Abstract
Ischemic stroke is a serious disease leading to significant morbidity and mortality. Multifocal and recurrent strokes are usually caused by embolic diseases, i.e. atrial fibrillation, but rare causes like cerebral vasculitis and clotting disorders are also well known. Here we report on two patients suffering from the very rare intravascular large B-cell lymphoma leading to multifocal and recurrent strokes in the brain and spinal cord as the prominent neurological symptom. The difficulties and the need for diagnostic brain biopsy in making an ‘in vivo’ diagnosis in this particular disease are outlined. Furthermore, the prerequisite for an interdisciplinary approach in these patients is strongly emphasized. Delayed diagnosis for several reasons was the most probable cause for cerebral relapse leading to death in one patient a few months after diagnosis. Conversely, early initiation of immunochemotherapy with a classical lymphoma schedule (R-CHOP) led to long-lasting remission of the disease in the other patient. With this report we like to improve alertness to intravascular large B-cell lymphoma as a cause for multifocal and recurrent strokes.
Key words: Intravascular large B-cell lymphoma, Stroke, Cerebral vasculitis, Brain biopsy, R-CHOP
Introduction
Ischemic stroke is the leading cause for morbidity and mortality worldwide [1]. Large- and small-vessel arteriopathy as well as atrial fibrillation are the prominent risk factors for cerebral stroke. However, besides other rare etiologies, occlusion of small brain arteries is very rarely caused by the malignant intravascular lymphoma, which was first described in 1959 as ‘angioendotheliomatosis proliferans systemisata’ [2]. It is characterized by intravascular growth of neoplastic CD20-positive large B cells [3]. Clotting of neoplastic lymphoid cells especially in the capillaries leads to disseminated ischemic lesions in different organs, i.e. in the brain [4]. The exclusively intravascular localization of this lymphoma is attributed to a deficient property of extravasation, most probably due to a loss of the two adhesion molecules ICAM-1 (CD54) and beta-1 integrin (CD29). Since 2001, the intravascular large B-cell lymphoma (IVLBCL) has been recognized as an entity in the WHO classification. The estimated annual incidence is about 0.5–1/1,000,000 population. In half of the patients the diagnosis can only be made postmortem. CNS and skin are by far the most frequently affected sites (68%). Liver, spleen and bone marrow are involved to a much lesser extent. The reason of this neuroectodermal tropism remains elusive. A limited form with only cutaneous involvement exists chiefly in younger women. They have a far better prognosis than patients with multiorganic disease [3]. In contrast to the ‘Western’ type of IVLBCL, the ‘Asian’ variant shows preferentially hepatosplenic involvement and is associated with a hemaphagocytic syndrome accompanied by prominent thrombocytopenia. Interestingly, CNS and skin are rarely involved in the ‘Asian’ type of IVLBCL, suggesting a different spectrum of the disease. To improve the alertness among neurologists for this rare disease, we report two cases initially suffering from stroke symptoms, where the challenging ‘in vivo’ diagnosis of IVLBCL was histologically proven due to an interdisciplinary crusade.
Case Report
Case 1
A 56-year-old formerly healthy man developed a progressive diffuse encephalopathic syndrome including cognitive impairment, disorientation, left hemiparesis and symptomatic epilepsy over more than eight month. Initial brain MRI showed disseminated subcortical T2-hyperintense lesions without gadolinium enhancement. CSF analysis revealed a normal protein level and borderline pleocytosis. The patient was treated with steroids, but repeated tapering provoked recurrence of the encephalopathic syndrome. After acute deterioration of the left hemiparesis suggesting an acute stroke, he was admitted to our hospital. Brain MRI now demonstrated numerous acute and subacute as well as old ischemic infarcts in different arterial territories. Laboratory workup showed an elevated lactate dehydrogenase (LDH) level (355 U/l), red and white cell count and platelets were unremarkable, but CSF now contained slightly increased total protein levels (0.68 g/l). No other organ alteration was found by CT scans of the lung and abdomen. In the next days, the patient suffered from multiple further strokes in different arterial territories despite therapy with aspirin, low-dose heparin and high doses of steroids. The follow-up brain MRI examination showed new strokes again in different arterial territories (fig. 1a–c). ECG and echocardiography were unremarkable. Conventional cerebral angiography was not suggestive of vasculitis. Because of the progressive multifocal cerebral manifestations, we decided to perform a brain biopsy of the right parietal lobe ten days after admission. This biopsy revealed the surprising diagnosis of an IVLBCL (fig. 1d). Standard immunochemotherapy including rituximab (375 mg/m2) combined with cyclophosphamide, vincristine, doxorubicin and prednisolone (R-CHOP-14) as standard regimen for peripheral diffuse large B-cell lymphoma led to complete remission accompanied by a dramatic neurological improvement after six cycles of R-CHOP-14 and two additional cycles of rituximab. Of note, due to the intravascular localization of the lymphoma, neither intrathecal nor systemic chemotherapy with significant penetration into the CNS (such as high-dose cytarabine or methotrexate) were applied. Complete remission is currently ongoing 26 month after the initial diagnosis.
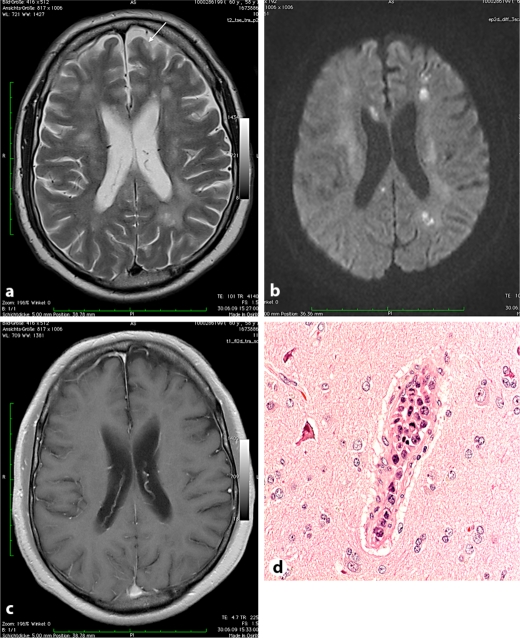
Fig. 1.
a–c Case 1. a T2-weighted axial MR image with diffuse leukoencephalopathy, older cortical infarct (arrow). b Diffusion-weighted image showing multiple acute and subacute medullary infarcts in both hemispheres. c Contrast-enhanced T1-weighted image demonstrating intact blood-brain barrier and absent enhancement of ischemic areas. d Brain biopsy (case 1): blastic lymphoid tumor cells within intracerebral vessels but not in the brain tissue (two mitotic figures).
Case 2
A 61-year-old woman was admitted to the emergency department with an acute onset of a left hemiparesis and a right-sided hemianopsia. Contrast-enhanced CT scan of the brain including a CT perfusion study revealed a perfusion deficit in the territory of the left posterior artery without evidence of arterial occlusion on CT angiography. Furthermore she experienced a complex partial seizure with myoclonic jerks of the right hand and a speech arrest. Under the suspicion of an ischemic stroke, i.v. thrombolysis with rtPA (0.9 mg/kg) was immediately administered and antiepileptic therapy with lorazepam and levetiracetam installed. Laboratory workup showed thrombocytopenia (131 G/l), elevated LDH (580 U/l) and elevated liver enzymes (GGT 55 U/l, AST 50 U/l). Weight loss of about 10 kg in the previous 6 months and a depression was reported. She had a history of smoking. Previous diagnostic workup revealed unspecific infiltrations of the lung parenchyma on a chest CT scan without evidence of lung cancer. Hepatosplenomegaly and suspicious abdominal skin thickening were additionally described. On the next day she had fully recovered from the neurological symptoms. An MRI scan of the brain showed a wedge-shaped hyperintense lesion in the right parietal lobe without gadolinium enhancement (fig. 2a–c). Surprisingly, it additionally showed an asymptomatic T2 hyperintensity of the pontine brainstem (not shown). No common risk factor for central pontine myelinolysis was present. Under the working diagnosis of a multi-organ disease, bronchoscopic lung biopsy, hepatic and abdominal skin biopsy, CSF analysis and multiple laboratory workup including infectious and paraneoplastic parameters did not lead to a definite diagnosis. Unfortunately, a brain, liver or spleen biopsy was refused. With the hypothesis of a systemic autoimmune disease, she was symptomatically treated with tapered doses of steroids and her condition greatly improved. After four weeks she was readmitted with an acute conus medullaris syndrome with urine incontinence, flaccid paraparesis and areflexia and was treated with repeated cycles of plasmapheresis under the diagnosis of an accompanying acute inflammatory demyelinating polyneuropathy (AIDP). Later on, the spinal affection turned out to be most probably of ischemic origin. As her condition worsened, diagnostic splenectomy was now performed and revealed the diagnosis of an IVLBCL (fig. 2d). Immediately, she was treated with intravenous immunochemotherapy (R-CHOP-14) [4]. Despite a serological response of the serum LDH, the neurological condition showed only minor improvements. After 5 cycles she died a few months after her first stroke due to a fulminant cerebral relapse.
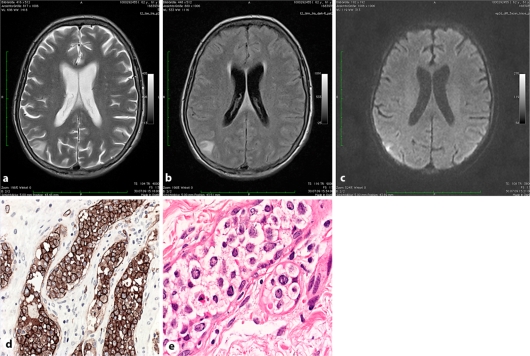
Fig. 2.
a–c Case 2. T2-weighted (a), fluid-attenuated inversion recovery (b) and diffusion-weighted (c) images showing a triangular shaped cortically based lesion with moderately restricted diffusion in the right parietal lobe suggestive of a subacute ischemic lesion. d Splenectomy specimen: blastic lymphoid tumor cells strictly confined to hilar blood vessels showing strong immunoreactivity with the pan-B cell marker CD20. e Skin biopsy: IVBCL infiltration in the deeper dermis.
Discussion
Western type IVLBCL is a very rare disease characterized by a multifocal CNS and multisystemic clinical presentation. Subacute diffuse and unspecific symptoms make the diagnosis of IVLBCL ‘in vivo’ a real challenge. The hallmark of IVLBCL with cerebral involvement is the triad of a subacute encephalopathy, multiple strokes and elevated serum LDH. Cerebral vasculitis, septic encephalopathy or infections of the CNS are the main differential diagnoses, while IVLBCL is mostly not under consideration in the first place.
Patients suffering from clinical syndromes as described are often treated with steroids based on the clinical and radiological diagnosis of a cerebral vasculitis, which is rarely proven by brain biopsy. In patients with IVLBCL, as presented here, blind immunosuppressive treatment without biopsy may critically delay the final diagnosis of IVLBCL due to a transient remission of neurological symptoms. Moreover, corticosteroids alone are clearly insufficient to substantially treat this disease as demonstrated by our presented cases.
Brain biopsy is the diagnostic procedure of choice in suspected IVLBCL affecting the CNS, as CSF analysis, brain MRI/CT scans, MR or CT angiography and even conventional cerebral angiography lack specificity to differentiate IVLBCL from other diseases like cerebral vasculitis [6]. This has especially been taken into account since immunosuppressive therapy without prior histological diagnosis has been reported to lead to fatal outcome in overlooked infectious brain diseases [7].
In contrast to other organs, brain biopsy is considerably more rarely performed. Despite low morbidity even in specialized centers, only 4–5 brain biopsies are reported per year for suspected nonneoplastic disease [8]. The diagnostic yield of brain biopsy in multifocal CNS diseases like cerebral vasculitis is reported to be as high as 75% [9]. In a retrospective analysis of 64 patients over a time period of 15 years, a specific diagnosis was made in 34 patients (53%) [8]. Although half of the brain biopsies did not lead to a definite diagnosis, this procedure, at least, excludes a CNS infection. This is of utmost importance according to the choice of the appropriate immunosuppressive therapy.
IVLBCL and primary CNS lymphoma (PCNSL) both represent rare entities of high grade B-cell lymphomas affecting the CNS. Due to the different localization of both entities – PCNSL growing on the parenchymal side protected by the blood-brain barrier (BBB) and IVLBCL growing inside the vessel – the adequate chemotherapeutic approach is strikingly different. The therapy of choice in PCNSL consist of high-dose methotrexate and/or cytarabine, with or without whole-brain radiotherapy as these drugs penetrate into the brain parenchyma by crossing the BBB in cytotoxic concentrations [5]. In contrast, the intravascular location of IVLBCL does not require drugs which are able to cross the BBB. Therefore, and given the favorable toxicity profile, standard R-CHOP chemo-immunotherapy without the use of high-dose methotrexate and/or cytarabine is an adequate and active therapy with favorable toxicity for patients suffering from CNS manifestations of IVLBCL [4].
In our patients, ischemic strokes were the prominent clinical condition which covered the diagnosis of the underlying IVLBCL for a considerably long time. Multiple acute and subacute strokes prompted us to perform a brain biopsy in case 1. Missing the exact diagnosis by a refused brain or spleen biopsy in case 2 led to a dramatic neurological decline with the emergence of a fatal conus medullaris syndrome. Despite of numerous cycles of R-CHOP-14 therapy the patient died due to fulminant cerebral progression. Unfortunately, a former skin biopsy from this patient retrospectively turned out to show diagnostic characteristics of IVLBCL (fig. 2e). Therefore, earlier appropriate treatment might have changed the outcome in a more favorable direction.
In conclusion, IVLBCL affecting the brain is an extremely rare but life-threatening disease. It is characterized by multifocal and recurrent strokes. Early brain biopsy is of paramount importance to achieve the correct diagnosis. It can be performed with acceptable risks and may lead to the diagnosis of a potentially curative condition that would otherwise be missed with the consequence of a vital threat to the patient. Immunochemotherapy with R-CHOP-14, omitting classical chemotherapy regimens used for PCNSL, can substantially lead to the prevention of devastating strokes as well as ischemias in other organs and may induce durable complete remission as demonstrated in one case presented here. As IVLBCL is an extremely rare disease and the clinical presentation is unspecific, the correct diagnosis can easily be overlooked even in biopsies as demonstrated in case 2. Of note, the most important step to immediate and appropriate treatment of the patients with a CNS involvement is to obtain and thoroughly evaluate brain tissue for a workup involving neurodegenerative, immunological, infectious and neoplastic aspects.
References
- 1.Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors, 2001:systematic analysis of population health data. Lancet. 2006;367:1747–1757. doi: 10.1016/S0140-6736(06)68770-9. [DOI] [PubMed] [Google Scholar]
- 2.Pfleger L, Tappeiner J. On the recognition of systematized endotheliomatosis of the cutaneous blood vessels (reticuloendotheliosis?) Hautarzt. 1959;10:359–363. [PubMed] [Google Scholar]
- 3.Ferreri AJ, Campo E, Seymour JF, Willemze R, Ilariucci F, Ambrosetti A, Zucca E, Rossi G, Lopez-Guillermo A, Pavlovsky MA, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127:173–183. doi: 10.1111/j.1365-2141.2004.05177.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009;10:895–902. doi: 10.1016/S1470-2045(09)70140-8. [DOI] [PubMed] [Google Scholar]
- 5.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, Roth A, Hertenstein B, von Toll T, Hundsberger T, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010;11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 6.Alrawi A, Trobe JD, Blaivas M, Musch DC. Brain biopsy in primary angiitis of the central nervous system. Neurology. 1999;53:858–860. doi: 10.1212/wnl.53.4.858. [DOI] [PubMed] [Google Scholar]
- 7.Berlit P. Diagnosis and treatment of cerebral vasculitis. Ther Adv Neurol Disord. 2010;3:29–42. doi: 10.1177/1756285609347123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong SH, Jenkinson MD, Faragher B, Thomas S, Crooks D, Solomon T. Brain biopsy in the management of neurology patients. Eur Neurol. 2010;64:42–45. doi: 10.1159/000315032. [DOI] [PubMed] [Google Scholar]
- 9.Pulhorn H, Quigley DG, Bosma JJ, Kirollos R, du Plessis DG, Jenkinson MD. Impact of brain biopsy on the management of patients with nonneoplastic undiagnosed neurological disorders. Neurosurgery. 2008;62:833–837. doi: 10.1227/01.neu.0000318168.97966.17. [DOI] [PubMed] [Google Scholar]