Abstract
Background: This study was carried out to determine whether humoral and cellular immune responses would be provoked by cutaneous administration of keyhole limpet hemocyanin (KLH) and in particular by scarification of the skin (SS). Methods: This was an unblinded, single-center, 8-week pilot study in healthy young adults. Twenty-four subjects assigned to 4 groups completed the study. Each group was immunized twice, with a 3-week interval, either by SS or intradermally (ID), with an SS dose of 50 or 250 μg and an ID dose of 100 or 250 μg. Serum was collected for antibody assays at baseline and 3 weeks after both the first and second immunizations. Delayed-type hypersensitivity (DTH) testing was performed before the first immunization and 3 weeks after the second. Results: In the 250-μg SS group, there was a significant increase from day 0 to day 47 in anti-KLH IgG (p = 0.02; day 0: 3.46 ± 5.49 mg/dl, day 47: 7.54 ± 8.87 mg/dl) and anti-KLH IgA (p = 0.04; day 0: 4.78 ± 9.15 mg/dl, day 47: 11.42 ± 13.62 mg/dl). One subject in each treatment group showed a positive DTH test result representing 20% (50-μg SS), 10% (250-μg SS), 25% (100-μg ID) and 20% (250-μg ID) of the subjects. Conclusions: It was possible to induce both humoral and cellular immune responses by SS administration despite the limited antigenic potency of the low-molecular-weight KLH preparation. This approach may be useful for studying the mechanisms of immune response in allergic skin diseases such as atopic dermatitis.
Key Words: Keyhole limpet hemocyanin, Atopic dermatitis/eczema, Immune response, Skin scarification, Vaccine
Introduction
A main objective of this pilot study was to determine whether the administration of a protein antigen by skin scarification (SS) would induce immune responses in healthy subjects. SS has been used for centuries to immunize against smallpox with a live vaccine, but an inactivated smallpox vaccine given to unimmunized human subjects was shown ineffective following SS administration [1]. Cutaneous dendritic cells (DC) and Langerhans cells residing in the skin are highly efficient antigen-presenting cells. The DC system applies the recognition repertoire of T cells that number in billions, all with distinct randomly arranged antigen receptors. DC are being considered in the design of vaccines to deliver antigens to specific receptors [2, 3]. For example, the CD205 receptor, abundant on DC in human lymphoid tissues, delivers antigen for processing onto both MHC class I and class II, increasing presentation efficiency more than 100-fold [4]. DC induce T cell-mediated immune responses and enhance antibody production by B cells through CD4+ T cell modulation [2, 3]. Recent studies have targeted these cells to enhance the immunogenicity of vaccines [5]. Liu et al. [6] studied mice immunized with live vaccinia virus delivered by SS. They reported that immunization of mice by SS with attenuated vaccinia virus generated more interferon gamma-producing CD8+ T cells, and increased humoral responses compared to immunization by subcutaneous, intradermal (ID), or intramuscular (IM) injection. Kenney et al. [7] and Belshe et al. [8] found that in human volunteers ID administration of a fraction of the standard dose of inactivated influenza virus vaccine elicited immune responses similar or superior to those of a full dose given by IM administration. Our current pilot study, conducted with the support of the NIH/NIAID-funded Atopic Dermatitis Vaccinia Network, examined immune responses to keyhole limpet hemocyanin (KLH), a novel (i.e. previously never encountered) protein antigen for human subjects. A main objective of this study was to determine whether the administration of a protein antigen by SS would induce immune responses in healthy subjects. The SS and ID routes of KLH administration in human subjects were compared for the potency of their immune response.
Subjects and Methods
Subjects
Healthy, nonatopic adult volunteers aged 18–40 years with negative delayed-type hypersensitivity (DTH) tests to KLH were randomized to 1 of 4 treatment groups: low dose by SS (50 μg, n = 5), high dose by SS (250 μg, n = 10), low dose by ID (100 μg, n = 5) and high dose by ID (250 μg, n = 5). For SS, Vacmune® (20 mg/ml) was administered using a bifurcated needle (each needle calibrated to deliver 0.0025 ml; 3 jabs with 1 needle for the 50-μg dose and 15 jabs with 5 needles for the 250-μg dose) [9]. The needles were examined under a magnifying glass after loading to confirm that they were full and after use to confirm that they were empty. Immucothel® was used for ID injections. All subjects were immunized twice, 21 days apart (i.e. on days 5 and 26). Written informed consent was obtained from all subjects with approval from the National Jewish Health Institutional Review Board.
KLH Production
Low molecular weight KLH was obtained from Biosyn Corp., Carlsbad, Calif., USA. It consists of two immunologically distinct subunits, both composed of eight domains. The molecular weight of both subunits is 400 kDa [10]. Two purified and standardized clinical grade KLH preparations were used. The manufacturer provided assurance that the two preparations were antigenically identical. Vacmune, a solubilized preparation, was only available in 20-mg quantities, while lyophilized Immucothel was available in more convenient and more cost-effective 1- and 10-mg quantities. Immucothel was used for ID immunization and DTH testing, and Vacmune was used only for SS. The high concentration of Vacmune (20 mg/ml) enabled us to administer 50- and 250-μg doses with 3 and 15 jabs with bifurcated needles by methods identical to those used for smallpox vaccination [9]. To preserve antigenicity all KLH was used on the day that the vials were opened.
Anti-KLH Antibody Assays
Serum anti-KLH IgG, IgM and IgA were measured by ELISA at baseline (day 0), day 26 and day 47 [11]. Ninety-six-well microtiter plates (Nunc, Rochester, N.Y., USA) were coated with 1 μg of KLH (Biosyn) in 100 μl of carbonate-coating buffer per well and incubated for 2 h at 37°C, then overnight at 4°C. Plates were washed 4 times with phosphate-buffered saline-Tween 20 (PBS-T) and blocked with 1% dry skim milk in PBS. Plates were washed 4 more times with PBS-T and 100 μl of diluted serum (1:50 dilution) was added. Plates were incubated for 45 min and washed 6 more times with PBS-T. One hundred microliters of horseradish peroxidase-conjugated anti-human IgG (DAKO, Carpinteria, Calif., USA), anti-human IgM (DAKO) or anti-human IgA (Invitrogen) was added to the appropriate plates at a concentration of 1:500 in 1% dry skim milk in PBS-T and incubated for 45 min. Plates were washed 6 more times with PBS-T, and 100 μl of tetramethylbenzidine substrate solution (SurModics, Eden Prairie, Minn., USA) was added to all wells. Plates were incubated for 5–20 min and read at an optical density of 450 nm with a reference wavelength of 550 nm [11].
DTH Tests
DTH is an in vivo assay of cell-mediated immune function. DTH reactions occur in two phases – the sensitization phase, when the antigen is introduced into the skin, and the challenge phase or ID skin testing that typically follows 4–6 weeks later. The presence of the DTH response is shown by redness and induration of the skin. The immune response is assessed by measuring the diameter of the induration 48–72 h after the injection of the antigen [12]. Aliquots of 0, 1, 5 and 10 μg KLH in 0.10 ml normal saline were administered on the volar aspects of both arms at baseline (day 0) and 3 weeks after the second immunization (day 47). The diameters of induration were measured at 1, 2, 3 and 5 days after administration. Subjects were only eligible for participation in the study if they had had a negative DTH test result at baseline. After the second immunization, a DTH test was considered positive if the measured diameter of induration was at least 5 mm.
Statistics
Differences in the baseline characteristics among groups were compared using a χ2 test for the categorical measures (gender, race, ethnicity) and an ANOVA test for continuous measures (age). Adverse events were collected throughout the study and summarized for each treatment group. Individual subject antibody responses at day 0 and day 47, including anti-KLH IgG, IgG1–3, IgM and IgA, were log10 transformed and summarized for each treatment group. A value of 0.1 was added to all values in order to facilitate the log transformation of zero values. Changes in log-transformed antibody levels from baseline to day 47 were compared to zero using a signed-rank test and compared between treatment groups using a Wilcoxon test. The number and percent of subjects who had a doubling of their anti-KLH antibodies were summarized for each treatment group. Only subjects who completed the study were included in the analysis of immune response due to the need for efficacy measurements after the second vaccination.
Results
Subjects
Of the 26 subjects who consented and were eligible, 25 were randomized to 1 of the 4 treatments and 24 completed the study. One subject (100-μg ID group) dropped out due to an adverse event unrelated to the study. The ages ranged from 22 to 36 years (mean ± SD 29 ± 3.84). Fifty percent of the subjects were male, 96% self-identified as white race and 12% self-identified as Hispanic or Latino. Overall tests of baseline characteristics, including gender, age, race and ethnicity showed no significant differences among the 4 treatment groups. Four subjects (2 in each ID group) reported a total of 5 adverse events. The adverse events consisted of: 1 urinary tract infection, 2 rashes, 1 swelling at the injection site and 1 instance of pruritus. The swelling and pruritus were the only events determined to be related to the intervention and none of the events was considered serious. Using the Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventative Vaccine Clinical Trials guidelines [13], 8 additional subjects experienced 13 abnormal vital or laboratory measurements [abnormal blood pressure readings (n = 3), abnormal neutrophil counts (n = 3), abnormal heart rate (n = 1) and abnormal respiration readings (n = 1)].
Anti-KLH Antibody Assays
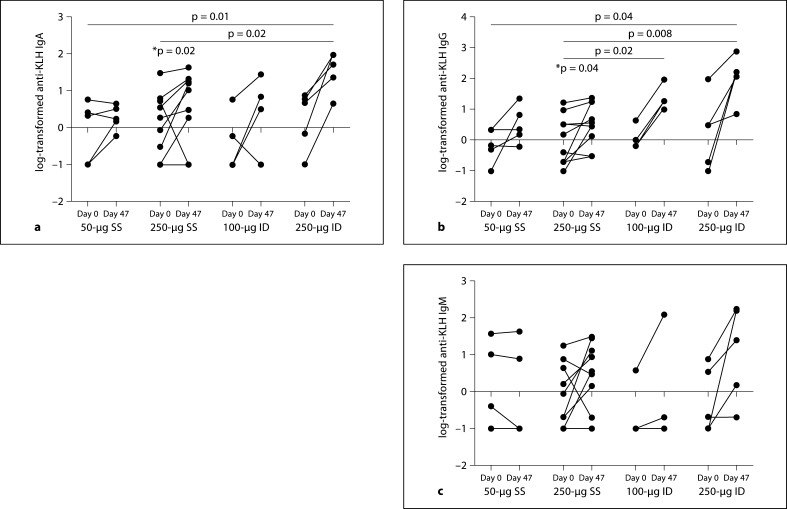
Individual subject log-transformed anti-KLH IgA, IgG and IgM at baseline and at day 47 are summarized in figure 1. There was a significant increase from day 0 to day 47 in anti-KLH IgG (p = 0.02; day 0: 3.46 ± 5.49 mg/dl, day 47: 7.54 ± 8.87 mg/dl) and anti-KLH IgA (p = 0.04; day 0: 4.78 ± 9.15 mg/dl, day 47: 11.42 ± 13.62 mg/dl) for the 250-μg SS group. No significant increases from baseline were observed for the other 3 groups (50-μg SS, 100-μg ID, 250-μg ID); however, power was limited due to the small sample size per group.
Fig. 1.
Individual subject values at baseline to day 47 in log10 anti-KLH IgA, IgG and IgM. Log10-transformed anti-KLH IgA (a), IgG (b) and IgM (c) by dose and administration group. * The 250-μg SS treatment arm had a statistically significant (p < 0.05) increase in anti-KLH IgA and IgG compared to no response (0) as determined by a signed-rank test. Bars indicate significant differences in the comparison of mean change from baseline between 2 treatment groups as determined by a Wilcoxon test. The p value associated with the comparison is included at the top of the bar. Antibody levels measured at 0 are set to −1 for the log transformation.
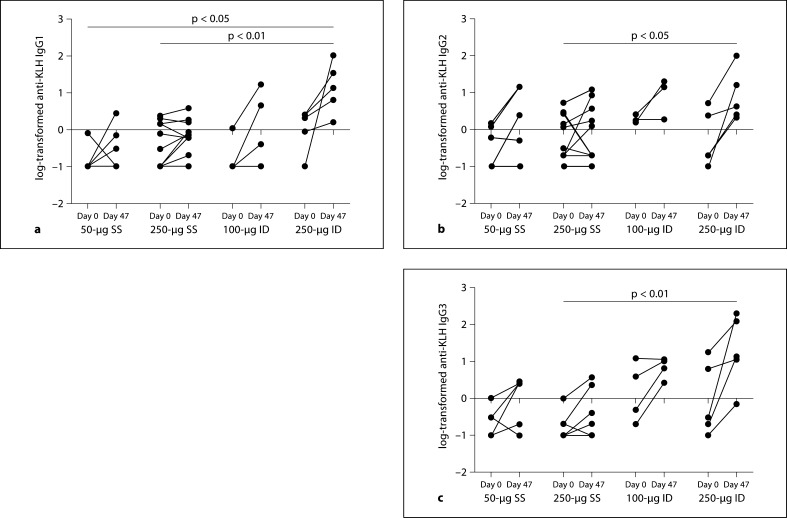
The magnitude of the increase from baseline to day 47 in the 250-μg ID group was significantly greater than the corresponding increase in anti-KLH IgA (50-μg SS: p = 0.01, 250-μg SS: p = 0.02) and in anti-KLH IgG (50-μg SS: p = 0.04, 250-μg SS: p = 0.008). Additionally, the increase in anti-KLH IgG for the 100-μg ID group was significantly greater than for the 250-μg SS group (p = 0.02). Similar to figure 1, figure 2 displays the individual responses in the anti-KLH subgroups IgG1, IgG2 and IgG3. No significant increases from baseline were observed for these antibodies, although all groups including the 2 SS groups showed a general increase from day 0 to day 47. The increase for the 250-μg ID group was significantly greater than the increase for the 250-μg SS group for anti-KLH IgG1 (p < 0.01), IgG2 (p < 0.05) and anti-KLH IgG3 (p < 0.01). The increase in IgG1 for the 250-μg ID group was also significantly greater than the increase for the 50-μg SS group (p < 0.05).
Fig. 2.
Individual subject values at baseline to day 47 in log anti-KLH IgG1, IgG2 and IgG3. Log10-transformed anti-KLH IgG1 (a), IgG2 (b) and IgG3 (c) by dose and administration group. Bars indicate significant differences in the comparison of mean change from baseline between 2 treatment groups as determined by a Wilcoxon test. The p value associated with the comparison is included at the top of the bar. Antibody levels measured at 0 are set to −1 for the log.
The percentage of subjects who doubled their anti-KLH antibody response (IgA, IgG, IgM, IgG1, IgG2 and IgG3) was similar across the SS and ID groups (table 1). None of the treatment groups exhibited an IgG4 response (data not shown).
Table 1.
Percentages (with numbers in parentheses) of subjects with a doubling in antibody from day 0 to day 47 in each treatment group
| Anti-KLH antibody | 50-μg SS (n = 5) | 250-μg SS (n = 10) | 100-μg ID (n = 4) | 250-μg ID (n = 5) |
|---|---|---|---|---|
| IgA | 40 (2) | 50 (5) | 75 (3) | 100 (5) |
| IgG | 60 (4) | 50 (5) | 100 (4) | 100 (5) |
| IgM | 0 (0) | 50 (5) | 50 (2) | 80 (4) |
| IgG1 | 60 (3) | 40 (4) | 75 (3) | 80 (4) |
| IgG2 | 60 (3) | 40 (4) | 67 (2 of 3) | 80 (4) |
| IgG3 | 80 (4) | 60 (6) | 75 (3) | 80 (4) |
DTH Tests
One subject in each treatment group showed a positive DTH test result representing 20% (50-μg SS), 10% (250-μg SS), 25% (100-μg ID) and 20% (250-μg ID) of the subjects.
Discussion
In this study, we show that low-molecular-weight KLH by ID or SS administration stimulates both humoral and cellular responses in some subjects. Ours is the first report of immune responses to scarification with a protein antigen in human subjects. We base this on an exhaustive PubMed search. Systemic immunization with KLH has been very useful for the assessment of the immune function of human subjects for many years. Our immunization strategy used doses of KLH in a range similar to earlier studies in healthy volunteers. We observed less potent immune responses to ID immunization than previously reported [14, 15, 16]. We attribute these results to the lower molecular weight and greater purity of the current clinical-grade KLH preparation. Our findings may be enhanced by the use of a more potent KLH preparation; even so, any immune signal after SS with a protein antigen such as KLH warrants further inquiry.
Hemocyanin obtained from the marine mollusk giant keyhole limpet, Megathura crenulata, has been in use as a crude or partially purified product for over 40 years [17]. Native KLH is composed of 12 subunits with a molecular weight of 9,000–10,000 kDa. Earlier studies with KLH acting as an immunogen used preparations consisting in large measure of native KLH manufactured by chemical suppliers. Intracel KLH, unavailable during our study, preserves the high-molecular-weight structure with greater than 95% purity [18]. The low-molecular-weight subunit preparation of KLH appears to be inherently less immunogenic than the high-molecular-weight native or partially purified KLH [19]. Miller et al. [19] induced potent IgM, IgG1 and IgG2 responses with high-molecular-weight Intracel KLH in healthy volunteers within 1 month of administration. When they switched to low molecular-weight Biosyn KLH, 1 mg administered subcutaneously, they were unable to induce significant humoral or cellular responses. However, when they emulsified Biosyn KLH with a synthetic incomplete Freund's adjuvant (Montanide ISA-51, Seppic Inc., Fairfield, N.J., USA), full immunogenicity was restored with 100% of the healthy volunteers developing a humoral response [19]. The fact that they were able to restore full immunogenicity with the use of an adjuvant suggests that the lack of response was not due to a loss of important epitopes, but rather that the intact high molecular-weight KLH product comprises adjuvant properties [19].
The route and the dose of the administered antigen determine the response. In a recent study, low-dose oral KLH induced an antigen-specific systemic CD4+ T cell response with a shift toward a Th2 cytokine pattern. Later parenteral immunization boosted B cell responses and shifted the cytokine pattern of KLH-specific CD4+ T cells from a Th1 towards a Th2 pattern, suggesting a novel effect of oral immunomodulation [20].
Cutaneous immunization is being explored as an alternative to conventional IM or subcutaneous vaccination [5, 6, 7, 21]. Liu et al. [6] recently immunized mice by SS with live vaccinia virus. These mice generated superior humoral responses to those immunized by conventional routes. Kenney et al. [7] and Belshe et al. [8] found that, in human volunteers, a fraction of the standard-dose-administered ID elicited immune responses similar or superior to those of a full dose of vaccine given IM. These results and ours, all using protein immunogens tested in humans, showed the best responses with ID administration [7, 8], while the live virus immunization (even if capable of only limited replication), used by Liu et al. [6] in mice, was most effective by SS.
Although this was a small pilot study, we demonstrated that it is possible to induce both humoral and cellular immune responses in some healthy subjects by either ID or SS administration of KLH. Both ID and SS administration of antigen may be useful in probing mechanisms that underlie the variability amongst human subjects in skin immune responses to different stimuli. An understanding of the range and dynamics of these responses is indispensable for the development of new therapies against cancer and autoimmune diseases. The information gained may also be used to develop new vaccines against important pathogens. Lastly, it may be possible to use immunization by SS to study the defects of immune protection in the skin.
Acknowledgements
Biotinylated subclass antibodies were kindly provided by Robert Hamilton, director of the Johns Hopkins Dermatology, Allergy and Clinical Immunology Reference Laboratory. We also thank Maureen Sandoval for her help in the preparation of the manuscript. This work was sponsored by the National Institute of Allergy and Infectious Disease, National Institutes of Health, Department of Health and Human Services, Contract N01 AI40029 and N01 AI40033 and supported in part by the Colorado Clinical Translational Science Award grant 1 UL1 RR025780 from the NCRR/NIH.
References
- 1.Giurca A, Topciu VL, Voiculescu D, Moldovan E, Plavosin L. Investigations on allergic and serological reactions following inoculation of inactivated smallpox vaccines by cutaneous scarification. Virologie. 1976;27:173–177. [PubMed] [Google Scholar]
- 2.Steinman RM, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–426. doi: 10.1038/nature06175. 27. [DOI] [PubMed] [Google Scholar]
- 4.Trumpfheller C, Finke JS, Lopez CB, Moran TM, Moltedo B, Soares H, et al. Intensified and protective CD4+ T cell immunity in mice with anti-dendritic cell HIV gag fusion antibody vaccine. J Exp Med. 2006;203:607–617. doi: 10.1084/jem.20052005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Montagne JR, Fauci AS. Intradermal influenza vaccination – can less be more? N Engl J Med. 2004;351:2330–2332. doi: 10.1056/NEJMe048314. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Zhong Q, Tian T, Dubin K, Athale SK, Kupper TS. Epidermal injury and infection during poxvirus immunization is crucial for the generation of highly protective T cell-mediated immunity. Nat Med. 2010;16:224–227. doi: 10.1038/nm.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–2301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 8.Belshe RB, Newman FK, Cannon J, Duane C, Treanor J, Van Hoecke C, Howe BJ, Dubin G. Serum antibody responses after intradermal vaccination against influenza. N Engl J Med. 2004;351:2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control Smallpox Vaccination Method, 2007. http://www.bt.cdc.gov/agent/smallpox/vaccination/vaccination-method.asp
- 10.Biosyn Corporation. Vacmune, Immucothel, 2010. http://www.biosyncorp.com/bc_downloads/vacmune.pdf (accessed July 6, 2010).
- 11.Smith TP, Kennedy SL, Fleshner M. Influence of age and physical activity on the primary in vivo antibody and T cell-mediated responses in men. J Appl Physiol. 2004;97:491–498. doi: 10.1152/japplphysiol.01404.2003. [DOI] [PubMed] [Google Scholar]
- 12.Sokal JE. Editorial: measurement of delayed skin-test responses. N Engl J Med. 1975;293:501–502. doi: 10.1056/NEJM197509042931013. [DOI] [PubMed] [Google Scholar]
- 13.USA Food and Drug Administration Draft Guidance for Industry: Toxicity Grading Scale for Healthy Adult and Adolescent Volunteers Enrolled in Preventive Vaccine Clinical Trials, April 2005.
- 14.Swanson MA, Schwartz RS. Immunosuppressive therapy: the relation between clinical response and immunologic competence. N Engl J Med. 1967;277:163–170. doi: 10.1056/NEJM196707272770401. [DOI] [PubMed] [Google Scholar]
- 15.Curtis JE, Hersh EM. The human secondary immune response to keyhole limpet haemocyanin. Clin Exp Immunol. 1972;10:171–177. [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis JE, Hersh EM, Butler WT, Rossen RD. Antigen dose in the human immune response. Dose-relationships in the human immune response to keyhole limpet hemocyanin. J Lab Clin Med. 1971;78:61–69. [PubMed] [Google Scholar]
- 17.Malley A, Saha A, Halliday WJ. Immunochemical studies of hemocyanin from the giant keyhole limpet (Megathura crenulata) and the horseshoe crab (Limulus polyhemus) J Immunol. 1965;95:141–147. [PubMed] [Google Scholar]
- 18.Intracel. BCI-ImmuneActivator™. http://www.intracel.com/pdf/I-BCI.pdf (accessed July 6, 2010).
- 19.Miller JS, Curtsinger J, Berthold M, Malvey K, Bliss RL, Le CT, Fautsch SK, Dudek AZ, Blazar BR, Panoskaltsis-Mortari A. Diminished neo-antigen response to keyhole limpet hemocyanin (KLH) vaccines in patients after treatment with chemotherapy or hematopoietic cell transplantation. Clin Immunol. 2005;117:144–151. doi: 10.1016/j.clim.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 20.Kapp K, Maul J, Hostmann A, Mundt P, Preiss JC, Wenzel A, et al. Modulation of systemic antigen-specific immune responses by oral antigen in humans. Eur J Immunol. 2010;40:3128–3137. doi: 10.1002/eji.201040701. [DOI] [PubMed] [Google Scholar]
- 21.Weniger BG, Papania M. Alternative vaccine delivery methods. In: Plotkin S, Orenstein W, Offit P, editors. Vaccines. ed 5. Philadelphia: Elsevier; 2008. [Google Scholar]