Abstract
The importance of antigen site density has been studied by means of a model passive hemagglutination system using human red cells coupled with sulfanilic acid groups. Relative site numbers were estimated from the covalent linkage of sulfanilic acid-35S to red cell membrane protein and the effective antigen site number was determined with 125I-labeled rabbit IgG antisulfanilic acid.
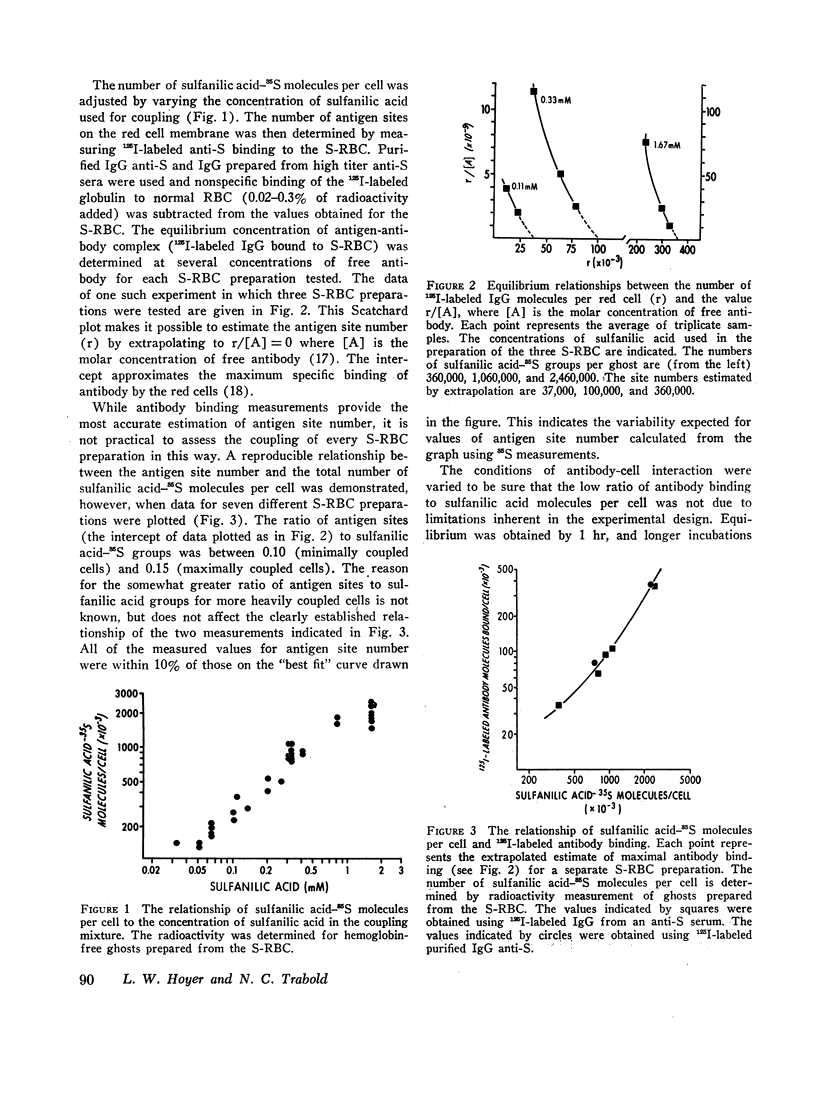
Cells which had fewer than 20,000 antigen sites per cell were not agglutinated. As greater numbers of sulfanilic groups were coupled to the red cells, the agglutination titers increased to maximum values with red fanilic groups were coupled to the red cells, the agglutination titers of purified IgM antibody were 10-20 times greater than IgG antibody when preparations with the same protein concentration were compared, but this difference was not noted when IgG antibody was measured by antiglobulin reactions.
These findings emphasize the need to consider differences in antigen site density when comparing blood group systems. They are consistent with the hypothesis that those blood group antigens which have a very low site number will not be detected by IgG antibodies in saline hemagglutination determinations.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALMEIDA J., CINADER B., HOWATSON A. THE STRUCTURE OF ANTIGEN-ANTIBODY COMPLEXES. A STUDY BY ELECTRON MICROSCOPY. J Exp Med. 1963 Sep 1;118:327–340. doi: 10.1084/jem.118.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- COHEN F., ZUELZER W. W., EVANS M. M. Identification of blood group antigens and minor cell populations by the fluorescent antibody method. Blood. 1960 Jun;15:884–900. [PubMed] [Google Scholar]
- Chesebro B., Bloth B., Svehag S. E. The ultrastructure of normal and pathological IgM immunoglobulins. J Exp Med. 1968 Mar 1;127(3):399–410. doi: 10.1084/jem.127.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPUY M. E., ELLIOT M., MASOUREDIS S. P. RELATIONSHIP BETWEEN RED CELL BOUND ANTIBODY AND AGGLUTINATION IN THE ANTIGLOBULIN REACTION. Vox Sang. 1964 Jan-Feb;9:40–44. doi: 10.1111/j.1423-0410.1964.tb03766.x. [DOI] [PubMed] [Google Scholar]
- Economidou J., Hughes-Jones N. C., Gardner B. Quantitative measurements concerning A and B antigen sites. Vox Sang. 1967 May;12(5):321–328. doi: 10.1111/j.1423-0410.1967.tb03362.x. [DOI] [PubMed] [Google Scholar]
- GREENBURY C. L., MOORE D. H., NUNN L. A. REACTION OF 7S AND 19S COMPONENTS OF IMMUNE RABBIT ANTISERA WITH HUMAN GROUP A AND AB RED CELLS. Immunology. 1963 Sep;6:421–433. [PMC free article] [PubMed] [Google Scholar]
- HELMKAMP R. W., GOODLAND R. L., BALE W. F., SPAR I. L., MUTSCHLER L. E. High specific activity iodination of gamma-globulin with iodine-131 monochloride. Cancer Res. 1960 Nov;20:1495–1500. [PubMed] [Google Scholar]
- HIGGINS H. G., HARRINGTON K. J. Reaction of amino acids and proteins with diazonium compounds. II. Spectra of protein derivatives. Arch Biochem Biophys. 1959 Dec;85:409–425. doi: 10.1016/0003-9861(59)90506-5. [DOI] [PubMed] [Google Scholar]
- HUGHES-JONES N. C., GARDNER B., TELFORD R. STUDIES ON THE REACTION BETWEEN THE BLOOD-GROUP ANTIBODY ANTI-D AND ERYTHROCYTES. Biochem J. 1963 Sep;88:435–440. doi: 10.1042/bj0880435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W., Borsos T., Rapp H. J., Vannier W. E. Heterogeneity of rabbit IgM antibody as detected by C'1a fixation. J Exp Med. 1968 Mar 1;127(3):589–603. doi: 10.1084/jem.127.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer L. W., Vannier W. E., Renfer L. Antibody elution from hapten-cellulose immunoadsorbents: the effects of hapten structure, pH and salt concentration. Immunochemistry. 1968 May;5(3):277–292. doi: 10.1016/0019-2791(68)90072-4. [DOI] [PubMed] [Google Scholar]
- INGRAHAM J. S. [Specific, complement-dependent hemolysis of sheep erythrocytes by antiserum to azo hapten groups]. J Infect Dis. 1952 Nov-Dec;91(3):268–275. doi: 10.1093/infdis/91.3.268. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leddy J. P. Immunological aspects of red cell injury in man. Semin Hematol. 1966 Jan;3(1):48–73. [PubMed] [Google Scholar]
- MASOUREDIS S. P. Relationship between RhO(D) genotype and quantity of 1131 anti-RhO(D) bound to red cells. J Clin Invest. 1960 Sep;39:1450–1462. doi: 10.1172/JCI104164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage R., Dray S. Persistent altered phenotypic expression of allelic gamma-G-immunoglobulin allotypes in heterozygous rabbits exposed to isoantibodies in fetal and neonatal life. J Immunol. 1965 Sep;95(3):525–535. [PubMed] [Google Scholar]
- Mäkelä O., Ruoslahti E., Ehnholm C. Subtypes of human ABO blood groups and subtype-specific antibodies. J Immunol. 1969 Mar;102(3):763–771. [PubMed] [Google Scholar]
- NETER E., BERTRAM L. F., ZAK D. A., MURDOCK M. R., ARBESMAN C. E. Studies on hemagglutination and hemolysis by escherichia coli antisera. J Exp Med. 1952 Jul;96(1):1–15. doi: 10.1084/jem.96.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoue K., Tanigaki N., Yagi Y., Pressman D. IgM and IgG anti-hapten antibody: hemolytic, hemagglutinating and precipitating activity. Proc Soc Exp Biol Med. 1965 Nov;120(2):340–346. doi: 10.3181/00379727-120-30531. [DOI] [PubMed] [Google Scholar]
- POLLACK W., HAGER H. J., RECKEL R., TOREN D. A., SINGHER H. A STUDY OF THE FORCES INVOLVED IN THE SECOND STAGE OF HEMAGGLUTINATION. Transfusion. 1965 Mar-Apr;5:158–183. doi: 10.1111/j.1537-2995.1965.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Pasanen V. J., Mäkelä O. Effect of the number of haptens coupled to each erythrocyte on haemolytic plaque formation. Immunology. 1969 Mar;16(3):399–407. [PMC free article] [PubMed] [Google Scholar]
- RAPP H. J., BORSOS T. EFFECTS OF LOW IONIC STRENGTH ON IMMUNE HEMOLYSIS. J Immunol. 1963 Dec;91:826–832. [PubMed] [Google Scholar]
- ROBBINS J. B., KENNY K., SUTER E. THE ISOLATION AND BIOLOGICAL ACTIVITIES OF RABBIT GAMMA M- AND GAMMA G-ANTI-SALMONELLA TYPHIMURIUM ANTIBODIES. J Exp Med. 1965 Aug 1;122:385–402. doi: 10.1084/jem.122.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENFIELD R. E., SZYMANSKI I. O., KOCHWA S. IMMUNOCHEMICAL STUDIES OF THE RH SYSTEM. 3. QUANTITATIVE HEMAGGLUTINATION THAT IS RELATIVELY INDEPENDENT OF SOURCE OF RH ANTIGENS AND ANTIBODIES. Cold Spring Harb Symp Quant Biol. 1964;29:427–434. doi: 10.1101/sqb.1964.029.01.044. [DOI] [PubMed] [Google Scholar]
- Rega A. F., Weed R. I., Reed C. F., Berg G. G., Rothstein A. Changes in the properties of human erythrocyte membrane protein after solubilization by butanol extraction. Biochim Biophys Acta. 1967 Oct 23;147(2):297–312. doi: 10.1016/0005-2795(67)90408-4. [DOI] [PubMed] [Google Scholar]
- Small P. A., Jr, Lamm M. E. Polypeptide chain structure of rabbit immunoglobulins. I. gamma-G-immunoglobulin. Biochemistry. 1966 Jan;5(1):259–267. doi: 10.1021/bi00865a034. [DOI] [PubMed] [Google Scholar]



