Abstract
Purpose
The purpose of this study was to investigate the effect of recombinant human bone morphogenetic protein-7 (rhBMP-7) with or without osteogenic differentiation medium (ODM) on osteogenic differentiation of primary human bone-marrow-derived mesenchymal stem cells (hBMSCs) in vitro.
Method
The hBMSCs were isolated from medullary reaming tissue. At 80% confluence, hBMSCs were treated with different concentrations of rhBMP-7 with and without ODM. Alkaline phosphatase (ALP) activity, calcium deposition and messenger RNA (mRNA) expression of osteocalcin (OC) and osteopontin (OPN) were examined.
Results
ALP activity and calcium deposits in hBMSC culture were significantly increased by rhBMP-7 at 0.1 μg/ml (0.23 ± 0.07 IU and 28.9 ± 4.2 mg/dl) and 1.0 μg/ml (0.32 ± 0.03 IU and 38.7 ± 3.0 mg/dl), respectively, in the presence of ODM, showing a clearly dose-dependent osteoblastic differentiation. However, the same dose of 0.1 μg/ml rhBMP-7 without ODM and ODM alone induced low level of ALP and calcium deposits, indicating a synergistic effect of rhBMP-7 and ODM on committed osteogenic differentiation. Quantitative real-time reverse-transcriptase polymerase chain reaction (RT-PCR) analysis showed up-regulated OC and OPN mRNA levels, corroborating the synergistic effect of rhBMP-7 and ODM.
Conclusion
Our study showed that rhBMP-7 with ODM created a synergistic effect on up-regulation of osteogenic genes as well as osteogenic differentiation of primary hBMSCs in vitro. In the presence of ODM, the lowest concentration of rhBMP-7 needed to induce significant osteogenic differentiation of hBMSCs was 0.1 μg/ml.
Introduction
During the past decade, recombinant human bone morphogenetic protein-7 (rhBMP-7) has been used to promote healing of bone defects in various animal models and in clinical trials for therapeutic purposes [1–6]. rhBMP-7 is commercially available for treatment of clinically defined bone disorders [7, 8]. However, the clinical treatment protocol for bone healing calls for a supraphysiological dose of rhBMP-7 (1,500 μg/ml), whereas endogenous levels of the protein are at least 1,000-fold lower. Unfortunately, the adverse side effects seen in clinical practice may be in part related to the supraphysiological dosage of rhBMP-7 [9].
The roles of BMPs in stimulating or promoting differentiation of mesenchymal stem cells (MSCs) towards osteogenic cell linage have been extensively studied in recent years [10]. Some studies demonstrated a dose-dependent osteogenic differentiation under the influence of BMP-7 and in vitro bone formation by using rat or mouse bone-marrow-derived stem cells (BMSCs) [11, 12], as well as a human osteosarcoma cell line [13]. However, the dose effect of rhBMP-7 on osteogenic differentiation of human BMSCs (hBMSCs) is largely unknown. In addition, osteogenic differentiation medium (ODM) demonstrated strong osteoinductivity to hBMSCs in vitro [14]. However, little is known about its complementary role in osteogenic differentiation of hBMSCs in the presence of rhBMP-7.
In this study, we investigated in vitro osteogenic differentiation of hBMSCs under the influence of a different concentration of rhBMP-7 in the presence or absence of ODM. In vitro osteogenic biomarkers, such as the level of alkaline phosphatase (ALP) and cumulative calcification, were examined. The mRNA expression level of the specific osteogenic cytokines osteocalcin (OC) and osteopontin (OPN) were also quantified using quantitative real-time reverse-transcriptase polymerase chain reaction (RT-PCR). The purpose of the study was twofold: (1) to investigate the synergistic effect of rhBMP-7 and ODM on osteogenic differentiation of primary hBMSCs in vitro, and (2) to identify the minimal-effective dose threshold of rhBMP7 for osteogenic differentiation of primary hBMSCs.
Materials and methods
Primary hBMSCs isolation and culture
Primary hBMSCs from healthy donors were isolated and characterised as described [15]. Briefly, primary hBMSCs were initially cultured and maintained in mesenchymal stem-cell growth medium (MGM) Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad, USA) containing 10% foetal bovine serum (FBS) (Invitrogen) and 4 ng/ml fibroblast growth factor-2 (FGF-2) (Millipore, Billerica, USA) at 37°C with 5% humidified carbon dioxide (CO2). Ethical approval was obtained from the Ethical Committee at the university to use human bone reamings.
Treating hBMSCs with rhBMP-7 and osteogenic differentiation medium
The ODM used for hBMSC cell culture consisted of DMEM with 10% FBS, 10 nM dexamethasone, 5 mM β-glycerophosphate and 50 mg/L L-ascorbic acid-2-phosphate (all from Sigma, Oakville, Canada). Primary hBMSCs were seeded at a density of 3,000 ~ 3,500 cells/cm2 in six-well plates and cultured in 2.0 ml of MGM at 37°C with 5% of CO2 until they reached 70–80% confluence. Six study groups were designed as follows: MGM alone (1), MGM with 0.1 μg/ml rhBMP-7 (Stryker Biotech, Hopkinton, USA) (2), ODM alone (3) and ODM with rhBMP-7 concentrations of 0.01 μg/ml (4), 0.1 μg/ml (5) and 1 μg/ml (6). Media were changed twice a week, and hBMSCs were harvested 17 days after treatment to assess osteogenic differentiation biomarkers, including ALP activity, and OC and OPN mRNA levels. hBMSCs were also harvested for quantitative measurement of calcium deposition using a colorimetric assay at 17 and 35 days.
Alkaline phosphatase assay
ALP activity was quantitatively measured by a colorimetric assay (BioAssay Systems, Hayward, USA) following the manufacturer’s instruction. The cells in triplicate cultures were collected with a lysis buffer containing 1% Triton, 100 mM Tris-hydrochloride (HCl) and 10 mM magnesium chloride (MgCl2). The lysate was then transferred to a 96-well plate and incubated with ALP substrate at 37°C for 30 min, and then the reaction was halted with the stop buffer. The p-nitrophenol formed by enzymatic hydrolysis of the p-nitrophenyl-phospate substrate was measured at 405 nm using a plate reader.
Calcium assay and staining
Cells and the extracellular matrix were demineralised by adding 1 ml of 0.5 N hydrochloric acid to each well and incubated at 4°C on a shaker overnight. Supernatants were collected after being centrifuged at 10,000 rpm for 10 min. Calcium concentrations were quantified using the QuantiChrom Calcium Assay Kit (BioAssay Systems) according to manufacturer’s instructions. The amount of calcium deposited was expressed in the concentration as milligrams per decilitre. On the 35th day of osteogenic induction, alizarine red staining was performed to characterise calcium deposition. Samples were first washed with phosphate-buffered saline (PBS) three times and fixed by 75% ethanol at 4°C for one hour. Samples were then washed with water three times, stained by 40 mM alizarine red solution (Sigma) for 10 min and washed with water three times to remove dissociative dye.
Total RNA isolation and reverse transcription
Total RNA was isolated from attached cells using Trizol (Invitrogen). Reverse transcription was performed using the single-step iScript complementary DNA (cDNA) synthesis kit (Bio-Rad, Mississauga, Canada). Reactions were set up in 20 μl of final volume with the following protocol: incubate at 25°C for 5 min, followed by 42°C for 5 min and 85°C for 5 min, and store at 4°C for further experiment.
Quantitative real-time PCR
Real-time PCR reactions were run in duplicate with final volume of 25 μl using the iQ5 system (Bio-Rad) in which fluorescence intensity data were collected and transformed. The reaction mixture contained 10 ng cDNA from RT-PCR of individual sample, 200 nM each of the primers and 1x iQ SYBR Green supermix (Bio-Rad). The specific primers for each target gene were designed at the junction between two exons or within two exons separately. The nucleotide sequences of OC and OPN primers and their references are shown in Table 1. Real-time PCR was performed with the following conditions: denature at 95°C for 3 min, run 45 thermal cycles with the denature at 95°C for 10 s, annealing at 58°C for 20 s and extension at 72°C for 10 s. Specificity of the PCR products was evaluated using the melting curve analysis. Triplicate reactions for each sample were assessed. glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an endogenous reference gene (amplified with GAPDHF/GAPDHR) , and a 700-bp DNA fragment of GAPDH (amplified with GAPDHFC/GAPDHRC) with known copy numbers was used as the external standard for quantification (Table 1) .
Table 1.
Description of the designed primers
| Candidate gene | Sequence label | Sequence (5′-3′) | Length (nt) | Product length(bp) |
|---|---|---|---|---|
| GAPDH | GAPDHFC | CGCTGAGTACGTCGTGGAGT | 20 | 704 |
| GAPDHRC | CCACCACCCTGTTGCTGTAG | 20 | ||
| GAPDH | GAPDHF | GGACTCATGACCACAGTCCAT | 21 | 109 |
| GAPDHR | CAGGGATGATGTTCTGGAGAG | 21 | ||
| OPN | OPNF2 | ATGGCCGAGGTGATAGTGTG | 20 | 146 |
| OPNR2 | GATGGCCTTGTATGCACCAT | 20 | ||
| OC | OCF | GAAGCCCAGCGGTGCA | 16 | 70 |
| OCR | CACTACCTCGCTGCCCTCC | 19 |
GAPDH glyceraldehyde 3-phosphate dehydrogenase, OPN osteopontin, OC osteocalcin
Statistical analysis
Data are presented as mean with standard deviation (SD). The overall statistical significance of any difference resulting from treatment was determined by analysis of variance (ANOVA). Bonferroni post hoc test was used to determine significant difference between different groups using the statistical package SPSS 17.0 for windows (SPSS, Chicago, IL, USA). The significance between different treatment groups is considered as P < 0.05 (all tests were two-sided).
Results
Morphological changes of primary hBMSCs were observed under treatment with rhBMP-7 and ODM. Primary hBMSCs cultured in MGM showed spindle-shaped morphology that then turned into cuboidal-shaped cells with rich cytoplasm after they were exposed to rhBMP-7, ODM and ODM/rhBMP-7. There was no sign of cell detachment from the bottom of culture wells up to the 35th day of the experiment.
Alkaline phosphatase activity and calcium deposit in vitro
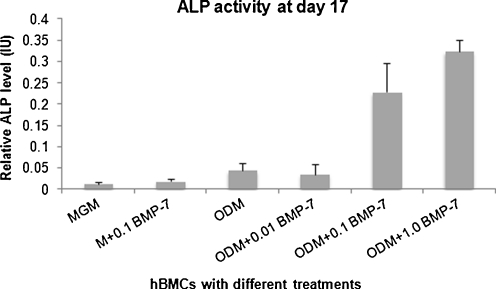
The level of ALP activity in cultured hBMSCs at at the 17th day did not increase noticeably in MGM alone, MGM with 0.1 μg/ml rhBMP-7, ODM alone or ODM with 0.01 μg/ml rhBMP-7. With 0.1 μg/ml rhBMP-7, a significantly higher level of ALP activity was found in the group with than in the group without ODM (0.23 ±0.07 IU vs 0.02 ± 0.01 IU, P < 0.001, Fig. 1). Moreover, ALP activity in the ODM group with 0.1 μg/ml rhBMP-7 was also higher than that of 0.01 μg/ml rhBMP-7 (0.23 ±0.07 IU vs 0.04 ± 0.02 IU, P < 0.001). In the ODM group with the highest rhBMP-7 concentration of 1.0 μg/ml, the level of ALP activity was further enhanced (0.32 ± 0.03 IU) compared with activity in the ODM with 0.1 μg/ml of rhBMP-7, clearly showing a dose-dependent effect of rhBMP-7 on osteogenic differentiation of primary hBMSCs.
Fig. 1.
Alkaline phosphatase activity (ALP) according to six treatment groups
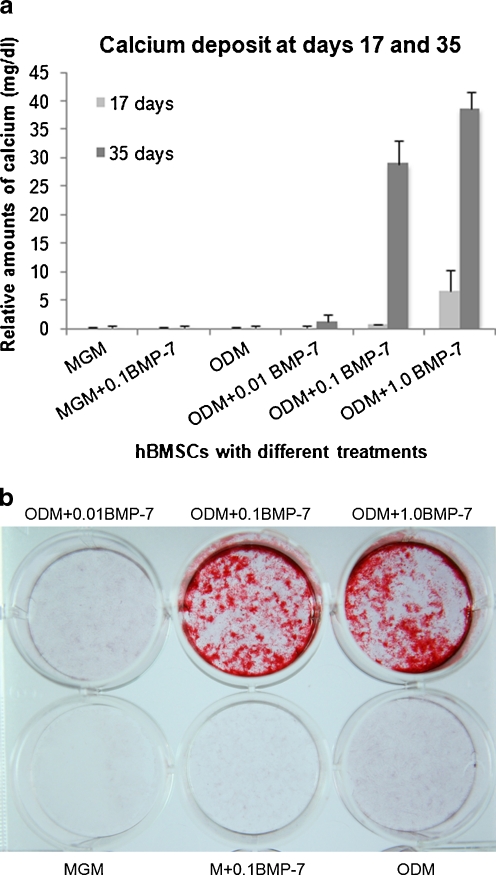
There was no apparent calcium deposition in any treatment group except for the ODM group with 1.0 μg/ml rhBMP-7 (6.6 ± 3.74 mg/dl) at day 17 of the culture (Fig. 2a). At day 35, calcium deposit was remarkably augmented by ODM with 0.1 μg/ml rhBMP-7 (28.9 ± 4.2 mg/dl) and 1.0 μg/ml rhBMP-7 (38.7 ± 3.0 mg/dl) in comparison with the other treatment groups (all P < 0.001). Calcium content in the ODM + 1.0 μg/ml rhBMP-7 group was significantly higher than that of the ODM + 0.1 μg/ml rhBMP-7 group at both day 17 and 35 (P ≤ 0.002). No apparent calcium deposition was found in MGM + 0.1 μg/ml rhBMP-7, ODM alone or ODM + 0.01 μg/ml rhBMP-7. Alizarine red staining also showed apparent calcification in ODM groups treated with 0.1 and 1.0 μg/ml rhBMP-7 at day 35 (Fig. 2B).
Fig. 2.
a Cumulative calcium deposit at both day 17 and day 35 according to six treatment groups. b Calcium staining at day 35 according to six treatment groups
mRNA expression of osteocalcin and osteopontin of hBMSC in vitro
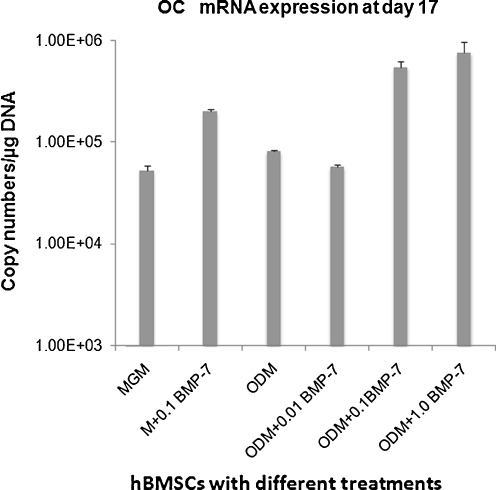
Quantitative analysis on the level of gene expression in the terms of copy numbers of mRNA using real-time RT-PCR demonstrated that mRNA expression of OC was significantly up-regulated in the ODM group with 0.1 μg/ml rhBMP-7 (5.43E + 05 ± 7.61E + 04) and the ODM group with 1.0 μg/ml rhBMP7 (7.48E + 05 ± 2.18E + 05) in comparison with the other groups (P ≤ 0.012, Fig. 3).
Fig. 3.
Quantitative analysis on the level of osteocalcin (OC) gene expression in the terms of copy numbers of messenger RNA (mRNA) using real-time reverse-transcriptase polymerase chain reaction (RT-PCR) according to six treatment groups
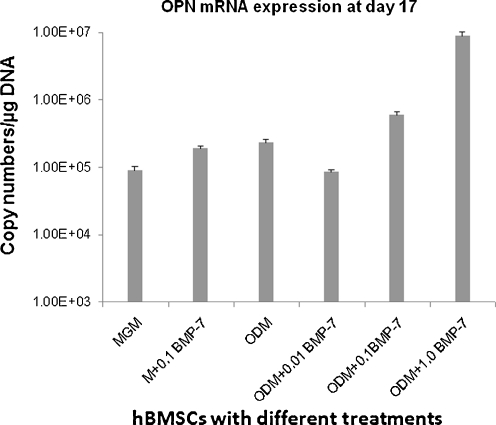
Significant up-regulation of OPN mRNA expression was also observed in the ODM group treated with 1.0 μg/ml rhBMP-7 (8.77E + 07 ± 1.52E + 06) in comparison with the 0.1 μg/ml rhBMP7( 5.96E + 06 ± 7.15E + 04) group and the other groups (P < 0.001, Fig. 4).
Fig. 4.
Quantitative analysis on the level of osteopontin (OPN) gene expression in the terms of copy numbers of messenger RNA (mRNA) using quantitative real-time reverse-transcriptase polymerase chain reaction (RT-PCR) according to six treatment groups
Discussion
Osteogenesis is an orchestrated process with engagement of committed mesenchymal stem cells, osteoprogenitors, osteoblasts and osteoclasts and is characterised by sequential expression of a cascade of relevant genes. The level of the tissue nonspecific isoform of ALP has been recognised as a functional biomarker of osteoblast differentiation [16] and calcium deposition in the extracellular matrix as an indicator of subsequent endochondral bone formation. In our study, ALP activity in hBMSC culture was significantly increased at 0.1 μg/ml rhBMP-7 and was further enhanced at the concentration of 1.0 μg/ml in the presence of ODM, showing a clear dose-dependent increment of osteoblastic differentiation in vitro. However, the same dose of 0.1 μg/ml rhBMP-7 failed to induce high levels of ALP response when primary hBMSCs were cultured in MGM medium in the absence of ODM. This observation suggests a synergistic effect of rhBMP-7 and ODM on committed osteogenic differentiation of hBMSCs. It appears that an rhBMP-7 concentration of ≤0.01 μg/ml was not sufficient to increase ALP activity and thus indicates that there is a minimal dose threshold of BMP-7 required for inducing differentiation of hBMSCs towards osteogenic lineage in vitro.
The osteoinductive ingredients in ODM include β-glycerophosphate (5 mM), L-ascorbic acid (50 mg/l) and dexamethasone (10 nM). Previous studies reported that BMP-2, 4, 5, 6 and 7 with two concentrations (0.05 and 0.1 μg/ml) induced high levels of ALP activity of primary adult rat bone marrow cells at ten days when β-glycerophosphate (5 mM) and L-ascorbic acid (100 mg/l) were added to the culture medium [11]. Although ODM without rhBMP-7 increased ALP activity above the baseline level, the ODM with 0.1 μg/ml rhBMP-7 induced a five-fold higher ALP level comparatively. There was also a significant difference of rhBMP-7-induced ALP levels between 0.01 μg/ml and 0.1 μg/ml rhBMP-7 concentrations in the presence of ODM. Yeh et al. reported that the dose threshold was >0.2 μg/ml BMP-7 for differentiation of mouse pluripotent precursor cell line C2C12 into osteoblasts [12]. Our study demonstrated that the minimal dose for inducing in vitro osteoblastic differentiation of hBMSCs was ≥ 0.1 μg/ml rhBMP-7 under synergistic effect of ODM. We observed that rhBMP-7 did not promote calcium deposit in vitro culture of primary hBMSCs at an early stage (17 days), except for a moderate increase in calcium content in the ODM group with 1.0 μg/ml rhBMP-7. However, calcium deposit was significantly enhanced with rhBMP-7 at concentrations of 0.1 and 1.0 μg/ml at the later stage (35 days) of osteogenic differentiation in the presence of ODM. The time frame for calcium deposition corresponds well with the clinical timing of bone healing in humans. Calcium content in hBMSC culture was 30% higher in the 1.0 μg/ml rhBMP-7 group than that in the 0.1 μg/ml rhBMP-7 group, indicating a dose-dependent effect. The synergistic effect of rhBMP-7 and ODM on the increase of calcium content in hBMSC culture was also evidenced, as rhBMP-7 at 0.1 μg/ml in MGM had no effect at all. Our results support the conclusion that the effect of BMP-2, 4, 5, 6 and 7 on mineralised bone-nodule formation in vitro is facilitated by ascorbic acid and β-glycerophosphate, the components of ODM [11].
Dexamethasone is another factor that may interplay with rhBMP-7 to create the synergistic effect of ODM and rhBMP-7 observed in this study. Although the effect of dexamethasone on osteogenic differentiation of bone marrow MSCs has been extensively studied for several decades [17–21], the synergistic effect between dexamethasone and BMPs was recently examined. Jäger et al. reported that BMP-2 enhanced ODM-induced osteogenic differentiation in human mesenchymal bone marrow cells [22]. A later study conducted in rat dental follicle progenitor cells showed that the joint application of dexamethasone and BMP-2 achieved the highest ALP activity, calcium and phosphonium and osteocalcin content compared with the groups treated with dexamethasone or BMP-2 alone [23]. The synergistic effect of dexamethasone and BMP-2 on in vitro matrix mineralisation was then observed in mouse C3H10T1/2 pluripotent stem cells [24] and clonal rat mesenchymal progenitor cell line ROB-C26 (C26) [25]. Compared with the extensive study on the synergistic effect of dexamethasone and BMP-2 on osteoinduction, only a few studies have reported the synergistic effect between dexamethasone and BMP-7. Recently, Hu and colleagues reported that BMP-7 itself had little effect on in vitro osteogenic differentiation of human embryonic stem-cell-derived MSCs; however, there was a synergic effect between dexamethasone (100 nM) and BMP-7 in promoting osteogenesis [26]. In conclusion, we believe the synergistic effect between OGM and rhBMP-7 on osteoinduction in our study is highly likely due to the interplay of dexamethasone and rhBMP-7.
OC is a noncollagenous protein present in bone and secreted by osteoblasts [27]. OC plays an active role in bone mineralisation and is often used as a biomarker for the bone formation process. OPN, one of the extracellular matrix molecules in bone, is another common biomarker for bone formation [28]. The results of our study showed that ODM alone and ODM with 0.01 μg/ml rhBMP-7 had no effect on mRNA expression levels of either OC or OPN after 17 days of culture (i.e. equivalent to baseline level of OC and OPN in MGM). Our findings are consistent with Laflamme and Rouabhia’s study, which reported no significant effect on OC mRNA expression of MG-63 human osteosarcoma cell line with 0.01 or 0.1 μg/ml BMP7 compared with the control group. However, OC mRNA expression increased significantly when osteoblasts were stimulated with a combination of BMP-2 and BMP-7 [13]. The synergistic effect of rhBMP-7 and ODM on OC mRNA levels of hBMSCs was revealed at 0.1 μg/ml rhBMP-7 in our study. The magnitude of dose-dependent up-regulation of OC mRNA level was not obvious in comparison with OPN, in which OPN mRNA level increased 14 times when rhBMP-7 dose increased from 0.1 μg/ml to 1.0 μg/ml in the presence of ODM. The up-regulation of mRNA expression level of OC and OPN provided further evidence of the critical dose of rhBMP-7 in this study.
Taken together, rhBMP-7 and ODM posed a synergistic effect on up-regulation of osteogenic genes as well as an increase in the level of biomarkers for osteogenic differentiation of primary hBMSCs in vitro. In the presence of simple chemical molecules contained in ODM, the functional dose of rhBMP-7 for bone induction was much less than rhBMP-7 alone. The mechanism of interaction between those molecules and rhBMP-7 on committed osteogenic differentiation of hBMSCs remains largely unknown. A complicated signalling and regulatory pathway at cellular or receptor levels are expected to be involved during BMP-induced endochondral bone formation. In conclusion, rhBMP-7 induced osteogenic differentiation of primary hBMSCs derived from middle-aged adults in a dose-dependent manner in the presence of ODM. Human BMSCs were not very responsive to low-dose rhBMP-7 stimulation in vitro without other osteogenic components or otherwise required higher dosage of rhBMP-7. Further investigation of rhBMP-7 synergy with other molecules in improving osteogenic differentiation of hBMSCs may be warranted, ultimately with aim of reducing costs and complications related to superphysiological dosage of rhBMPs in therapeutic protocols.
Acknowledgements
This study was supported in part by grants from Edmonton Orthopaedic Research Committee and Edmonton Civic Employee Fund.
Conflict of interest The authors declare that they have no conflict of interest.
Footnotes
Investigation performed at the Department of Surgery, Faculty of Medicine and Dentistry; University of Alberta, Edmonton, Alberta, Canada
References
- 1.Pecina M, Giltaij LR, Vukicevic S. Orthopaedic applications of osteogenic protein-1 (BMP-7) Int Orthop. 2001;25:203–208. doi: 10.1007/s002640100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bilic R, Simic P, Jelic M, Stern-Padovan R, Dodig D, Meerdervoort HP, Martinovic S, Ivankovic D, Pecina M, Vukicevic S. Osteogenic protein-1 (BMP-7) accelerates healing of scaphoid non-union with proximal pole sclerosis. Int Orthop. 2006;30:128–134. doi: 10.1007/s00264-005-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. doi: 10.1177/0363546504263703. [DOI] [PubMed] [Google Scholar]
- 4.Friedlaender GE, Perry CR, Cole JD, Cook SD, Cierny G, Muschler GF, Zych GA, Calhoun JH, LaForte AJ, Yin S. Osteogenic protein-1 (bone morphogenetic protein-7) in the treatment of tibial nonunions. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S151–S158. [PMC free article] [PubMed] [Google Scholar]
- 5.Geesink RG, Hoefnagels NH, Bulstra SK. Osteogenic activity of OP-1 bone morphogenetic protein (BMP-7) in a human fibular defect. J Bone Joint Surg Br. 1999;81:710–718. doi: 10.1302/0301-620X.81B4.9311. [DOI] [PubMed] [Google Scholar]
- 6.Makino T, Hak DJ, Hazelwood SJ, Curtiss S, Reddi AH. Prevention of atrophic nonunion development by recombinant human bone morphogenetic protein-7. J Orthop Res. 2005;23:632–638. doi: 10.1016/j.orthres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Issack PS, DiCesare PE. Recent advances toward the clinical application of bone morphogenetic proteins in bone and cartilage repair. Am J Orthop. 2003;32:429–436. [PubMed] [Google Scholar]
- 8.White AP, Vaccaro AR, Hall JA, Whang PG, Friel BC, McKee MD. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31:735–741. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mehrara BJ, Saadeh PB, Steinbrech DS, Dudziak M, Spector JA, Greenwald JA, Gittes GK, Longaker MT. Adenovirus-mediated gene therapy of osteoblasts in vitro and in vivo. J Bone Miner Res. 1999;14:1290–1301. doi: 10.1359/jbmr.1999.14.8.1290. [DOI] [PubMed] [Google Scholar]
- 10.Diefenderfer DL, Osyczka AM, Garino JP, Leboy PS. Regulation of BMP-induced transcription in cultured human bone marrow stromal cells. J Bone Joint Surg Am. 2003;85-A(Suppl 3):19–28. doi: 10.2106/00004623-200300003-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul S, Lee JC, Yeh LC. A comparative study on BMP-induced osteoclastogenesis and osteoblastogenesis in primary cultures of adult rat bone marrow cells. Growth Factors. 2009;27:121–131. doi: 10.1080/08977190802707324. [DOI] [PubMed] [Google Scholar]
- 12.Yeh LC, Tsai AD, Lee JC. Osteogenic protein-1 (OP-1, BMP-7) induces osteoblastic cell differentiation of the pluripotent mesenchymal cell line C2C12. J Cell Biochem. 2002;87:292–304. doi: 10.1002/jcb.10315. [DOI] [PubMed] [Google Scholar]
- 13.Laflamme C, Rouabhia M. Effect of BMP-2 and BMP-7 homodimers and a mixture of BMP-2/BMP-7 homodimers on osteoblast adhesion and growth following culture on a collagen scaffold. Biomed Mater. 2008;3:15008. doi: 10.1088/1748-6041/3/1/015008. [DOI] [PubMed] [Google Scholar]
- 14.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997;64:295–312. doi: 10.1002/(SICI)1097-4644(199702)64:2<295::AID-JCB12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 15.Jiang H, Secretan C, Gao T, Bagnall K, Korbutt G, Lakey J, Jomha NM. The development of osteoblasts from stem cells to supplement fusion of the spine during surgery for AIS. Stud Health Technol Inform. 2006;123:467–472. [PubMed] [Google Scholar]
- 16.Orimo H, Shimada T. The role of tissue-nonspecific alkaline phosphatase in the phosphate-induced activation of alkaline phosphatase and mineralization in SaOS-2 human osteoblast-like cells. Mol Cell Biochem. 2008;315:51–60. doi: 10.1007/s11010-008-9788-3. [DOI] [PubMed] [Google Scholar]
- 17.Friedenstein AJ, Chailakhyan RK, Latsinik NV, Panasyuk AF, Keiliss-Borok IV. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation. 1974;17:331–340. doi: 10.1097/00007890-197404000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Friedenstein AJ, Chailakhyan RK, Gerasimov UV. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987;20:263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- 19.Owen M, Friedenstein AJ. Stromal stem cells: marrow-derived osteogenic precursors. Ciba Found Symp. 1988;136:42–60. doi: 10.1002/9780470513637.ch4. [DOI] [PubMed] [Google Scholar]
- 20.Leboy PS, Beresford JN, Devlin C, Owen ME. Dexamethasone induction of osteoblast mRNAs in rat marrow stromal cell cultures. J Cell Physiol. 1991;146:370–380. doi: 10.1002/jcp.1041460306. [DOI] [PubMed] [Google Scholar]
- 21.Locklin RM, Williamson MC, Beresford JN, Triffitt JT, Owen ME. In vitro effects of growth factors and dexamethasone on rat marrow stromal cells. Clin Orthop Relat Res. 1995;313:27–35. [PubMed] [Google Scholar]
- 22.Jäger M, Fischer J, Dohrn W, Li X, Ayers DC, Czibere A, Prall WC, Lensing-Hohn S, Krauspe R. Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro. J Orthop Res. 2008;26:1440–1448. doi: 10.1002/jor.20565. [DOI] [PubMed] [Google Scholar]
- 23.Xu LL, Liu HC, Wang DS ELL, Xu L, Jin ZL, Duan YZ. Effects of BMP-2 and dexamethasone on osteogenic differentiation of rat dental follicle progenitor cells seeded on three-dimensional beta-TCP. Biomed Mater. 2009;4:065010. doi: 10.1088/1748-6041/4/6/065010. [DOI] [PubMed] [Google Scholar]
- 24.Mikami Y, Asano M, Honda MJ, Takagi M. Bone morphogenetic protein 2 and dexamethasone synergistically increase alkaline phosphatase levels through JAK/STAT signaling in C3H10T1/2 cells. J Cell Physiol. 2010;223:123–133. doi: 10.1002/jcp.22017. [DOI] [PubMed] [Google Scholar]
- 25.Mikami Y, Lee M, Irie S, Honda MJ. Dexamethasone modulates osteogenesis and adipogenesis with regulation of osterix expression in rat calvaria-derived cells. J Cell Physiol. 2011;226:739–748. doi: 10.1002/jcp.22392. [DOI] [PubMed] [Google Scholar]
- 26.Hu J, Smith LA, Feng K, Liu X, Sun H, Ma PX. Response of human embryonic stem cell-derived mesenchymal stem cells to osteogenic factors and architectures of materials during in vitro osteogenesis. Tissue Eng A. 2010;16:3507–3514. doi: 10.1089/ten.tea.2010.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi A, Komori T, Suda T. Regulation of osteoblast differentiation mediated by bone morphogenetic proteins, hedgehogs, and Cbfa1. Endocr Rev. 2000;21:393–411. doi: 10.1210/er.21.4.393. [DOI] [PubMed] [Google Scholar]
- 28.Morinobu M, Ishijima M, Rittling SR, Tsuji K, Yamamoto H, Nifuji A, Denhardt DT, Noda M. Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res. 2003;18:1706–1715. doi: 10.1359/jbmr.2003.18.9.1706. [DOI] [PubMed] [Google Scholar]