes involved in the control of cell energy metabolism.[23,24,34] The studies of Johns et al.[35] and Daley et al.[36] indicate that hypoxia activates lung macrophages to release FIZZ1 (also called RELMα or hypoxia-induced mitogenic factor [HIMF]) which promotes pulmonary arteriolar SMC growth. One of the early experimental studies linking chronic hypoxia-induced lung vessel remodeling and inflammation is the study by Ono et al.[37] In the lungs from patients with COPD/emphysema we find evidence of endothelial cell apoptosis[38] and endothelial cell dysfunction;[39,40] there is a loss of a number of proteins expressed in normal endothelium[20] which can explain both endothelial cell loss and dysfunction (Fig. 2). The schematic (Fig. 2) pays tribute to the modern ideas about hypoxia and lung vessel remodeling; this figure also illustrates that the loss of the expression of enzyme proteins like prostacyclin synthase and nitric oxide synthase and loss of the expression of Vascular Endothelial Growth Factor (VEGF) and VEGF receptor proteins, may lead to EC dysfunction, EC apoptosis, and intima fibrosis. The events which lie upstream are unclear, but oxidant and endoplasmic reticulum stress (ERS) are candidates. A critical controller of lung endothelial cell growth and survival is VEGF.[25,41] How endothelial cell dysfunction causes the intima to become a thrombogenic surface is not yet understood, but in situ thrombosis[6] and bronchial artery thrombosis have been described in the COPD/ emphysematous lungs.
The topic of bronchial endothelial cell dysfunction has been recently reviewed.[42] Upstream triggers for both pulmonary vascular and bronchial vascular endothelial cell damage and dysfunction are likely oxidative and endoplasmatic reticulum stress (ERS)[43,44] and activation of immune responses. Our understanding of how immune cells and their cytokines shape pulmonary vascular remodeling is still in its infancy.[45,46]
Effect of cigarette smoke on pulmonary vessels
Altered pulmonary vascular morphology in emphysema attributable to cigarette smoking has been reported by several investigators,[47–49] and these findings are supported by animal studies and experiments demonstrating the effect of cigarette smoke extracts on cultured EC. Yamato et al.[26] reported that cigarette smoke exposure of guinea pigs induced emphysema associated with a diffusely reduced lung capillary density, and Wright et al. showed that cigarette smoke increases the expression of vasoactive mediators in pulmonary arteries[50] and causes rapid changes in gene expression in the pulmonary arteries.[51] Lee et al.[52] reported induction of endothelin-1 in pulmonary artery EC by cigarette smoke extract and Nana Sinkam et al. reported loss of prostacyclin synthase expression.[53] Cigarette smoke extract (CSE) can induce superoxide in EC, which inactivates NO and generates peroxynitrite[54] and this oxidative and nitrosative stress inactivates VEGFR2 signaling;[43] CSE induces p53-dependent pulmonary EC apoptosis[55] and sildenafil can protect against CSE-stimulated EC apoptosis.[56]
Pulmonary hypertension
We have known since the pioneering studies of Benjamin Burrows that the degree of pulmonary hypertension in patients with COPD at rest and during exercise is very variable,[57] and these early findings have been confirmed by a number of larger studies,[58] in particular by the work of E. Weitzenblum and his group in Strassbourg, France.[59] Whereas most patients with COPD/emphysema have small elevations of the pulmonary artery pressure at rest, a small subgroup of the COPD patients (around 1%) presents with pulmonary artery pressures at rest that are in the range usually observed in patients with idiopathic forms of PH.[59] The pathobiology of the PH in these patients remains unexplored and lung histological studies have not been reported. Because echocardiographic evaluation of patients with hyperinflated lungs is problematic, hemodynamic assessment of these patients is necessary. It should become the standard procedure to evaluate exercise hemodynamics in these patients, in particular if the clinician wants to rule out PH as a cause of dyspnea. Obesity, airtrapping and auto-PEEP effects, a limited venous return and cardiac output,[60] are other reasons for dyspnea in these patients (Fig. 3).
Figure 3.
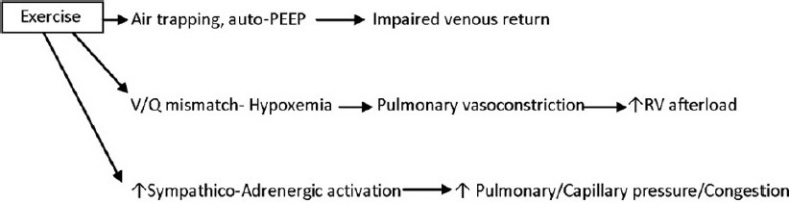
How exercise affects the lung circulation.
Still today, the interplay of mechanical aspects of impaired lung function (stretch) and the cellular and molecular functional consequences of inflammation and oxidative stress for pulmonary vascular tone regulation and cardiac performance (see below) are largely unexplored. The extent of daily physical activities in patients with COPD/emphysema decreases with the severity of lung function impairment (GOLD Classification II-IV) and the degree of hyperinflation.[29] The desire to predict the presence and severity of PH in COPD/emphysema by using non-invasive lung function and/or blood gas variables is understandable, yet the assumptions are that COPD is a homogeneous disease and that patients do not have also thromboembolic or left heart disease. Correlations between the systolic pulmonary artery pressure PAP and the PaO2[59] and the mean Pap and the O2saturation[61] have been published and the large scatter of the data points makes it impossible to predict the Pa pressure in the individual patient. COPD patients living at altitude and those with the diagnosis of sleep apnea are more likely to have PH.
Cor pulmonale
The late David Flenley used to say that, “COPD patients die with cor pulmonale, not of cor pulmonale”.[17,18] It is unclear whether this statement is correct given the more recent epidemiological data which demonstrate that most COPD/emphysema patients die not from respiratory failure but from cardiovascular causes. Cor pulmonale is also an interesting topic of discussion because we do not really understand the pathobiology[17,18] and because some clinicians believe that it (cor pulmonale) has disappeared as a consequence of supplemental oxygen treatment of patients with COPD.
Whereas we distinguish cor pulmonale, the involvement of the heart in the setting of chronic lung diseases, from right ventricular failure in the setting of severe forms of lung vascular diseases, the pathobiology and pathohistology of the lung vessels may determine the cardiac response. We have recently demonstrated experimentally that chronic elevation of the right ventricular afterload alone generates adaptive RV hypertrophy but not RV failure. The right ventricle failed when there was angioobliterative lung vessel involvement, but not when the lung vessels were muscularized but patent.[62] Right heart failure in the setting of chronic left ventricular failure, or associated with angioproliferative PAH, causes death and is a strong predictor of survival.[58] We have recently proposed that a “sick lung circulation” (lung vascular endothelial cell apoptosis and/or proliferation) affects the heart. This “bad lung humor” hypothesis posits that information from the sick lung vessels is carried to the myocardium resulting in cardiac capillary rarefaction and fibrosis.[63] Information carriers could be cytokines, microparticles and microRNA contained in the blood exiting from the pulmonary veins and entering the coronary circulation. The schematic (Fig. 4) is an attempt to illustrate this concept.
Figure 4.

Comparison between pulmonary hypertension in the setting of COPD/emphysema (left) and pulmonary hypertension due to left ventricular dysfunction (right). Both conditions affect the lung vessels and can lead to right heart failure. The loss of myocardial capillaries is emphasized. *Diastolic dysfunction as a consequence of myocardial fibrosis.
At present, we have no histological or molecular studies that would refute such a concept, and the discovery of microparticles and circulating microRNAs[64] now makes it attractive to begin to investigate this hypothesis. Products released from a sick lung circulation would be expected to affect — via the coronary circulation — both ventricles of the heart, and indeed, several studies over the years reported impaired left ventricular function in patients with COPD; we refer the reader to a recent publication by the Vienese group which had shown left ventricular diastolic dysfunction also in COPD patients without PH.[60,65] Diastolic dysfunction can be mechanistically explained as a consequence of endothelial cell to mesenchymal transition (EMT). At least in mice TGF-β can transform cardiac capillary EC into myofibroblasts resulting in cardiac fibrosis and greater stiffness of the ventricles, as shown by Zeisberg et al.[66] In short, the “sick lung circulation” hypothesis postulates that the endothelial cell disease of the lung causes endothelial cell dysfunction and capillary loss in the heart.[58]
Treatment of pulmonary hypertension in COPD
Supplemental oxygen therapy, now well established in the treatment plan of many COPD patients, may reduce oxygen-and endoplasmic reticulum stress and improve EC function.[39,40,44] Interestingly such a hypothesis has not been explored to date.
Can the lung vasculature be or become a target for the treatment of COPD/emphysema?[67] And, if so, then could it be that the treatment may not be restricted to the lung vessels but aimed at the improvement of general endothelial cell dysfunction? Yasuo et al.[25] demonstrated, after analyzing lung samples from patients with severe emphysema, a reduction of expressed HDAC2, HIF-1α, and VEGF proteins, as well as a reduction in the expressed phosphoAkt. Recognizing that not a single protein, but a pattern—or signature — characterizes the destruction of the lung vessel maintenance program, where chromatin structure modification and growth factor signaling are all impaired (Fig. 5), it appears that there may not be a single molecular target for therapy.
Figure 5.
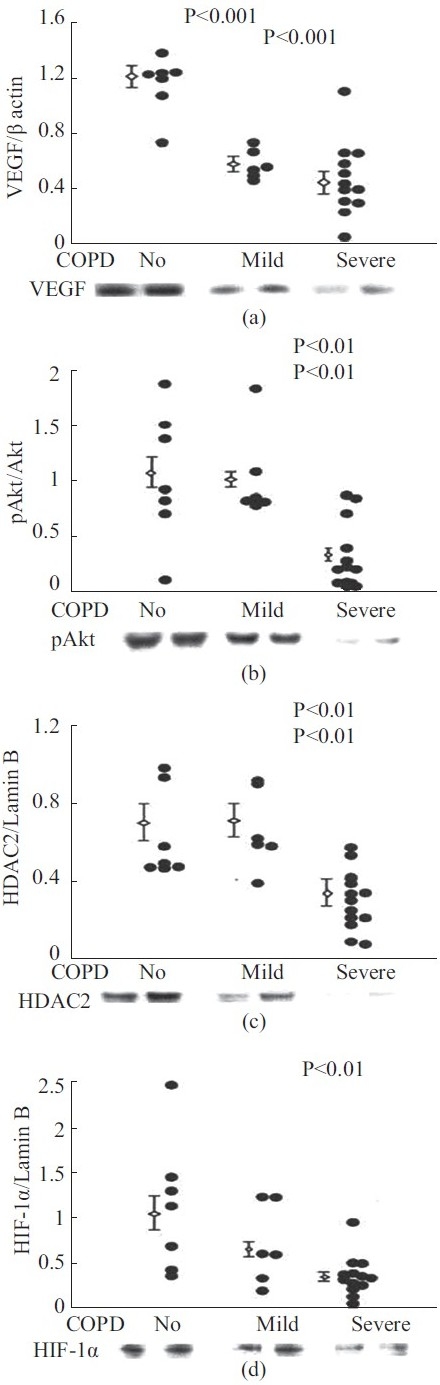
Analysis of lung tissue samples from patients with normal lung function (no COPD) and samples from patients with mild and severe COPD. Western blot protein expression data reproduced with permission.[21]
The data shown in Figure 5 likely reflect a generalized impairment of lung cellular repair — not only the impairment of vascular maintenance. Examples of strategies which may protect the lung microvessels are simvastatin treatment, which protected against experimental cigarette smoke exposure-induced emphysema and PH,[68] the protective effect of the prostacyclin analog beraprost[69] or of endothelin receptor 1 blockade.[70] The report of a reduction in the cardiovascular mortality of COPD patients treated with the anticholinergic tiotropium (UPLIFT trial)[71] raises perhaps the question whether long-acting anticholinergic agents also have a vascular protective effect.
CONCLUSIONS
Pulmonary vascular involvement is part of many chronic and progressive lung parenchyma diseases and this includes COPD/emphysema and chronic left heart failure. Oswald-Mammosser et al.[53] reported 15 years ago that the survival of COPD patients was worse in patients older than 63 and having a mean Pap >25 mmHg. Both the pathobiology of PH in patients with COPD/emphysema and the consequences for cardiac performance are incompletely understood. New concepts for both have been proposed in this review and these may encourage studies which make use of the disease-relevant tissues and modern cell biology and molecular tools.
ACKNOWLEDGMENTS
This work has been supported by the Victoria Johnson Laboratory for Obstructive Lung Disease Research. The authors want to thank Mrs. Leslee Key for her expert help with the preparation of this manuscript.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Voelkel NF. Historical overview of emphysema. Chronic Obstructive Lung Diseases. In: Voelkel NF, Macnee W, editors. Hamilton: BC Decker; 2002. pp. 1–6. [Google Scholar]
- 2.Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80:67–93. doi: 10.1164/arrd.1959.80.1P2.67. [DOI] [PubMed] [Google Scholar]
- 3.Wright GW, Kleinerman J. The J. Burns Amberson Lecture 1-4. A consideration of the etiology of emphysema in terms of contemporary knowledge. Am Rev Respir Dis. 1963;88:605–20. doi: 10.1164/arrd.1963.88.5.605. [DOI] [PubMed] [Google Scholar]
- 4.Continuous or nocturnal oxygen therapy in hypoxemic chronic obstruction lung disease: A clinical trial. Ann Intern Med. 1980;93:391–8. doi: 10.7326/0003-4819-93-3-391. [DOI] [PubMed] [Google Scholar]
- 5.Kessler R, Faller M, Weitzenblum E, Chaouat A, Aykut A, Ducoloné A, et al. “Natural history” of pulmonary hypertension in a series of 131 patients with chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:221–4. doi: 10.1164/ajrccm.164.2.2006129. [DOI] [PubMed] [Google Scholar]
- 6.Voelkel NF, Cool CD. Pulmonary vascular improvement in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2003;46:28s–32s. doi: 10.1183/09031936.03.00000503. [DOI] [PubMed] [Google Scholar]
- 7.Peinado VI, Pizarro S, Barbera JA. Pulmonary vascular involvement in COPD. Chest. 2008;134:808–14. doi: 10.1378/chest.08-0820. [DOI] [PubMed] [Google Scholar]
- 8.Alessandri C, Basili S, Violi F, Ferroni P, Gassaniga PP, Cordova C. Hypercoagulability stage in patients with chronic obstructive pulmonary disease. Thromb Haemost. 1994;72:343–6. [PubMed] [Google Scholar]
- 9.Erelel M, Cuhadaroglu C, Ece T, Arseven O. The frequency of deep venous thrombosis and pulmonary embolus in acute exacerbation of chronic obstructive pulmonary disease. Respir Med. 2002;96:515–8. doi: 10.1053/rmed.2002.1313. [DOI] [PubMed] [Google Scholar]
- 10.Snider GL, Kleinerman J, Thurlbeck WM, Bangali ZH. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases Workshop. Am Rev Respir Dis. 1985;132:182–5. doi: 10.1164/arrd.1985.132.1.182. [DOI] [PubMed] [Google Scholar]
- 11.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, et al. An official American Thoracic Society public policy statement: Novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 12.Vestbo J. Epidemiology. Chronic Obstructive Lung Diseases 2. In: Voelkel NF, Macnee W, editors. Hamilton: BC Decker; 2008. pp. 17–31. [Google Scholar]
- 13.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–53. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 14.Grange DK, Kaler SG, Albers GM, Petterchak JA, Thorpe CM, DeMello DE. Severe bilateral panlobular emphysema and pulmoanry arterial hypoplasia: Unusual manifestations of Menkes disease. Am J Med Genet A. 2005;139:159–5. doi: 10.1002/ajmg.a.31001. [DOI] [PubMed] [Google Scholar]
- 15.La Fontaine S, Firth SD, Lockhart PJ, Brooks H, Camakaris J, Mercer JF. Intracellular localization and loss of copper responsiveness of Mnk, the murine homologue of the Menkes protein in cells: Blotchy (Mo blo) and brindled (Mo br) mouse mutants. Hum Mol Genet. 1999;8:1069–75. doi: 10.1093/hmg/8.6.1069. [DOI] [PubMed] [Google Scholar]
- 16.de Menthon M, Dusser DJ, Guillevin L, Burgel PR. Undiagnosed coeliac disease in patients with emphysema: A fortuitous association? Eur Respir J. 2010;36:453–6. doi: 10.1183/09031936.00020210. [DOI] [PubMed] [Google Scholar]
- 17.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease.Part One. Am J Respir Crit Care Med. 1994;150:833–52. doi: 10.1164/ajrccm.150.3.8087359. [DOI] [PubMed] [Google Scholar]
- 18.MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease.Part two. Am J Respir Crit Care Med. 1994;150:1158–68. doi: 10.1164/ajrccm.150.4.7921453. [DOI] [PubMed] [Google Scholar]
- 19.Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:20–2. doi: 10.1513/pats.200407-037MS. [DOI] [PubMed] [Google Scholar]
- 20.Barbera JA, Peinado VI, Santos S. Pulmonary hypertension in chronic obstructive pulmonary disease. Eur Respir J. 2003;21:892–905. doi: 10.1183/09031936.03.00115402. [DOI] [PubMed] [Google Scholar]
- 21.Higenbottam T. Pulmonary hypertension and chronic obstructive pulmonary disease: A case for treatment. Proc Am Thorac Soc. 2005;2:12–9. doi: 10.1513/pats.200411-053SF. [DOI] [PubMed] [Google Scholar]
- 22.Girgis RE, Mathai SC. Pulmonary hypertension associated with chronic respiratory disease. Clin Chest Med. 2007;28:219–32, x. doi: 10.1016/j.ccm.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:588–97. doi: 10.1164/rccm.200901-0159PP. x. [DOI] [PubMed] [Google Scholar]
- 24.Barr RG, Bluemke DA, Ahmed FS, Carr JJ, Enright PL, Hoffman EA, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362:217–27. doi: 10.1056/NEJMoa0808836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yasuo M, Mizuno S, Kraskauskas D, Bogaard HJ, Natarajan R, Cool CD, et al. Hypoxia inducible factor-1. in human emphysema lung tissue? Eur Respir J. 2011;37:775–83. doi: 10.1183/09031936.00022910. [DOI] [PubMed] [Google Scholar]
- 26.Yamato H, Sun JP, Churg A, Wright JL. Cigarette smoke-induced emphysema in guinea pigs is associated with diffusly decreased capillary density and capillary narrowing. Lab Invest. 1996;75:211–9. [PubMed] [Google Scholar]
- 27.Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–8. doi: 10.1152/ajplung.00378.2006. [DOI] [PubMed] [Google Scholar]
- 28.Frid MG, Li M, Gnanasekharan M, Burke DL, Fragoso M, Strassheim D, et al. Sustained hypoxia leads to the emergence of cells with enhanced growth, migratory, and promitogenic potentials within the dilated pulmonary artery wall. Am J Physiol Cell Mol Physiol. 2009;297:L1059–72. doi: 10.1152/ajplung.90611.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garcia-Rio F, Lores V, Mediano O, Rojo B, Hernanz A, Lopez-Collazo E, et al. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am J Respir Crit Care Med. 2009;180:506–12. doi: 10.1164/rccm.200812-1873OC. [DOI] [PubMed] [Google Scholar]
- 30.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, et al. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–11. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eltzschig HK, Carmeliet P. Hypoxia and Inflammation. N Engl J Med. 2011;364:656–65. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimoda LA, Semenza GL. HIF and the lung: Role of hypoxia-inducible factors in pulmonary development and disease. Am J Respir Crit Care Med. 2011;183:152–6. doi: 10.1164/rccm.201009-1393PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, et al. p53 gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L753–61. doi: 10.1152/ajplung.00286.2010. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. Hypoxia-inducible factor 1: Regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–8. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamaji-Kegan K, Su Q, Angelini DJ, Myers AC, Cheadle C, Johns RA. Hypoxia-induced mitogenic factor (HIMF/FIZZ1/RELMalpha) increases lung inflammation and activates pulmonary microvascular endothelial cells via an IL-4-dependent mechanism. J Immunol. 2010;185:5539–48. doi: 10.4049/jimmunol.0904021. [DOI] [PubMed] [Google Scholar]
- 36.Daley E, Emson C, Guignabert C, de Waal MR, Louten J, Kurup VP, et al. Pulmonary arterial remodeling induced by a Th2 immune response. J Exp Med. 2008;205:361–72. doi: 10.1084/jem.20071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ono S, Westcott JY, Voelkel NF. PAF antagonists inhibit pulmonary vascular remodeling induced by hypobaric hypoxia in rats. J Appl Physiol. 1992;73:1084–92. doi: 10.1152/jappl.1992.73.3.1084. [DOI] [PubMed] [Google Scholar]
- 38.Kasahara Y, Tuder RM, Cool CD, Lynch DA, Flores SC, Voelkel NF. Endothelial cell death and decreased expression of vascular endothelial growth factor and vascular endothelial growth factor receptor 2 in emphysema. Am J Respir Crit Care Med. 2001;163:737–44. doi: 10.1164/ajrccm.163.3.2002117. [DOI] [PubMed] [Google Scholar]
- 39.Peinado VI, Barbera JA, Ramirez J, Gomez FP, Roca J, Jover L, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274:L908–13. doi: 10.1152/ajplung.1998.274.6.L908. [DOI] [PubMed] [Google Scholar]
- 40.Dinh-Xuan AT, Higenbottam TW, Clelland CA, Pepke-Zaba J, Cremona G, Butt AY, et al. Impairment of endothelium-dependent pulmonaryartery relaxation in chronic obstructive lung disease. N Engl J Med. 1991;324:1539–47. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- 41.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–21. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 42.Wanner A, Mendes ES. Airway endothelial dysfunction in asthma and chronic obstructive pulmonary disease: A challenge for future research. Am J Respir Crit Care Med. 2010;182:1344–51. doi: 10.1164/rccm.201001-0038PP. [DOI] [PubMed] [Google Scholar]
- 43.Edirisinghe I, Arunachalam G, Wong C, Yao H, Rahman A, Phipps RP, et al. Cigarette-smoke-induced oxidative/nitrosative stress impairs. Antioxid Redox Signal. 2010;12:1355–69. doi: 10.1089/ars.2009.2874. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Malhotra D, Thimmulappa R, Vij N, Navas-Acien A, Sussan T, Merali S, et al. Heightened endoplasmic reticulum stress in the lungs of patients with chronic obstructive pulmonary disease: The role of Nrf2-regulated proteasomal activity. Am J Respir Crit Care Med. 2009;180:1196–207. doi: 10.1164/rccm.200903-0324OC. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, Moore M, Sullivan A, Nicolls MR, et al. An animal model of autoimmune emphysema. Am J Respir Crit Care Med. 2005;171:734–42. doi: 10.1164/rccm.200409-1275OC. [DOI] [PubMed] [Google Scholar]
- 46.Sullivan AK, Simonian PL, Falta MT, Mitchell JD, Cosgrove GP, Brown KK, et al. Oligoclonal CD4+ T cells in the lungs of patients with severe emphysema. Am J Respir Crit Care Med. 2005;172:590–6. doi: 10.1164/rccm.200410-1332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sekhon HS, Wright JL, Churg A. Cigarette smoke causes rapid cell proliferation in small airways and associated pulmonary arteries. Am J Physiol. 1994;267:L557–63. doi: 10.1152/ajplung.1994.267.5.L557. [DOI] [PubMed] [Google Scholar]
- 48.Santos S, Peinado VI, Ramirez J, Melgosa T, Roca J, Rodriguez-Roisin R, et al. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur Respir J. 2002;19:632–8. doi: 10.1183/09031936.02.00245902. [DOI] [PubMed] [Google Scholar]
- 49.Kubo K, Ge RL, Koizumi T, Fujimoto K, Yamanda T, Haniuda M, et al. Pulmonary artery remodeling modifies pulmonary hypertension during exercise in severe emphysema. Respir Physiol. 2000;120:71–9. doi: 10.1016/s0034-5687(00)00090-6. [DOI] [PubMed] [Google Scholar]
- 50.Wright JL, Tai H, Churg A. Cigarette smoke induces persisting increases of vasoactive mediators in pulmonary arteries. Am J Respir Cell Mol Biol. 2004;31:501–9. doi: 10.1165/rcmb.2004-0051OC. [DOI] [PubMed] [Google Scholar]
- 51.Wright JL, Tai H, Dai J, Churg A. Cigarette smoke induces rapid changes in gene expression in pulmonary arteries. Lab Invest. 2002;82:1391–8. doi: 10.1097/01.lab.0000032806.45023.08. [DOI] [PubMed] [Google Scholar]
- 52.Lee SD, Lee DS, Chun YG, Shim TS, Lim CM, Koh Y, et al. Cigarette smoke extract induces endothelin-1 via protein kinase C in pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281:L403–11. doi: 10.1152/ajplung.2001.281.2.L403. [DOI] [PubMed] [Google Scholar]
- 53.Nana-Sinkam SP, Lee JD, Sott-Santiago S, Stearman RS, Keith RL, et al. Prostacyclin prevents pulmonary endothelial cell apoptosis induced by cigarette smoke. Am J Respir Crit Care Med. 2007;175:676–85. doi: 10.1164/rccm.200605-724OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peluffo G, Calcerrada P, Piacenza L, Pizzano N, Radi R. Superoxidemediated inactivation of nitric oxide and peroxynitrite formation by tobacco smoke in vascular endothelium: Studies in cultured cells and smokers. Am J Physiol Heart Circ Physiol. 2009;296:H1781–92. doi: 10.1152/ajpheart.00930.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, et al. p53 Mediates Cigarette Smoke-induced Apoptosis of Pulmonary Endothelial Cells: Inhibitory Effects of Macrophage Migration Inhibitor Factor. Am J Respir Cell Mol Biol. 2011;44:323–32. doi: 10.1165/rcmb.2009-0379OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milara J, Juan G, Ortiz JL, Guijarro R, Losada M, Serrano A, et al. Cigarette smoke-induced pulmonary endothelial dysfunction is partially suppressed by sildenafil. Eur J Pharm Sci. 2010;39:363–72. doi: 10.1016/j.ejps.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 57.Burrows B, Kettel LJ, Niden AH, Rabinowitz M, Diener CF. Patterns of cardiovascular dysfunction in chronic obstructive lung disease. N Engl J Med. 1972;286:912–8. doi: 10.1056/NEJM197204272861703. [DOI] [PubMed] [Google Scholar]
- 58.Oswald-Mammosser M, Weitzenblum E, Quoix E, Moser G, Chaouat A, Charpentier C, et al. Prognostic factors in COPD patients receiving longterm oxygen therapy.Importance of pulmonary artery pressure. Chest. 1995;107:1193–8. doi: 10.1378/chest.107.5.1193. [DOI] [PubMed] [Google Scholar]
- 59.Chaouat A, Bugnet AS, Kadaoui N, Schott R, Enache I, Ducolone A, et al. Severe pulmonary hypertension and chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:189–94. doi: 10.1164/rccm.200401-006OC. [DOI] [PubMed] [Google Scholar]
- 60.Watz H, Waschki B, Meyer T, Kretschmar G, Kirsten A, Claussen M, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: Role of hyperinflation. Chest. 2010;138:32–8. doi: 10.1378/chest.09-2810. [DOI] [PubMed] [Google Scholar]
- 61.Holverda S, Bogaard HJ, Groepenhoff H, Postmus PE, Boonstra A, Vonk- Noordegraaf A. Cardiopulmonary exercise test characteristics in patients with chronic obstructive pulmonary disease and associated pulmonary hypertension. Respiration. 2008;76:160–7. doi: 10.1159/000110207. [DOI] [PubMed] [Google Scholar]
- 62.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–60. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 63.Voelkel NF, Natarajan R, Drake JI, Bogaard HJ. Right ventricle in pulmonary hypertension. Compre Physiol. 2011;1:595–610. doi: 10.1002/cphy.c090008. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Q, Kandic I, Kutryk MJ. Dysregulation of angiogenesis-related microRNAs in endothelial progenitor cells from patients with coronary artery disease. Biochem Biophys Res Commun. 2011;405:42–6. doi: 10.1016/j.bbrc.2010.12.119. [DOI] [PubMed] [Google Scholar]
- 65.Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008;133:1354–9. doi: 10.1378/chest.07-2685. [DOI] [PubMed] [Google Scholar]
- 66.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–61. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 67.Golpon HA, Voelkel NF. The vasculature as a target in the treatment of pulmonary emphysema. Curr Drug Targets. 2006;7:737–41. doi: 10.2174/138945006777435335. [DOI] [PubMed] [Google Scholar]
- 68.Lee JH, Lee DS, Kim EK, Choe KH, Oh YM, Shim TS, et al. Simvastatin inhibits cigarette smoking-induced emphysema and pulmonary hypertension in rat lungs. Am J Respir Crit Care Med. 2005;172:987–93. doi: 10.1164/rccm.200501-041OC. [DOI] [PubMed] [Google Scholar]
- 69.Chen Y, Hanaoka M, Chen P, Droma Y, Voelkel NF, Kubo K. Protective effect of beraprost sodium, a stable prostacyclin analog, in the development of cigarette smoke extract-induced emphysema. Am J Physiol Lung Cell Mol Physiol. 2009;296:L648–56. doi: 10.1152/ajplung.90270.2008. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, Hanaoka M, Droma Y, Chen P, Voelkel NF, Kubo K. Endothelin-1 receptor antagonists prevent the development of pulmonary emphysema in rats. Eur Respir J. 2010;35:904–12. doi: 10.1183/09031936.00003909. [DOI] [PubMed] [Google Scholar]
- 71.Celli B, Decramer M, Kesten S, Liu D, Mehra S, Tashkin DP. Mortality in the 4-year trial of tiotropium (UPLIFT) in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180:948–55. doi: 10.1164/rccm.200906-0876OC. [DOI] [PubMed] [Google Scholar]


