Abstract
Long-term aging of potato (Solanum tuberosum) seed-tubers resulted in a loss of patatin (40 kD) and a cysteine-proteinase inhibitor, potato multicystatin (PMC), as well as an increase in the activities of 84-, 95-, and 125-kD proteinases. Highly active, additional proteinases (75, 90, and 100 kD) appeared in the oldest tubers. Over 90% of the total proteolytic activity in aged tubers was sensitive to trans-epoxysuccinyl-l-leucylamido (4-guanidino) butane or leupeptin, whereas pepstatin was the most effective inhibitor of proteinases in young tubers. Proteinases in aged tubers were also inhibited by crude extracts or purified PMC from young tubers, suggesting that the loss of PMC was responsible for the age-induced increase in proteinase activity. Nonenzymatic oxidation, glycation, and deamidation of proteins were enhanced by aging. Aged tubers developed “daughter” tubers that contained 3-fold more protein than “mother” tubers, with a polypeptide profile consistent with that of young tubers. Although PMC and patatin were absent from the older mother tubers, both proteins were expressed in the daughter tubers, indicating that aging did not compromise the efficacy of genes encoding PMC and patatin. Unlike the mother tubers, proteinase activity in daughter tubers was undetectable. Our results indicate that tuber aging nonenzymatically modifies proteins, which enhances their susceptibility to breakdown; we also identify a role for PMC in regulating protein turnover in potato tubers.
Potato (Solanum tuberosum) seed-tubers are a model system for studying the process of aging in plants. The tubers can be stored (at 4°C and 95% RH) for to 3 years without a loss of viability. However, storage (aging) beyond about 8 months effects a progressive decline in apical dominance, rooting ability, and sprout vigor (Kumar and Knowles, 1993a). In addition to changes in growth potential, aging is accompanied by increased respiration of tubers (Kumar and Knowles, 1996a), oxidative stress (Kumar and Knowles, 1996b), lipid peroxidation (Kumar and Knowles, 1993b), and decreased protein content (Kumar and Knowles, 1993c). Although protein loss is partly due to reduced synthesis (Kumar and Knowles, 1993c), the contribution of proteolysis and the mechanisms by which proteins become damaged and subsequently targeted for degradation with advancing age are unknown. Processes that may lead to protein degradation during aging include (a) increased accessibility of proteins to proteinases resulting from decompartmentation, (b) molecular modifications to polypeptides that enhance proteolysis, and (c) increased activity of proteinases (Dalling, 1987).
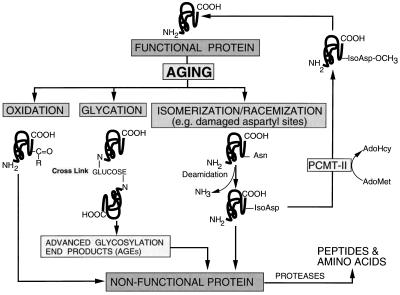
Oxidation, glycation, and isomerization/racemization of amino acid residues of proteins have been identified as nonenzymatic mechanisms that can adversely affect structure and function (Fig. 1), rendering proteins more susceptible to proteolysis during aging (Dalling, 1987; Stadtman, 1992; Luthra and Balasubramanian, 1993; Eckardt and Pell, 1995). Oxidative stress contributes to the formation of carbonyl derivatives on amino acid residues of proteins (Dalling, 1987; Oliver et al., 1987; Levine et al., 1990). For example, carbonyl content and susceptibility of Rubisco to proteolysis increased during oxidative stress (Ferriera and Shaw, 1989; Penarrubia and Moreno, 1990; Garcia-Ferris and Moreno, 1993; Eckardt and Pell, 1995). Similarly, oxidative stress caused by the inhibition of catalase by aminotriazole in maize seedlings resulted in a 2-fold increase in protein carbonyl content (Prasad, 1997). The increased oxidative stress accompanying aging of potato tubers may provide an ideal environment for oxidation of proteins.
Figure 1.
Schematic diagram showing several nonenzymatic mechanisms that could affect protein structure and function in aging potato tubers. Protein modifications that may accompany aging include oxidation (increased carbonyl groups), glycation (reaction of amino acids with reducing sugars leading to protein cross-linking), and deamidation/isomerization/racemization of asparaginyl and aspartyl residues. Although these molecular modifications can target proteins for proteolysis, deamidation-mediated increases in isoaspartyl residues creates substrates for PCMT-II, which can restore function of the affected proteins. Effects of tuber age on such molecular modifications are presented in Figures 9 and 10 and Table III.
Amino groups of proteins can react with aldehyde or keto groups of reducing sugars through a Schiff-base reaction, yielding brown fluorescent pigments known as advanced glycation end products (Luthra and Balasubramaniyan, 1993). Proteins thus modified tend to form cross-links (Fig. 1) that can destroy protein function (Wettlaufer and Leopold, 1991). A number of age-related diseases in humans are attributed to protein glycation. For example, in diabetics, elevated blood Glc is associated with cataracts (Monnier et al., 1979), accelerated aging, and vascular narrowing (Brownlee et al., 1986; Cerami et al., 1987). In light of the substantial increase in reducing sugar concentration of tubers during aging (Kumar and Knowles, 1993b), it was of interest to determine the extent of age-induced protein glycation.
Proteins are also susceptible to nonenzymatic modification by deamidation-mediated conversion of l-asparaginyl to l-isoaspartyl residues (Fig. 1). Although proteins containing isomerized residues can be targeted for degradation, they are also substrates for PCMT (type II), which can restore protein function. Repair to such damaged proteins involves methylation, using AdoMet as a methyl donor. PCMT is a cytosolic “housekeeping” enzyme with specificity for the recognition and repair of altered aspartyl residues (Galletti et al., 1995), and has been detected in 45 plant species belonging to 23 families (Mudgett et al., 1997). Changes in PCMT activity with advancing tuber age were thus characterized as an indicator of deamidation-mediated damage to proteins.
In addition to reduced protein synthesis and enhanced susceptibility of proteins to proteolysis, advancing tuber age may contribute to loss in the ability to synthesize proteinase inhibitors and thus to protein catabolism. Potato tubers contain a proteinase inhibitor, PMC (Rodis and Hoff, 1984; Walsh and Strikland, 1993). With its multiple inhibitory domains, PMC (85 kD) has the capacity for simultaneous inhibition of several Cys-proteinase molecules (Walsh and Strickland, 1993). The effect of aging on PMC and proteinase levels is unknown. Using potato as a model system, we examined potential mechanisms for age-induced protein loss and the extent to which proteins become nonenzymatically modified during aging.
MATERIALS AND METHODS
Tuber Aging
Certified potato (Solanum tuberosum L. cv Russet Burbank) seed-tubers, obtained directly from a local grower at harvest, were aged for 3 to 30 months in storage (at 4°C and 95% RH). These storage conditions were inhibitory to sprouting. Seed-tuber age was calculated from harvest. For discussion, 3- to 6-month-old tubers are considered to be physiologically “young” and tubers stored beyond 12 months are “old.”
Protein Extraction and Analysis
Soluble proteins were extracted from the apical portion (periderm included) of 6-, 18-, and 30-month-old tubers by homogenizing 20 g of fresh tuber tissue with 20 mL of 50 mm potassium phosphate buffer, pH 7.5, containing 2 μg mL−1 leupeptin, 2 μg mL−1 antipain, 5 μg mL−1 aprotinin, 2 mm PMSF, 0.5% (w/v) polyvinyl polypyrrolidone, and 2 mm Na2S2O5. The homogenate was filtered through Miracloth (Calbiochem) and centrifuged at 30,000g for 30 min. All manipulations were at 4°C. Supernatants were stored at −80°C.
After determination of soluble protein (Bradford, 1976), proteins from each of the three ages of tubers were mixed with equal volumes of SDS-sample preparation buffer (62.5 mm Tris, pH 6.8, containing 2% [w/v] SDS, 5% [v/v] β-mercaptoethanol, 25% [v/v] glycerol, and 0.01% [w/v] bromphenol blue) and were compared after electrophoresis (Laemmli, 1970) on 10% polyacrylamide gels (27 μg of protein per lane).
Oxidized and Glycated Protein Determinations
Protein carbonyl content was determined as an index of protein oxidation. Following derivitization of carbonyl groups with DNPH, oxidized proteins in the 60% ammonium sulfate fraction were determined spectrophotometrically. Carbonyl content was quantified using an extinction coefficient of 22,000 m−1 cm−1 (Oliver et al., 1987; Levine et al., 1990). Qualitative determination of oxidized proteins via western analysis followed the methods of Levine et al. (1994). DNPH-treated protein samples (60 μg of protein per lane) were resolved by SDS-PAGE (10% acrylamide gels) and electroblotted to nitrocellulose membrane (Laemmli, 1970; Kumar and Knowles, 1996a). Oxidized proteins were probed with alkaline phosphatase-conjugated monoclonal anti-DNP antibody (1:2,500, Sigma).
Glycated proteins (Amadori products) were quantified spectrophotometrically (A435) using the method of Wettlaufer and Leopold (1991). Quantification of glycated protein was based on a standard curve using 0 to 15 μg of 5-hydroxymethyl-2-furaldehyde (Sigma).
Glycated proteins were also examined by boronate-affinity chromatography. Tuber tissue (10 g fresh weight, as described above) from 3-, 15-, and 27-month-old tubers was homogenized using a mortar and pestle with 10 mL of Tris buffer (100 mm, pH 7.4, containing 0.5 m NaCl, 1 mm EDTA, 0.1% [w/v] NaN3, 1 mm DTT, 0.25 mm PMSF, and 2 mm Na2S2O5), filtered through Miracloth, and centrifuged at 30,000g for 30 min. All manipulations were performed at 4°C. Glycated proteins in the supernatant were isolated using a Glyco-Gel II column that contained immobilized m-aminophenylboronic acid cross-linked to 6% beaded agarose according to the manufacturer's instructions (Pierce). The column (10-mL bed volume) was washed with 50 mL of 0.5% (v/v) acetic acid and equilibrated with 50 mL of binding buffer (250 mm ammonium acetate, pH 8.05, containing 50 mm MgCl2, 0.02% NaN3, and 0.25 mm PMSF). The tuber extract (1000 μL) was loaded on the column, and nonglycated proteins were removed by washing with 110 mL of binding buffer. Glycated proteins were eluted with 50 mL of Tris buffer (100 mm, pH 8.5) containing 200 mm sorbitol and 0.05% (w/v) NaN3. Protein in glycated and nonglycated fractions was quantified by a modified Lowry method (Bensadoun and Weinstein, 1976).
PCMT Activity
PCMT, a substrate-inducible enzyme (Mudgett and Clarke, 1994, 1996), was assayed in the absence of synthetic peptide substrate as an index of endogenous proteins containing deamidated, isomerized, and/or racemized asparaginyl and/or aspartyl residues in 6-, 18-, and 30-month-old tubers. PCMT activity was assessed in tubers stored at 4°C and in tubers acclimated to 23°C for 24 h. Ten grams (fresh weight) of tuber tissue from the apical end (see above) was homogenized with 10 mL of 100 mm Hepes buffer, pH 7.5, containing 1 mm PMSF, 1 mm DTT, 5 g of hydrated polyvinyl polypyrrolidone, 2 mm Na2S2O5, and 10 mm Na2S2O4. The homogenate was filtered through Miracloth and centrifuged at 30,000g for 30 min. All manipulations were performed at 4°C. The supernatant was frozen in liquid nitrogen and stored at −80°C.
PCMT activity assays were according to the method of Mudgett and Clarke (1993) with minor modifications. The reaction medium (40 μL), consisting of 12 μL of enzyme extract, 10 μm [3H-methyl]AdoMet (1.8 Ci mmol−1, NEN; 70–80 Ci mmol−1), and, where indicated, 500 μm synthetic peptide substrate Val-Tyr-Pro-(l-isoAsp)-His-Ala (synthesized by the Macromolecular Structure Analysis Facility, University of Kentucky, Lexington) was incubated at 30°C for 60 min. The reaction was stopped with 40 μL of 200 mm NaOH, containing 1% (w/v) SDS, and 60 μL of the reaction mixture was spotted onto 1.5- × 8-cm pleated filter paper (3MM, Whatman) suspended in the neck of a 20-mL scintillation vial containing 5 mL of Bio-Safe II scintillation cocktail (Research Products International, Mount Prospect, IL). After 2 h the paper was removed and the [3H]methanol captured in the scintillation solution from the vapor phase was determined (Kester et al., 1997).
Proteinase Extraction and Activity
Proteolytic enzymes were extracted from tuber tissue (a composite of three tubers cut from the apical ends, periderm included) with 50 mm potassium citrate buffer, (pH 6.6, 1 g fresh weight mL−1) containing 20 mm KCl, 2 mm MgCl2, 5% (w/v) Suc, 1 mm DTT, 0.5% PVP, and 2 mm Na2S2O5 (Belles et al., 1991) with a homogenizer. The homogenate was filtered through Miracloth, centrifuged at 30,000g for 30 min, and the supernatant was stored at −80°C for subsequent proteinase activity assays. Insoluble protein was also quantified from these tuber extracts. The pellet was suspended in 5 mL of buffer, as described above, and starch was removed by centrifuging twice at 200g for 5 min each time. The insoluble protein remaining in the supernatant was pelleted at 30,000g for 30 min, and solubilized in 1 mL of extraction buffer (described above), containing 0.2% (w/v) Triton X-100. Protein was determined by the Bradford (1976) assay.
Proteolytic activity was assessed spectrofluorometrically with FITC-casein (Sigma) as a substrate (Vera and Conejero, 1988; Belles et al., 1991). The reaction mixture (2.6 mL), containing 150 mm potassium citrate buffer, pH 6.1, 1.5 mm DTT, 513 μg of FITC-casein, and 800 μL of enzyme extract (2.1–2.9 mg of protein), was incubated at 37°C in the dark. At 0, 15, 30, 45, 60, and 90 min, 350 μL of the reaction medium was transferred to Eppendorf tubes containing 100 μL of 40% TCA to stop the reaction. The samples were held on ice for 30 min and centrifuged at 1640g for 20 min. The supernatant (300 μL) was mixed with 3 mL of 500 mm Tris-HCl buffer, pH 8.5, and emission at 520 nm (excitation at 500 nm) was measured on a spectrofluorometer (model SF 330, Varian Instruments, Palo Alto, CA). The pH optimum for FITC-casein hydrolysis by extracts from 30-month-old tubers was determined by profiling activity from pH 4.0 to 9.0 in sodium acetate/potassium citrate/Mes/Tricine (37.5 mm each) buffer.
While FITC-casein-hydrolyzing activity was relatively low in extracts from 6-month-old tubers, activity was high in extracts from 18- and 30-month-old tubers. The ability of extract from 6-month-old tubers to inhibit the FITC-casein-hydrolyzing activity of extract from older tubers was examined. Assays were as described above with the addition of 6-month-old tuber extract as a source of proteinase inhibitor. Extracts from 6-month-old tubers were included, such that the ratio of protein in 18- and 30-month-old extracts to inhibitor protein (6-month-old extract) remained constant at 2.7:1. In an additional study, the heat lability (at 95°C for 10 min) of inhibitory factor(s) in crude extracts from 6-month-old tubers was compared with that of PMC in inhibiting FITC-casein hydrolysis by extracts from 30-month-old tubers.
The relative contributions of different proteinases to proteolytic activity was estimated using class-specific proteinase inhibitors. The inhibition of FITC-casein hydrolysis by 1 mm PMSF, 1 mm 4-(2-aminoethyl) benzenesulfonyl fluoride, 25 μm E-64, 180 μm leupeptin, 70 μm pepstatin, or 2 mm EDTA was assessed by first incubating (at 4°C for 30 min) each inhibitor separately with extracts from 6-, 18-, and 30-month-old tubers. FITC-casein, DTT, and potassium citrate buffer (see above) were then added to the samples and the reactions allowed to continue at 37°C for 60 min. The proteinase inhibitor concentrations indicated were concentrations before the addition of the substrate and buffer. Reactions were stopped with 10% (w/v) TCA, and FITC was quantified spectrofluorometrically as described above. All inhibitors except PMSF and EDTA (Sigma) were prepared according to the manufacturer's instructions (Calbiochem). PMSF and EDTA were prepared in DMSO and distilled water, respectively.
Proteinase activities were also compared on 10% polyacrylamide gels (Laemmli, 1970) containing 0.1% (w/v) gelatin (Heussen and Dowdle, 1980; Michaud et al., 1993, 1994). Enzyme extracts were diluted 2-fold in a sample preparation buffer (Michaud et al., 1994), incubated for 10 min at 37°C, and subjected to SDS-PAGE at 4°C (10% running gel) for 60 or 120 min to resolve the low- and high-molecular-mass proteinases, respectively (72 μg of protein per lane). The gels were then washed in 2.5% (w/v) Triton X-100 for 30 min (23°C), and incubated in 150 mm potassium citrate buffer, pH 6.1, containing 5 mm l-Cys and 0.1% (w/v) Triton X-100 for 20 h (at 37°C). The gels were stained with Coomassie blue, and gelatinolytic activity was assessed as achromatic zones on a blue background following destaining.
Isolation of Patatin and PMC
Patatin was isolated from 6-month-old tubers to assess the effects of tuber age on patatinolytic activity. Protein was precipitated from tuber extract (see above) with 80% ammonium sulfate, solubilized in SDS-PAGE sample preparation buffer (Laemmli, 1970), and 120 to 165 mg was loaded onto a column (model 491, Prep Cell, Bio-Rad) containing 10% acrylamide. Electrophoresis was at a constant 200 V for 20 h. Fractions were collected and analyzed for patatin by running 10-μL aliquots on minigels. Fractions containing patatin were pooled and concentrated by ultrafiltration. PMC was isolated from the peel of young (5- to 7-month-old) tubers according to the method of Rodis and Hoff (1984). Purity of the isolated PMC was verified via SDS-PAGE (Laemmli, 1970) and western analysis (Walsh and Strickland, 1993).
PMC Inhibition of Age-Induced Patatinolytic Activity
We examined the ability of extracts from 26-month-old tubers to degrade exogenous patatin in the presence and absence of PMC. The reaction medium (250 μL) consisted of 100 μL of 150 mm potassium citrate buffer, pH 6.1, 145 μg of patatin (see above), and 0 or 36 μg of PMC. The reaction was started by adding 50 μL (122 μg of protein) of the 26-month-old tuber extract, incubated at 37°C, and terminated at 0, 15, and 30 min with 10% (w/v) TCA (final concentration). The protein was then pelleted at 1640g for 20 min and separated by SDS-PAGE (Laemmli, 1970).
Tissue Prints of PMC
The tissue-printing technique of Varner (1992) was used to detect PMC in 6-, 18-, and 30-month-old tubers. One-millimeter-thick longitudinal sections of tubers were cut and placed immediately on 0.25 μm nitrocellulose for 5 min. The blots were then blocked and probed for PMC with anti-PMC antibody (Walsh and Strickland, 1993).
Mother/Daughter Tuber System
Tubers that had been stored for 29 months at 4°C (95% RH) were placed in the dark at 23°C for approximately 2 months to develop daughter tubers. This system was then used to assess whether the older mother tubers have the ability to produce daughter tubers with polypeptide profiles, patatin content, proteinase activities, and PMC levels comparable to those of younger mother tubers.
RESULTS AND DISCUSSION
Tuber Age Affects Total Protein, Patatin, and PMC Content
Soluble protein concentration (on a fresh-weight basis) decreased, whereas insoluble protein increased as tubers aged from 6 to 30 months (Table I). The loss of soluble proteins was not simply a consequence of age-induced changes in tuber fresh weight, as similar effects have been documented on a dry-weight basis (Kumar and Knowles, 1993c). The increase in insoluble protein suggests that aging may induce molecular modifications to proteins that effect reduced solubility (e.g. nonenzymatic glycation).
Table I.
Changes in soluble and insoluble protein with advancing tuber age
| Tuber Age | Protein Fraction
|
||
|---|---|---|---|
| Soluble | Insoluble | Total | |
| months | mg protein g−1 fresh wt | ||
| 6 | 3.75 | 0.103 | 3.85 |
| 18 | 3.52 | 0.186 | 3.73 |
| 30 | 2.64 | 0.192 | 2.83 |
Soluble and insoluble protein were quantified in 30,000g supernatants and destarched pellets, respectively. Insoluble protein was solubilized with Triton X-100. Linear trends were significant (P < 0.01) for soluble, insoluble, and total protein.
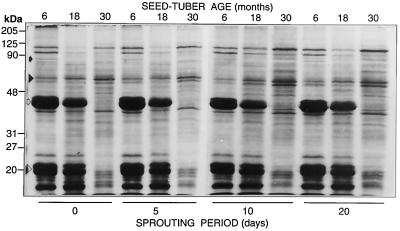
Changes in soluble proteins over 20 d of sprouting were age dependent. Tuber age influenced polypeptides with molecular masses of 110, 98, 85, 58, 40, 35, 25, and 20 kD and below (Fig. 2). The greatest effect of age was on loss of patatin (40 kD, Fig. 2), the major storage glycoprotein that makes up 20% to 40% of soluble protein in tubers (Galliard, 1971; Racusen and Foote, 1980; Paiva et al., 1983; Bohac, 1991; Strickland et al., 1995). Patatin is also a nonspecific lipid acyl hydrolase with activity similar to that of the phospholipases A1, A2, B, glycolipase, sulfolipase, monoacylglycerol lipase, and esterase (Hirayama et al., 1975; Galliard, 1980; Strickland et al., 1995).
Figure 2.
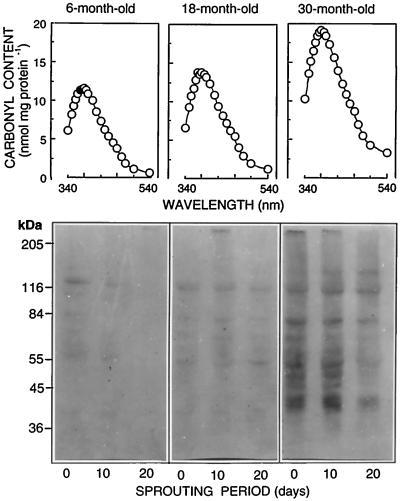
SDS-PAGE of soluble proteins (27 μg/lane) from 6-, 18-, and 30-month-old tubers during sprouting. Note the loss of the 85-kD PMC (black arrow) and 40-kD patatin (white arrow) with advancing tuber age. PMC was present in 6-month-old tubers and nondetectable in 18- and 30-month-old tubers, as confirmed by western analysis with anti-PMC (see Fig. 4B, inset). Aging also effected an increase in glutathione reductase (58 kD, black arrowhead) as well as the loss of several other unidentified proteins (e.g. 20 kD, white arrowhead).
Considering its multiple roles as a storage protein and as a potential antioxidant (Al-Saikhan et al., 1995) and its enzymatic involvement in lipid metabolism, the loss of patatin may contribute to many of the changes in tuber physiology that have been associated with advancing age. These changes include increased peroxidation of membrane lipids and reduced membrane integrity (Knowles and Knowles, 1989; Kumar and Knowles, 1993b), increased oxidative stress (Kumar and Knowles, 1996b), and loss of sprouting vigor (Kumar and Knowles, 1993a). Increases in the relative levels of specific polypeptides with advancing tuber age (e.g. 110, 58, and 35 kD; Fig. 2) could reflect a gel-loading bias caused by the age-induced loss of patatin, because the gels were loaded on an equal-protein basis. However, increases in the concentrations of these proteins over the sprouting interval were unique to the 30-month-old tubers. The 58-kD polypeptide has been previously identified as glutathione reductase (Kumar and Knowles, 1996b).
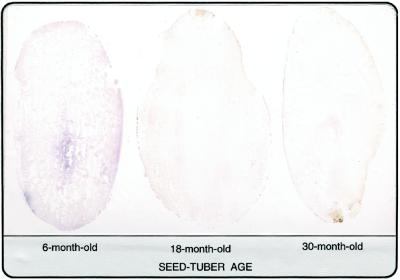
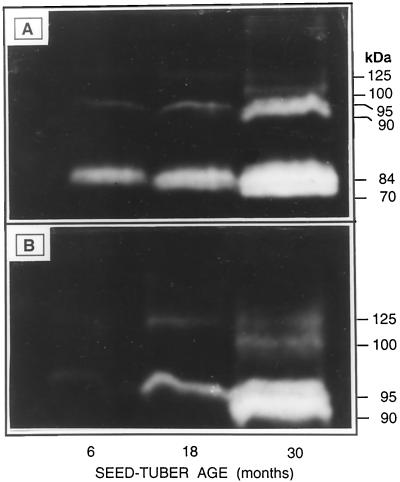
Another polypeptide lost during tuber aging was PMC (85 kD; Fig. 2), a Cys-proteinase inhibitor (Rodis and Hoff, 1984) that can simultaneously bind and inhibit the activities of as many as eight proteinase molecules (Walsh and Strickland, 1993). The age-induced loss of PMC was verified by tissue printing (Fig. 3) and western analysis (see blot in Fig. 4B). Consistent with results from SDS-PAGE (Fig. 2), PMC was present in the tissue print and western blot from 6-month-old tubers, but was undetectable in those from 18- and 30-month-old tubers (Figs. 3 and 4).
Figure 3.
Tissue prints of PMC in longitudinal sections of 6-, 18-, and 30-month-old tubers. PMC is indicated by purple coloration. Note the absence of PMC in the 18- and 30-month-old tubers.
Figure 4.
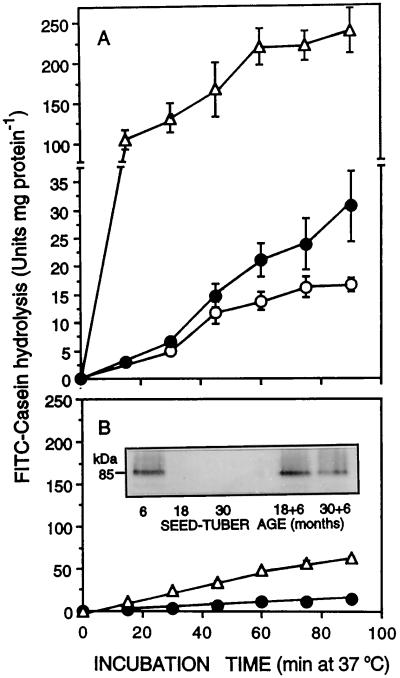
A, FITC-casein hydrolyzing proteinase activity of extracts from 6- (○), 18- (•), and 30-month-old (▵) tubers. B, Inhibition of the proteinase activity in extracts from 18- (•) and 30-month-old (▵) tubers with extracts from 6-month-old tubers. Data are averages ± se of three replicates (se values are eclipsed by the symbols in B). Inset shows immunoblots of PMC (32 μg of protein per lane) in extracts from tubers, before and after the addition of 6-month-old extract to extracts from 18- and 30-month-old tubers.
Our previous studies have shown that tuber aging is accompanied by a decrease in the capacity for protein synthesis and an increase in free amino nitrogen (Kumar and Knowles, 1993c), suggesting that the age-induced decline in protein content is mediated by increased proteolysis. The loss of PMC could be a controlling factor in this process. Therefore, we investigated the contribution of proteolysis to the age-induced loss of proteins in general, and patatin in particular. We compared the proteolytic activity in extracts from 6-, 18-, and 30-month-old tubers. Extracts from 6-month-old tubers hydrolyzed FITC-casein relatively slowly (Fig. 4A). In contrast, FITC-casein-hydrolyzing activity in extracts from 18- and 30-month-old tubers was substantial and much greater than what could be accounted for by age-induced declines in nonproteinase tuber proteins (e.g. selective loss of patatin from older tubers). When compared on a tuber fresh-weight basis, proteinase activity from 18- and 30-month-old tubers was 1.6- and 10-fold greater, respectively, than those from 6-month-old tubers (data not shown).
Higher proteolytic activity in older tubers could have been the result of increased synthesis and/or activation of proteinases, loss of proteolytic inhibitors such as PMC, or a combination of these during aging. Extracts from 6-month-old tubers effectively inhibited the FITC-casein-hydrolyzing activities of extracts from 18- and 30-month-old tubers (Fig. 4B), reflecting the presence of a proteinase inhibitor or inhibitors in younger tubers. Western analysis indicated that PMC was present in 6-month-old tubers but was absent from 18- and 30-month-old tubers (Fig. 4B). To determine whether the inhibitory effect of extracts from 6-month-old tubers was due to PMC, purified PMC from 6-month-old tubers was assessed for its ability to inhibit FITC-casein hydrolysis by extracts from 30-month-old tubers. PMC was as effective as crude extracts from 6-month-old tubers in inhibiting the proteinase activity in extracts from 30-month-old tubers (Table II). Moreover, heat treatment of crude extracts and PMC rendered both completely ineffective at inhibiting the age-induced proteinase activity. The heat-labile nature of PMC has been previously documented (Rodis and Hoff, 1984). Collectively, these results indicate that the age-induced increase in proteinase activity is a consequence of PMC loss during the aging process.
Table II.
Inhibition of proteinase activity in extracts from 30-month-old tubers by nonheated and heated crude extracts and PMC from 6-month-old tubers
| Source of Inhibitor | FITC-Casein Hydrolysis | |
|---|---|---|
| units mg−1 protein h−1 | % inhibition | |
| No inhibitor | 80.3 | 0 |
| 6-month-old tuber extract | 12.8 | 84 |
| Heat-treated 6-month-old tuber extract | 72.9 | 9 |
| PMC from 6-month-old tubers | 10.5 | 87 |
| Heat-treated PMC from 6-month-old tubers | 76.3 | 5 |
FITC-casein hydrolysis was determined by incubating extracts from 30-month-old tubers at 37°C (60 min) with or without inhibitors. Inhibitors included heat-treated (95°C, 10 min) or nonheated crude extract (160 μg of protein equivalents) and PMC (65 μg of protein equivalents) from 6-month-old tubers. lsd0.05 = 15.8 units mg−1 protein h−1.
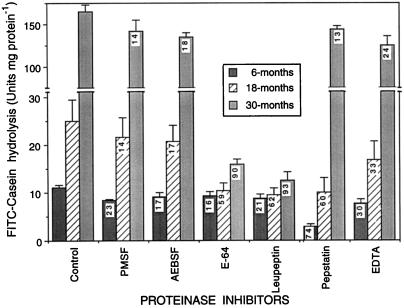
Characterization of the proteinases in 6-, 18-, and 30-month-old tubers provided further evidence that the age-induced increase in proteolytic activity is primarily due to loss of PMC. Commercial Cys-type proteinase inhibitors (E-64 and leupeptin) were most effective at inhibiting FITC-casein-hydrolyzing activity of extracts from 30-month-old tubers, reducing activity by 90% to 93% (Fig. 5). Furthermore, the pH optimum for FITC-casein hydrolysis by older tuber extracts was 6.1 (data not shown), which is characteristic of potato Cys-type proteinases (Kitamura and Maruyama, 1985). These results agree with those of Isola and Franzoni (1993), who showed that the Cys-proteinases, which are leupeptin-sensitive, were most active in protein degradation during aging of potato slices. In contrast, Asp-type proteinases (pepstatin-sensitive) dominated (74%) the total proteinase activity of 6-month-old tubers. Total proteinase activity in 18-month-old tubers was equally sensitive to Asp- and Cys-type inhibitors. A shift in the predominant proteinase from Asp- to Cys-type therefore occurs during tuber aging, reflecting the age-induced loss of PMC.
Figure 5.
Effects of class-specific proteinase inhibitors on the FITC-casein-hydrolyzing activities of extracts from 6-, 18-, and 30-month-old tubers. FITC-casein was added to tuber extracts containing each inhibitor and hydrolysis was assessed after 60 min of incubation at 37°C. Spectrofluorometric units are emission at 520 nm following excitation at 500 nm. Proteinase inhibitors include PMSF and AEBSF (Ser-type), E-64 and leupeptin (Cys-type), pepstatin (Asp-type), and EDTA (metallo-type). Data are averages ± se of three replicates. Numbers in bars are percent inhibition relative to control (no inhibitors present).
The age-induced loss of PMC and patatin, concomitant with increased proteinase activity, suggests a role for PMC in the regulation of tuber protein/patatin content. PMC and patatin were isolated from young tubers (Fig. 6A). To determine whether PMC can inhibit the patatinolytic activity of older tubers, extracts from 26-month-old tubers were spiked with purified patatin with or without PMC, and patatinolytic activity was assessed by SDS-PAGE. Extracts from older tubers effectively degraded exogenous patatin within 30 min of incubation (Fig. 6B). This proteolytic activity of the older tuber extracts was substantially inhibited when PMC was included in the assay medium. Under similar conditions, extracts from younger tubers did not catabolize patatin (data not shown), indicating a potential role for PMC in regulating the degradation of patatin and other tuber proteins.
Figure 6.
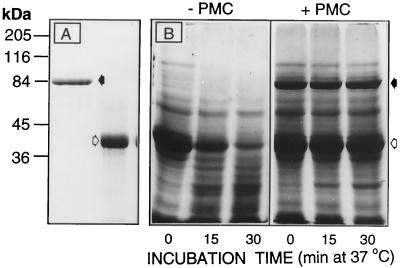
Ability of PMC to inhibit the patatinolytic activity of extracts from 26-month-old tubers. A, SDS-PAGE of purified PMC (black arrow) and patatin (white arrow) (3.5 and 7.6 μg per lane, respectively) isolated from 6-month-old tubers. B, Patatin (white arrow) was added to extracts from 26-month-old tubers, which were then incubated for 0, 15 and 30 min (37°C) in the absence or presence of PMC (black arrow). A total of 100 μg of protein containing 48 μg of patatin and 12 μg of PMC (where indicated) at time zero was loaded in each lane.
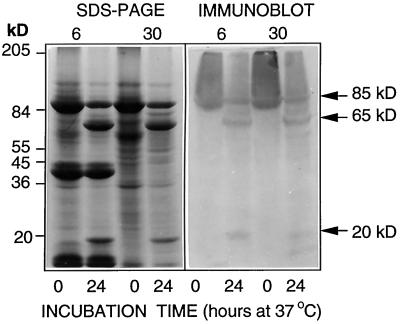
If PMC is involved in preventing patatinolytic activity in vivo, its loss should precede that of patatin during the aging process. Evidence for this is provided in Figures 2, 3, and 4B. As tuber age advanced from 6 to 18 months, PMC decreased to nondetectable levels (Figs. 3 and 4B), while patatin, though significantly reduced in concentration, was still present (Fig. 2). The mechanism by which PMC was lost during tuber aging is unknown; however, while the proteinases in 30-month-old tuber extracts did not inhibit the effects of exogenous PMC in preventing FITC-casein-hydrolysis (Fig. 4B) and patatin degradation (Fig. 6B) in short-term studies, extracts from 6- and 30-month-old tubers were equally effective in degrading PMC into 65- and 20-kD polypeptides over a 24-h incubation period (Fig. 7). Such partial proteolysis, however, did not greatly alter the ability of PMC to inhibit patatin degradation, presumably because PMC has multiple inhibitory domains and its subunits are capable of retaining inhibitory activity even after partial proteolysis (Walsh and Strickland, 1993). The age-induced loss of PMC from tubers, which probably facilitates patatin breakdown, could be due to a combination of reduced synthesis, complete proteolysis, and/or inactivation of PMC through a number of nonenzymatic mechanisms (e.g. glycation, oxidation, or isomerization/racemization).
Figure 7.
SDS-PAGE and immunoblots comparing the ability of extracts from 6- and 30-month-old tubers to degrade PMC into 65- and 20-kD subunits. Extracts were spiked with 10 μg of PMC and incubated for 0 and 24 h at 37°C prior to electrophoresis (64 μg/lane).
The nature of increased proteolytic activity associated with aging was further examined by electrophoretic analysis of proteinases. Two gelatinolytic proteinases (approximately 84 and 95 kD) were common among the 6-, 18-, and 30-month-old tubers, increasing with advancing age (Fig. 8). Tubers of all three ages also had a 125-kD proteinase (Fig. 8B), but its activity in 6-month-old tubers was below photographic resolution. In 30-month-old tubers, additional proteinases with greatly enhanced activity were observed at approximately 75, 90, and 100 kD. Similar increases in gelatinolytic activity were evident when gels were loaded on a tuber fresh-weight basis, eliminating gel-loading bias as an explanation for the increased proteinase activity in older tubers. Age-induced proteinases could not be detected in extracts from young tubers, even when protein loads were 3-fold greater than that of 30-month-old tuber extracts. All six proteinases effectively degraded patatin on patatin-containing gels (data not shown). Therefore, increased proteolytic activity in older tubers was due to activation and/or synthesis of existing and novel proteinases during aging, and protein loss with advancing tuber age was correlated with the loss of PMC and increased activities of Cys-type proteinases.
Figure 8.
Gelatinolytic proteinase activities of extracts from 6-, 18-, and 30-month-old tubers (resolved by SDS-PAGE in gels containing 0.1% gelatin). Proteins were electrophoresed for 60 min (A) and 120 min (B) to resolve the total and high-molecular-mass proteinases, respectively (72 μg/lane).
Age-Induced, Nonenzymatic Modifications of Tuber Proteins
Although proteolysis serves as a method for cellular housekeeping by degrading defective proteins (Goldberg and Dice, 1974; Finley and Chau, 1991; Hershko and Ciechanover, 1992), relatively few modifications that target abnormal proteins for degradation have been adequately documented. Defective proteins can arise through a number of mechanisms, including biosynthetic errors (Jentsch, 1996), spontaneous denaturation, and free-radical attack (Stadtman, 1992). Depending on the type of damage, repair and restoration of protein conformation and function are possible (Deshaies et al., 1988; Rothman, 1989); however, degradation is the only fate for proteins synthesized from defective mRNAs (Jentsch, 1996). Although defective proteins arise spontaneously in every cell, the continued assault by free radicals on the transcription/translation machinery (Desimone et al., 1996) of cells under high oxidative stress produces unabated generation of abnormal proteins. Failure to degrade such proteins results in their accumulation to toxic levels (Vierstra, 1993) and limits the size of the amino acid pool for protein turnover. An increase in free amino acids with advancing tuber age, along with a reduced ability for protein synthesis (Kumar and Knowles, 1993c) and increased proteolytic activity (Figs. 4, 6 and 8), indicates that tuber aging is associated predominantly with protein catabolism rather than with repair.
Nonenzymatic molecular modifications to proteins adversely affect their structure and function, and have been associated with aging in mammalian and other systems (Furth, 1988; Stadtman, 1988, 1992; Ota and Clarke, 1990). For this reason, we examined the effects of tuber age on protein oxidation, glycation, and susceptibility as substrates for PCMT activity. Older tubers respire at a significantly higher rate than younger tubers (Kumar and Knowles, 1996a) and are therefore subject to increased oxidative stress (Kumar and Knowles, 1993b, 1996b) from the accumulation of reactive oxygen species, emanating in part from increased electron transport in mitochondria (Boveris et al., 1976; Forman and Boveris, 1982). Reactive oxygen species effectively damage various macromolecules, including proteins (Stadtman, 1992; Gebicki and Gebicki, 1993; Sohal and Weindruch, 1996). Aging in animal systems is accompanied by the accumulation of less active and in some cases totally inactive enzymes, much of which has been attributed to oxidative modifications (Oliver et al., 1987).
Protein oxidation is characterized by increased carbonyl content (Fig. 1) and, in general, such modifications target proteins for proteolysis (Kyle et al., 1984; Dalling, 1987; Stadtman, 1992). For example, increased carbonyl content resulting from oxidative stress effected increased proteolysis of Rubisco (Ferriera and Shaw, 1989; Penarrubia and Moreno, 1990; Garcia-Ferris and Moreno, 1993; Eckardt and Pell, 1995; Desimone et al., 1996). Carbonyl content of proteins from 18- and 30-month-old tubers was 17% and 61% higher, respectively, than that of 6-month-old tubers (Fig. 9, compare maxima at A380), reflecting increased protein oxidation with advancing age. Western analysis showed progressively greater proportions of oxidized proteins in the soluble fraction as tubers aged from 6 to 30 months (Fig. 9). In fact, most of the soluble polypeptides from 30-month-old tubers had increased carbonyl content, reflecting the nonspecificity of protein oxidation. Oxidation is therefore one mechanism by which proteins could be targeted for degradation in aging tubers.
Figure 9.
Absorption spectra and immunoblots depicting the effects of tuber age on protein oxidation. Carbonyl groups of oxidized proteins were derivitized with DNPH and quantified at A380. Oxidized proteins in the soluble-protein fraction of 6-, 18-, and 30-month-old tubers over 20 d of sprouting are shown in the western blot, where protein-carbonyl-DNP derivatives were probed with monoclonal anti-DNP antibody.
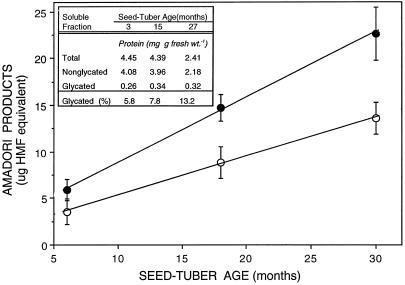
Another nonenzymatic modification that effects decreased protein function and increased catalysis in aging systems is glycation (Fig. 1), a consequence of free amino groups on proteins reacting with the free carbonyl groups of reducing sugars. The resulting glycated proteins often cross-link, leading to the accumulation of insoluble, advanced-glycation end products (Furth, 1988). Tuber proteins may be particularly susceptible to this type of damage, because reducing sugars increase during aging, presenting the ideal environment for protein glycation (Kumar and Knowles, 1993b). A linear increase in glycated proteins (Amadori products) was evident as tubers aged from 6 to 30 months (Fig. 10). Increased glycation was not a manifestation of the declining protein content associated with aging, because isolation of the glycated protein by boronate affinity chromatography showed that older tubers had 23% to 31% more glycated protein than younger tubers, and the glycated fraction increased as a percentage of total soluble protein on a tuber fresh-weight basis (Fig. 10, inset). According to Wettlaufer and Leopold (1991), the nonenzymatic glycation of proteins during accelerated aging of soybean seeds plays an important role in seed deterioration. These authors also suggested that reducing sugars in seeds can reduce viability by enhancing the glycation process. The negative correlation between protein glycation and vigor of true seeds during aging is also apparent for potato. The insoluble protein that accumulates in aging tubers may consist of advanced glycation products, although this remains to be established.
Figure 10.
Effect of tuber age on protein glycation as determined by boronate affinity chromatography (inset) and thiobarbituric acid assay. The TBA data are averages ± se of three replicates. Micrograms of hydroxymethyl furaldehyde (HMF) equivalents are expressed per gram fresh weight (○) and per milligram protein (•).
Molecular alterations affecting protein charge and/or structure also yield dysfunctional proteins that may be targeted for degradation or repair. One such nonenzymatic modification is the deamidation of asparaginyl and the isomerization/racemization of aspartyl residues, resulting in the formation of isomerized l-isoaspartyl or racemized d-aspartyl residues (Fig. 1). Proteins with such adducts accumulate in mammalian systems during aging (Galletti et al., 1995). Deamidation affects Ca+2-binding domains of calmodulin (Johnson et al., 1989) and results in functional inactivation (Johnson et al., 1985, 1987). Functionality of deamidated proteins, however, can be restored by methylation of isoaspartyl residues by PCMT (Johnson et al., 1987; Li and Clarke, 1992), a substrate-inducible enzyme (Mudgett and Clarke, 1994, 1996).
When PCMT activity was measured with a saturating level of l-isoaspartyl-containing peptide substrate (VYP-[l-isoAsp]-HA), activities from 6- and 30-month-old tubers were comparable (30.5 pmol min−1 mg−1 protein on average). However, in the absence of the synthetic peptide substrate, endogenous PCMT activity increased linearly as tubers aged from 6 to 30 months (Table III). Thus, the enhanced PCMT activity in aged tubers was due to increased protein substrates containing altered amino acid residues and not to changes in the level of PCMT, as determined by total activity measurements with an artificial substrate. This is in direct contrast to aging of barley seeds, in which PCMT activity decreased with a concomitant increase in l-isoaspartylated proteins over a 17-year period (Mudgett, et al., 1997). Accelerated aging of tomato seeds also lowered PCMT activity, which was correlated with a loss of vigor (Kester et al., 1997). To our knowledge, our study is the first report of an increase in PCMT activity during aging of a vegetative plant tissue, a consequence of the increasing level of damaged peptide substrate.
Table III.
PCMT activity as affected by age and acclimation temperature of potato tubers
| Tuber Age | PCMT-II Activity
|
|
|---|---|---|
| 4°C | 23°C | |
| months | pmol CH3 h−1 mg−1 protein | |
| 6 | 17.5 | 15.2 |
| 18 | 29.1 | 19.1 |
| 30 | 33.5 | 21.7 |
PCMT was extracted from tubers directly from cold storage (4°C) and after acclimation of tubers to 23°C for 24 h. Assays were conducted at 30°C without exogenous polypeptide substrate. Main effects of age (linear) and temperature were significant (P < 0.05). The age × temperature interaction was not significant.
Mother/Daughter Tuber System
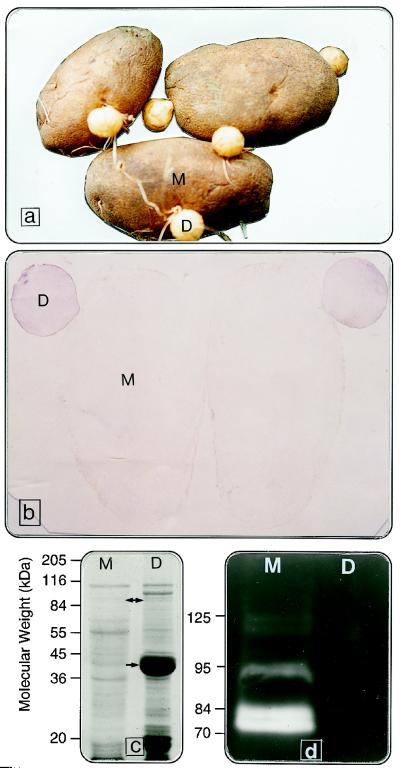
Tubers stored for more than 20 months at 4°C developed daughter tubers directly when placed in the dark at 23°C, providing an ideal system for studying the effects of age on protein content and metabolism (Fig. 11a). Daughter tubers contained 3-fold more protein (on a fresh-weight basis) than their 29-month-old mother tubers, and the polypeptide profile of daughter tubers (Fig. 11c) was comparable to that of 6-month-old tubers (Fig. 2). Tissue prints (Fig. 11b) and SDS-PAGE (Fig. 11c) showed that mother tubers lacked PMC and patatin, whereas daughter tubers contained both proteins. Because daughter tubers are clones of mother-tuber tissue, aging did not compromise the fidelity of genes coding for PMC and patatin. Our results suggest a new role for PMC in regulating the levels of patatin and other tuber proteins through inhibiting proteolysis. Although gelatinolytic proteinase activity of extracts from 29-month-old mother tubers was consistent with that shown previously for 30-month-old tubers, proteolytic activity was absent from daughter tubers (compare Figs. 11d and 8A). The loss of PMC during aging probably contributes to proteolysis of patatin and other tuber proteins.
Figure 11.
a, Mother/daughter tuber system for studying the effects of tuber age on protein metabolism. Mother tubers (M) were stored at 4°C for 29 months, transferred to 23°C, and incubated in the dark for approximately 2 months to induce daughter (D) tubers. b, Tissue prints of PMC from longitudinal sections of 29-month-old mother and daughter tubers. Purple color indicates the presence of PMC. c, SDS-PAGE of proteins from mother and daughter tubers. Note the absence of PMC (double-headed arrow) and patatin (arrow) from the 29-month-old mother tuber, whereas daughter tubers contain high levels of both proteins. d, Gelatinolytic proteinase activities in extracts from mother tubers and their absence in extracts from daughter tubers.
In summary, our results demonstrate that: (a) the loss of patatin contributes substantially to the age-induced decline in soluble protein content of potato tubers; (b) aging results in an increase in proteolytic activity and the appearance of several novel proteinases; (c) PMC, a Cys-proteinase inhibitor effective against patatinolytic proteinases in vitro, is lost with advancing seed-tuber age; (d) age-enhanced proteolytic activity is mainly due to increases in Cys-proteinases; (e) as tubers age there is a shift from Asp- to Cys-type proteinases; and (f) the observed increases in protein glycation, oxidation, and deamidation/isomerization/racemization with advancing tuber age are potential targeting mechanisms for protein degradation. To our knowledge, this is the first demonstration of the plurality of nonenzymatic protein modifications that can be definitively associated with aging in a nonsenescent plant system.
ACKNOWLEDGMENT
We thank T.A. Walsh (Biotechnology Laboratory, Dow-Elanco, Indianapolis, IN) for providing PMC antisera.
Abbreviations:
- AdoMet
S-adenosyl-l-Met
- DNPH
2,4-din-itrophenyl hydrazine
- E-64
trans-epoxysuccinyl-l-leucylamido (4-guanidino) butane
- FITC
fluorescein isothiocyanate
- PCMT
protein carboxylmethyl transferase
- PMC
potato multicystatin
Footnotes
This research was funded by the Natural Sciences and Engineering Research Council of Canada (grant to N.R.K.) and by the U.S. Department of Energy (grant no. DE-FG05-92ER26075 to R.L.H.).
LITERATURE CITED
- Al-Saikhan MS, Howard LR, Miller JC. Antioxidant activity and total phenolics in different genotypes of potato (Solanum tuberosum L.) J Food Sci. 1995;60:341–344. [Google Scholar]
- Belles JM, Carbonell J, Conejero V. Polyamines in plants infected by citrus exocartis viroid or treated with silver ions and ethephon. Plant Physiol. 1991;96:1053–1059. doi: 10.1104/pp.96.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensadoun A, Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Bohac JR. A modified method to purify patatin from potato tubers. J Agric Food Chem. 1991;39:1411–1415. [Google Scholar]
- Boveris A, Cadenas E, Stoppani AOM. The role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232:1629–1632. doi: 10.1126/science.3487117. [DOI] [PubMed] [Google Scholar]
- Cerami A, Vlassara H, Brownlee M. Glucose and aging. Sci Am. 1987;256:90–96. doi: 10.1038/scientificamerican0587-90. [DOI] [PubMed] [Google Scholar]
- Dalling MJ (1987) Proteolytic enzymes and leaf senescence. In Thomson WW, Nothnagel EA, Huffaker RC, eds, Plant Senescence: Its Biochemistry and Physiology. American Society of Plant Physiologists, Rockville, MD, pp 54–80
- Deshaies RJ, Koch BD, Shekman R. The role of stress proteins in membrane biogenesis. Trends Biochem Sci. 1988;13:384–388. doi: 10.1016/0968-0004(88)90180-6. [DOI] [PubMed] [Google Scholar]
- Desimone M, Henke A, Wagner E. Oxidative stress induces partial degradation of the large subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase in isolated chloroplasts of barley. Plant Physiol. 1996;111:789–796. doi: 10.1104/pp.111.3.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt NA, Pell EJ. Oxidative modification of rubisco from potato foliage in response to ozone. Plant Physiol Biochem. 1995;33:273–282. [Google Scholar]
- Ferriera RB, Shaw NM. Effect of osmotic stress on protein turnover in Lemma minor fronds. Planta. 1989;179:456–465. doi: 10.1007/BF00397585. [DOI] [PubMed] [Google Scholar]
- Finley D, Chau V. Ubiquitination. Annu Rev Cell Biol. 1991;7:25–69. doi: 10.1146/annurev.cb.07.110191.000325. [DOI] [PubMed] [Google Scholar]
- Forman HJ, Boveris A (1982) Superoxide radical and hydrogen peroxide in mitochondria. In WA Pryor, ed, Free Radicals in Biology and Medicine, Vol 5. Academic Press, New York, pp 65–90
- Furth A. Sweet peril for proteins. New Scientist. 1988;149:58–62. [Google Scholar]
- Galletti P, Ingrosso D, Manna C, Clemente G, Zappia V. Protein damage and methylation-mediated repair in the erythrocyte. Biochem J. 1995;306:313–325. doi: 10.1042/bj3060313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T. The enzymatic deacylation of phospholipids and galactolipids in plants. Biochem J. 1971;121:379–390. doi: 10.1042/bj1210379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galliard T (1980) Degradation of acyl lipids: hydrolytic and oxidative enzymes. In PK Stumpf, ed, The Biochemistry of Plants, Vol 4. Academic Press, New York, pp 85–116
- Garcia-Ferris C, Moreno J. Redox regulation of enzymic activity and proteolytic suceptibility of ribulose-1,5-bisphosphate carboxylase/oxygenase from Euglena gracilis. Photosynth Res. 1993;35:55–56. doi: 10.1007/BF02185411. [DOI] [PubMed] [Google Scholar]
- Gebicki S, Gebicki JM. Formation of peroxides in amino acids and proteins exposed to oxygen free radicals. Biochem J. 1993;289:743–749. doi: 10.1042/bj2890743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg AL, Dice JF. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43:835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Heussen C, Dowdle EB. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- Hirayama O, Matsuda H, Takeda H, Maenaka K, Takatsuka H. Purification and properties of a lipid acyl-hydrolase from potato tubers. Biochim Biophys Acta. 1975;384:127–137. doi: 10.1016/0005-2744(75)90102-3. [DOI] [PubMed] [Google Scholar]
- Isola MC, Franzoni L. Studies on proteolytic enzymes of potato tuber. Plant Physiol Biochem. 1993;31:169–174. [Google Scholar]
- Jentsch S. When proteins receive deadly messages at birth. Science. 1996;271:955–956. doi: 10.1126/science.271.5251.955. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Freitag NE, Aswad DW. Protein carboxyl methyltransferase selectively modifies an atypical form of calmodulin: evidence for methylation at deamidated asparagine residues. J Biol Chem. 1985;260:10913–10916. [PubMed] [Google Scholar]
- Johnson BA, Langmack EL, Aswad DW. Partial repair of deamidation-damaged calmodulin by protein carboxyl methyltransferase. J Biol Chem. 1987;262:12283–12287. [PubMed] [Google Scholar]
- Johnson BA, Shirokawa JM, Aswad DW. Deamidation of calmodulin at neutral and alkaline pH: quantitative relationships between ammonia loss and the susceptibility of calmodulin to modification by protein caboxyl methyltransferase. Arch Biochem Biophys. 1989;268:276–286. doi: 10.1016/0003-9861(89)90589-4. [DOI] [PubMed] [Google Scholar]
- Kester ST, Geneve RL, Houtz RL. Priming and accelerated ageing affect l-isoaspartyl methyltransferase activity in tomato (Lycopersicon esculentum Mill.) J Exp Bot. 1997;48:943–949. [Google Scholar]
- Kitamura N, Maruyama Y. Cysteine endopeptidase activity in sprouting potato tubers. Agric Biol Chem. 1985;49:1591–1597. [Google Scholar]
- Knowles NR, Knowles LO. Correlations between electrolyte leakage and degree of saturation of polar lipids from aged potato (Solanum tuberosum L.) tuber tissue. Ann Bot. 1989;63:331–338. [Google Scholar]
- Kumar GNM, Knowles NR. Involvement of auxin in the loss of apical dominance and plant growth potential accompanying aging of potato seed tubers. Can J Bot. 1993a;71:541–550. [Google Scholar]
- Kumar GNM, Knowles NR. Changes in lipid peroxidation and lipolytic and free-radical scavenging enzyme activities during aging and sprouting of potato seed-tubers. Plant Physiol. 1993b;102:115–124. doi: 10.1104/pp.102.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GNM, Knowles NR. Age of potato seed-tubers influences protein synthesis during sprouting. Physiol Plant. 1993c;89:262–270. [Google Scholar]
- Kumar GNM, Knowles NR. Nature of enhanced respiration during sprouting of aged potato seed-tubers. Physiol Plant. 1996a;97:228–236. [Google Scholar]
- Kumar GNM, Knowles NR. Oxidative stress results in increased sinks for metabolic energy during aging and sprouting of potato seed-tubers. Plant Physiol. 1996b;112:1301–1313. doi: 10.1104/pp.112.3.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyle DJ, Ohad I, Arntzen CF. Membrane protein damage and repair: selective loss of quinone-protein function in chloroplast membranes. Proc Natl Acad Sci USA. 1984;81:4070–4074. doi: 10.1073/pnas.81.13.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine RL, Garland D, Oliver CN, Amici A, Climent I, Anke G, Ahn B-W, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–485. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346–357. doi: 10.1016/s0076-6879(94)33040-9. [DOI] [PubMed] [Google Scholar]
- Li C, Clarke S. A protein methyltransferase specific for altered aspartyl residues is important in Escherichia coli stationary-phase survival and heat-shock resistance. Proc Natl Acad Sci USA. 1992;89:9885–9889. doi: 10.1073/pnas.89.20.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra M, Balasubramanian D. Nonenzymatic glycation alters protein structure and stability: a study of eye lens crystallins. J Biol Chem. 1993;268:18119–18127. [PubMed] [Google Scholar]
- Michaud D, Faye L, Yelle S. Electrophoretic analysis of plant cystein and serine proteinases using gelatin-containing polyacrylamide gels and class specific proteinase inhibitors. Electrophoresis. 1993;14:94–98. doi: 10.1002/elps.1150140117. [DOI] [PubMed] [Google Scholar]
- Michaud D, Nguyen-Quoc B, Bernier-Vadnais N, Faye L, Yelle S. Cysteine proteinase forms in sprouting potato tuber. Physiol Plant. 1994;90:497–503. [Google Scholar]
- Monnier VM, Stevens VJ, Cerami A. Nonenzymatic glycosylation, sulfhydryl oxidation, and aggregation of lens proteins in experimental sugar cataracts. J Exp Med. 1979;150:1098–1107. doi: 10.1084/jem.150.5.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudgett MB, Clarke S. Characterization of plant l-isoaspartyl methyltransferases that may be involved in seed survival: purification, cloning and sequence analysis of the wheat germ enzyme. Biochemistry. 1993;32:11100–11111. doi: 10.1021/bi00092a020. [DOI] [PubMed] [Google Scholar]
- Mudgett MB, Clarke S. Hormonal and environmental responsiveness of a developmentally regulated protein repair l-isoaspartyl methyltransferase in wheat. J Biol Chem. 1994;269:25605–25612. [PubMed] [Google Scholar]
- Mudgett MB, Clarke S. A distinctly regulated protein repair l-isoaspartyl methyltransferase from Arabidopsis thaliana. Plant Mol Biol. 1996;30:723–737. doi: 10.1007/BF00019007. [DOI] [PubMed] [Google Scholar]
- Mudgett MB, Lowenson JD, Clarke S. Protein repair l-isoaspartyl methyltransferase in plants. Phylogenetic distribution and the accumulation of substrate proteins in aged barley seeds. Plant Physiol. 1997;115:1481–1489. doi: 10.1104/pp.115.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver CN, Anh B-W, Moerman EJ, Goldstein S, Stadtman ER. Age-related changes in oxidized proteins. J Biol Chem. 1987;262:5488–5491. [PubMed] [Google Scholar]
- Ota JM, Clarke S. The function and enzymology of protein d-aspartyl (l-isoaspartyl methyltransferases) in eukaryotic and prokaryotic cells. In: Paik WK, Kim S, editors. Protein Methylation. Boca Raton, FL: CRC Press; 1990. pp. 179–194. [Google Scholar]
- Paiva E, Lister RM, Park WD. Induction and accumulation of major tuber proteins of potato in stems and petioles. Plant Physiol. 1983;71:161–168. doi: 10.1104/pp.71.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penarrubia L, Moreno J. Increased susceptibility of ribulose-1,5-bisphosphate carboxylase/oxygenase to proteolytic degradation caused by oxidative treatments. Arch Biochem Biophys. 1990;281:319–323. doi: 10.1016/0003-9861(90)90450-d. [DOI] [PubMed] [Google Scholar]
- Prasad TK. Role of catalase in inducing chilling tolerance in pre-emergent maize seedlings. Plant Physiol. 1997;114:1369–1376. doi: 10.1104/pp.114.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racusen D, Foote M. A major soluble glycoprotein of potato tubers. J Food Biochem. 1980;4:43–52. [Google Scholar]
- Rodis P, Hoff JE. Naturally occurring protein crystals in the potato. Inhibitor of papain, chymopapain and ficin. Plant Physiol. 1984;74:907–911. doi: 10.1104/pp.74.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE. Polypeptide chain binding proteins: catalysis of protein folding and related processes in cell. Cell. 1989;59:591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. Oxidative stress, caloric restriction and aging. Science. 1996;273:59–63. doi: 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman ER. Biochemical markers of aging. J Gerontol. 1988;43:B112–B120. doi: 10.1016/0531-5565(88)90036-8. [DOI] [PubMed] [Google Scholar]
- Stadtman ER. Protein oxidation and aging. Science. 1992;257:1220–1224. doi: 10.1126/science.1355616. [DOI] [PubMed] [Google Scholar]
- Strickland JA, Orr GL, Walsh TA. Inhibition of larval growth by patatin, the lipid acyl hydrolase from potato tubers. Plant Physiol. 1995;109:667–674. doi: 10.1104/pp.109.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner JE (1992) Visualization of enzyme activity. In PD Reid, RF Pont-Lezica, eds, Tissue Printing: Tools for the Study of Anatomy, Histochemistry and Gene Expression. Academic Press, New York, pp 59–70
- Vera P, Conejero V. Pathogenesis-related proteins of tomato. P-69 as an alkaline endoproteinase. Plant Physiol. 1988;87:58–63. doi: 10.1104/pp.87.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vierstra RD. Protein degradation in plants. Annu Rev Plant Physiol. 1993;44:385–410. [Google Scholar]
- Walsh TA, Strickland JA. Proteolysis of the 85-kilodalton crystalline cysteine proteinase inhibitor from potato releases functional cystatin domains. Plant Physiol. 1993;103:1227–1234. doi: 10.1104/pp.103.4.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wettlaufer SH, Leopold AC. Relevance of Amadori and Maillard products to seed deterioration. Plant Physiol. 1991;97:165–169. doi: 10.1104/pp.97.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]