Abstract
Purpose
We evaluated the biomechanical characteristics of the transiliac internal fixator (TIFI) as compared to two well-established methods of internal posterior pelvic ring fixation.
Methods
Six freshly frozen human pelves were used for simulated single-leg stance loading of an AO type C injury model (pubic symphysis diastasis and unilateral sacroiliac joint disruption). The symphysis rupture was stabilized with a dynamic compression plate. Afterwards the three internal stabilization systems (TIFI, iliosacral screws and ventral plate osteosynthesis) were analysed. Fragment movement was measured in a contact-free manner with a stereophotometric infrared system.
Results
No significant differences in the three-dimensional deformation tolerated by the TIFI as compared to the other internal fixation systems were found.
Conclusions
The transiliac internal fixator provides the same biomechanical stability as the other reference implants tested. We suggest the use of this device as a suitable alternative to the other implants.
Introduction
AO type C pelvic ring injuries (complete disruption of posterior arch with vertical and rotational instability [1]) have an increasing but low incidence representing 3–8% of all fractures; however, these injuries are frequently lethal (5–20% of cases) [2]. Unstable pelvic ring injuries are usually caused by high-energy trauma and often combined with other serious injuries. There is an urgent requirement for a quick and effective surgical procedure [3, 4]. Extended soft-tissue damage and lengthy surgical procedures complicate the decision-making process [5–7]. The most frequent reason for a fatal outcome is a pelvic mass haemorrhage, which can only be controlled by compression [8] with a stable pelvic ring as counter-bearing force [9, 10].
A great variety of techniques exist to stabilize the dorsal pelvic ring and the sacroiliac joint. In 1987, Simpson published a method to stabilize the unstable sacroiliac joint with an anterior approach using two v-placed neutralizing plates [11]. Thread bars used as transiliac bars from a dorsal approach are described by Tile [1]. In the early 1930s, Lehmann first demonstrated the successful use of a screw implanted directly through the sacroiliac joint [12]. This approach is currently aided by CT scans or alternative navigation devices [13]. Yinger et al. was able to demonstrate in a biomechanical setting [14] that many of these currently used techniques show no discernible improvement with regard to stiffness; deciding upon the best method for treatment is therefore a multifactorial decision-making process.
In clinical use there is a tendency towards percutaneous screw fixation as a minimally invasive technique. In cases which require an open reduction the use of two plates through an anterior approach is still one of the standard treatment modalities [15].
We have demonstrated an alternative method in this study, namely, a transiliac internal fixator (TIFI). Two pedicle screws are inserted in the alae of the ileum, with an entry point slightly cranial to the posterior superior iliac spine and transfixed with a connection rod. With this quick and minimally invasive technique, in a preliminary clinical study soft tissue damage could be minimized, wound infection remained low and no neurovascular lesions were caused [16]. While it seems that there are some clinical benefits to the use of this implant, it is still unclear if this device has the same biomechanical stability as other well-established methods. The aim of this study was to compare two commonly used implants and the TIFI with regard to biomechanical behaviour.
Materials and methods
Single-leg stance loading of an AO type C injury (pubic symphysis diastasis and unilateral sacroiliac joint disruption) was used as a load transmission model. Ten freshly frozen, nonembalmed human pelvises with unruptured capsules and ligament systems including the fifth lumbar vertebra were used [17]. Four of these pelvic rings were used for pre-experimental data collection; six were used in the main experiment. Each pelvic ring was re-used for all implants. The sequence of the implants used was randomized at each pelvis. Twelve hours before testing, the pelvises were thawed and checked to verify integrity of the sacroiliac ligaments; a search for undetected fractures was conducted as well. While undergoing testing procedures the specimens were kept moist by the frequent spraying of an isotonic saline solution. The intact six pelvises were used as a control group. Afterwards, disruption of the symphysis as well as displacement of the sacroiliac joint were created by deep cuts with a scalpel [18]. The symphysis rupture was stabilized with a four-hole AO dynamic compression plate. Afterwards the following three internal posterior stabilization systems were tested gradually in randomized order:
Two three-hole dynamic, 4.5 compression plates (Synthes) were placed in a v-form pattern, extending from the ventral, crossing the sacroiliac joint.
Two 6.5-mm cannulated screws (Synthes) were placed transsacroiliacally, using a 32-mm thread and a washer.
The transiliac internal fixator (Universal Spine System, Synthes) was positioned with 6-mm diameter screws and a 5-mm diameter connection rod (Fig. 1).
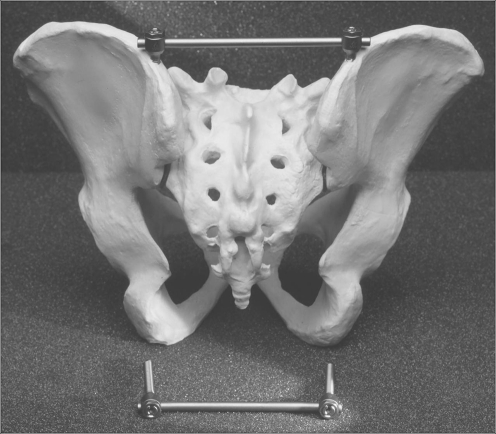
Fig. 1.
The TIFI (transiliac internal fixator) in its correct implantation site bridging the sacroiliac region on a hard plastic pelvic model
The femur was imitated using a steel tube with a femur shaft prosthesis on its top. This device was positioned with an antetorsion angle of 15 degrees. The acetabulum was simulated using an implanted polyethylene hip prosthesis cup (48er-Inlay, PROTEK); the diameter of the mounted head was 28 mm. The inclination direction of the pelvis was at a physiological angle of approximately 50 degrees was considered. Because of the importance in preventing the sacrum from tipping over horizontally, we imitated the gluteal muscles with a system of three fine-linked chains that were evenly spaced, with metal inserts through the bone between the anterior superior iliac spine and the posterior superior iliac spine. The inserts met at the height of the former greater trochanter parallel to the femoral shaft-mimicking tube. Using a tension spring mechanism, a readjustment was possible every time the pelvis rotated out of the correct starting position. Force was applied in a physiological cranio/caudal direction over the fifth lumbar vertebra. Load transmission was performed directly through the top plate of the fifth vertebral body, which was prepared with an iron plate on top. This iron plate had a diameter of 4 cm, a height of 1 cm and a centrally-welded metal ball. The lower surface was layered with methylacrylate to adjust for irregularities, and a load cell was installed for visualization of the applied force (Fig. 2). Preload was 25 N and three cycles of 70% of the former body mass were performed. Prior to the main experiments, each pelvic ring was tested in three cycles as an intact pelvic ring to eliminate a setting effect and to decrease the development of loading artifacts. Each experimental cycle afterwards included three mass loadings and lasted for 10 seconds, reaching maximum load at 5 seconds and falling back to the initial load at 10 seconds.
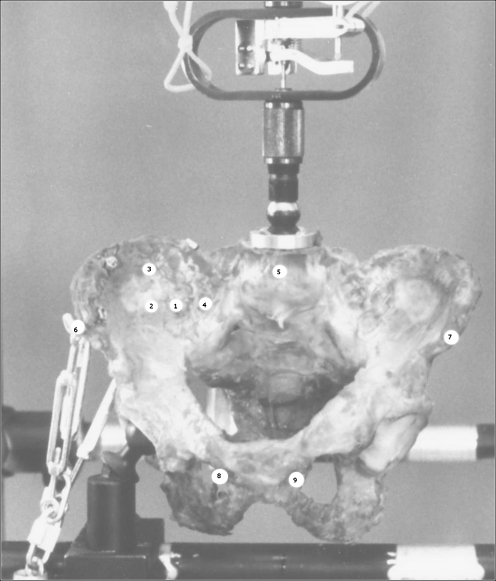
Fig. 2.
A human pelvis was positioned in the experimental device to measure three-dimensional fragment movements. The chains on the left mimic the muscular forces on the pelvic ring. Nine points of interest were marked by Kirschner wires and metal balls with special reflector paint were placed on top
Nine metal balls (5 mm in diameter) were used to detect three-dimensional fragment movements. These balls were coated with special reflector paint (number 885; 3M, Minnesota, USA). Cameras were installed 6 m away from the testing device. Nine points of interest were marked in this way. The positions studied in detail were:
Right ilium, ventral and 1 cm lateral from the SI-joint at the height of S1
Right ilium, ventral and 3 cm lateral from the SI-joint at the height of S1
Right ilium, ventral and 3 cm cranial from marker point 2
Sacrum, ventral and 1 cm medial from the SI-joint at the height of S1
L5, middle point of L5 in the frontal plane
Right ilium, anterior superior iliac spine
Left ilium, anterior superior iliac spine
Right pubis, ventral and 1 cm lateral of the symphysis
Left pubis, ventral and 1 cm lateral of the symphysis
The settings of the makers, the correct implantation angles of the implants and the correct setting of the data collection system were verified through data collected pre-experimentally using hard plastic pelvic models (Synbone, Davos, Switzerland).
To investigate the complex deformations of the pelvic ring, we used a three-dimensional analysing tool based on a contactless stereophotometric digital infrared analysing video recording system (Qualisys, Sävedalen, Sweden). With this tool we were able to detect minor fragment movements, with dissolution of 0.01 mm in all three dimensions. These factors allowed the simultaneous use of nine markers. The frequency of data acquisition reached 1000 Hz. Statistical analysis was performed using the analysis of variance test (ANOVA) with repeated measurements for multiple comparisons. A commercially available statistical program (version 11.0, SPSS Inc.) was used. Statistical significance was set at p < 0.05.
Results
Our pre-experimental data revealed a consistent model. None of the materials for osteosynthesis failed, nor were fracture sites other than the induced disruptions found. Symphyseal gapping was not measured in detail but there was no apparent evidence of displacement of the symphysis. The applied plate osteosynthesis remained in place in each experiment.
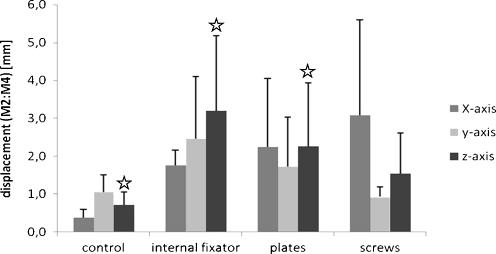
Differences in displacement along the x- and y-axes at the sacroiliac joint did not reach statistical significance. A notably larger standard deviation was observed along the x-axis for the screw treatment. All treatment groups tolerated more movement in the y-axis than the intact pelvic ring, which showed absolute values under 1 mm. The differences, when using plates or the internal fixator, were significant (p < 0.05) compared to control but not in comparison to the screw treatment group (Fig. 3).
Fig. 3.
Displacement in millimetres (mm) of marker points two and four measuring the movement in the ruptured sacroiliac joint. Data shown are medium values from six different specimens after treatment with three loading cycles each. The three osteosyntheses are compared to the control pelvic ring without defects, p < 0.05
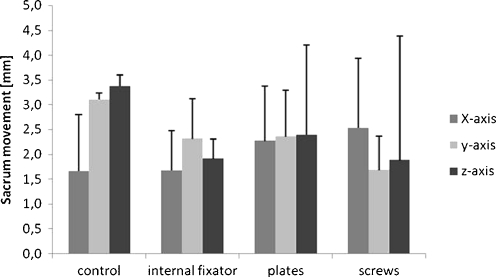
Linear displacement of the sacrum compared to the ileum ranged between 1.5 mm and 3.5 mm in all groups (Fig. 4). The internal fixator showed the most constant values with least standard deviation in all treatment groups. Notably the pelvises with osteosyntheses tolerated less movement as compared to the control group. However, these differences were not significant.
Fig. 4.
Sacrum movement measured in millimetres (mm) along three different axes. Data shown are median values from six different specimens after treatment with three loading cycles each. The three osteosyntheses are compared to the control pelvic ring without defects, p < 0.05
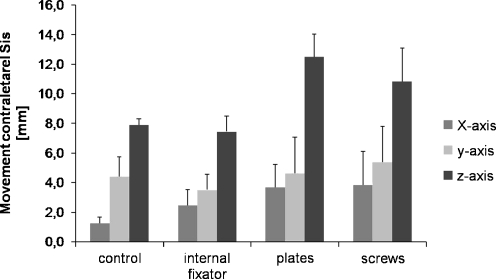
The movement of the contralateral spina iliaca anterior superior correlates with greater movement of the pelvic ring. Screws and plates demonstrated significant deviation in the z-axis when compared to intact pelvic rings (p < 0.05, 12 mm vs. 8 mm total displacement, respectively). The internal fixator allowed the same displacement as the control pelvises. No statistically significant difference between the osteosyntheses groups and the control group was detected along the x- and y-axes (Fig. 5).
Fig. 5.
Movement in millimetres (mm) of the contralateral anterior superior iliac spine (Sis), which served as an index for the greater movement of the pelvic ring. Data shown are medium values from six different specimens after treatment with three loading cycles each. The three osteosyntheses are compared to the control pelvic ring without defects, p < 0.05
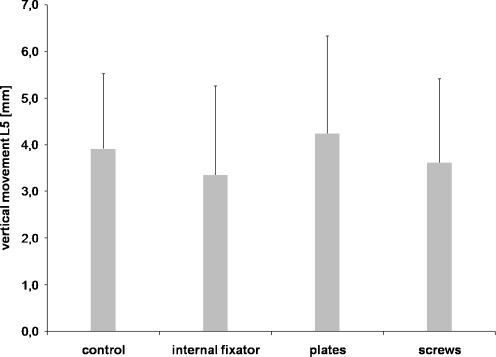
There was no significant displacement or rotation of the spinal column with respect to the entire ileum in all treatment groups. After three loading cycles, maximal vertical movement was 4 mm. No difference between the groups was evident (Fig. 6).
Fig. 6.
Vertical movement in millimetres (mm) of the fifth lumbar vertebra (L5), which served as an index for greater movement of the pelvic ring towards the spinal column. Data shown are median values from six different specimens after treatment with three loading cycles each. The three osteosyntheses are compared to the control pelvic ring without defects, p < 0.05
The power of the performed statistic analysis was 0.5 and therefore below the desired power of 0.8. We performed a priori power analysis and with the given results a number of over 300 samples had to be included to reach a test power of over 0.8. Given the precious nature of the sample pelvises this could not be conducted.
Discussion
In part due to lifestyle changes that incorporate dangerous leisure activities and increasing motorization [19, 20], the number of pelvic fractures is increasing. There is an obvious ongoing need for a quick and effective method of surgical treatment. Various methods exist but iatrogenic harm is not uncommon. For example, Kellam et al. [21] found wound healing problems or infections in one quarter of the patients treated with ilioiliac bars.
Among clinicians there is an intense discussion regarding whether patients will benefit from minimally invasive techniques in the field of pelvic trauma. Giannoudis investigated percutaneous fixation of the pelvic ring and concluded that only experienced surgeons with extensive knowledge of the pelvic region should perform this procedure [22].
While many biomechanical studies show that the various stabilization systems exhibit similar stiffness [14], it might be advantageous to use a method with minor risks during surgical implantation. In a preliminary clinical study the TIFI has proven to be a system with a low complication rate, as far as iatrogenic vessel or nerve damage [16, 23]. In this experimental setting we found that the biomechanical characteristics of the TIFI are comparable to those of established methods.
We used a model of symphysis displacement combined with a ruptured sacroiliac joint due to the high incidence of this injury type. An alternative is the creation of a lateral fracture of the sacrum. In our opinion, a rupture of the sacroiliac joint is much easier to reproduce in an experimental setting and therefore favourable.
Literature review reveals a wide range of opinions about which testing device and which model are suitable. Most studies in this field use freshly frozen specimens or plastic sawbones. In sawbone models the question of whether the implants anchor sufficiently in the model is not yet established. This was our primary concern because the TIFI contains pedicular screws that are susceptible to axial tear when not fixed in place with proper counter-bearings. We used cadaveric human pelves with an unstable right hemipelvis; notably, it has been shown that freshly frozen bones have nearly the same biomechanical characteristics as non-frozen specimens [24, 25].
Our setting of a single-leg stance model is suitable to simulate the clinical application of stabilization devices [26]. The injured areas are subject to high forces. In contrast, when using a two-leg stance model, these loads are compensated by the uninjured part of the pelvis and do not simulate the maximum forces during normal patient activities. The aim of this study was to focus on the primary stability achieved by the different implants. We therefore employed a high primary load and less repetition.
In a single-leg stance model the pelvis has to bear the full bodyweight minus the load of the weight bearing leg. We believe that therefore approximately 70% of former body weight is suitable for this matter [27]. However the load settings in the literature differ immensely [17].
Our rate of linear displacement at the sacroiliac joint was slightly higher than in previous studies [17]. But the model used differed significantly in the way the load was applied.
Statistically relevant results were observed in the vertical direction alone. Authors have suggested that measuring displacement in the vertical direction alone is sufficient [27]. In our opinion, measuring and recording in three dimensions is necessary—only this approach meets the demands of the complex pelvic ring structure.
According to Tornetta and Matta, a reduction after dorsal pelvic ring disruption is sufficient if a gap under 10 mm is achieved [28]. These clinical findings are achieved with radiological diagnostics under static conditions. In our dynamic experimental setting these values were not significantly exceeded and therefore we draw the conclusion that none of the tested devices permit a relevant mobility for the clinical situation.
Our usage of contactless measurement devices yielded highly reproducible results. The force-measuring tapes typically used frequently show marked deviation when the fixation device is swapped during experiments. To keep the intraspecimen deviation to a minimum, maximum compressive force was set at 70% of former body mass. No significant loading effects were detected using this arrangement during pretest conditions.
Conclusion
The presented data showed that the TIFI has similar biomechanical stability to other tested devices. We suggest the TIFI as an alternative to established internal fixation systems for unstable dorsal pelvic ring injuries. Additional clinical studies will have to clarify whether the results regarding patient safety and long-term outcome validate our experimental findings.
Acknowledgments
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Tile M. Pelvic ring fractures: should they be fixed? J Bone Joint Surg Br. 1988;70(1):1–12. doi: 10.1302/0301-620X.70B1.3276697. [DOI] [PubMed] [Google Scholar]
- 2.Culemann U, Tosounidis G, Reilmann H, Pohlemann T. Injury to the pelvic ring. Diagnosis and current possibilities for treatment. Unfallchirurg. 2004;107(12):1169–1181. doi: 10.1007/s00113-004-0898-4. [DOI] [PubMed] [Google Scholar]
- 3.Pape HC, Giannoudis PV, Krettek C, Trentz O. Timing of fixation of major fractures in blunt polytrauma: role of conventional indicators in clinical decision making. J Orthop Trauma. 2005;19(8):551–562. doi: 10.1097/01.bot.0000161712.87129.80. [DOI] [PubMed] [Google Scholar]
- 4.Giannoudis PV, Pape HC. Damage control orthopaedics in unstable pelvic ring injuries. Injury. 2004;35(7):671–677. doi: 10.1016/j.injury.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Nerlich M, Maghsudi M. Algorithms for early management of pelvic fractures. Injury. 1996;27(Suppl 1):S-A29–S-A37. doi: 10.1016/0020-1383(96)83792-2. [DOI] [PubMed] [Google Scholar]
- 6.Pohlemann T, Tscherne H, Baumgartel F, Egbers HJ, Euler E, Maurer F, Fell M, Mayr E, Quirini WW, Schlickewei W, Weinberg A. Pelvic fractures: epidemiology, therapy and long-term outcome. Overview of the multicenter study of the Pelvis Study Group. Unfallchirurg. 1996;99(3):160–167. [PubMed] [Google Scholar]
- 7.Connor GS, McGwin G, Jr, MacLennan PA, Alonso JE, Rue LW., 3rd Early versus delayed fixation of pelvic ring fractures. Am Surg. 2003;69(12):1019–1023. [PubMed] [Google Scholar]
- 8.Pohlemann T, Culemann U, Gansslen A, Tscherne H. Severe pelvic injury with pelvic mass hemorrhage: determining severity of hemorrhage and clinical experience with emergency stabilization. Unfallchirurg. 1996;99(10):734–743. doi: 10.1007/s001130050049. [DOI] [PubMed] [Google Scholar]
- 9.Thannheimer A, Woltmann A, Vastmans J, Buhren V. The unstable patient with pelvic fracture. Zentralbl Chir. 2004;129(1):37–42. doi: 10.1055/s-2004-44882. [DOI] [PubMed] [Google Scholar]
- 10.Bottlang M, Simpson T, Sigg J, Krieg JC, Madey SM, Long WB. Noninvasive reduction of open-book pelvic fractures by circumferential compression. J Orthop Trauma. 2002;16(6):367–373. doi: 10.1097/00005131-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Simpson LA, Waddell JP, Leighton RK, Kellam JF, Tile M. Anterior approach and stabilization of the disrupted sacroiliac joint. J Trauma. 1987;27(12):1332–1339. doi: 10.1097/00005373-198712000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann J. Luxation einer Beckenhälfte. Zentralbl Chir. 1934;37:2149–2152. [Google Scholar]
- 13.Vanderschot P, Meuleman C, Lefevre A, Broos P. Trans iliac-sacral-iliac bar stabilisation to treat bilateral lesions of the sacro-iliac joint or sacrum: anatomical considerations and clinical experience. Injury. 2001;32(7):587–592. doi: 10.1016/S0020-1383(01)00039-0. [DOI] [PubMed] [Google Scholar]
- 14.Yinger K, Scalise J, Olson SA, Bay BK, Finkemeier CG. Biomechanical comparison of posterior pelvic ring fixation. J Orthop Trauma. 2003;17(7):481–487. doi: 10.1097/00005131-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Kabak S, Halici M, Tuncel M, Avsarogullari L, Baktir A, Basturk M. Functional outcome of open reduction and internal fixation for completely unstable pelvic ring fractures (type C): a report of 40 cases. J Orthop Trauma. 2003;17(8):555–562. doi: 10.1097/00005131-200309000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Fuechtmeier B, Maghsudi M, Neumann C, Hente R, Roll C, Nerlich M. The minimally invasive stabilization of the dorsal pelvic ring with the transiliacal internal fixator (TIFI)–surgical technique and first clinical findings. Unfallchirurg. 2004;107(12):1142–1151. doi: 10.1007/s00113-004-0824-9. [DOI] [PubMed] [Google Scholar]
- 17.Zwienen CM, Bosch EW, Snijders CJ, Kleinrensink GJ, Vugt AB. Biomechanical comparison of sacroiliac screw techniques for unstable pelvic ring fractures. J Orthop Trauma. 2004;18(9):589–595. doi: 10.1097/00005131-200410000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Saiki K, Hirabayashi S, Horie T, Tsuzuki N, Inokuchi K, Tsutsumi H. Anatomically correct reduction and fixation of a Tile C-1 type unilateral sacroiliac disruption using a rod and pedicle screw system between the S1 vertebra and the ilium: experimental and clinical case report. J Orthop Sci. 2002;7(5):581–586. doi: 10.1007/s007760200104. [DOI] [PubMed] [Google Scholar]
- 19.Mucha P, Jr, Farnell MB. Analysis of pelvic fracture management. J Trauma. 1984;24(5):379–386. doi: 10.1097/00005373-198405000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Ragnarsson B, Olerud C, Olerud S. Anterior square-plate fixation of sacroiliac disruption. 2–8 years follow-up of 23 consecutive cases. Acta Orthop Scand. 1993;64(2):138–142. doi: 10.3109/17453679308994554. [DOI] [PubMed] [Google Scholar]
- 21.Kellam JF, McMurtry RY, Paley D, Tile M. The unstable pelvic fracture operative treatment. Orthop Clin North Am. 1987;18(1):25–41. [PubMed] [Google Scholar]
- 22.Giannoudis PV, Tzioupis CC, Pape HC, Roberts CS. Percutaneous fixation of the pelvic ring: an update. J Bone Joint Surg Br. 2007;89(2):145–154. doi: 10.1302/0301-620X.89B2.18551. [DOI] [PubMed] [Google Scholar]
- 23.Hao T, Changwei Y, Qiulin Z. Treatment of posterior pelvic ring injuries with minimally invasive percutaneous plate osteosynthesis. Int Orthop. 2009;33(5):1435–1439. doi: 10.1007/s00264-009-0756-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linde F, Sorensen HC. The effect of different storage methods on the mechanical properties of trabecular bone. J Biomech. 1993;26(10):1249–1252. doi: 10.1016/0021-9290(93)90072-M. [DOI] [PubMed] [Google Scholar]
- 25.Panjabi MM, Krag M, Summers D, Videman T. Biomechanical time-tolerance of fresh cadaveric human spine specimens. J Orthop Res. 1985;3(3):292–300. doi: 10.1002/jor.1100030305. [DOI] [PubMed] [Google Scholar]
- 26.Shaw JA, Mino DE, Werner FW, Murray DG. Posterior stabilization of pelvic fractures by use of threaded compression rods. Case reports and mechanical testing. Clin Orthop Relat Res. 1985;192:240–254. [PubMed] [Google Scholar]
- 27.Ponsen KJ, Hoek van Dijke GA, Joosse P, Snijders CJ. External fixators for pelvic fractures: comparison of the stiffness of current systems. Acta Orthop Scand. 2003;74(2):165–171. doi: 10.1080/00016470310013897. [DOI] [PubMed] [Google Scholar]
- 28.Tornetta P, 3rd, Matta JM. Outcome of operatively treated unstable posterior pelvic ring disruptions. Clin Orthop Relat Res. 1996;329:186–193. doi: 10.1097/00003086-199608000-00022. [DOI] [PubMed] [Google Scholar]