Abstract
Isolated pulmonary artery involvement by large vessel vasculitis is rare. This case report describes two patients with large vessel pulmonary vasculitis initially thought to have chronic thromboembolic pulmonary hypertension who had their diagnosis revised following pulmonary endarterectomy surgery. Advances in imaging techniques such as positron emission tomography and magnetic resonance imaging have permitted complementary radiological methods of diagnosis and follow up of large vessel disease and these are discussed in conjunction with the immunosuppressive and operative management of these patients.
Keywords: large vessel vasculitis, pulmonary artery, pulmonary endarterectomy
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is associated with the obstruction of the pulmonary arteries by organized thrombo-embolic material resulting in restriction of blood flow to affected segmental branches and vascular remodelling in the unobstructed pulmonary arteries. The definitive treatment is pulmonary endarterectomy (PEA), which has proven symptomatic and survival benefit.[1]
As the national PEA center, our institution has carried out over 700 PEA operations. Many more patients have been referred for assessment of possible CTEPH or other causes of pulmonary artery obstruction. A small proportion of patients undergoing PEA have had alternative diagnoses made at or following surgery. We describe two patients who had a revised diagnosis of isolated large vessel pulmonary artery vasculitis following attempted PEA surgery.
CASE REPORT
Case 1
A 40-year-old Caucasian male was referred with a 2-month history of exertional breathlessness and had New York Heart Association (NYHA) Class II performance status at presentation. Prior to referral, he had been therapeutically anti-coagulated for 5 months with a possible diagnosis of CTEPH based on imaging at his local hospital. Repeat CT Pulmonary Angiogram (CTPA) (Fig. 1) demonstrated extensive proximal narrowing of the right and left pulmonary artery wall with concentric soft tissue thickening, and occlusion of the right upper lobe and left upper lobe apical segmental branches. Full blood count and renal function were normal. C-reactive protein (CRP) was 77 mg/L with an erythrocyte sedimentation rate (ESR) of 79 mm/hr. Anti nuclear antibodies (ANA) and anti-neutrophil cytoplasmic antibodies (ANCA) were negative. MRI pulmonary angiogram confirmed the distribution of disease and also demonstrated smooth plaque like focal intimal thickening in both proximal main pulmonary arteries. Due to concerns over the possible diagnosis of a pulmonary artery sarcoma, positron emission tomography CT (PET/CT) was performed (Fig. 2). This showed intermediate uptake in the distal right main pulmonary artery. Pre-operative RHC demonstrated a mean pulmonary artery pressure (mPAP) of 32 mmHg with a cardiac index of 2 l/min/m2.
Figure 1.
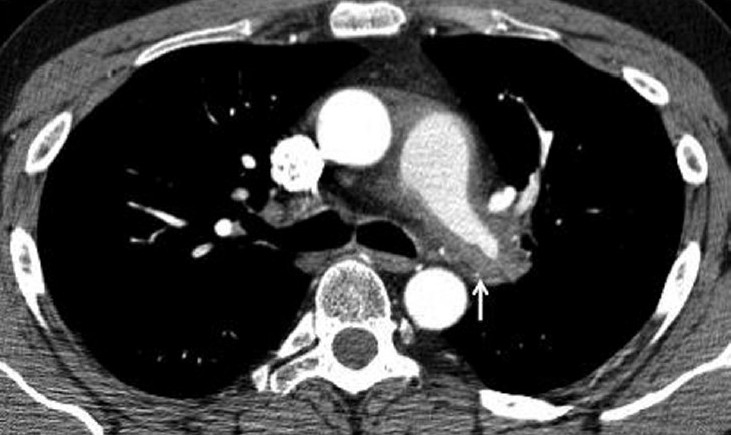
Axial CTPA image showing concentric soft tissue thickening around the left pulmonary artery (arrow).
Figure 2.
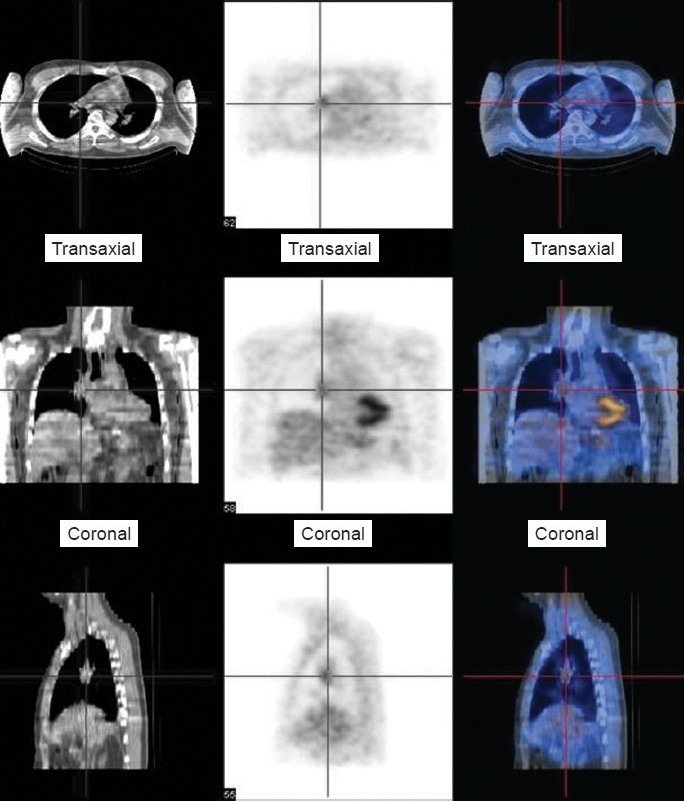
Selected images from 18 FDG-positron emission tomographic study demonstrates increased tracer uptake in the right pulmonary artery (red cross bars) in keeping with active phase of the vasculitic process.
He underwent PEA for symptomatic benefit and to provide a histological diagnosis. During surgery, the pulmonary arteries were thick walled and fibrotic, making the operation technically difficult. A limited endarterectomy was performed bilaterally. The mPAP fell to 25 mmHg on Day One post-operation. Histology of the excised material revealed transmural lymphohistocytic infiltration with a giant cell granulomatous vasculitis (Fig. 3). There was no evidence of acid-fast bacilli or fungi. Subsequent HIV testing and syphilis serology were negative. Coronary angiography revealed good flow with no coronary artery aneurysms. Ear nose and throat, renal and ophthalmological evaluations excluded systemic vasculitic involvement.
Figure 3.
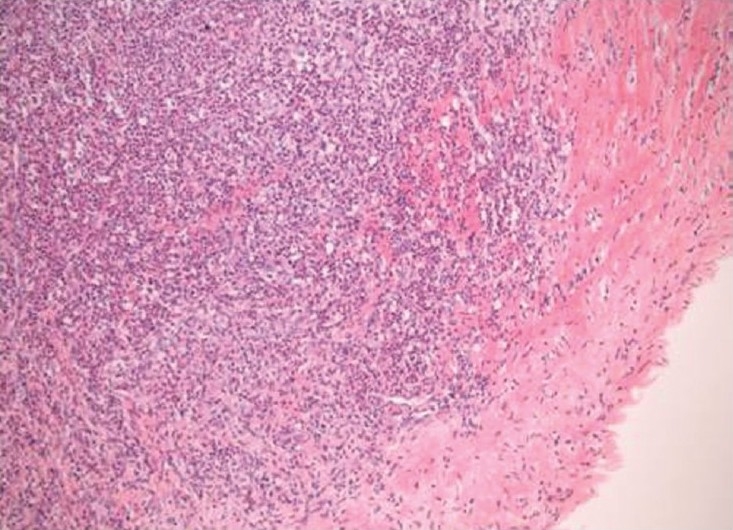
Haematoxylin and eosin stain (×100) showing a dense inflammatory cell infiltrate including acute and chronic inflammatory cells with histiocytes and focal giant cells involving the vascular intima and extending into the rim of media included in the specimen.
As treatment for his pulmonary artery vasculitis, this patient was commenced on 1 mg/kg of prednisolone and pulsed cyclophosphamide, which was subsequently substituted for azathioprine (1.5 mg/kg). Prednisolone was subsequently tapered down monitoring symptoms and MRI appearance. PET/CT scan 15 months after his PEA showed low-level uptake in the lateral wall of the ascending aorta and the medial wall of the pulmonary artery. A repeat MRI angiogram demonstrated new 3 mm soft tissue thickening in the ascending aorta and the aortic arch, as well as persisting fusiform narrowing of the right main pulmonary artery with proximal segment occlusions of the right upper and left lower lobes, suggesting non-progression of the pulmonary changes on a background of systemic large vessel involvement. In view of these changes and persistently raised inflammatory markers his immunosuppression was changed to mycophenolate mofetil (MMF) with the subsequent addition of infliximab.
His condition has been stable for the last three years with normal ESR and CRP. Subsequent MRI pulmonary angiography continues to show the previously described narrowing and occlusion of the pulmonary arteries, presumably representing “burnt out” vasculitis. He continues on anticoagulation and low dose a combination of infliximab (3 mg/kg 8 weekly) and MMF (3 g/day).
Case 2
A 53-year-old woman was referred with a two year history of increasing breathlessness and initial investigations suggested multiple pulmonary emboli with ventilation perfusion (VQ) scintigraphy demonstrating perfusion defects in the right mid zone and left mid and lower zones. There was no significant improvement with anticoagulation and her performance status was NYHA class III. An echocardiogram revealed a dilated right ventricle with impaired function.
Right heart catheterisation (RHC) demonstrated a mPAP of 41 mmHg with a cardiac index of 1.7 l/min/m2. CTPA (Fig. 4) demonstrated extravascular soft tissue thickening adjacent to the main pulmonary arteries. In addition, there were proximal stenosis of the right and left main pulmonary arteries with additional soft tissue encasement and occlusion of the proximal lingular and lower lobe arteries. Catheter directed pulmonary angiogram (Fig. 5) confirmed the CTPA findings and also demonstrated a marked stretching of the right main pulmonary artery with concentric soft tissue thickening. Baseline ESR was 25 mm/hr. and autoantibody screen was negative.
Figure 4.
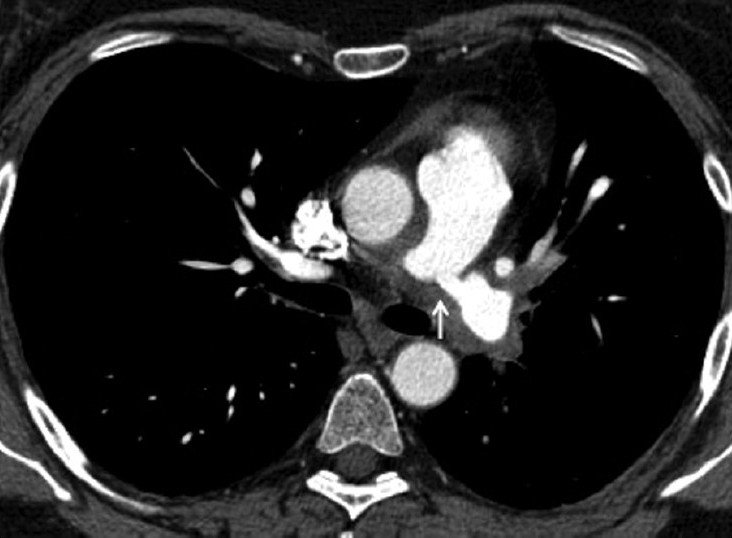
Axial multi-detector CT image showing concentric thickening around the pulmonary artery with a stenosis at the origin of the left main pulmonary artery (arrow).
Figure 5.
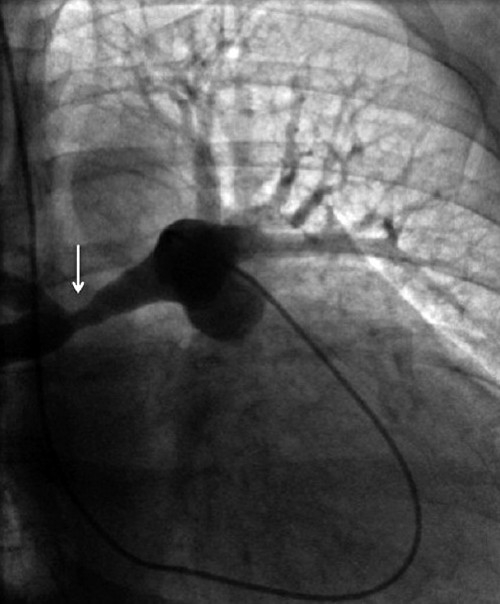
Still image from catheter pulmonary angiogram demonstrates concentric soft tissue thickening around the right pulmonary artery resulting in stretching of the artery and luminal irregularity (arrow).
A PEA was performed. At operation there was a rigid mass of fibrous tissue encasing the pulmonary trunk and both main pulmonary arteries, extending out into the left sub-segmental vessels. This was dissected out and the central pulmonary arteries enlarged with a bovine pericardial patch. Histology of the excised pulmonary artery wall demonstrated a chronic inflammatory lymphoplasmacytic mass, with no evidence of giant cells or granulomas, extending into the tunica media, implying a vasculitic process. RHC three months after PEA showed a mPAP of 30 mmHg, with cardiac index of 2.36 l/min/m2. PET/CT scanning undertaken at the same time showed increased uptake in the aorta and left lower lobe which corresponded to an area which could not be removed at operation. A diagnosis of large vessel vasculitis was made. She was commenced on prednisolone (1 mg/kg) and azathioprine (1.5 mg/kg), with subsequent weaning of the prednisolone dose. At 12 month follow up her six-minute walk had improved from 260 m preoperatively to 380 m.
DISCUSSION
We have described two patients with large vessel vasculitis who presented with breathlessness in the absence of systemic vasculitic manifestations, with initial imaging demonstrating isolated pulmonary pathology. Raised inflammatory markers were present at the time of assessment. Both patients were Caucasian and in their fifth or sixth decade of life. The causes of chronic obstruction of the pulmonary arteries can be subdivided into endoluminal disease (such as chronic thromboembolic disease or tumor) or extrinsic compression (such as lymphadenopathy or fibrosing mediastinitis). Large vessel pulmonary arteritis can cause either endoluminal blockage or extrinsic compression and therefore may give a similar radiological and clinical presentation to that of chronic thromboembolic disease.[2]
The incidence of isolated pulmonary artery vasculitis is unknown but is thought to be rare. Takayasu arteritis can produce isolated pulmonary artery vasculitis.[3,4] The incidence of Takayasu arteritis in the UK is 0.8/ million/ year.[5] The incidence of pulmonary artery involvement in Takayasu arteritis ranges from 14.3%[6] to 86%;[7] involvement is often subclinical.[6] Pulmonary artery involvement has also been reported due to giant cell arteritis and Behçet's disease, although the latter tends to cause pulmonary artery aneurysms or mass lesions rather than vascular obstruction.[8]
Radiological features on CTPA that may suggest large vessel pulmonary vasculitis rather than CTEPH include wall thickening and contrast enhancement in the early phases, with mural calcium deposition and luminal stenosis in more chronic phases. Circumferential arterial wall thickening is not present in CTEPH.[8] Aortic wall thickening with mural enhancement on gadolinium contrast MRI in Takayasu arteritis has been described which may correlate with disease activity[9] and MRI was utilized for follow up in Case 1. PET scanning has an established role for diagnosis and is included with MRI in contemporary large vessel vasculitis guidelines,[10] but its role in disease monitoring is less well defined. The evidence base for MRI and PET follow up is mostly in aortitis and it is likely that their role has not been specifically studied in isolated pulmonary vasculitis due to the rarity of the condition.
In cases from Japan the diagnosis is based on certain radiological features and HLA types associated with Takayasu's.[3,4] It is unclear how applicable this approach is to patients of European background and a requirement for a tissue diagnosis is likely to be present in most cases. We have used PEA (with bovine patch enlargement of the pulmonary artery in Case 2) as the operation for symptom relief and diagnosis. There are case reports of a pericardial patch to enlarge the pulmonary artery stenosis,[3] or the use of a bypass graft.[4]
CONCLUSIONS
In summary, isolated large vessel pulmonary artery vasculitis may mimic CTEPH. Entertaining the diagnosis preoperatively is of benefit as the patient can be informed about the differential diagnosis and the surgeon can plan to undertake a reconstructive procedure of a pulmonary artery as well as PEA. Evidence of a pulmonary arterial vasculitis should lead to examination of systemic large vessels to define the extent and severity of disease. Large vessel vasculitis responds to immunosuppressive therapy which arrests disease progression and may improve pulmonary haemodynamics. Ideally reconstructive surgery should be undertaken when the disease is in remission[10] and so the timing of any surgery could be influenced by disease activity and response to therapy. The possibility of these patients subsequently developing a more widespread vasculitis is important to consider. On PET/CT scan, both of our patients were later found to have asymptomatic aortitis. Finally, the advent of modern imaging techniques such as MRI and PET has provided clinicians with novel tools allowing a greater ability to diagnose and monitor response to treatment in patients with this uncommon condition.
ACKNOWLEDGMENTS
The authors acknowledge the contributions of Prof. David Scott (Norfolk and Norwich University Hospitals) and Prof. Andrew Peacock and Dr. Martin Johnson (Golden Jubilee Hospital, Glasgow) who are involved in the continued care of the patients.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Condliffe R, Kiely DG, Gibbs JS, Corris PA, Peacock AJ, Jenkins DP, et al. Improved outcomes in medically and surgically treated chronic thrombolic pulmonary hypertension. Am J Resp Crit Care Med. 2008;177:1122–7. doi: 10.1164/rccm.200712-1841OC. [DOI] [PubMed] [Google Scholar]
- 2.Kerr KM, Auger WR, Fedullo PF, Channick RH, Yi ES, Moser KM. Large vessel pulmonary arteritis mimicking chronic thromboembolic disease. Am J Resp Crit Care Med. 1995;152:367–73. doi: 10.1164/ajrccm.152.1.7599847. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki I, Ichikawa Y, Ishii M, Hamada T, Kajiwara H. Surgical case of isolated pulmonary Takayasu's arteritis. Circ J. 2005;69:500–2. doi: 10.1253/circj.69.500. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima N, Masuda M, Imamaki M, Ishida A, Tanabe N, Kuriyama T. A case of pulmonary artery bypass surgery for a patient with isolated Takayasu pulmonary arteritis and a review of the literature. Ann Thorac Cardiovasc Surg. 2007;13:267–71. [PubMed] [Google Scholar]
- 5.Watts R, Al-Taiar A, Mooney J, Scott D, Macgregor A. The epidemiology of Takayasu arteritis in the UK. Rheumatology. 2009;48:1008–11. doi: 10.1093/rheumatology/kep153. [DOI] [PubMed] [Google Scholar]
- 6.Sharma S, Kamalakar T, Rajani M, Talwar KK, Shrivastava S. The incidence and patterns of pulmonary artery involvement in Takayasu's arteritis. Clin Radiol. 1990;42:177–81. doi: 10.1016/s0009-9260(05)81929-4. [DOI] [PubMed] [Google Scholar]
- 7.Yamato M, Lecky JW, Hiramatsu K, Kohda E. Takayasu arteritis: Radiographic and angiographic findings in 59 patients. Radiology. 1986;161:329–34. doi: 10.1148/radiology.161.2.2876459. [DOI] [PubMed] [Google Scholar]
- 8.Marthen K, Schnyder P, Schirg E, Prokop M, Rummeny EJ, Engelke C. Pattern-based differential diagnosis in pulmonary vasculitis using volumetric CT. AJR Am J Roentgenol. 2005;184:720–33. doi: 10.2214/ajr.184.3.01840720. [DOI] [PubMed] [Google Scholar]
- 9.Choe YH, Han BK, Koh EM, Kim DK, Do YS, Lee WR. Takayasu's arteritis: Assessment of disease activity with contrast enhanced MR imaging. Am J Roent. 2000;175:505–11. doi: 10.2214/ajr.175.2.1750505. [DOI] [PubMed] [Google Scholar]
- 10.Mukhtyar C, Guillevin L, Cid MC, Dasgupta B, de Groot K, Gross W, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis. 2009;68:318–23. doi: 10.1136/ard.2008.088351. [DOI] [PubMed] [Google Scholar]


