Abstract
Neuroblastoma is the most common extra-cranial solid tumor in children. Despite advances in the treatment of childhood cancer, outcomes for children with advanced-stage neuroblastoma remain poor. Here we reported that 2-methoxyestradiol (2-ME) inhibited the proliferation and induced apoptosis in human neuroblastoma SK-N-SH and SH-SY5Y cells. 2-ME treatment also resulted in the generation of ROS and the loss of mitochondrial membrane potential in SK-N-SH and SH-SY5Y, indicating that 2-ME-induced apoptosis is mediated by ROS. This is supported by the results that have shown that co-treatment with antioxidants, VC, L-GSH and MitoQ10, decreased 2-ME-induced generation of ROS and the loss of the mitochondrial membrane potential, increased the Bcl-2/Bax ratio, decreased 2-ME-induced activation of caspase-9 and caspase-3 and the up-regulation of apoptosis-inducing factor (AIF), and prevented 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells. These results suggested that oxidative stress plays an important role in 2-ME-induced apoptotic death of human neuroblastoma cells.
Keywords: 2-Methoxyestradiol, Neuroblastoma, Reactive oxygen species, Apoptosis
1. Introduction
Neuroblastoma is the most common extra-cranial solid tumor in children and exhibits very complex biological and clinical heterogeneities [1]. While the benign form of the disease can achieve spontaneous and complete recession, the morbidity and mortality rates of the malignant form is very high [2, 3]. Despite dramatic increases in the intensity of therapy provided, the cure rates among children with high-risk neuroblastoma have shown only modest improvement [4–7]. Therefore, extensive efforts have been directed toward the development of new drugs to treat high-risk neuroblastoma.
2-Methoxyestradiol (2-ME) is a natural metabolite of 17β-estradiol. Unlike its parent compounds and their other metabolites, 2-ME has minimal estrogenic activity due to its low affinity for estrogen receptors (ERs) [8]. However, 2-ME has been shown, in a variety of in vitro and in vivo models, to possess therapeutic effects against angiogenesis and tumor growth [9–13]. Because of its low toxicity and broad-spectrum anti-cancer activity, 2-ME has been considered as a promising anticancer drug candidate. It is in phase I and II clinical trials for the treatment of a variety of cancers, including multiple myeloma; breast; glioblastoma multiforme carcinoid, and prostate tumors [14]. However, the molecular mechanism of 2-ME is not fully understood [15].
Growing evidence suggests that oncogenic stimulation, increased metabolic activity, and mitochondrial malfunction can result in increased intrinsic ROS stress in cancer cells. The increased ROS generation in cancer cells can lead to the stimulation of cellular proliferation, promotion of mutations and genetic instability, and alterations in drug sensitivity [16–18]. Despite these negative impacts of ROS generation in cancer cells, excessive production of ROS may inflict damage to various cellular components, including DNA, protein and lipid members, which trigger apoptosis in cancer cells [19], suggesting that it is possible to develop novel therapeutic strategies to kill cancer cells through ROS-mediated mechanisms. Therefore, ROS-generating compounds and/or antioxidant inhibitors are proposed as new therapeutic agents for cancers [16].
The increase in ROS levels makes cancer cells highly dependent on antioxidants to cope with the ROS stress. It has been suggested that impairment of antioxidant system in cancer cells underlies anticancer effects of some anticancer agents. Huang et al. proposed that 2-ME inhibits tumor growth by superoxide dismutase (SOD) inhibition [20]. However, studies from other investigators have suggested that while 2-ME does increase intracellular superoxide levels in tumor cells, it does not inhibit SOD but rather interferes with the SOD assay [21]. In addition, the effects of 2-ME on cellular superoxide levels appear to be cell type specific. For instance, the ability of 2-ME to inhibit proliferation in a murine pre-B cell line transfected with Bcr-Abl was found to be independent of elevations in cellular superoxide [22]. Although 2-ME has been proved to be effective in the treatment of neural cancers [23–26], the role of ROS in anticancer activity of 2-ME against neuroblastoma is unknown.
In the present study, human neuroblastoma cell lines, SK-N-SH and SH-SY5Y, were used to examine whether 2-ME can inhibit proliferation and induce apoptosis in human neuroblastoma cells. The roles of ROS in 2-ME-induced apoptosis and in anticancer activity of 2-ME against neuroblastoma are also determined by directly measuring the ROS generation and the changes in mitochondria membrane potential and by examining whether antioxidants can attenuate the pro-apoptotic effects of 2-ME. The results from this study demonstrated that 2-ME can inhibit the proliferation and induce apoptosis in human neuroblastoma SK-N-SH and SH-SY5Y cells and that oxidative stress plays an important role in 2-ME-induced apoptotic death of human neuroblastoma cells.
2. Materials and methods
2.1. Cell culture and reagents
Human neuroblastoma SK-N-SH and SH-SY5Y cells were kindly provided by Dr. Ying Peng at Peking Union Medical College and Dr. Lin-lin Yi at Xuanwu Hospital of Capital Medical University, China, respectively. SK-N-SH and SH-SY5Y cells were maintained in Dulbecco’s Modified Eagle Medium (DMEM) or DMEM/F12 (HyClone, Beijing, China), respectively, supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 10% fetal bovine serum at 37 °C with a 5% CO2 atmosphere in a humidified incubator.
2-ME was synthesized by Zheng Zhou University School of Pharmaceutical science (Zhengzhou, China). The purity of the 2-ME used in this study is 99.5%, as determined by HPLC. Trypan blue and sulphorhodamine (SRB) dye were purchased from Sigma (USA). Vitamin C (Ascorbic Acid, VC) was obtained from Zhuofeng Biotechnology (Zhengzhou, China). L-Glutathione (Reduced) (L-GSH) was purchased from Solarbio (Beijing, China). MitoQ10 was provided by Dr. Murphy (Mitochondrial Biology Unit, UK). Cell Apoptosis Rhodamine123 Detection Kit and an Apoptotic Cell Hoechst 33258 Detection Kit were purchased from KeyGEN Biotech Ltd. (Nanjing, China). ROS Detection Kit was purchased from Beyotime Institute of Biotechnology (Nanjing, China). BCA protein Detection Kit was purchased from DingGuo Biotech Ltd (Beijing, China). The rabbit anti-caspase-3, anti-caspase-9, anti-Bcl-2, and the anti-Bax antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-AIF antibody was purchased from Beyotime Institute of Biotechnology (Nanjing, China). All other chemicals were purchased from Sigma Chemical Co. (USA).
2.2. SRB assay
Cells (2.5×104 cells /mL) were plated in media containing 10% FBS and were allowed to attach overnight before the drugs were added to the wells at indicated concentrations. Each dose was tested in at least six replicate wells. At the end of the incubation period, the cells were fixed with 10% trichloroacetic acid (TCA) (4°C) for 1 h, and then were washed five times with tap water to remove TCA. Air-dried TCA-fixed cells were stained for 15 min with 0.4% (w/v) SRB dissolved in 1% acetic acid. At the end of staining period, SRB was removed and cultures were rinsed with 1% acetic acid to remove unbound dye. The cultures were air dried and bound dye was dissolved with 10 mM Tris base (pH 10.5). The plates were shaken for 5 min until the dye was uniformly distributed and then were measured using an Emax Precision Plate Reader at 515 nm.
2.3. Trypan Blue assay
Exponentially growing cells (1×104 cells/well) were seeded onto 12- well plates. After overnight incubation, cells were exposed to 5 μM 2-ME at 37°C for 1–8 days. Cells were harvested at the indicated times and were centrifuged for 5 min at 1000 rpm. After the pellet was washed with PBS, centrifuged and resuspended in complete culture media, a small aliquot of the cell suspension was mixed with 0.4% Trypan blue solution. The live (bright) and dead (blue) cells were counted using a hemocytometer. Cell viability was expressed as the percentage of surviving cells compared to the total number of cells. The experiment was performed in triplicate.
2.4. Nuclear staining with Hoechst 33258
For detecting the apoptosis with Hoechst 33258, cells were seeded in 6-well plates. After treatment with 2-ME for 48 or 72 h, cells were fixed with 4% paraformaldehyde for 10 min at 4°C, and then washed twice with Buffer A. Hoechst 33258, a DNA fluorochrome, was added to the fixed cells, and the cells were stained for 10 min at room temperature. After washing with double distilled water, the cells were observed and analyzed by Nikon 80i fluorescent microscope with excitation at 340 nm. Cells that exhibited reduced nuclear size, chromatin condensation, intense fluorescence, and nuclear fragmentation were considered as apoptotic cells. The percentage of apoptotic cells was calculated as the ratio of apoptotic cells to total cells. At least 300 cells were counted from five random microscopic fields by two observers.
2.5. Measurement of reactive oxygen species levels
ROS generation was measured using a 2′,7′-dichlorodihydrofluorescein diacetate (DCHF-DA) assay. Control and treated cells were washed twice with PBS, and then incubated with 10 μM DCFH-DA for 20 min at 37°C. Cells were washed three times with free serum DMEM to remove extracellular DCFH-DA. The fluorescence intensity was measured under an excitation at 488 nm and emission at 525 nm using a fluorophotometer (RF-5301PC, Japan). ROS levels were expressed as fold change over control.
2.6. Detection of mitochondrial membrane potential (Δ Ψm)
To determine the effects of 2-ME on mitochondrial membrane potential, control and treated cells were washed twice with PBS, and then incubated with 10 μg/mL rhodamine-123 (Molecular Probes) for 10 min. After the cells were washed three times, the cells were added to the six well plates and cultured for 60 min. The fluorescence intensity was measured under an excitation at 488 nm and emission at 530 nm using a fluorophotometer (RF-5301PC, Japan). The mitochondrial membrane potential was expressed as fold change over control.
2.7. Western blot analyses
After treatment of SK-N-SH cells with 2-ME, the collected cells were washed twice with cold PBS, incubated in RIPA buffer and protease inhibitor PMSF mixtures for 30 min on ice. Insoluble debris was precipitated by centrifugation at 12,300 × g for 15 min at 4°C, and the supernatants were collected and assayed for protein concentration using the BCA reagent kit. Western blots were performed by standard protocols. The levels of Bcl-2, Bax, caspase-9, caspase-3, and AIF proteins were analyzed with the following antibodies: rabbit anti-Bcl-2 (1:200), rabbit anti-Bax (1:200), rabbit anti-caspase-3 (1:200), rabbit anti-caspase-9 (1:200), and anti- AIF (1:1,000), respectively, followed by detection with ECL Western-blotting detection reagents.
2.8. Statistical analysis
All data are presented as mean ± SE. Statistical comparisons between groups were analyzed by the analysis of variance (ANOVA)-single factor model. Multiple comparison post-tests between groups were conducted using Turkey’s test. Differences between groups were considered significant at P < 0.05.
3. Results
3.1. Inhibition of SK-N-SH and SH-SY5Y cell proliferation by 2-ME
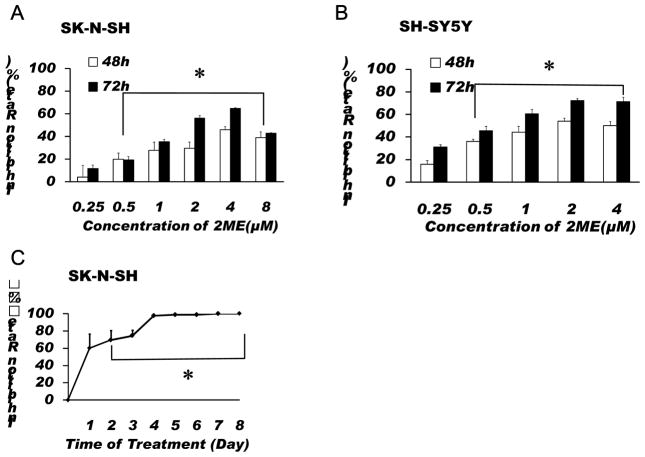
To determine whether 2-ME can inhibit the proliferation of SK-N-SH cells, cells were treated with 2-ME at different concentrations for 48 or 72 h, or 5 μM 2-ME for 1 to 8 days, respectively. As shown in Fig. 1A, 2-ME significantly inhibited the proliferation of SK-N-SH cells. We found that the maximum inhibition of proliferation was observed in the cells treated with 4 μM 2-ME. This result was further confirmed by the results from the experiments using another neuroblastoma cell line, SH-SY5Y, which have shown that 2-ME can also significantly decreased the proliferation of SH-SY5Y cells (Fig.1B). In addition, over 90% of SK-N-SH cells were found to be dead after treatment with 5 μM 2-ME for 4 days (Fig. 1C). These results indicate that 2-ME is a potent inhibitor of SK-N-SH and SH-SY5Y cell proliferation.
Fig.1.
Treatment with 2-ME significantly inhibited the proliferation of SK-N-SH and SH-SY5Y cells. SK-N-SH (A) and SH-SY5Y (B) cells were treated with 2-ME at indicated concentrations for 48 or 72 h. Cell viability was tested by SRB assay as described in the Methods. (C) SK-N-SH cells were treated with 5 μM 2-ME for 1–8 days. Cell viability was detected by Trypan blue exclusion assay. The data are expressed as inhibition rate and represent the mean ± SEM of three separate experiments. *P < 0.05 vs. control.
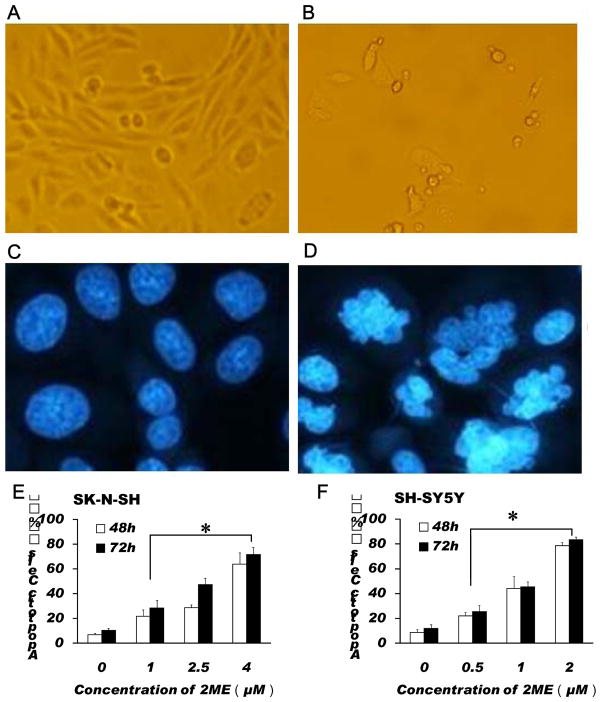
Fig.2.
2-ME treatment induced apoptosis in SK-N-SH and SH-SY5Y cells. Morphological changes (A, B) and Hoechst 33258 staining (C, D) of SK-N-SH cells were observed in the cells cultured in absence (A, C) and presence (B, D) of 2-ME (4 μM) for 72 h. (E) Dose response of apoptosis induction in SK-N-SH cells treated with 2-ME for 48 or 72 h. (F) Dose response of apoptosis induction in SH-SY5Y cells treated with 2-ME for 48 or 72 h. The data are expressed as percentage of apoptotic cells in the total cells and represent the mean ± SEM of five separate experiments. *P < 0.05 vs. control.
3.2. 2-ME treatment induced apoptosis in SK-N-SH and SH-SY5Y cells
To determine the mechanism of growth inhibition by 2-ME in SK-N-SH and SH-SY5Y cells, we examined the effect of 2-ME on apoptosis. As shown in Fig. 2, 2-ME treatment induced apoptosis in SK-N-SH cells (Fig. 2, A–E). DNA fragmentation and chromatin condensation were observed in the cells treated with 4 μM 2-ME for 72 h, indicating that 2-ME can induce apoptosis in SK-N-SH cells (Fig. 2, A–D). We also found that 2-ME increased apoptosis in SH-SY5Y cells (Fig. 2, F) and that 2-ME -induced apoptosis in both SK-N-SH and SH-SY5Y cells is dose-dependent. For example, after treatment with 1, 2.5, or 4 μM 2-ME, the apoptotic rates of SK-N-SH cells were increased from 6.88% to 21.63%, 28.64% and 63.83%; respectively in the cells treated with 2-ME for 48 h, and from 10.38 % to 28.39%, 47.32% and 71.56 % in the cells treated with 2-ME for 72 h (Fig. 2, E, F).
3.3. Treatment with 2-ME resulted in significant increases in ROS generation and the loss of the mitochondrial membrane potential in SK-N-SH and SH-SY5Y cells
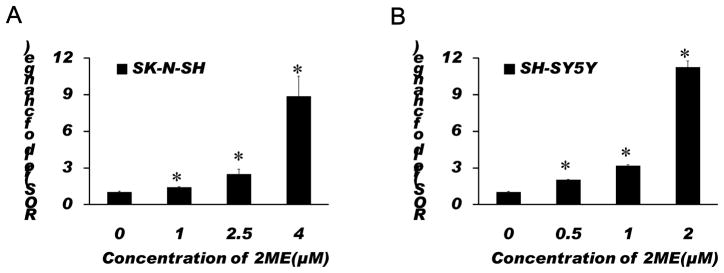
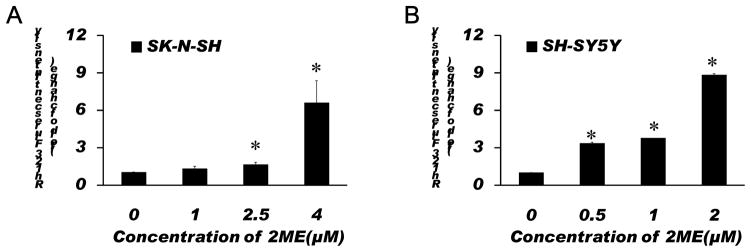
To examine whether ROS and ROS-induced mitochondrial injury are involved in the 2-ME-induced anti-proliferation and apoptosis, the effects of 2-ME on ROS generation and the mitochondrial membrane potential were determined in SK-N-SH and SH-SY5Y cells. We found that 2-ME treatment significantly increased the ROS generation and the loss of the mitochondrial membrane potential in a dose-dependent manner (Figs. 3, 4). While 1 μM 2-ME moderately increased the ROS generation in SK-N-SH cells, 2-ME at this concentration did not significantly increase the mitochondrial membrane potential. However, 2.5 and 4 μM 2-ME significantly increased both the ROS generation and the mitochondrial membrane potential in SK-N-SH cells. In SH-SY5Y cells, 2-ME at all doses tested significantly increased the ROS generation and the loss of the mitochondrial membrane potential. These results suggest that ROS and ROS-induced mitochondrial injury are involved in 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells.
Fig. 3.
Treatment with 2-ME resulted in significant increases in ROS generation in SK-N-SH (A) and SH-SY5Y (B) cells. Cells were treated with 2-ME at indicated concentrations for 72 h. Analysis of ROS was performed using a fluorophotometer as described in Methods. The data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. *P < 0.05 vs. control.
Fig. 4.
Treatment with 2-ME resulted in significant increases in the loss of the mitochondrial membrane potential in SK-N-SH (A) and SH-SY5Y (B) cells. Cells were treated with 2-ME at indicated concentrations for 72 h. Analysis of the mitochondrial membrane potential was performed using a fluorophotometer as described in Methods. The data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. *P < 0.05 vs. control.
3.4. Antioxidants attenuated anti-proliferation and apoptotic effects of 2-ME on SK-N-SH and SH-SY5Y cells
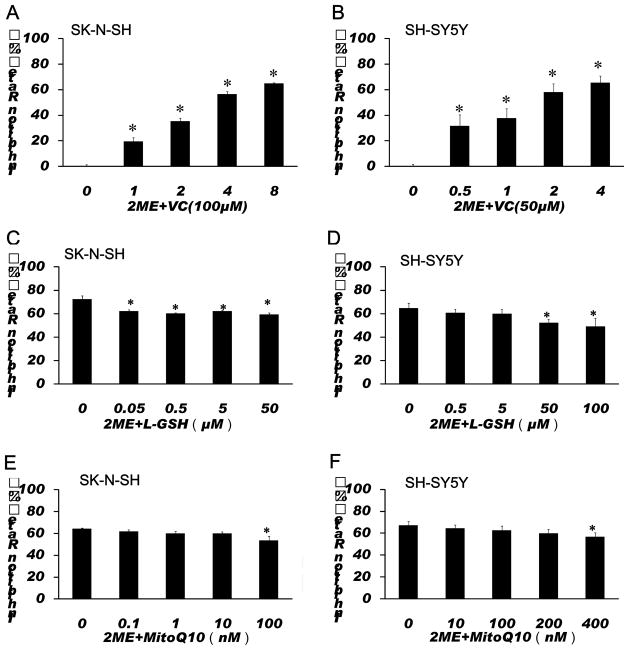
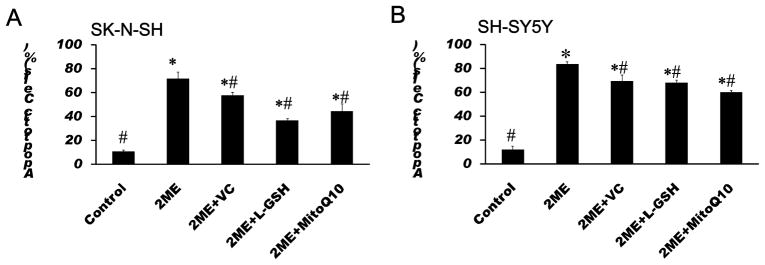
To further test whether ROS are involved in 2-ME-induced anti-proliferation and apoptotic effects, antioxidants, VC, L-GSH and a mitochondria-targeted antioxidant MitoQ10 were used to test whether inhibition of oxidative stress can attenuate the anti-proliferation and apoptotic effects of 2-ME. Co-treatment with VC significantly decreased the inhibition rate of proliferation in 2-ME exposed SK-N-SH and SH-SY5Y cells. Co-treatment with L-GSH and MitoQ10 also significantly decreased anti-proliferation effects of 2-ME in SK-N-SH and SH-SY5Y cells (Fig. 5). In addition, treatment with VC, L-GSH and MitoQ10 significantly decreased 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells (Fig. 6).
Fig. 5.
Antioxidants attenuated anti-proliferation effects of 2-ME on SK-N-SH (A, C, E) and SH-SY5Y (B, D, F) cells. SK-N-SH cells were treated with 2-ME at indicated concentrations in combination with 100 μM VC for 72 h (A), or treated with 4 μM 2-ME alone, or in combination with L-GSH or MitoQ10 at indicated concentrations for 72 h (C, E). SH-SY5Y cells were treated with 2-ME at indicated concentrations in combination with 50 μM VC for 72 h (B), or treated with 2 μM 2-ME alone, or in combination with L-GSH or MitoQ10 at indicated concentrations for 72 h (D, F). Cell viability was then tested by SRB assay as described in Methods. The data are expressed as inhibition rate over control and represent the mean ± SEM of six separate experiments. *P<0.05 vs. control.
Fig. 6.
Antioxidants attenuated apoptotic effects of 2-ME on SK-N-SH (A) and SH-SY5Y (B) cells. SK-N-SH cells were treated with 4 μM 2-ME alone, or in combination with 100 μM VC, 50 μM L-GSH or 100 nM MitoQ10 for 72 h. SH-SY5Y cells were treated with 2 μM 2-ME alone, or in combination with 50 μM VC, 100 μM L-GSH or 400 nM MitoQ10 for 72 h. The apoptosis was identified using Hoechst 33258 staining as described in Method. The data are expressed as percentage of apoptotic cells in the total cells and represent the mean ± SEM of five separate experiments. *P < 0.05 vs. control, # P < 0.05 vs. 2-ME treated group.
3.5. Antioxidants diminished 2-ME-induced ROS generation and the loss of mitochondrial membrane potential in 2-ME treated SK-N-SH and SH-SY5Y cells
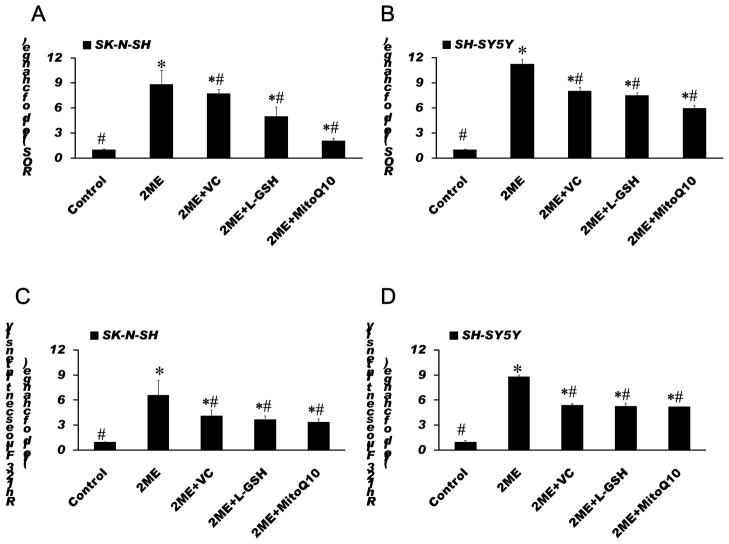
To investigate whether the attenuation of effects of 2-ME on proliferation and apoptosis by antioxidants are mediated by the prevention of ROS generation and mitochondrial injury, the effects of antioxidants, VC, L-GSH and MitoQ10, on 2-ME-induced ROS generation and the loss of mitochondrial membrane potential were determined. We found that all antioxidants tested significantly diminished the 2-ME-induced elevation of ROS and the loss of mitochondrial membrane potential in the cells exposed to 2-ME (Fig. 7).
Fig. 7.
Antioxidants diminished 2-ME-induced ROS generation (A, B) and decreased the loss of the mitochondrial membrane potential (C, D) in 2-ME treated SK-N-SH (A, C) and SH-SY5Y (B, D) cells. SK-N-SH cells were treated with 4 μM 2-ME alone, or in combination with 100 μM VC, 50 μM L-GSH or 100 nM MitoQ10 for 72 h. SH-SY5Y cells were treated with 2 μM 2-ME alone, or in combination with 50 μM VC, 100 μM L-GSH or 400 nM MitoQ10 for 72 h. Analysis of ROS and the mitochondrial membrane potential were performed using a fluorophotometer as described in Methods. The data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. *P < 0.05 vs. control, # P < 0.05 vs. 2-ME treated group.
3.6. MitoQ10 significantly increased the Bcl-2/Bax ratio, decreased the activation of caspase-9 and caspase-3, and the up-regulation of AIF in 2-ME exposed SK-N-SH cells
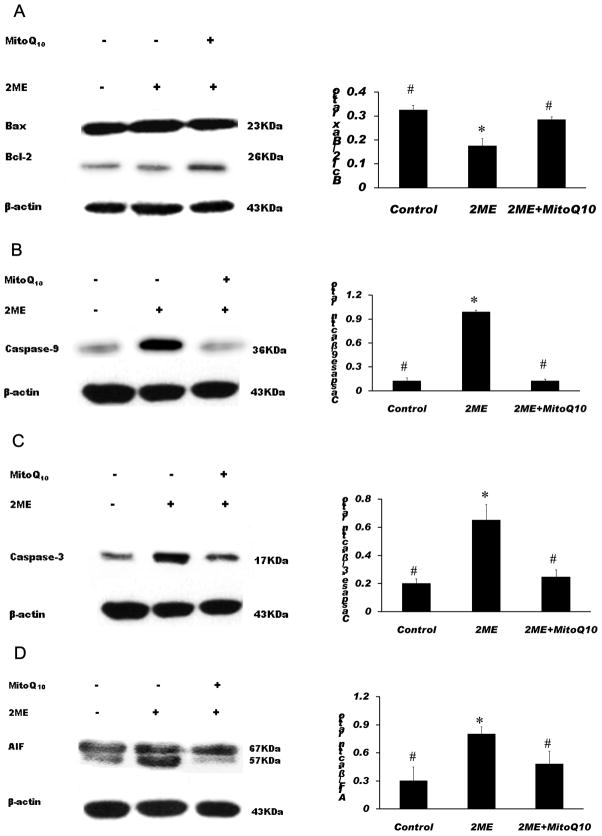
To further confirm the role of ROS in 2-ME-induced apoptosis, the effects of MitoQ10 on the expression of anti-apoptotic and pro-apoptotic proteins were determined in SK-N-SH cells. Western blot analysis showed that MitoQ10 significantly increased the Bcl-2/Bax ratio and decreased the activation of caspase-3 and 9 in 2-ME exposed SK-N-SH cells (Fig. 8, A-C), indicating that MitoQ10 can reverse the down-regulation of anti-apoptotic protein Bcl-2 and up-regulation of pro-apoptotic protein Bax as well as the activation of caspase-9 and caspase-3 induced by 2-ME. In addition, the expression of AIF was also analyzed in SK-N-SH cells to determine whether AIF is involved in 2-ME-induced apoptosis. We found that treatment with 2-ME resulted in a significant increase in AIF expression and that MitoQ10 can significantly reduce 2-ME-induced increase in AIF expression (Fig. 8, D).
Fig. 8.
MitoQ10 significantly increased the Bcl-2/Bax ratio (A), decreased the activation of caspase-9 (B) and caspase-3 (C), and decreased the expression of AIF (D) in 2-ME exposed SK-N-SH cells. Cells were treated with 5 μM 2-ME alone or in combination with 100 nM MitoQ10 for 48 h. Western blot was performed to analyze the levels of Bcl-2, Bax, caspase-9, caspase-3 and AIF proteins. Western blots show cleavage fragments for caspase-9 (36 KDa) (B) and caspase-3 (17 KDa)(C). The data are expressed as the Bcl-2/Bax ratio (A), ratio of caspase-9/β-actin (B), ratio of caspase-3/β-actin (C), or ratio of AIF/ β-actin (D), respectively and represent the mean ± SEM of three separate experiments. *P < 0.05 vs. control, # P < 0.05 vs. 2-ME treated group.
4. Discussion
Cancer is a disease characterized by proliferation disorder and apoptosis obstacle, and as such, the inhibition of proliferation and induction of apoptosis have been considered to be one of the most efficient methods of cancer therapy. Some anticancer agents inhibit proliferation through interfering with the processes of cell cycle, and others induce cell death by apoptosis. In this study, the results of cell viability assay indicated that 2-ME significantly inhibited SK-N-SH and SH-SY5Y cell proliferation. In addition, 2-ME significantly increased apoptosis in SK-N-SH and SH-SY5Y cells. These results support that 2-ME can effectively reduce the malignancy and suppressed the regeneration potential of neuroblastoma cells.
Because cancer cells generate high levels of ROS from their active metabolism associated with the dysregulation of various cellular events and thus are under the intrinsic oxidative stress, it has been suggested that anticancer agents can kill cancer cells by either inducing further ROS generation or impairing antioxidant systems [17, 27, 28]. A numbers of anticancer agents currently used for cancer treatment have been shown to induce an increase in ROS generation [29–32]. However, 2-ME was the first agent which has been shown to increase ROS by inhibiting SOD, one of the most important antioxidant enzymes in cells [20]. While the effects on 2-ME on SOD activity are controversial [21] and may be cell type specific, 2-ME-induced increases in intracellular ROS in cancer cells were confirmed by many groups [33–36]. Studies have shown that 2-ME exerted a minimal cytotoxic effect in normal lymphocytes, which exhibited lower levels of endogenous ROS generation and tolerate treatment with 2-ME [37]. Moreover, the degree of 2-ME-induced apoptosis in primary leukemia cells from CLL patients is significantly correlated with both the basal level of cellular superoxide and the extent of drug-induced increase in superoxide [38]. In addition, studies have shown that the 2-Me-induced increases in peroxide and superoxide anions were correlated with G(2)/M-cycle arrest [39] and that the tubulin filaments in cytoplasm remain intact in 2-ME-treated HK-1 cells pretreated with superoxide dismutase mimetic TEMPO [40]. However, it has also been reported that while targeting of microtubules is the major mechanism of action of 2-ME, induction of cellular ROS may not be critical for 2-ME’s action in epithelial cancer cells [41]. In the present study, 2-ME dose-dependently increased ROS and the loss of mitochondrial membrane potential in SK-N-SH and SH-SY5Y cells, indicating that 2-ME can increase oxidative stress and damage the mitochondria membrane. These findings are consistent with a number of other studies that have demonstrated that 2-ME potently induced ROS generation and apoptosis in human leukemia cells [20, 42], Ewing sarcoma-derived cells [43] and gastric carcinoma cells [39].
In this study, we have also shown that 2-ME inhibited Bcl-2 expression and increased Bax expression, leading to the decrease in the Bcl-2/Bax ratio. 2-ME treatment also increased the activation of caspase-9 and caspase-3 in SK-N-SH cells. It is well known that mitochondria is the major source of ROS in the cells and play an important role in apoptosis [44–46]. Bcl-2 family of proteins, including anti-apoptotic protein Bcl-2 and pro-apoptotic protein Bax plays an important role in mitochondrial dependent caspase activation [47–49]. The results from this study strongly suggest that 2-ME-induced ROS can down regulate Bcl-2 and up regulate Bax, which induces the outer mitochondrial membrane permeabilization and the loss of mitochondrial potential, and eventually activates caspase-9 and caspase-3 and results in apoptotic death of human neuroblastoma cells.
While a numbers of studies have demonstrated that caspase activation is involved in 2-ME-induced apoptosis [39, 43], a study using the pan caspase inhibitor Z-VAD-FMK has shown that 2-ME-induced apoptosis in rat DS-sarcoma cells is independent of caspase activation [50]. In the present study, we have shown that antioxidant MitoQ10 decreased both 2-ME-induced caspase-3 and 9 activation and apoptosis, thus providing evidence for a role of caspase activation in 2-ME-induced apoptosis in SK-N-SH cells. In addition, the fact that MitoQ10 can also significantly diminished 2-ME-induced up-regulation of AIF suggests that both caspase-dependent and caspase-independent mechanisms contribute to the pro-apoptotic effects of 2-ME.
That the antioxidants, VC, L-GSH and MitoQ10 were shown to decrease 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells further supports a role of ROS in 2-ME-induced apoptosis in neuroblastoma cells. L-GSH is an intracellular antioxidant and is the most easily absorbed form of glutathione. GSH not only directly confers an antioxidant protection against ROS but also contributes to the maintenance of the antioxidant activity of other antioxidant enzymes. MitoQ10 is a recently developed mitochondria-targeted antioxidant, which can be directly delivered to the mitochondria [51]. In mitochondria, MitoQ10 can be reduced to its active ubiquinol form, which is an effective antioxidant that prevents lipid peroxidation and mitochondrial damage [52,53]. MitoQ10 is also orally active, has been found in rat tissues after administration in the drinking water and has been shown to be able to protect against tissue damage [53, 54]. In the present study, we found that VC, L-GSH and MitoQ10 diminished the 2-ME-induced elevation of ROS and decreased the loss of the mitochondrial membrane potential in the cells exposed to 2-ME. MitoQ10 also significantly increased the Bcl-2/Bax ratio and decreased the activation of caspase-9 and caspase-3 and the up-regulation of AIF in 2-ME exposed SK-N-SH cells. In addition, VC, L-GSH and MitoQ10 significantly attenuated the antiproliferation effects of 2-ME and prevented 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells. These data showed strong evidence that ROS played an important role in 2-ME-induced apoptosis in neuroblastoma cells.
In summary, the results of this study demonstrate that 2-ME has a significant anticancer effect and can induce proliferation inhibition and apoptosis in human neuroblastoma cells. The findings from this study also suggest that ROS is involved in 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells. This hypothesis is further supported by the results that have shown that co-treatment with antioxidants decreased the generation of ROS and the loss of the mitochondrial membrane potential, increased the Bcl-2/Bax ratio, decreased 2-ME-induced activation of caspase-9 and caspase-3, and the up-regulation of AIF, and prevented 2-ME-induced apoptosis in neuroblastoma cells. The discovery of ROS as a novel mechanism involved in 2-ME-induced apoptosis in SK-N-SH and SH-SY5Y cells provides a novel framework for developing therapeutic treatments for human neuroblastoma.
Research highlights.
2-ME inhibited the proliferation and induced apoptosis in human neuroblastoma cells.
2-ME treatment also resulted in the generation of ROS and mitochondrial injury.
Co-treatment with antioxidants decreased 2-ME-induced ROS and mitochondrial injury.
Treatment with antioxidants prevented 2-ME-induced apoptosis in neuroblastoma cells.
Acknowledgments
This work is supported by the Henan Science and Technology Innovation of Outstanding Talents Project. Dr. Shao-yu Chen is supported by NIH grant AA017446.
Footnotes
Conflict of Interest Statement None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brodeur GM. Neuroblastoma: biological insights into a clinical enigma. Nat Rev Cancer. 2003;3:203–216. doi: 10.1038/nrc1014. [DOI] [PubMed] [Google Scholar]
- 2.Goldsby RE, Matthay KK. Neuroblastoma: evolving therapies for a disease with many faces. Paediatr Drugs. 2004;6:107–122. doi: 10.2165/00148581-200406020-00004. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369:2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 4.De Bernardi B, Nicolas B, Boni L, Indolfi P, Carli M, Cordero L, Montezemolo Di, Donfrancesco A, Pession A, Provenzi M, di Cataldo A, Rizzo A, Tonini GP, Dallorso S, Conte M, Gambini C, Garaventa A, Bonetti F, Zanazzo A, D’Angelo P, Bruzzi P. Disseminated neuroblastoma in children older than one year at diagnosis: comparable results with three consecutive high-dose protocols adopted by the Italian Co-Operative Group for Neuroblastoma. J Clin Oncol. 2003;21:1592–1601. doi: 10.1200/JCO.2003.05.191. [DOI] [PubMed] [Google Scholar]
- 5.Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children’s Cancer Group. N Engl J Med. 1999;341:1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 6.Sawada T, Kidowaki T, Sakamoto I, Hashida T, Matsumura T, Nakagawa M, Kusunoki T. Neuroblastoma. Mass screening for early detection and its prognosis. Cancer. 1984;53:2731–2735. doi: 10.1002/1097-0142(19840615)53:12<2731::aid-cncr2820531232>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 7.Nishi M, Miyake H, Takeda T, Shimada M, Takasugi N, Sato Y, Hanai J. Effects of the mass screening of neuroblastoma in Sapporo City. Cancer. 1987;60:433–436. doi: 10.1002/1097-0142(19870801)60:3<433::aid-cncr2820600326>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 8.Zhu BT, Conney AH. Is 2-methoxyestradiol an endogenous estrogen metabolite that inhibits mammary carcinogenesis? Cancer Res. 1998;58:2269–2277. [PubMed] [Google Scholar]
- 9.Pribluda VS, Gubish ER, Jr, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer Metastasis Rev. 2000;19:173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- 10.Fotsis T, Zhang Y, Pepper MS, Adlercreutz H, Montesano R, Nawroth PP, Schweigerer L. The endogenous oestrogen metabolite 2-methoxyoestradiol inhibits angiogenesis and suppresses tumour growth. Nature. 1994;368:237–239. doi: 10.1038/368237a0. [DOI] [PubMed] [Google Scholar]
- 11.Mooberry SL. New insights into 2-methoxyestradiol, a promising antiangiogenic and antitumor agent. Curr Opin Oncol. 2003;15:425–430. doi: 10.1097/00001622-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Dubey RK, Jackson EK. Potential vascular actions of 2-methoxyestradiol. Trends Endocrinol Metab. 2009;20:374–379. doi: 10.1016/j.tem.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fotopoulou C, Baumunk D, Schmidt SC, Schumacher G. Additive growth inhibition after combined treatment of 2-methoxyestradiol and conventional chemotherapeutic agents in human pancreatic cancer cells. Anticancer Res. 2010;30:4619–4624. [PubMed] [Google Scholar]
- 14.Kulke MH, Chan JA, Meyerhardt JA, Zhu AX, Abrams TA, Blaszkowsky LS, Regan E, Sidor C, Fuchs CS. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2010 doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller VM. Estrogen metabolomics: a physiologist’s perspective. Hypertension. 2010;56:816–818. doi: 10.1161/HYPERTENSIONAHA.110.154385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pelicano H, Carney D, Huang P. ROS stress in cancer cells and therapeutic implications. Drug Resist Updat. 2004;7:97–110. doi: 10.1016/j.drup.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 17.Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer Cell. 2006;10:175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Waris G, Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, Marrack P. Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100:15035–15040. doi: 10.1073/pnas.1936213100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang P, Feng L, Oldham EA, Keating MJ, Plunkett W. Superoxide dismutase as a target for the selective killing of cancer cells. Nature. 2000;407:390–395. doi: 10.1038/35030140. [DOI] [PubMed] [Google Scholar]
- 21.Kachadourian R, Liochev SI, Cabelli DE, Patel MN, Fridovich I, Day BJ. 2-methoxyestradiol does not inhibit superoxide dismutase. Arch Biochem Biophys. 2001;392:349–353. doi: 10.1006/abbi.2001.2455. [DOI] [PubMed] [Google Scholar]
- 22.Sattler M, Quinnan LR, Pride YB, Gramlich JL, Chu SC, Even GC, Kraeft SK, Chen LB, Salgia R. 2-methoxyestradiol alters cell motility, migration, and adhesion. Blood. 2003;102:289–296. doi: 10.1182/blood-2002-03-0729. [DOI] [PubMed] [Google Scholar]
- 23.Fajardo I, Quesada AR, Nunez de Castro I, Sanchez-Jimenez F, Medina MA. A comparative study of the effects of genistein and 2-methoxyestradiol on the proteolytic balance and tumour cell proliferation. Br J Cancer. 1999;80:17–24. doi: 10.1038/sj.bjc.6690315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manca P, Chisu V. 2-methoxyestradiol induces morpho-functional changes and impairs the microtubular system in mouse neuroblastoma and rat glioma cells. Arch Ital Biol. 2010;148:11–21. [PubMed] [Google Scholar]
- 25.Wassberg E. Angiostatic treatment of neuroblastoma. Ups J Med Sci. 1999;104:1–24. doi: 10.3109/03009739909178953. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa-Yagi Y, Ogane N, Inoki Y, Kitoh N. The endogenous estrogen metabolite 2-methoxyestradiol induces apoptotic neuronal cell death in vitro. Life Sci. 1996;58:1461–1467. doi: 10.1016/0024-3205(96)00116-6. [DOI] [PubMed] [Google Scholar]
- 27.Pan J, She M, Xu ZX, Sun L, Yeung SC. Farnesyltransferase inhibitors induce DNA damage via reactive oxygen species in human cancer cells. Cancer Res. 2005;65:3671–3681. doi: 10.1158/0008-5472.CAN-04-2744. [DOI] [PubMed] [Google Scholar]
- 28.Pani G, Colavitti R, Bedogni B, Fusco S, Ferraro D, Borrello S, Galeotti T. Mitochondrial superoxide dismutase: a promising target for new anticancer therapies. Curr Med Chem. 2004;11:1299–1308. doi: 10.2174/0929867043365297. [DOI] [PubMed] [Google Scholar]
- 29.Atsumi T, Tonosaki K, Fujisawa S. Comparative cytotoxicity and ROS generation by curcumin and tetrahydrocurcumin following visible-light irradiation or treatment with horseradish peroxidase. Anticancer Res. 2007;27:363–371. [PubMed] [Google Scholar]
- 30.Meng LH, Ding J. Salvicine, a novel topoisomerase II inhibitor, exerts its potent anticancer activity by ROS generation. Acta Pharmacol Sin. 2007;28:1460–1465. doi: 10.1111/j.1745-7254.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- 31.Benhar M, Dalyot I, Engelberg D, Levitzki A. Enhanced ROS production in oncogenically transformed cells potentiates c-Jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol Cell Biol. 2001;21:6913–6926. doi: 10.1128/MCB.21.20.6913-6926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asumendi A, Morales MC, Alvarez A, Arechaga J, Perez-Yarza G. Implication of mitochondria-derived ROS and cardiolipin peroxidation in N-(4-hydroxyphenyl)retinamide-induced apoptosis. Br J Cancer. 2002;86:1951–1956. doi: 10.1038/sj.bjc.6600356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin HL, Liu TY, Chau GY, Lui WY, Chi CW. Comparison of 2-methoxyestradiol-induced, docetaxel-induced, and paclitaxel-induced apoptosis in hepatoma cells and its correlation with reactive oxygen species. Cancer. 2000;89:983–994. [PubMed] [Google Scholar]
- 34.Mates JM, Sanchez-Jimenez FM. Role of reactive oxygen species in apoptosis: implications for cancer therapy. Int J Biochem Cell Biol. 2000;32:157–170. doi: 10.1016/s1357-2725(99)00088-6. [DOI] [PubMed] [Google Scholar]
- 35.She MR, Li JG, Guo KY, Lin W, Du X, Niu XQ. Requirement of reactive oxygen species generation in apoptosis of leukemia cells induced by 2-methoxyestradiol. Acta Pharmacol Sin. 2007;28:1037–1044. doi: 10.1111/j.1745-7254.2007.00604.x. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Hileman EO, Plunkett W, Keating MJ, Huang P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood. 2003;101:4098–4104. doi: 10.1182/blood-2002-08-2512. [DOI] [PubMed] [Google Scholar]
- 37.Chauhan D, Catley L, Hideshima T, Li G, Leblanc R, Gupta D, Sattler M, Richardson P, Schlossman RL, Podar K, Weller E, Munshi N, Anderson KC. 2-Methoxyestradiol overcomes drug resistance in multiple myeloma cells. Blood. 2002;100:2187–2194. doi: 10.1182/blood-2002-02-0376. [DOI] [PubMed] [Google Scholar]
- 38.Gao N, Rahmani M, Dent P, Grant S. 2-Methoxyestradiol-induced apoptosis in human leukemia cells proceeds through a reactive oxygen species and Akt-dependent process. Oncogene. 2005;24:3797–3809. doi: 10.1038/sj.onc.1208530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin HL, Liu TY, Wu CW, Chi CW. 2-Methoxyestradiol-induced caspase-3 activation and apoptosis occurs through G(2)/M arrest dependent and independent pathways in gastric carcinoma cells. Cancer. 2001;92:500–509. doi: 10.1002/1097-0142(20010801)92:3<500::aid-cncr1348>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Ting CM, Lee YM, Wong CK, Wong AS, Lung HL, Lung ML, Lo KW, Wong RN, Mak NK. 2-Methoxyestradiol induces endoreduplication through the induction of mitochondrial oxidative stress and the activation of MAPK signaling pathways. Biochem Pharmacol. 2010;79:825–841. doi: 10.1016/j.bcp.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Chua YS, Chua YL, Hagen T. Structure activity analysis of 2-methoxyestradiol analogues reveals targeting of microtubules as the major mechanism of antiproliferative and proapoptotic activity. Mol Cancer Ther. 2010;9:224–235. doi: 10.1158/1535-7163.MCT-09-1003. [DOI] [PubMed] [Google Scholar]
- 42.Gao N, Rahmani M, Shi X, Dent P, Grant S. Synergistic antileukemic interactions between 2-medroxyestradiol (2-ME) and histone deacetylase inhibitors involve Akt down-regulation and oxidative stress. Blood. 2006;107:241–249. doi: 10.1182/blood-2005-06-2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djavaheri-Mergny M, Wietzerbin J, Besancon F. 2-Methoxyestradiol induces apoptosis in Ewing sarcoma cells through mitochondrial hydrogen peroxide production. Oncogene. 2003;22:2558–2567. doi: 10.1038/sj.onc.1206356. [DOI] [PubMed] [Google Scholar]
- 44.Guessous I, Cornuz J, Paccaud F. Lung cancer screening: current situation and perspective. Swiss Med Wkly. 2007;137:304–311. doi: 10.4414/smw.2007.11582. [DOI] [PubMed] [Google Scholar]
- 45.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 46.Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 47.Attalla H, Westberg JA, Andersson LC, Adlercreutz H, Makela TP. 2-Methoxyestradiol-induced phosphorylation of Bcl-2: uncoupling from JNK/SAPK activation. Biochem Biophys Res Commun. 1998;247:616–619. doi: 10.1006/bbrc.1998.8870. [DOI] [PubMed] [Google Scholar]
- 48.Gao Z, Shao Y, Jiang X. Essential roles of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J Biol Chem. 2005;280:38271–38275. doi: 10.1074/jbc.M506488200. [DOI] [PubMed] [Google Scholar]
- 49.Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, Maundrell K, Gambale F, Sadoul R, Martinou JC. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- 50.Lambert C, Apel K, Biesalski HK, Frank J. 2-methoxyestradiol induces caspase-independent, mitochondria-centered apoptosis in DS-sarcoma cells. Int J Cancer. 2004;108:493–501. doi: 10.1002/ijc.11579. [DOI] [PubMed] [Google Scholar]
- 51.Graham D, Huynh NN, Hamilton CA, Beattie E, Smith RA, Cocheme HM, Murphy MP, Dominiczak AF. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 52.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 53.Murphy MP, Smith RA. Drug delivery to mitochondria: the key to mitochondrial medicine. Adv Drug Deliv Rev. 2000;41:235–250. doi: 10.1016/s0169-409x(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 54.Smith RA, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]