Abstract
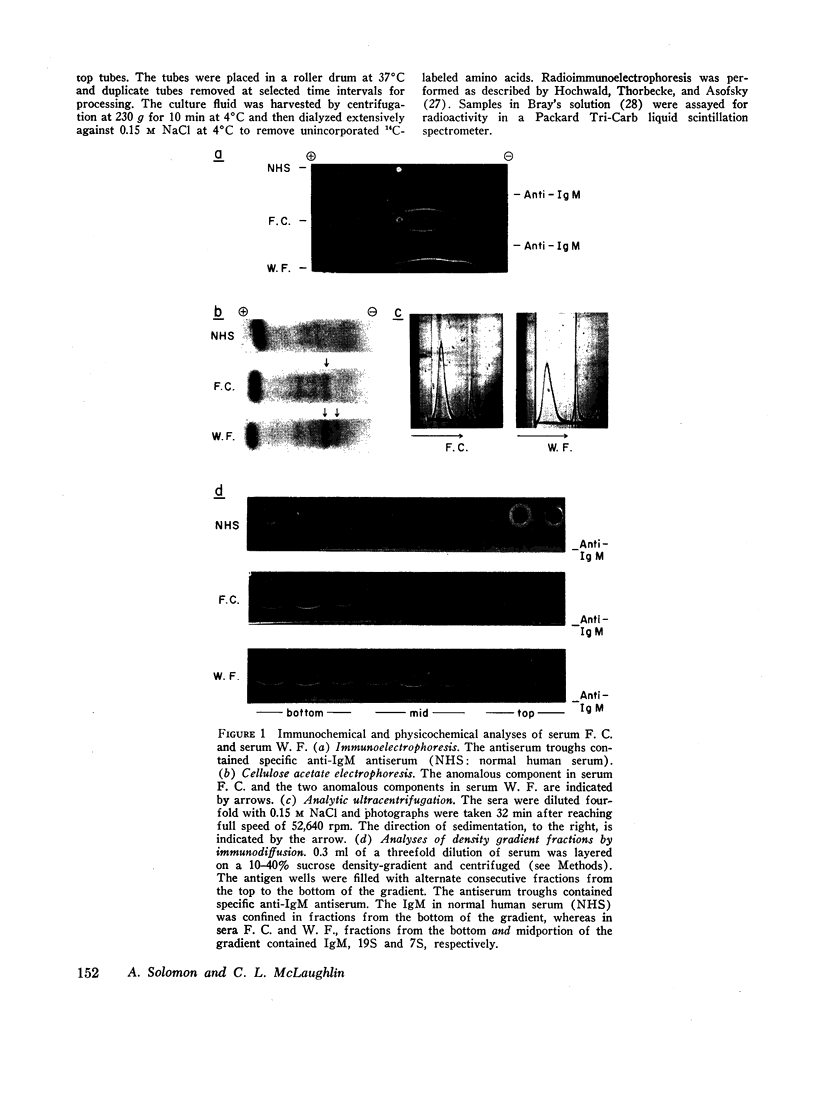
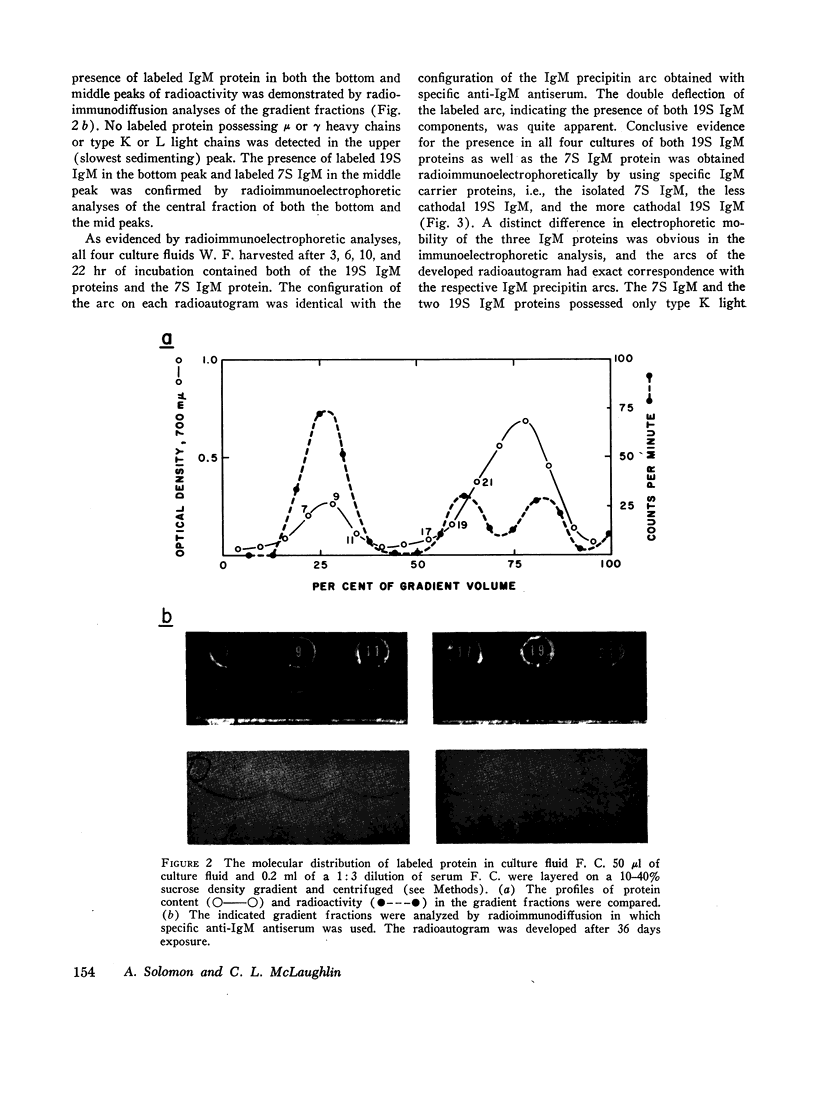
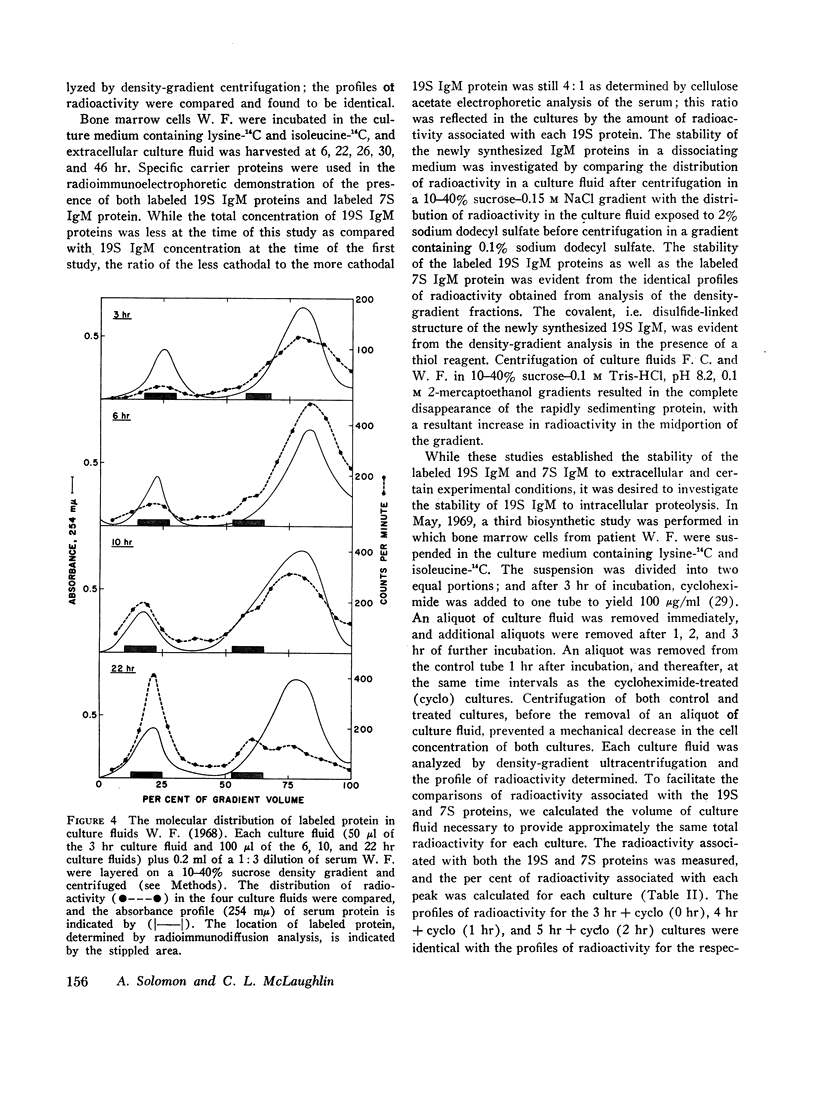
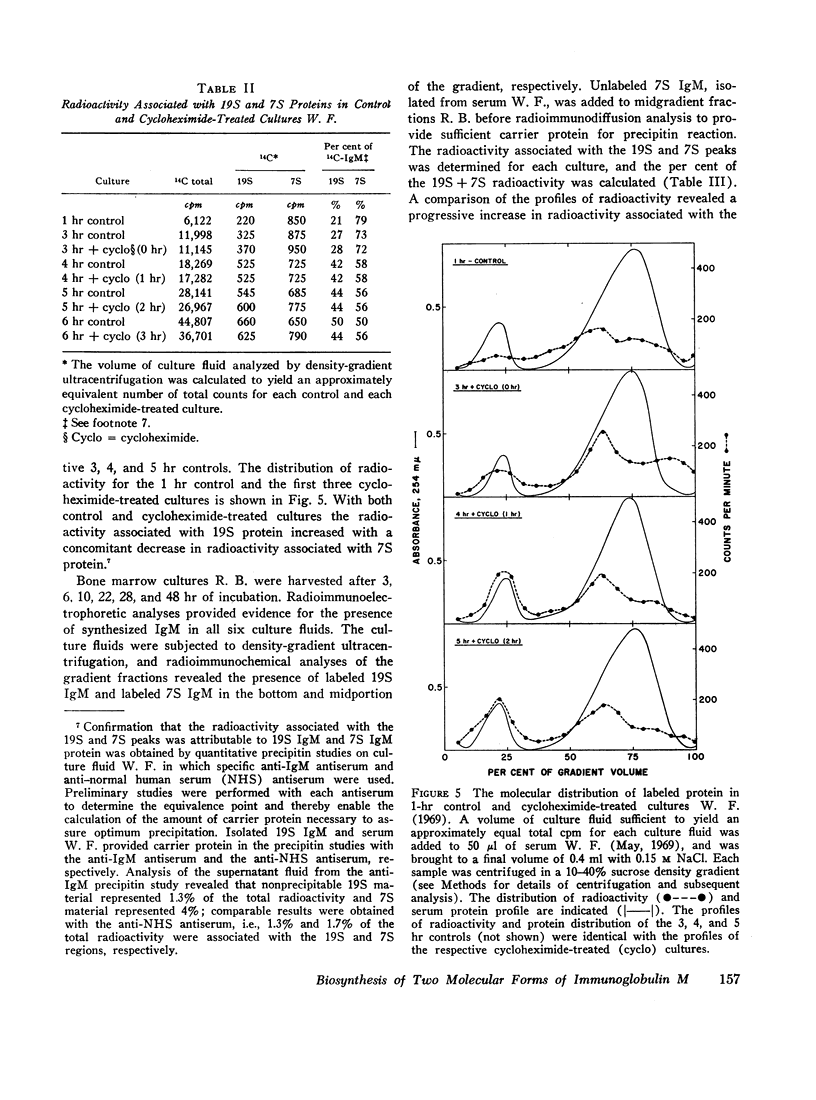
The class of immunoglobulin M (IgM) characterized by high molecular weight proteins with a sedimentation coefficient of 19S, includes a smaller molecular form with an S20,[unk] of approximately 7. The synthetic origin of the 7S IgM was investigated by biosynthetic studies on bone marrow cells from three patients with macroglobulinemia whose sera contained 7S IgM and 19S IgM. Labeled 7S IgM and 19S IgM were identified in extracellular culture fluids by radioimmunochemical techniques. The separation of the two molecular forms of IgM by density-gradient ultracentrifugation of the culture fluids before radioimmunochemical analyses permitted the identification of both the labeled 7S IgM and 19S IgM. One patient's serum contained two separate and distinct 19S IgM proteins as well as 7S IgM. The use of specific isolated carrier IgM proteins permitted the radioimmunochemical detection of labeled 7S IgM and both 19S IgM proteins. The introduction of cycloheximide into a culture system effects the cessation of protein synthesis. The analyses of culture fluids harvested at timed intervals after the addition of cycloheximide revealed not only the stability of 19S IgM to intracellular proteolysis, but also provided evidence for a possible precursor-product relationship between the 7S IgM and the 19S IgM. The demonstration that the labeled 7S IgM is neither an in vitro breakdown product of 19S IgM nor a resultant of 19S IgM intracellular catabolism substantiated the synthetic origin of 7S IgM in human sera.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel C. A., Grey H. M. Carboxy-terminal amino acids of gamma-A and gamma-M heavy chains. Science. 1967 Jun 23;156(3782):1609–1610. doi: 10.1126/science.156.3782.1609. [DOI] [PubMed] [Google Scholar]
- Bush S. T., Swedlund H. A., Gleich G. J. Low molecular weight IgM in human sera. J Lab Clin Med. 1969 Feb;73(2):194–201. [PubMed] [Google Scholar]
- Clem I. W., De Boutaud F., Sigel M. M. Phylogeny of immunoglobulin structure and function. II. Immunoglobulins of the nurse shark. J Immunol. 1967 Dec;99(6):1226–1235. [PubMed] [Google Scholar]
- Clem L. W., Small P. A., Jr Phylogeny of immunoglobulin structure and function. I. Immunoglobulins of the lemon shark. J Exp Med. 1967 May 1;125(5):893–920. doi: 10.1084/jem.125.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEUTSCH H. F., MORTON J. I. Dissociation of human serum macroglobulins. Science. 1957 Mar 29;125(3248):600–601. doi: 10.1126/science.125.3248.600. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Singer S. J., Metzger H. Evolution of immunoglobulin polypeptide chains: carboxy-terminal of an IgM heavy chain. Science. 1966 Dec 23;154(3756):1561–1562. doi: 10.1126/science.154.3756.1561. [DOI] [PubMed] [Google Scholar]
- FAHEY J. L., MCLAUGHLIN C. PREPARATION OF ANTISERA SPECIFIC FOR 6.6 S GAMMA-GLOBULINS, BETA 2A-GLOBULINS, GAMMA-1.-MACROGLOBULINS, AND FOR TYPE I AND II COMMON GAMMA-GLOBULIN DETERMINANTS. J Immunol. 1963 Oct;91:484–497. [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Gleich G. J., Uhr J. W., Vaughan J. H., Swedlund H. A. Antibody formation in dysgammaglobulinemia. J Clin Invest. 1966 Aug;45(8):1334–1340. doi: 10.1172/JCI105440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILLANDER J. SEPARATION OF HUMAN IMMUNOGLOBULINS BY GEL FILTRATION AND ZONE ELECTROPHORESIS. Acta Soc Med Ups. 1963;68:230–244. [PubMed] [Google Scholar]
- KUNKEL H. G. Zone electrophoresis. Methods Biochem Anal. 1954;1:141–170. doi: 10.1002/9780470110171.ch6. [DOI] [PubMed] [Google Scholar]
- Klein F., Mattern P., Radema H., Van Zwet T. L. Slowly sedimenting serum components reacting with anti-IgM sera. Immunology. 1967 Dec;13(6):641–647. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MILLER F., METZGER H. CHARACTERIZATION OF A HUMAN MACROGLOBULIN. I. THE MOLECULAR WEIGHT OF ITS SUBUNIT. J Biol Chem. 1965 Aug;240:3325–3333. [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis). J Exp Med. 1965 Sep 1;122(3):601–618. doi: 10.1084/jem.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Polypeptide chains of immunoglobulins from the smooth dogfish (Mustelus canis). Science. 1966 Dec 23;154(3756):1567–1568. doi: 10.1126/science.154.3756.1567. [DOI] [PubMed] [Google Scholar]
- Merrill D., Hartley T. F., Claman H. N. Electroimmunodiffusion (EID): a simple, rapid method for quantitation of immunoglobulins in dilute biological fluids. J Lab Clin Med. 1967 Jan;69(1):151–159. [PubMed] [Google Scholar]
- Morris J. E., Inman F. P. Isolation of the monomeric subunit of immunoglobulin M with its interchain disulfide bonds intact. Biochemistry. 1968 Aug;7(8):2851–2857. doi: 10.1021/bi00848a022. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Perchalski J. E., Clem L. W., Small P. A., Jr 7S gamma-M immunoglobulins in normal human cord serum. Am J Med Sci. 1968 Aug;256(2):107–111. doi: 10.1097/00000441-196808000-00006. [DOI] [PubMed] [Google Scholar]
- ROTHFIELD N. F., FRANGIONE B., FRANKLIN E. C. SLOWLY SEDIMENTING MERCAPTOETHANOL-RESISTANT ANTINUCLEAR FACTORS RELATED ANTIGENICALLY TO M IMMUNOGLOBULINS (GAMMA-1M-GLOBULIN) IN PATIENTS WITH SYSTEMIC LUPUS ERYTHEMATOSUS. J Clin Invest. 1965 Jan;44:62–72. doi: 10.1172/JCI105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- SOLOMON A., FAHEY J. L., MALMGREN R. A. Immunohistologic localization of gamma-1-macroglobulins, beta-2A-myeloma proteins, 6.6 S gamma-myeloma proteins and Bence Jones proteins. Blood. 1963 Apr;21:403–423. [PubMed] [Google Scholar]
- Solomon A., Kunkel H. G. A "monoclonal" type, low molecular weight protein related to gamma-M-macroglobulins. Am J Med. 1967 Jun;42(6):958–967. doi: 10.1016/0002-9343(67)90076-9. [DOI] [PubMed] [Google Scholar]
- Solomon A. Molecular heterogeneity of immunoglobulin-M (gammaM-globulin). J Immunol. 1969 Feb;102(2):496–506. [PubMed] [Google Scholar]
- Stobo J. D., Tomasi T. B. A Low Molecular Weight Immunoglobulin Antigenically Related to 19 S IgM. J Clin Invest. 1967 Aug;46(8):1329–1337. doi: 10.1172/JCI105625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T., Deutsch H. F. Dissociation, reaggregation, and subunit structure studies of some human gamma-M-globulins. J Biol Chem. 1967 Jun 10;242(11):2725–2738. [PubMed] [Google Scholar]
- Swedlund H. A., Gleich G. J., Chodirker W. B. Comparison of certain properties of naturally occurring low molecular weight Gamma M and the Gamma M monomer derived by reduction and alkylation of 19S Gamma M. J Immunol. 1968 Jun;100(6):1296–1303. [PubMed] [Google Scholar]
- WETTSTEIN F. O., NOLL H., PENMAN S. EFFECT OF CYCLOHEXIMIDE ON RIBOSOMAL AGGREGATES ENGAGED IN PROTEIN SYNTHESIS IN VITRO. Biochim Biophys Acta. 1964 Jul 22;87:525–528. doi: 10.1016/0926-6550(64)90131-8. [DOI] [PubMed] [Google Scholar]
- van Furth R., Schuit H. R., Hijmans W. The formation in vitro of paraproteins in multiple myeloma and Waldenström's macroglobulinaemia. Br J Haematol. 1966 Mar;12(2):202–211. doi: 10.1111/j.1365-2141.1966.tb05626.x. [DOI] [PubMed] [Google Scholar]






