Abstract
Cereal aleurone responses to gibberellic acid (GA3) include activation of synthesis of hydrolytic enzymes and acidification of the external medium. We have studied the effect of the pH of the incubation medium on the response of wheat (Triticum aestivum) aleurone cells to GA3. De-embryonated half grains show the capacity for GA3-activated medium acidification when incubation is carried out at pH 6.0 to 7.0 but not at lower pHs. In addition, the activating effect of GA3 on the expression of carboxypeptidase III and thiol protease genes is more efficient when the hormone treatment is carried out at neutral pH. In situ pH staining showed that starchy endosperm acidification takes place upon imbibition and advances from the embryo to the distal part of the grain. In situ hybridization experiments showed a similar pattern of expression of a carboxypeptidase III gene, which is up-regulated by GA3 in aleurone cells. However, aleurone gene expression precedes starchy endosperm acidification. These findings imply that in vivo GA perception by the aleurone layer takes place at neutral pH and suggest that the acidification of the starchy endosperm is regulated by GA3 in germinated wheat grains.
During the development of cereal grain endosperm cells differentiate into two different tissues, the starchy endosperm and the aleurone layer. Although they share a common origin, only the aleurone cells remain alive in the mature grain, whereas the starchy endosperm cells accumulate storage compounds (predominantly starch and proteins) and are not alive in the mature grain (Fincher, 1989).
Following germination, the storage nutrients accumulated in the starchy endosperm are mobilized to provide nutrients for the growing seedling. This degradative process is carried out by hydrolytic enzymes (amylases, proteases, glucanases, and others) that are synthesized and secreted by the aleurone layer. GAs that diffuse from the embryo to the starchy endosperm (Appleford and Lenton, 1997) play an important activating role in the production of hydrolytic enzymes (Fincher, 1989; Jacobsen et al., 1995; Bethke et al., 1997; Bewley, 1997). An acidic pH (4.9–5.1) is maintained in the starchy endosperm of germinated barley grains (Mikola and Virtanen, 1980). The degradation that takes place in the starchy endosperm of germinating cereal grains requires an acidic pH for several reasons. Proteases participating in the mobilization of storage proteins show optimal activity at acid pH (Jacobsen and Varner, 1967; Mikola and Mikola, 1980), the solubilization of storage compounds of the starchy endosperm is also favored at acidic pH (Hamabata et al., 1988), which facilitates the dissociation of endogenous inhibitors from their respective hydrolases (Halayko et al., 1986). When the hydrolases have produced sugars, peptides, and amino acids from starch and storage proteins, the transport of these nutrients to the scutellum requires an acidic pH (Sopanen et al., 1980; Salmekalio and Sopanen, 1989; Hardy and Payne, 1991).
In the barley grain an accumulation of malic acid at late stages of grain development is thought to be responsible for starchy endosperm acidification (Macnicol and Jacobsen, 1992). However, in wheat (Triticum aestivum) grains no acidification has been observed during grain development (Jacobsen et al., 1995). In previous reports we described the content of proteolytic enzymes in both developing and germinating wheat grains (Domínguez and Cejudo, 1995, 1996). Surprisingly, we found a similar amount of neutral and acid proteases in the starchy endosperm of germinating grains, which suggests the coexistence of neutral and acidic compartments in the starchy endosperm of wheat grains following germination.
After Mikola and Virtanen (1980) found that isolated aleurone layers from germinated barley grains could acidify the external medium, several reports described differing effects of the hormones GA3 and ABA on this capacity of the aleurone layer. Drozdowicz and Jones (1995) found an activating effect of GA3 on the acidification capacity of dissected barley aleurone layers; however, Heimovaara-Dijkstra et al. (1994) found no activating effect of GA3 on barley aleurone protoplasts. Hamabata et al. (1988) found that dissected wheat aleurone layers acidified the external medium, but acidification was not affected by the presence of GA3. Sinjorgo et al. (1993) found that a reduction of medium pH enhanced GA3 response of isolated barley aleurone layers.
In this study we analyzed the effect of the pH of the incubation medium on the response of wheat aleurone cells to GA3. Our results show a more efficient response at neutral than at acidic pH. In agreement with this result, in situ pH staining of the starchy endosperm and in situ hybridization of a carboxypeptidase III gene showed that in vivo perception of GA by the aleurone cells takes place at neutral pH in germinated grains.
MATERIALS AND METHODS
Plant Material
Wheat (Triticum aestivum cv Chinese Spring) grains or de-embryonated half grains were sterilized in 2% (v/v) NaOCl for 20 min and washed twice with sterile water, once with 0.01 m HCl, and then thoroughly again with sterile distilled water. Sterile grains were allowed to germinate at room temperature on sterile filter paper soaked with water for as long as 6 d. Hormone treatments were carried out on sterile de-embryonated half grains that were incubated on filter paper soaked with different buffers: 20 mm sodium acetate or 20 mm citrate phosphate, pH 5.0 and 5.5, 20 mm sodium phosphate, pH 6.0, 6.5, and 7.0, or 20 mm Mops-KOH, pH 7.0. All buffers were supplemented with 10 mm CaCl2. When required, incubation medium was supplemented with 5 μm GA3, 25 μm ABA, or both. Hormone treatments were carried out in the dark at 25°C. GA3 and ABA were purchased from Sigma.
pH and Titratable Acidity
The pH of the medium in which de-embryonated half grains had been incubated was measured using a pH meter (model 071, Beckman). Titratable acidity was measured after a sample (0.5 mL) of the incubation medium was diluted 1:5 with distilled water and titrated with 0.1 n NaOH to pH 7.0 as previously reported by Drozdowicz and Jones (1995).
In Situ pH Staining and in Situ Hybridization
Wheat grains were allowed to soak for up to 6 d. After the shoots and roots were cut, grains were longitudinally dissected with a razor blade. Dissected grains were then incubated for 15 min at room temperature in a solution containing 0.1 g of the pH indicator Bromcresol purple (Sigma) in 250 mL of water and 37 μL of 5 n NaOH. Dissected grains were then washed in water for 2 min, observed under a stereoscopic microscope, and photographed in dark field.
In situ hybridization was carried out as previously reported (Cejudo et al., 1992b). Wheat grains that had been soaked for 14, 24, or 48 h were longitudinally dissected, and after the starchy endosperm was removed they were immediately fixed in FAE (50% ethanol, 5% acetic acid, and 3.7% formaldehyde) with an occasional vacuum. After dehydration tissues were embedded in Paraplast Plus (Sigma). Sections (10 μm thick) were hybridized with 35S-labeled RNA. Riboprobes for in situ hybridization were labeled with uridine 5′-[α-thio][35S]triphosphate (Amersham). 2437 cDNA (Baulcombe et al., 1987) subcloned in pBluescriptII KS was amplified by PCR using standard M13 reverse and forward primers. About 1 μg of the PCR product was used as the template to synthesize 35S-labeled RNA with T7 RNA polymerase (antisense probe) or T3 RNA polymerase (sense probe). After the samples were hybridized and washed, slides were coated with a Hypercoat LM-1 nuclear emulsion (Amersham) and exposed at 4°C for 5 to 7 d before development.
Northern-Blot Hybridization
Total RNA was extracted from liquid nitrogen-frozen tissue (Baulcombe and Buffard, 1983), fractionated on agarose-formaldehyde gels, and blotted onto Hybond-N filters (Amersham) according to the manufacturer's instructions. Hybridization was performed as described by Sambrook et al. (1989) with 32P-labeled probes. RNA loading was checked by hybridization of filters with 32P-labeled radish 18S rRNA (Grellet et al., 1989).
RESULTS
Effect of GA3 and External pH on Medium Acidification and Gene Expression by De-Embryonated Wheat Grains
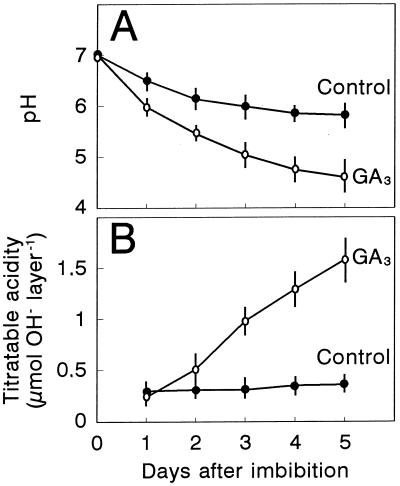
To test whether aleurone cells are responsible for starchy endosperm acidification and to evaluate the role that GA plays in this process, we studied the effect of GA3 treatment on incubation medium acidification by de-embryonated half grains that were incubated at different initial pHs. Table I shows that de-embryonated half grains incubated for 5 d at an initial pH of 6.0 to 7.0 significantly lowered the pH of the external medium, and the acidification was enhanced by GA3 treatment (up to 1 pH unit at an initial pH of 7.0). No acidification was observed at an initial pH of 5.0 to 5.5 independently of the presence of the hormone. The trend of incubation medium acidification with time was studied when the initial pH was 7.0 by measuring either the variation of pH in the external medium (Fig. 1A) or the titratable acidity (Fig. 1B). GA3 treatment hastened acidification, which was clearly observed after the 1st d of treatment and continued during 5 d. These results confirm the activating effect of GA3 on the acidification capacity of wheat aleurone cells. The fact that this activating effect of GA3 was favored as pH approached neutrality and did not take place at pH 5.0 to 5.5 shows that this GA3 response of the aleurone layer is pH dependent.
Table I.
Effect of initial pH and GA3 treatment on incubation medium acidification by de-embryonated wheat grains
| Initial pH | Final pH
|
ΔpHa | |
|---|---|---|---|
| −GA3 | +GA3 | ||
| 5.0 | 5.49 ± 0.23 | 5.44 ± 0.23 | 0.05 |
| 5.5 | 6.07 ± 0.06 | 5.97 ± 0.08 | 0.10 |
| 6.0 | 5.17 ± 0.20 | 4.62 ± 0.30 | 0.55 |
| 6.5 | 5.55 ± 0.15 | 4.89 ± 0.29 | 0.66 |
| 7.0 | 5.47 ± 0.32 | 4.42 ± 0.17 | 1.05 |
De-embryonated half grains were incubated for 5 d at 25°C in the dark on filter paper soaked with either of the following buffers: 20 mm sodium acetate, pH 5.0 or 5.5, or 20 mm sodium phosphate, pH 6.0, 6.5, or 7.0. All buffers were supplemented with 10 mm CaCl2. GA3 was added at 5 μm final concentration.
ΔpH is pH (−GA3) − pH (+GA3).
Figure 1.
Effect of GA3 treatment on external medium acidification by de-embryonated half grains. Wheat de-embryonated half grains were incubated on filter paper soaked with 20 mm sodium phosphate, pH 7.0, containing 10 mm CaCl2 and supplemented (○) or not (•) with 5 μm GA3. At the indicated days after imbibition acidification was recorded by measuring the pH of the external medium (A) or titratable acidity (B). Results are means ± se of three independent determinations.
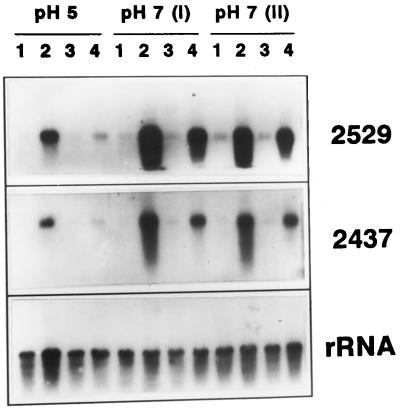
The influence of the initial pH of the incubation medium on GA3 activation of gene expression was also analyzed. De-embryonated half grains were incubated for 2 d in the presence of different hormones (GA3, ABA, or GA3 plus ABA) at an initial pH of 5.0 and 7.0, and the accumulation of transcripts of two GA-regulated genes encoding proteolytic enzymes, a thiol protease- (2529 cDNA; Cejudo et al., 1992b), and a carboxypeptidase II- (2437 cDNA; Baulcombe et al., 1987), was studied by northern-blot hybridization. A greater accumulation of both transcripts occurred when GA3 treatment was carried out at pH 7.0, using either phosphate buffer (I) or Mops buffer (II; Fig. 2). ABA treatment, which itself did not show any positive effect on the level of transcript accumulation, did counteract the activating effect of GA3 at both pHs, although this effect was more evident at pH 5.0, when the level of transcript accumulation was lower.
Figure 2.
Effect of external pH on hormonal regulation of carboxypeptidase III (2437 cDNA) and thiol protease (2529 cDNA) transcript accumulation. Wheat de-embryonated half grains were incubated for 2 d on filter paper soaked with 20 mm sodium acetate, pH 5.0; 20 mm sodium phosphate, pH 7.0 (I); or 20 mm Mops-NaOH, pH 7.0 (II), supplemented with 10 mm CaCl2. Hormone treatments were as follows: lanes 1, no hormone; lanes 2, 5 μm GA3; lanes 3, 25 μm ABA; lanes 4, 5 μm GA3 plus 25 μm ABA. RNA samples (10 μg) were electrophoresed, blotted onto Hybond-N filters, and hybridized with 32P-labeled 2529 cDNA (thiol protease) or 2437 cDNA (carboxypeptidase III). Even loading was checked by rehybridizing filters with 32P-labeled 18S rRNA.
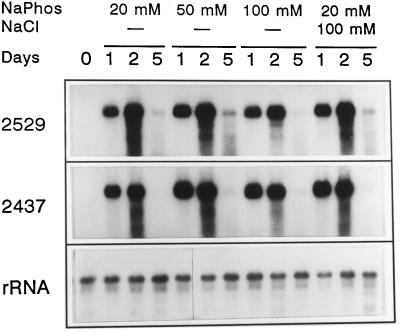
Since GA3 treatment of de-embryonated grains at neutral pH promotes acidification of the incubation medium (Fig. 1), we tested whether this GA3-promoted acidification had any effect on GA3 activation of gene expression. For this purpose the buffer concentration of the incubation medium was increased to 100 mm sodium phosphate and transcript accumulation after 1, 2, and 5 d of treatment with GA3 was analyzed by northern-blot hybridization (Fig. 3). GA3 treatment of de-embryonated grains for 5 d in 100 mm sodium phosphate lowered the external pH from 7.0 to 6.3; in contrast, when treatment was carried out in 20 mm sodium phosphate (pH 7.0), the pH of the incubation medium decreased to 4.4 (Table I). In spite of this variation of the incubation medium pH, GA3 treatment promoted a clear activation of transcript accumulation in all of the conditions analyzed (Fig. 3, lanes 1 and 2). To test for a possible effect of the high ionic strength on the response of aleurone cells to GA3, hormone treatment was also carried out in the presence of 20 mm sodium phosphate (pH 7.0) supplemented with 100 mm NaCl. A similar level of transcript accumulation in response to GA3 was observed in these conditions (Fig. 3). It is worth mentioning that after 5 d of GA3 treatment the mRNA of both genes analyzed was almost undetectable in all of the conditions tested (Fig. 3, lanes 5). This result suggests that regulation of protease gene expression in aleurone cells may also involve mRNA stability.
Figure 3.
Effect of buffer concentration on GA3 activation of carboxypeptidase III (2437 cDNA) and thiol protease (2529 cDNA) transcript accumulation at neutral pH. De-embryonated half grains were incubated for 0, 1, 2, or 5 d on filter paper soaked with sodium phosphate buffer (NaPhos) at the indicated concentration and pH 7.0, supplemented with 10 mm CaCl2 and 5 μm GA3. RNA samples (10 μg) were loaded, electrophoresed, blotted onto Hybond-N filters, and hybridized with 32P-labeled 2529 cDNA (thiol protease) or 2437 cDNA (carboxypeptidase III). Even loading was checked by rehybridizing filters with 32P-labeled 18S rRNA.
Pattern of Starchy Endosperm Acidification in Wheat Grains following Germination
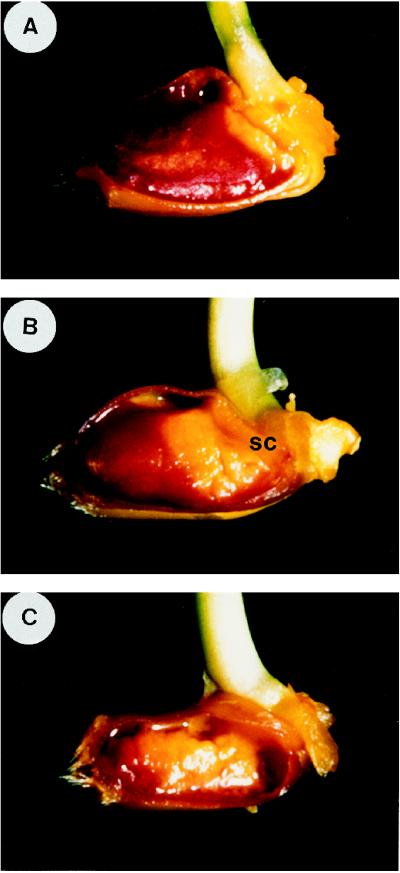
The observation that the pH of the incubation medium influences the response of wheat aleurone cells to GA3 prompted us to study in situ the acidification of the starchy endosperm in germinated grains. A tissue stain was devised using the pH indicator Bromcresol purple to study in situ the variation of pH in the starchy endosperm. This pH indicator shows brick red at pH 6.8 or above and yellow at pH 5.2 or below, permitting distinction between neutral and acidic pH. Figure 4 shows the pattern of acidification of the starchy endosperm of wheat grains following germination. In grains that had been soaked for 3 d, most of the starchy endosperm appeared brick red, indicating neutral pH. A yellow stain was observed, however, in the area adjacent to the embryo, which is indicative of acidic pH (Fig. 4A). Four days after imbibition, the acidity advanced progressively toward the distal part of the grain (Fig. 4B); by 6 d after imbibition, most of the starchy endosperm showed acidic pH and was clearly degraded (Fig. 4C). From these results it may be concluded that the pH of the starchy endosperm is neutral in the mature wheat grain and that acidification takes place during germination and seedling growth.
Figure 4.
pH staining of the starchy endosperm of wheat grains following germination. Wheat grains that were soaked for 3 d (A), 4 d (B), or 6 d (C) were longitudinally sectioned and stained with the pH indicator Bromcresol purple for 15 min. A yellow color corresponds to pH 5.2 or below and a brick red color corresponds to pH 6.8 or above. sc, Scutellum.
Pattern of Expression of a Carboxypeptidase III Gene in Germinating Grains
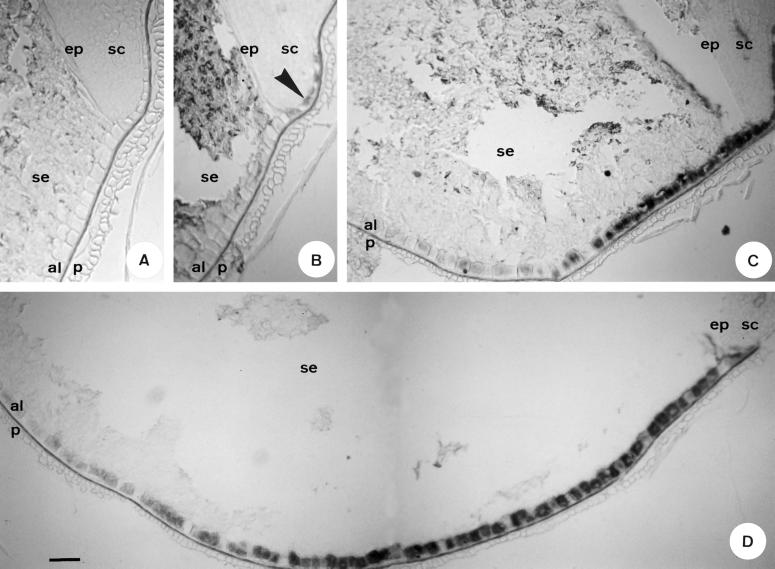
Based on the fact that the expression of genes encoding hydrolytic enzymes in aleurone cells is activated by GA3 treatment, we used one of these genes, encoding a carboxypeptidase III (Baulcombe et al., 1987), as a sensor of in vivo GA perception by the aleurone cells following grain germination. The pattern of expression of this gene was studied by in situ hybridization. When in situ hybridization was carried out on sections of grains after 14 h of imbibition with the 35S-labeled antisense probe, expression was restricted to aleurone cells adjacent to the scutellum (Fig. 5B, arrowhead). In grains that had been soaked for 24 h (Fig. 5C) or 48 h (Fig. 5D), it was observed that, as germination proceeded, carboxypeptidase III expression in the aleurone layer progressed toward the distal part of the grain. No signal above background was detected in control sections hybridized with the 35S-labeled sense probe (Fig. 5A), i.e. starchy endosperm acidification and gene expression show a similar pattern advancing from the embryo to the distal part of the grain. However, 2 d after imbibition, when most of the starchy endosperm showed neutral pH, the expression of the carboxypeptidase III gene had progressed significantly toward the distal part of the grain (Fig. 5D). Although it must be taken into account that the pH staining of the starchy endosperm does not allow us to precisely determine the environmental pH at the plasma membrane of aleurone cells, this result shows that in vivo GA perception by the aleurone cells takes place when the pH of the surrounding starchy endosperm is still neutral. This finding is in agreement with the above-mentioned results showing a better response of aleurone cells to GA3 at neutral than at acidic pH.
Figure 5.
Histological localization of carboxypeptidase III mRNA (2437 cDNA) in germinating wheat grains. In situ hybridizations were carried out with sense (A) or antisense 35S-labeled riboprobes on longitudinal sections (10 μm thick) of grains that had been soaked for 14 h (B), 24 h (C), or 48 h (D). al, Aleurone layer; ep, epithelium; p, pericarp; sc, scutellum, se, starchy endosperm. Bar corresponds to 100 μm.
DISCUSSION
The results presented in this report show a clear effect of the pH of the incubation medium on the response of wheat aleurone cells to GA3. The two aleurone responses to GA3 that we analyzed, incubation medium acidification and protease transcript accumulation, are favored at neutral pH. In agreement with these results, the comparison of the spatio-temporal pattern of starchy endosperm acidification (Fig. 4) and carboxypeptidase III expression (Fig. 5) allows us to conclude that in vivo GA perception by the aleurone cells takes place when the pH of the surrounding starchy endosperm is neutral. This result was unexpected, since it has been described that the starchy endosperm of postgerminative cereal grains is maintained at acidic pH (Briggs, 1968; Mikola and Virtanen, 1980). In fact, based on this observation, most studies of GA3 regulation of gene expression in wheat aleurone cells were carried out at pH 5.2 (Baulcombe and Buffard, 1983; Huttly and Baulcombe, 1989; Cejudo et al., 1992a, 1992b).
GAs are weak hydrophobic acids with pKa values of 4.0 to 4.2 (Hooley, 1994); therefore, at acidic pH an increased passive entrance to the aleurone cells, as undissociated molecules, would be expected. There is consistent evidence that the site of GA perception by the aleurone cells is the plasma membrane and not a receptor inside the cells (Hooley et al., 1991; Gilroy and Jones, 1994). Therefore, it would be expected that neutral pH, which increases the dissociated form of GA and which is unable to passively enter the aleurone cell membrane, is more efficient in promoting the aleurone response than acidic pH. This view is supported by the results reported from this study.
It appears that wheat and barley grains behave differently with regard to starchy endosperm acidification. During late stages of barley grain development, the starchy endosperm is acidified by the accumulation of malic acid (Macnicol and Jacobsen, 1992). This is not the case in the wheat grain, as shown by the pH-staining experiments described in this report (Fig. 4). In addition, the in situ pH staining shows a clear pattern of starchy endosperm acidification following wheat grain germination. Initial acidification takes place in the area of the starchy endosperm adjacent to the embryo, implicating the scutellum in this process. In barley (Drozdowicz and Jones, 1995), it has been shown that isolated scutella are able to acidify the external medium because of the release of citric and succinic acids. We carried out a study of the expression and localization of PEP carboxylase in germinating wheat grains. Immunolocalization experiments showed an increase of PEP carboxylase in the scutellar epithelium of grains 24 h after imbibition (González et al., 1998) that might account for the organic acid produced by the scutellum at early stages of germination and, therefore, explain the acidification taking place near the embryo.
The acidification of the starchy endosperm and the expression of carboxypeptidase III following germination show a similar pattern. In agreement with Mikola and Virtanen (1980) and Drozdowicz and Jones (1995), we showed that the capacity of aleurone cells to acidify the external medium is enhanced by GA3 (Table I; Fig. 1). These results suggest that the progression of acidification toward the distal part of the grain involves aleurone cells and is a process regulated by GA. Treatment of isolated barley aleurone layers with GA3 results in accumulation of phosphoric and malic acid in the external medium (Drozdowicz and Jones, 1995), which has been proposed to be the cause of acidification. We tested the possibility that PEP carboxylase activity of aleurone cells might be involved in acidification. Although these cells contain appreciable amounts of PEP carboxylase (González et al., 1998), no effect of GA3 was observed on its expression (M.C. González and F.J. Cejudo, unpublished results), suggesting that this enzyme is not involved in the acidification capacity of the aleurone cells.
Upon wheat grain imbibition, GA1 and GA3, which are synthesized in the scutellum and diffuse to the starchy endosperm (Appleford and Lenton, 1997), promote the metabolic activation of aleurone cells through a complex signaling cascade that includes Ca2+, internal pH, cGMP, and calmodulin as signaling molecules (for review, see Bethke et al., 1997). This metabolic activation includes the synthesis and secretion of hydrolytic enzymes and the acidification of the starchy endosperm, which is essential for storage compound degradation since most hydrolytic enzymes show maximal activity at acidic pH.
The different processes activated by GA in aleurone cells after grain imbibition must be perfectly ordered and coordinated in time (Bethke et al., 1997). To explain the pattern of starchy endosperm acidification and carboxypeptidase III expression in germinated wheat grains, we propose that the response of any single aleurone cell to GA depends on its location in the wheat grain: aleurone cells proximal to the embryo are the first to perceive GA and activate the synthesis of hydrolytic enzymes and acidification of the surrounding starchy endosperm. As GAs diffuse from the embryo (Appleford and Lenton, 1997), they are perceived by aleurone cells located in the more distal part of the grain, and acidification and production of hydrolases is extended to this part of the grain.
ACKNOWLEDGMENTS
The photographic work of M.J. Cubas and Dr. J. García, who prepared Figure 4, is appreciated. We thank SENASA for providing cv Chinese Spring grains.
Footnotes
This work was supported by grant BIO97-1205 from Comision Interministerial de Ciencia y Tecnologia, Ministerio de Educación y Cultura, and grant no. CVI 118 from Junta de Andalucía (Spain).
LITERATURE CITED
- Appleford NEJ, Lenton JR. Hormonal regulation of α-amylase gene expression in germinating wheat (Triticum aestivum) grains. Physiol Plant. 1997;100:534–542. [Google Scholar]
- Baulcombe DC, Barker RF, Jarvis MG. A gibberellin responsive wheat gene has homology to yeast carboxypeptidase Y. J Biol Chem. 1987;262:13726–13735. [PubMed] [Google Scholar]
- Baulcombe DC, Buffard D. Gibberellic-acid-regulated expression of α-amylase and six other genes in wheat aleurone layers. Planta. 1983;157:493–501. doi: 10.1007/BF00396879. [DOI] [PubMed] [Google Scholar]
- Bethke PC, Schurink R, Jones RL. Hormonal signalling in cereal aleurone. J Exp Bot. 1997;48:1337–1356. [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs DE. α-Amylase in germinating decorticated barley. I. α-Amylase, conditions of growth, and grain constituents. Phytochemistry. 1968;7:513–529. [Google Scholar]
- Cejudo FJ, Ghose TK, Stabel P, Baulcombe DC. Analysis of the promoter of a wheat protease gene regulated by gibberellin in oat aleurone layers. Plant Mol Biol. 1992a;20:849–856. doi: 10.1007/BF00027156. [DOI] [PubMed] [Google Scholar]
- Cejudo FJ, Murphy G, Chinoy C, Baulcombe DC. A gibberellin-regulated gene from wheat with sequence homology to cathepsin B of mammalian cells. Plant J. 1992b;2:937–948. [PubMed] [Google Scholar]
- Domínguez F, Cejudo FJ. Pattern of endoproteolysis following wheat grain germination. Physiol Plant. 1995;95:253–259. [Google Scholar]
- Domínguez F, Cejudo FJ. Characterization of endoproteases appearing during wheat grain development. Plant Physiol. 1996;112:1211–1217. doi: 10.1104/pp.112.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drozdowicz YM, Jones RL. Hormonal regulation of organic and phosphoric acid release by barley aleurone layers and scutella. Plant Physiol. 1995;108:769–776. doi: 10.1104/pp.108.2.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fincher GB. Molecular and cellular biology associated with endosperm mobilization in germinating cereal grains. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:305–346. [Google Scholar]
- Gilroy S, Jones RL. Perception of gibberellin and abscisic acid at the external face of the plasma membrane of barley (Hordeum vulgare L) aleurone protoplasts. Plant Physiol. 1994;104:1185–1192. doi: 10.1104/pp.104.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González MC, Osuna L, Echevarría C, Vidal J, Cejudo FJ. Expression and localization of phosphoenolpyruvate carboxylase in developing and germinating wheat grains. Plant Physiol. 1998;116:1249–1258. doi: 10.1104/pp.116.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellet F, Delcasso-Tremousaygue D, Delseny M. Isolation and characterization of unusual repeated sequence from the ribosomal intergenic spacer of the crucifer Sisimbrium irio. Plant Mol Biol. 1989;12:695–706. doi: 10.1007/BF00044160. [DOI] [PubMed] [Google Scholar]
- Halayko AJ, Hill RD, Svensson B. Characterization of the interaction of barley α-amylase II with an endogenous α-amylase inhibitor from barley kernels. Biochim Biophys Acta. 1986;873:92–101. [Google Scholar]
- Hamabata A, García-Maya M, Romero T, Bernal-Lugo Y. Kinetics of the acidification capacity of aleurone layer and its effect upon solubilization of reserve substances from starchy endosperm of wheat. Plant Physiol. 1988;86:643–644. doi: 10.1104/pp.86.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Payne JW. Analysis of the peptide carrier in the scutellum of barley embryos by photoaffinity labelling. Planta. 1991;186:44–51. doi: 10.1007/BF00201496. [DOI] [PubMed] [Google Scholar]
- Heimovaara-Dijkstra S, Heistek JC, Wang M. Counteractive effects of ABA and GA3 on extracellular and intracellular pH and malate in barley aleurone. Plant Physiol. 1994;106:359–365. doi: 10.1104/pp.106.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley R. Gibberellins: perception, transduction and responses. Plant Mol Biol. 1994;26:1529–1555. doi: 10.1007/BF00016489. [DOI] [PubMed] [Google Scholar]
- Hooley R, Beale MH, Smith SJ. Gibberellin perception at the plasma membrane of Avena fatua aleurone protoplast. Planta. 1991;183:274–280. doi: 10.1007/BF00197799. [DOI] [PubMed] [Google Scholar]
- Huttly A, Baulcombe DC. A wheat α-Amy2 promoter is regulated by gibberellin in transformed oat aleurone protoplasts. EMBO J. 1989;8:1907–1913. doi: 10.1002/j.1460-2075.1989.tb03593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JV, Gubler F, Chandler PM. Gibberellin action in germinated cereal grains. In: Davis PJ, editor. Plant Hormones. Physiology, Biochemistry and Molecular Biology. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 246–271. [Google Scholar]
- Jacobsen JV, Varner JE. Gibberellic acid induced synthesis of proteases by isolated aleurone layers of barley. Plant Physiol. 1967;42:1596–1600. doi: 10.1104/pp.42.11.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnicol PK, Jacobsen JV. Endosperm acidification and related metabolic changes in the developing barley grain. Plant Physiol. 1992;98:1098–1104. doi: 10.1104/pp.98.3.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikola L, Mikola J. Mobilization of proline in the starchy endosperm of germinating barley grain. Planta. 1980;149:149–154. doi: 10.1007/BF00380876. [DOI] [PubMed] [Google Scholar]
- Mikola L, Virtanen M. Secretion of l-malic acid by barley aleurone layers (abstract no. 783) Plant Physiol. 1980;65:S-142. [Google Scholar]
- Salmenkallio M, Sopanen T. Amino acid and peptide uptake in the scutella of germinating barley and wheat. Plant Physiol. 1989;89:1285–1291. doi: 10.1104/pp.89.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning. A Laboratory Manual, Ed 2. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sinjorgo KMC, de Vries MA, Heistek JC, van Zeijl MJ, van der Veen SW, Douma AC. The effect of external pH on the gibberellic acid response of barley aleurone. J Plant Physiol. 1993;142:506–509. [Google Scholar]
- Sopanen T, Uuskallio M, Nyman S, Mikola J. Characterization and development of leucine transport activity in the scutellum of germinating barley grain. Plant Physiol. 1980;65:249–253. doi: 10.1104/pp.65.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]