Abstract
This study aimed to identify receptors mediating sphingosine-1-phosphate (S1P)-induced vasoconstriction in the normotensive and chronic hypoxia-induced hypertensive rat pulmonary circulation. In isolated perfused lungs from normoxic rats, infusion of S1P caused a sustained vasoconstriction, which was not reduced by combinational pretreatment with the dual S1P1 and 3 receptor antagonist VPC23019 and the S1P2 receptor antagonist JTE013. The S1P4 receptor agonists phytosphingosine-1-phospate and VPC23153, but not the dual S1P1 and 3 receptor agonist VPC24191, caused dose-dependent vasoconstrictions. In hypertensive lungs from chronically hypoxic rats, the vasoconstrictor responses to S1P and VPC23153 were markedly enhanced. The S1P4 receptor agonist VPC 23153 caused contraction of isolated pulmonary but not of renal or mesenteric arteries from chronically hypoxic rats. S1P4 receptor protein as well as mRNA were detected in both normotensive and hypertensive pulmonary arteries. In contrast to what has been reported in the systemic circulation and mouse lung, our findings raise the possibility that S1P4 receptor plays a significant role in S1P-induced vasoconstriction in the normotensive and hypertensive rat pulmonary circulation.
Keywords: pulmonary arteries, pulmonary hypertension, S1P4 receptor, sphingosine-1-phosphate
INTRODUCTION
Sphingosine-1-phosphate (S1P) is an active lipid mediator with regulatory roles in numerous physiological and pathological processes.[1–5] Extracellular signaling of S1P occurs through five known S1P receptors (S1P1-5) that couple to a variety of Ga proteins. While S1P is known to regulate systemic vascular tone via S1P1-3 receptors,[1,6–9] there has been minimal investigation of its effects on pulmonary vascular tone.[10] Two studies show that relatively high concentrations of S1P cause contraction of isolated rat[11] and porcine[12] pulmonary arteries. A recent study reports that S1P induces pulmonary vasoconstriction in mice, and the constriction is dependent on Rho kinase activation via the S1P2 receptor.[13] Nothing, however, has been reported about receptors mediating S1P-induced pulmonary vasoconstriction in the hypertensive pulmonary circulation. Given the emerging importance of S1P in systemic vasoregulation under physiological and pathological conditions,[1–5] it is likely that S1P also plays a vasoregulatory role in the normal and hypertensive pulmonary circulation. A better understanding of the pulmonary vasoactive effects of S1P is important in view of the consideration of the phospholipid as therapy for acute lung injury.[14] The purpose of this study, therefore, was to investigate which S1P receptors are responsible for S1P-induced vasoconstriction in the normotensive and chronic hypoxia-induced hypertensive rat pulmonary circulation.
MATERIALS AND METHODS
Animals
All experimental animal procedures were approved by the Animal Care and Use Committee of the University of South Alabama. Experiments were performed with two groups of male Sprague-Dawley rats (240- 400 g). The normoxic, pulmonary normotensive group was kept in room air. The chronically hypoxic, pulmonary hypertensive group was exposed to normobaric hypoxia (10% O2) for 3 to 4 weeks.
Right ventricular hypertrophy
To demonstrate the presence of pulmonary hypertension in the chronically hypoxic rats, the hearts were dissected and an index of right ventricular hypertrophy was calculated as the ratio of wet weight of right ventricular wall to wet weight of left ventricular wall plus septum (RV/LV+S).
Isolated perfused lungs
The techniques of lung isolation, ventilation, and constant-flow perfusion with physiological salt solution (PSS) have been described previously.[15] Because preliminary experiments showed that bolus injections of S1P into the lung perfusate caused only transient vasoconstrictions (presumably due to rapid degradation within the pulmonary vascular bed), S1P (Enzo Life Sciences) was infused via the pulmonary artery catheter at a constant rate (8.4 nmol/ min) for 10 minutes with and without combinational pretreatment (added 15 min. prior to S1P infusion) of the dual S1P1 and 3 receptor antagonist VPC23019 (3 mM; Avanti)[16] and the S1P2 receptor antagonist JTE013 (1 μM; Tocris)[17] in normotensive (NL) and hypertensive lungs (HL). In a separate set of experiments, effects of bolus administrations of the dual S1P1 and 3 receptor agonist VPC24191 (1-30 μM; Avanti) and two different S1P4 receptor agonists, pytosphingosine-1-phosphate (0.01-3 μM; Avanti)[18] and VPC23153 (0.01-3 μM; Avanti)[19] were examined in NL. In addition, pressor responses to VPC23153 were compared between NL and HL.
To test if the S1P- and S1P4-receptor agonist-induced pulmonary vasoconstrictions were mediated by similar intracellular signaling mechanisms, we examined the sequential effects of the Rho kinase inhibitor fasudil (10 μM)[15] and the Ca2+ channel blocker SKF93635 (50 mM)[20] on sustained, stable vasoconstrictions to continuous infusions of S1P and VPC23153 in both NL and HL.
Isolated arterial rings
Pulmonary (extra-lobar first branches), renal (extra-renal first branches), and mesenteric arteries (~1 mm in diameter) were isolated from chronically hypoxic rats and placed on steel wires attached to a force transducer, and suspended in baths containing 10 ml PSS at 37°C. Resting passive force was adjusted to a previously determined optimal tension (1.5 g for hypertensive pulmonary and 1 g for renal and mesenteric arterial rings).[15,21] Rings were gassed with 21% O2-5% CO2-74% N2, and allowed to equilibrate for 60 min. VPC23153 (0.1-10 μM) was added cumulatively to the organ baths at 15-min. intervals. After the final concentration, all rings were exposed to the nitric oxide synthase inhibitor Nωnitro-L-arginine (200 μM) to test for modulation of contraction by endogenous nitric oxide.
Immunohistochemical staining
A standard technique was used[22] with an anti-S1P4 receptor antibody (1:200; LIFESPAN Biosciences, LS-B513) as a primary antibody.
RNA isolation and quantitative RT-PCR
Intra-pulmonary arteries (200-400 μm in diameter) were isolated from NL and HL and snap frozen. Standard techniques were used for total RNA isolation and real-time PCR.[23] The sequences of primers were as follows: S1P4 f-primer: 5’-GGA AGG CCA TGA ACA TCA GT-3’, S1P4 r-primer: 5’-TGT AGT GCA GGA CGA TGA GC-3’, GAPDH f-primer: 5’-GGA AGG CCA TGA ACA TCA GT-3’, and GAPDH r-primer: 5’-GCT GGT GCT GAG TAT GTC GT-3’.
Statistical analysis
Values are reported as means±SE. Comparisons between groups were made with Student's t-test or analysis of variance (ANOVA) with Fisher's post-hoc test for multiple comparisons. Differences were considered significant at P<0.05.
RESULTS
RV hypertrophy
The presence of pulmonary hypertension in the chronically hypoxic rats was reflected in the RV/LV + S weight ratio, which averaged 0.49±0.01 (n=28) vs. 0.25±0.02 (n=46) in normoxic rats (P<0.05).
Vasoconstrictor effect of continuous infusion of S1P
Continuous infusion of S1P at 8.4 nmol/min. for 10 minutes caused a sustained and progressive vasoconstriction in isolated PSS-perfused lungs, the magnitude of which was much greater in HL than in NL (Fig. 1a). These vasoconstrictions were not reduced by the combinational pretreatment with the dual S1P1 and 3 receptor antagonist VPC23019 and the S1P2 antagonist JTE013. Pretreatment of the perfused lungs with the nitric oxide synthase inhibitor Nωnitro-L-arginine (200 μM) markedly enhanced the sustained S1P-induced vasoconstrictions in both NL and HL (from 3.8±1.1 to 15.0±7.6 mmHg in NL and from 22.4±3.0 to 52.9±11.4 mmHg in HL, n=3 each), indicating the response was moderated by endogenous nitric oxide.
Figure 1.
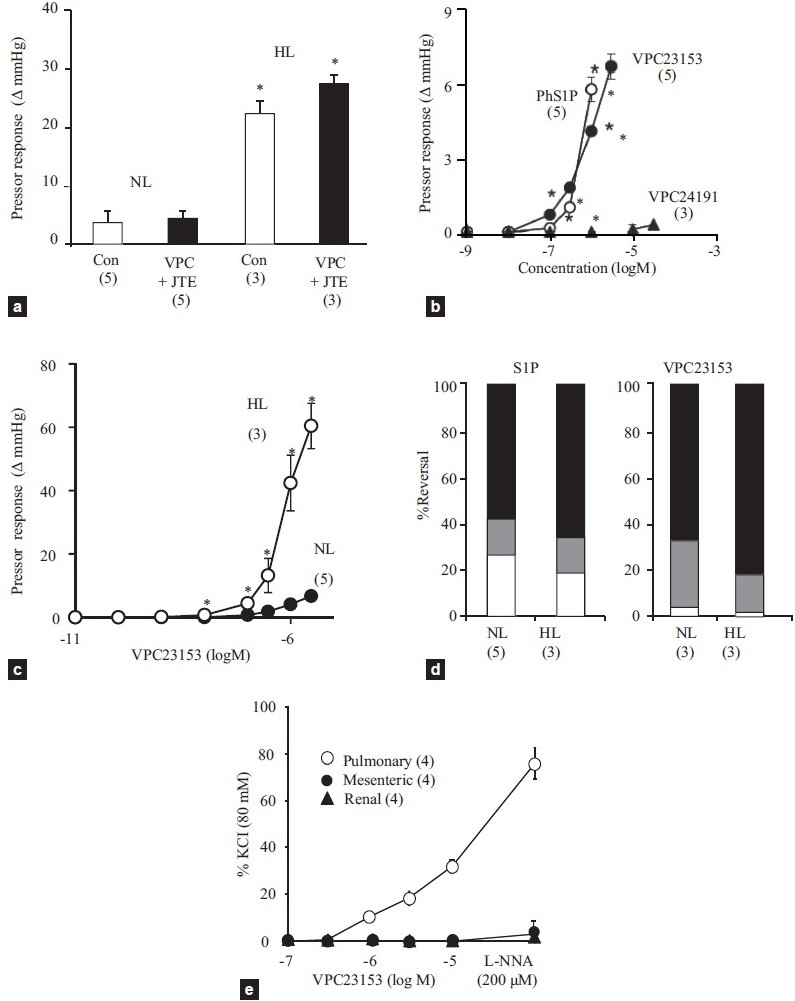
(a) Effects of combinational pretreatment of VPC23019 (3 μM) and JTE013 (1 μM) or the vehicle (dimethyl sulfoxide, Con) on S1P infusion (8.4 nmol/min.)-induced sustained vasoconstriction in normotensive (NL) and hypertensive lungs (HL). *<0.05 vs. NL. (b) Concentration response curves for bolus injections of VPC23153 (closed circle), phytosphingosine-1-phosphate (PhS1P, open circle), and VPC23019 (closed triangle) in normotensive lungs. *<0.05 vs. VPC23153. (c) Concentration response curves for VPC23153 in normotensive (NL, closed circle) and hypertensive lungs (HL, open circle). *P<0.05 vs. NL. (d) Percent reversal by fasudil (black area) and SKF 96365 (gray area) of S1P infusion- (left panel) and VPC23153 infusion-induced vasoconstriction (right panel) in normotensive (NL) and hypertensive lungs (HL). (e) Concentration-response curves for VPC23153 and effects of Nωnitro-L-arginine (L-NNA) in pulmonary, mesenteric and renal arteries isolated from pulmonary hypertensive rats. Sample size is indicated in parenthesis.
Effects of S1P receptor agonists
In PSS-perfused NL, bolus injections of two structurally different S1P4 receptor agonists, VPC23153 and phytoshingosine-1-phosphate, caused dose-dependent and, as compared to bolus S1P, more sustained vasoconstrictions. In contrast, the S1P1 and 3 agonist VPC24191 had no vasoconstrictor effect (Fig. 1b). Similar to the responses to S1P, the vasoconstrictor responsiveness to VPC23153 was markedly enhanced in HL as compared to NL (Fig. 1c).
Effects of Rho kinase and Ca2+channel blockers on S1P and VPC23153 responses
Because of the increased vasoconstrictor responsiveness to both S1P and VPC23153 in HL, lower rates of infusion were required to elicit stable, sustained pressor responses of ~5 mmHg in HL as compared to NL (S1P infusion = 0.6±0.2 nmol/min. in HL and 2.3±0.6 nmol/min. in NL, and VPC23153 infusion = 0.4±0.0 nmol/min. in HL and 4.9±0.7 nmol/min. in NL). As shown in Figure 1d, the sustained vasoconstrictor responses to both S1P and the S1P4 receptor agonist in both NL and HL were rapidly reversed by 60 to 70% with the Rho kinase inhibitor fasudil (10 μM) and an additional 10 to 20% by the Ca2+ entry blocker SKF93635 (50 μM).
Effects of VPC23153 in isolated pulmonary, renal, and mesenteric arteries
The S1P4 receptor agonist VPC23153 caused a concentration-dependent contraction in pulmonary but not in either renal or mesenteric arteries (Fig. 1e). Nωnitro-L-arginine markedly enhanced the S1P4 receptor agonist-induced contraction of pulmonary arteries, suggesting that the contraction was moderated by endogenously produced nitric oxide. The nitric oxide synthase inhibitor did not induce a contractile response to VPC23153 in the systemic arteries.
Immunohistochemical analysis
S1P4 receptor protein was expressed in pulmonary arterial media as well as airway epithelial and smooth muscle cells of NL (n=3) (Fig. 2a and b). S1P4 receptor protein was also detected in the thickened pulmonary arterial media of HL (n=4), but the intensity of the staining was not clearly greater than that in NL (Fig. 2b and c). In contrast to the positive staining for S1P4 receptor protein in pulmonary arteries, there was no positive staining in renal arterial media of normal rats (Fig. 2d).
Figure 2.
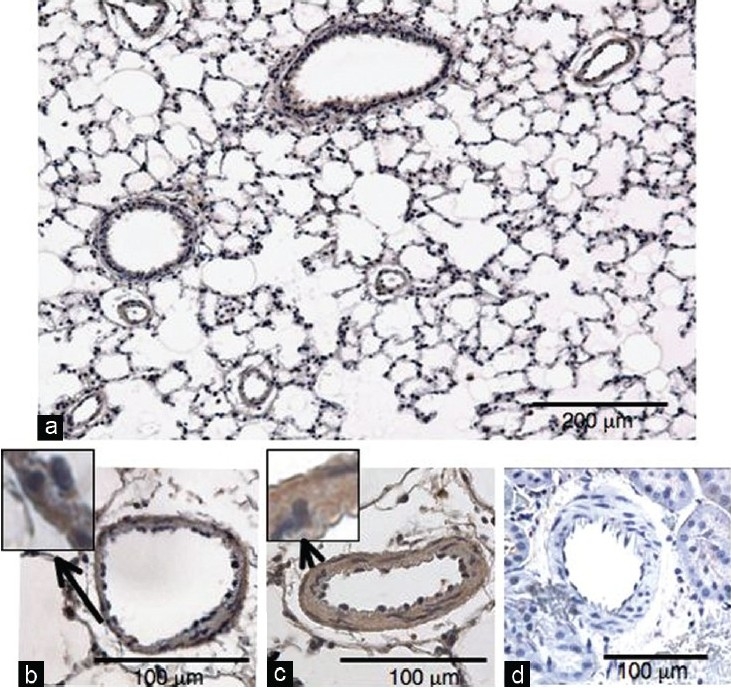
Immnohistochemical staining for S1P4 receptor. (a) A representative low magnification photo of normotensive lung. (b) Representative high magnification photos of small pulmonary arteries from normotensive and (c) hypertensive lungs and (d) renal artery from a normal rat.
S1P4 receptor mRNA expression
S1P4 receptor mRNA was detected in pulmonary arteries from NL and HL with RT-PCR in 2% agarose gel (Fig. 3a). Quantitative RT-PCR showed no statistically significant difference in S1P4 receptor mRNA levels between pulmonary arteries from NL and HL, although there was a tendency towards higher expression levels in HL arteries (Fig. 3b).
Figure 3.
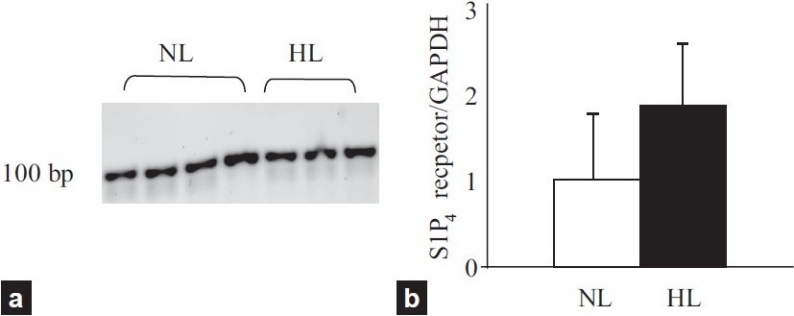
(a) S1P4 receptor mRNA expression in pulmonary arteries from normal (NL) and hypertensive lungs (HL) (b) Quantified values of S1P4 receptor mRNA expression in NL and HL. The amount of S1P4 receptor mRNA was normalized to that of GAPDH gene.
DISCUSSION
It is well documented that the S1P1, 2, and 3 receptors are widely distributed in the cardiovascular system, and several studies have shown that S1P2 and 3 receptors are the major subtypes responsible for S1P-induced constriction in various systemic arteries.[1,6–9] In fact, a recent study has shown that the S1P2 receptor is involved in S1P-induced pulmonary vasoconstriction in mice.[13] We found in this study in rats that infusion of S1P caused sustained vasoconstriction in isolated perfused NL, and the constriction was markedly augmented in HL. Surprisingly, however, our results show that S1P-induced pulmonary vasoconstriction in either NL or HL was not reduced by the pharmacological blockers of S1P1, 2 and 3 receptors. In addition, the dual S1P1 and 3 receptor agonist VPC24191 induced little increase in perfusion pressure. These results suggest that receptors other than S1P1, 2, and 3 are involved in the S1P-induced vasoconstriction in both the normotensive and hypertensive rat pulmonary circulation.
In contrast to widespread distribution of the S1P1, 2, and 3 receptors, expression of the S1P4 and 5 receptors is reported to be relatively restricted.[2,24] S1P4 receptors are found predominantly in lymphoid and hematopoietic tissues, while S1P5 receptors are primarily expressed in the white matter of the central nervous system. S1P4 receptor mRNA has been detected in human and mouse lungs[25,26] and cultured human airway smooth muscle cells.[27] However, little is known about the role of S1P4 receptors in lungs, especially with respect to regulation of pulmonary vascular tone. S1P4receptors are reported to couple to Gαi/0 and Gα12/13 but not to Gαq, and can activate phospholipase C and Rho kinase.[28] Thus, it is possible that activation of S1P4 receptors on pulmonary vascular smooth muscle cells induce contraction through increased cytosolic Ca2+ levels (via phospholipase C-mediated Ca2+mobilization and Ca2+ influx) and Rho kinase-mediated Ca2+ sensitization.[24,29] Our results demonstrate that two structurally distinct S1P4 receptor agonists, phytosphingosine-1-phosphate[18] and VPC23153,[19] caused concentration-dependent vasoconstrictions in isolated NL, and that, as was the case with S1P, the response to VPC23153 was markedly enhanced in HL. Other similarities between the sustained vasoconstrictor responses to infused S1P and VPC23153 in both NL and HL were that they were markedly augmented by inhibition of nitric oxide synthesis and substantially reversed by the inhibitor of Rho kinase fasudil and additionally reduced by the Ca2+ channel blocker SKF93635. In addition to eliciting vasoconstriction in perfused lungs, VPC23153 contracted pulmonary but neither mesenteric nor renal arteries isolated from pulmonary hypertensive rats. Also, our immunohistochemical analysis showed S1P4 receptor protein is expressed in the media of pulmonary arteries from pulmonary normotensive and hypertensive rats but not in that of renal arteries from pulmonary normotensive rats. Finally, we clearly detected S1P mRNA in normotensive and hypertensive pulmonary arteries. Collectively, these results support that S1P4receptors may play a key role in S1P-induced, Rho kinase- and Ca2+-mediated vasoconstriction in the normotensive and hypertensive rat pulmonary circulation.
Although VPC23153 at the concentrations used in this study has agonistic effects on the S1P1 receptor,[18] it is unlikely this receptor plays a major role in S1P-induced pulmonary vasoconstriction because we found that the dual S1P1 and 3 receptor agonist VPC24191 induced essentially no constriction in isolated NL. It should be noted, however, that the S1P4 receptor agonists phytosphingosine-1-phosphate and VPC23153 also have some affinity to S1P5 receptors,[17,18] and this study does not rule out the possible involvement of this receptor in the vasoconstriction. In contrast to the previous report that S1P-induced vasoconstriction in mouse lungs is reduced by the S1P2 receptor antagonist JTE013 at a concentration of 10 μM,[30] we did not observe inhibition at the more receptor-selective concentration of 1 μM.[31]
We found that vasoconstrictor responsiveness to S1P and the S1P4 receptor agonist was similarly and markedly enhanced in HL isolated from chronically hypoxic rats. Because the nitric oxide synthase inhibitor Nωnitro-L-arginine increased S1P-induced vasoconstriction in both NL and HL and did not eliminate the difference between them, the enhanced response in HL cannot be attributed to decreased activity of nitric oxide. It is also unlikely that upregulation of the S1P4 receptor plays a major role in this augmented response, since we did not detect a significant increase in its mRNA or protein expression in hypertensive as compared to normotensive pulmonary arteries. Increased vasoconstrictor and myogenic reactivity is generally a characteristic of the hypoxia-induced hypertensive rat pulmonary vasculature,[32,33] although it is unclear exactly why the responsiveness to S1P is so strikingly augmented in HL.
CONCLUSIONS
In summary, this study demonstrates that S1P is a vasoconstrictor in the normal rat pulmonary circulation, and that the constrictor response is markedly augmented in the chronically hypoxic hypertensive pulmonary circulation. In contrast to what has been observed in various systemic arteries and in the mouse lung, S1P-induced pulmonary vasoconstriction appears to be mediated largely by the S1P4 receptor in the rat. These findings raise the possibility that pulmonary vasoconstriction by endogenous S1P, produced either locally within the pulmonary arterial wall or by circulating platelets and/or erythrocytes,[10] could contribute to the pathogenesis of pulmonary hypertension. If so, and if S1P-induced constriction of human pulmonary arteries is also mediated by the S1P4 receptor, then a S1P4 receptor antagonist[34] might be clinically useful as a selective pulmonary vasodilator.
ACKNOWLEDGMENTS
The authors thank Boniface Obiako and Greg Holberg and Drs. Tetsutaro Nagaoka and Noriyuki Homma for technical assistance and Dr. Mark N. Gillespie for helpful editorial comment.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Alewijnse AE, Peters SL. Sphingolipid signalling in the cardiovascular system: Good, bad or both? Eur J Pharmacol. 2008;585:292–302. doi: 10.1016/j.ejphar.2008.02.089. [DOI] [PubMed] [Google Scholar]
- 2.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Maceyka M, Milstien S, Spiegel S. Sphingosine-1-phosphate: The Swiss army knife of sphingolipid signaling. J Lipid Res. 2009;50(Suppl):S272–6. doi: 10.1194/jlr.R800065-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rivera R, Chun J. Biological effects of lysophospholipids. Rev Physiol Biochem Pharmacol. 2008;160:25–46. doi: 10.1007/112_0507. [DOI] [PubMed] [Google Scholar]
- 5.Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res. 2009;50(Suppl):S293–8. doi: 10.1194/jlr.R800047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hemmings DG. Signal transduction underlying the vascular effects of sphingosine 1-phosphate and sphingosylphosphorylcholine. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:18–29. doi: 10.1007/s00210-006-0046-5. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi J, Michel T. Sphingosine-1-phosphate and modulation of vascular tone. Cardiovasc Res. 2009;82:212–20. doi: 10.1093/cvr/cvp064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levkau B. Sphingosine-1-phosphate in the regulation of vascular tone: A finely tuned integration system of S1P sources, receptors, and vascular responsiveness. Circ Res. 2008;103:231–3. doi: 10.1161/CIRCRESAHA.108.181610. [DOI] [PubMed] [Google Scholar]
- 9.Skoura A, Hla T. Regulation of vascular physiology and pathology by the S1P2 receptor subtype. Cardiovasc Res. 2009;82:221–8. doi: 10.1093/cvr/cvp088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uhlig S, Gulbins E. Sphingolipids in the lungs. Am J Respir Crit Care Med. 2008;178:1100–14. doi: 10.1164/rccm.200804-595SO. [DOI] [PubMed] [Google Scholar]
- 11.Thomas GD, Snetkov VA, Patel R, Leach RM, Aaronson PI, Ward JP. Sphingosylphosphorylcholine-induced vasoconstriction of pulmonary artery: Activation of non-store-operated Ca2+ entry. Cardiovasc Res. 2005;68:56–64. doi: 10.1016/j.cardiores.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao SH, Constable PD, Smith GW, Haschek WM. Effects of exogenous sphinganine, sphingosine, and sphingosine-1-phosphate on relaxation and contraction of porcine thoracic aortic and pulmonary arterial rings. Toxicol Sci. 2005;86:194–9. doi: 10.1093/toxsci/kfi167. [DOI] [PubMed] [Google Scholar]
- 13.Szczepaniak WS, Pitt BR, McVerry BJ. S1P2 receptor-dependent Rho-kinase activation mediates vasoconstriction in the murine pulmonary circulation induced by sphingosine 1-phosphate. Am J Physiol Lung Cell Mol Physiol. 2010;299:L137–45. doi: 10.1152/ajplung.00233.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang L, Dudek SM. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc Res. 2009;77:39–45. doi: 10.1016/j.mvr.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagaoka T, Morio Y, Casanova N, Bauer N, Gebb S, McMurtry I, et al. Rho/Rho kinase signaling mediates increased basal pulmonary vascular tone in chronically hypoxic rats. Am J Physiol Lung Cell Mol Physiol. 2004;287:L665–672. doi: 10.1152/ajplung.00050.2003. [DOI] [PubMed] [Google Scholar]
- 16.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem. 2005;280:9833–41. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 17.Ohmori T, Yatomi Y, Osada M, Kazama F, Takafuta T, Ikeda H, et al. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc Res. 2003;58:170–7. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- 18.Candelore MR, Wright MJ, Tota LM, Milligan J, Shei GJ, Bergstrom JD, et al. Phytosphingosine 1-phosphate: A high affinity ligand for the S1P(4)/Edg-6 receptor. Biochem Biophys Res Commun. 2002;297:600–6. doi: 10.1016/s0006-291x(02)02237-4. [DOI] [PubMed] [Google Scholar]
- 19.Clemens JJ, Davis MD, Lynch KR, Macdonald TL. Synthesis of benzimidazole based analogues of sphingosine-1-phosphate: Discovery of potent, subtype-selective S1P4 receptor agonists. Bioorg Med Chem Lett. 2004;14:4903–6. doi: 10.1016/j.bmcl.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Muramatsu M, Tyler RC, Rodman DM, McMurtry IF. Possible role of T-type Ca2+ channels in L-NNA vasoconstriction of hypertensive rat lungs. Am J Physiol. 1997;272:H2616–21. doi: 10.1152/ajpheart.1997.272.6.H2616. [DOI] [PubMed] [Google Scholar]
- 21.Duckles SP, Carter BJ, Williams CL. Vascular adrenergic neuroeffector function does not decline in aged rats. Circ Res. 1985;56:109–16. doi: 10.1161/01.res.56.1.109. [DOI] [PubMed] [Google Scholar]
- 22.Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. FASEB J. 2001;15:427–38. doi: 10.1096/fj.00-0343com. [DOI] [PubMed] [Google Scholar]
- 23.Chang CL, Ho MC, Lee PH, Hsu CY, Huang WP, Lee H. S1P(5) is required for sphingosine 1-phosphate-induced autophagy in human prostate cancer PC-3 cells. Am J Physiol Cell Physiol. 2009;297:C451–8. doi: 10.1152/ajpcell.00586.2008. [DOI] [PubMed] [Google Scholar]
- 24.Watterson KR, Ratz PH, Spiegel S. The role of sphingosine-1-phosphate in smooth muscle contraction. Cell Signal. 2005;17:289–98. doi: 10.1016/j.cellsig.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Graler MH, Bernhardt G, Lipp M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–9. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- 26.Ishii I, Friedman B, Ye X, Kawamura S, McGiffert C, Contos JJ, et al. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J Biol Chem. 2001;276:33697–704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- 27.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, et al. Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J. 2001;15:1212–4. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 28.Graler MH, Grosse R, Kusch A, Kremmer E, Gudermann T, Lipp M. The sphingosine 1-phosphate receptor S1P4 regulates cell shape and motility via coupling to Gi and G12/13. J Cell Biochem. 2003;89:507–19. doi: 10.1002/jcb.10537. [DOI] [PubMed] [Google Scholar]
- 29.Sward K, Mita M, Wilson DP, Deng JT, Susnjar M, Walsh MP. The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr Hypertens Rep. 2003;5:66–72. doi: 10.1007/s11906-003-0013-1. [DOI] [PubMed] [Google Scholar]
- 30.Salomone S, Potts EM, Tyndall S, Ip PC, Chun J, Brinkmann V, et al. Analysis of sphingosine 1-phosphate receptors involved in constriction of isolated cerebral arteries with receptor null mice and pharmacological tools. Br J Pharmacol. 2008;153:140–7. doi: 10.1038/sj.bjp.0707581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomone S, Waeber C. Selectivity and specificity of sphingosine-1-phosphate receptor ligands: caveats and critical thinking in characterizing receptor-mediated effects. Front Pharmacol. 2011;2:9. doi: 10.3389/fphar.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broughton BR, Walker BR, Resta TC. Chronic hypoxia induces Rho kinase-dependent myogenic tone in small pulmonary arteries. Am J Physiol Lung Cell Mol Physiol. 2008;294:L797–806. doi: 10.1152/ajplung.00253.2007. [DOI] [PubMed] [Google Scholar]
- 33.Shimoda LA, Sham JS, Sylvester JT. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res. 2000;49:549–60. [PubMed] [Google Scholar]
- 34.Oldstone M, Hodder P, Crisp M, Roberts E, Guerrero M, Urbano M, et al. Bethesda (MD): National Center for Biotechnology Information (US); 2010. Probe Development Efforts to Identify Novel Antagonists of the Sphingosine 1-phosphate Receptor 4 (S1P4). Probe Reports from the NIH Molecular Libraries Program [internet] [PubMed] [Google Scholar]


