Abstract
Microglia are major immunocompetent cells in the central nervous system and retain highly dynamic motility. The processes which allow these cells to move, such as chemotaxis and phagocytosis, are considered part of their functions and are closely related to purinergic signaling. Previously, we reported that the activation of the P2Y6 receptor by UDP stimulation in microglia evoked dynamic cell motility which enhanced their phagocytic capacity, as reported by Koizumi et al. (Nature 446(7139):1091–1095, 2007). These responses require actin cytoskeletal rearrangement, which is seen after UDP stimulation. However, the intracellular signaling pathway has not been defined. In this study, we found that UDP in rat primary microglia rapidly induced the transient phosphorylation at Ser157 of vasodilator-stimulated phosphoprotein (VASP). VASP, one of actin binding protein, accumulated at the plasma membrane where filamentous (F)-actin aggregated in a time-dependent manner. The phosphorylation of VASP was suppressed by inhibition of PKC. UDP-induced local actin aggregations were also abrogated by PKC inhibitors. The Rho inhibitor CT04 and the expression of p115-RGS, which suppresses G12/13 signaling, attenuated UDP-induced phosphorylation of VASP and actin aggregation. These results indicate that PKC- and Rho-dependent phosphorylation of VASP is involved in UDP-induced actin aggregation of microglia.
Keywords: UDP, Microglia, VASP, P2Y6, Actin
Introduction
Microglia play a central role in a variety of immunological responses in the central nervous system (CNS). Once they are activated, their morphology transforms through retraction of their processes and hypertrophy of their bodies. Activated microglia produce cytokines and growth factors, and increase their chemotactic and phagocytic capacity. Even in the healthy brain, they contribute positively to CNS immunity. In previous studies using in vivo two-photon imaging, it was determined that inactivated microglia also moved their processes around them as if they are searching for threats [2, 3]. These morphological changes and dynamic motility are critical to understanding microglial function, interactions with other cells and the mechanisms of microglia-related pathologies.
Extracellular nucleotides are known to regulate the microglial movement [4]. These nucleotides are released from damaged cells and act on their cognate receptors, P2X and P2Y. Microglia express ionotropic P2X4,7 and metabotropic P2Y6,12 receptors [1]. We previously showed that extracellular adenosine 5′-triphosphate (ATP) and adenosine 5′-diphosphate (ADP) induce membrane ruffling and chemotaxis by activation of P2Y12 receptors in microglia [1, 5–7]. Activation of the P2Y12 receptor also causes integrin-dependent microglial process extension [8]. In vivo imaging techniques revealed that the activation of the P2Y12 receptor was required to extend the processes toward a lesion site in damaged brain tissue [2, 9]. These studies indicate the prominent role of P2Y12 receptor activation in microglial motility. We also reported that P2Y6 receptor activation stimulated by its ligand uridine 5′-diphosphate (UDP) induced dynamic cytoskeletal arrangement and process movement in microglia, resulting in increase of microsphere phagocytosis [1]. Although the actin cytoskeletal rearrangement occurred in both cases of cellular movements, chemotaxis and phagocytosis, the signaling pathway of cytoskeletal regulation by P2 receptors in microglia remains to be understood.
Vasodilator-stimulated phosphoprotein (VASP) is an actin-binding protein that belongs to the enabled (Ena)/VASP family. Mena, EVL and VASP play pivotal roles in cell movement and shape changes in vertebrates. In various cell types, VASP constitutively or stimulus-dependently localizes at the filopodia or lamellipodia and regulates actin dynamics by promoting actin filament elongation and interacting with other actin regulatory factors [10]. Recently, it was reported that VASP exists functionally in the BV2 microglial cell line and regulates chemotaxis through P2Y12 receptor activation [11]. We also found involvement of VASP in UDP-induced alteration of cellular morphology in rat primary microglia and determined the relevant molecules involved in this response.
Materials and methods
Cell culture
Rat primary cultured microglia were prepared according to the method previously described [12, 13]. In summary, the mixed glial culture was prepared from neonatal Wistar rats and maintained for 9 to 15 days in DMEM (Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (Invitrogen). Floating microglia were collected by gentle shaking and transferred to the appropriate culture plate. The 293T cells were provided by RIKEN Bioresource Center (Tsukuba, Ibaraki, Japan).
Reagents, antibodies and plasmids
UDP sodium salt, ADP sodium salt, Reactive Blue 2 (RB2), Suramin and anti-β-actin mouse monoclonal antibodies were obtained from Sigma Chemical Co. (St. Louis, MO, USA). Bisindolylmaleimide I (Bis), Gö6976, Gö6983 were from Calbiochem (La Jolla, CA, USA). 3-Phenacyl-UDP (3-P-UDP) was from Tocris Bioscience (Ellisville, MO, USA). Texas-Red®-X phalloidin and Alexa Fluor 488 goat anti-rabbit antibody were purchased from Invitrogen. Phospho-VASP (Ser157) Antibody (pS157-VASP) was from Cell Signaling Technology (Beverly, MA, USA). Anti-mouse and anti-rabbit IgG HRP-linked whole antibody were from GE Healthcare (Piscataway, NJ, USA). Cell permeable C3 transferase recombinant human protein (CT04) was from Cytoskeleton Inc. (Denver, CO, USA). Plasmid vectors, pCMV-VSV-G-RSV-Rev, pCAG-HIVgp and CSII-EF-RfA were kindly provided by Dr. Hiroyuki Miyoshi (RIKEN BioResource Center, Tsukuba, Ibaraki, Japan). The cDNA encoding of the RGS domain of p115-RhoGEF (p115-RGS) and the negative mutant of p115-RGS (mut-RGS) was kindly provided by Dr. Hitoshi Kurose (Kyushu University, Fukuoka, Japan). The coding sequences of p115-RGS and mut-RGS were subcloned into pENTR plasmids which have the EGFP sequence under the IRES2 sequence. These plasmids were recombined with CSII-EF-RfA by a reaction using LR colonase (Invitrogen). Through this process, the lentiviral vectors (CSII-EF-p115-RGS and CSII-EF-mut-RGS) were obtained.
Immunocytochemistry
Microglia cells were seeded on aminopropyltriethoxysilane (APS)-coated glass (Matsunami, Osaka, Japan) at 3 × 104 cells/well and incubated for 1 h. The cells were washed two times with serum free medium and maintained in a serum-starved state for 1 h. Each inhibitor was pretreated for 10 min before UDP stimulation. UDP was added at a concentration of 100 μM and terminated 3 min after stimulation by changing the medium to 3.7% formaldehyde in phosphate-buffered saline (PBS). After 30 min of fixation time at room temperature, the cells were washed with PBS(−) and permeabilized and blocked with 3% normal goat serum in 0.1% Triton X-100/PBS(−) for 15 min at room temperature. Phospho-VASP antibody (1:50) was incubated at 4°C overnight. After washing the primary antibody, secondary antibody [Alexa Fluor 488 goat anti-rabbit antibody (1:1,000)] and Texas-Red phalloidin (1:100) were incubated for 1 h at room temperature with protection from light. Finally, the cells were placed under a coverslip in a Vectashield containing DAPI (Vector Laboratories, Burlingame, CA, USA). Observations were performed under a fluorescent confocal microscope (LSM510; Carl Zeiss, Jena, Germany).
Western blot analysis
The cells were plated in 60 mm culture dishes (~5 × 105 cells/dish) and incubated for 1 h. They were washed and starved for 1 h with serum-free DMEM. After stimulation with UDP, the cells were lysed, and the lysates were resolved in 10% SDS-PAGE gels and transferred to PVDF membranes. The membranes were blocked for 1 h in Tris-buffered saline containing 0.1% Tween-20 (TBS-T) and 5% BSA. The membranes were then incubated with primary antibodies [pS157-VASP (1:1,000) and β-actin (1:2,000) in TBS-T containing 5% BSA], overnight at 4°C. After washing with TBS-T, the membranes were incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibody (1:1,000 in TBS-T containing 5% BSA) for 1 h at room temperature. The membranes were washed three times and the proteins were visualized using an ECL Plus Western blot detection system (GE Healthscience) and analyzed using an LAS-3000 imaging system (Fujifilm, Tokyo, Japan).
Preparation of lentivirus and infection to microglia
The lentiviral vector plasmids (CSII-EF-p115-RGS or CSII-EF-mut-RGS) were cotransfected into 293T cells with packaging plasmids (pCAG-HIVgp, pCMV-VSV-G-RSV-Rev). The supernatant of the 293T cell culture was collected after 48 h and filtered through a 0.45-μm pore filter. Viral particles in the supernatant were concentrated by centrifuging in polyethylene glycol solution.
After the mixed culture preparation, viruses were added when the culture medium was changed the second time. After 1 week of infection, microglia were isolated by shaking the flasks and were plated on the appropriate dishes (~4.5–5 × 105 cells/dish) for the experiments. The expression efficacy of p115-RGS and mut-RGS was about 40%, as obtained by the fluorescent intensity of EGFP.
Results
UDP-induced local actin aggregation in microglia
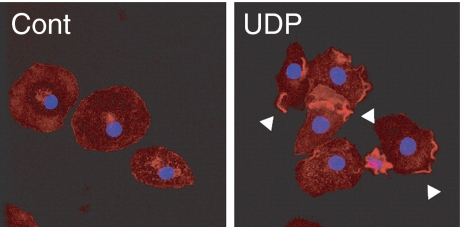
First, we analyzed UDP-induced actin polymerization by staining F-actin with Texas-Red phalloidin in primary rat microglia. After 3 min of UDP stimulation, actin aggregation was observed as concentrated phalloidin staining in a peripheral part of the cellular membrane (Fig. 1).
Fig. 1.
UDP induced local actin polymerization in microglia. Microglial cells were stimulated with or without UDP (100 μM) for 3 min. The cells were then fixed and stained for F-actin with Texas-Red phalloidin (red) and nuclei with DAPI (blue). In contrast to control cells (Cont), UDP induced F-actin aggregated in local cellular membranes, as indicated by arrowheads (UDP)
UDP induced the phosphorylation and translocation of VASP
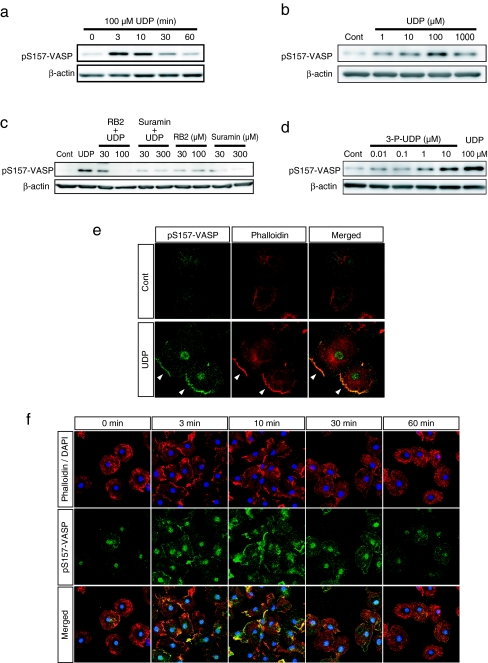
The actin binding protein VASP affects the actin-aggregating effect of UDP. Molecular functions of VASP are regulated by several phosphorylation sites [14, 15]. We found that UDP induced the transient phosphorylation of VASP at Ser157. The phosphorylation began 3 min after stimulation, peaked at 3 min and returned to basal level until 30 min (Fig. 2a). The phosphorylation of VASP at Ser157 after 3 min of UDP stimulation was detected at 1 μM and peaked at 100 μM (Fig. 2b). Therefore, we used 100 μM UDP for 3 min as the stimulation condition for subsequent experiments. Previous data showed that UDP-induced actin aggregation was mediated by P2Y6 receptor activation [1]. We examined the effects of P2 receptor inhibition on UDP-induced VASP phosphorylation. Both the P2Y receptor inhibitor RB2 and the P2 (X and Y) receptor inhibitor Suramin attenuated UDP-induced VASP phosphorylation (Fig. 2c). The P2Y6 receptor agonist 3-P-UDP also induced VASP phosphorylation at lower concentrations than UDP (Fig. 2d). Immunocytochemistry revealed that VASP phosphorylated at Ser157 (pS157-VASP) accumulated in the cell periphery where F-actin aggregated after UDP stimulation (Fig. 2e). The time course of actin aggregation was well correlated with the appearance of pS157-VASP (Fig. 2f). These results indicate the close relationship between UDP-induced actin aggregation and the phosphorylation of VASP.
Fig. 2.
UDP induced the phosphorylation and membrane accumulation of VASP. a The cells were stimulated with UDP (100 μM) for the indicated time and then lysed. The phosphorylated VASP in the extracted cellular protein was detected by Western blot analysis using the pS157-VASP antibody. The phosphorylation of VASP at Ser157 was increased from 3 min after UDP stimulation and returned to basal level around 30 min. b The cells were stimulated with UDP at indicated concentrations for 3 min. Phosphorylated VASP was increased from 1 μM UDP and peaked at 100 μM. c The cells were pretreated with P2 receptor inhibitors, RB2 and Suramin at indicated concentrations for 10 min and then stimulated with UDP (100 μM) for 10 min. Western blot analysis revealed that UDP-induced VASP phosphorylation was suppressed by each P2 receptor inhibitor. d The cells were stimulated with P2Y6 receptor agonist 3-P-UDP at indicated concentrations or UDP at 100 μM for 3 min. 3-P-UDP also induced VASP phosphorylation from the concentration of 1 μM; at 10 μM the increased phosphorylation was comparable to UDP stimulation. e The cells were stimulated with or without UDP (100 μM) for 3 min and stained with pS157-VASP antibody (green) and phalloidin (red). UDP stimulation induced the accumulation of phosphorylated VASP to the periphery of the cell where F-actin aggregated (as indicated by arrow heads). f Time course of phosphorylated VASP localization after UDP stimulation. UDP stimulated the accumulation of phosphorylated VASP to the plasma membrane in a time-dependent manner, which peaked at 3 to 10 min after stimulation and correlated with the time course of actin aggregation
UDP-induced actin aggregation and VASP phosphorylation were mediated by PKC
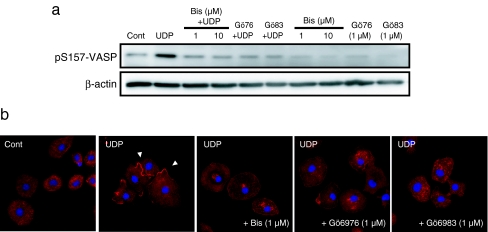
Although PKA is critical for Ser157 phosphorylation, it was reported that PKC is also capable of directly binding and phosphorylating the Ser157 site of VASP [16]. Stimulation of the P2Y6 receptor also leads to PKC activation by PLC signaling [17]. We investigated the effects of three PKC inhibitors, Bisindolylmaleimide I (Bis), Gö6976 (Gö76) and Gö6983 (Gö83), on both UDP-stimulated reactions, each with different selectivity for the PKC subtypes. All three PKC inhibitors completely abrogated the phosphorylation of VASP (Fig. 3a). Furthermore, UDP-induced actin aggregation was suppressed by pretreatment of each PKC inhibitor (Fig. 3b). This indicates that some PKC subtypes which are sensitive to all three PKC inhibitors are involved in UDP-induced VASP phosphorylation and actin aggregation.
Fig. 3.
a The cells were pretreated by each PKC inhibitor, Bisindolylmaleimide I (Bis), Gö6976 (Gö76) and Gö6983 (Gö83) at the indicated concentrations for 10 min and stimulated with UDP (100 μM). After 3 min of stimulation, the cells were lysed and cellular protein was extracted. Phosphorylated VASP was detected by the pS157-VASP antibody. UDP-induced VASP phosphorylations were completely suppressed by the pretreatment of each PKC inhibitor. b The cells were pretreated by each PKC inhibitor at indicated concentrations for 10 min and stimulated with UDP (100 μM). After 3 min of stimulation, the cells were fixed and stained with phalloidin (red) and DAPI (blue). UDP-stimulated local actin aggregation was inhibited by all of PKC inhibitors
UDP-induced actin aggregation and VASP phosphorylation were mediated by Rho activation
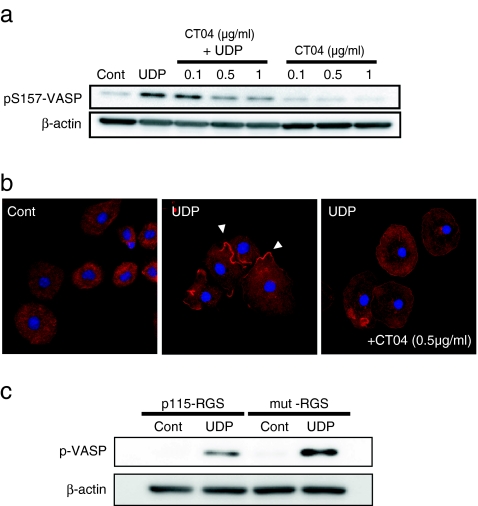
We examined the involvement of Rho GTPases because it was reported that PKC was a downstream target of RhoA [18] and VASP phosphorylation was regulated by RhoA activation [19]. We use the Rho inhibitor CT04 to inactivate RhoA, RhoB and RhoC. As a result, pretreatment with CT04 attenuated VASP phosphorylation at 0.5 μg/ml and this concentration of CT04 also abolished UDP-induced actin aggregation (Fig. 4a,b). UDP-induced VASP phosphorylation was also reduced by the expression of p115-RGS, which binds G12/13 and inhibits downstream signaling pathway, but not by the negative mutant form of p115-RGS (mut-RGS) (Fig. 4c) [20–23]. These data indicate that Rho GTPase is involved in UDP-induced VASP phosphorylation and actin aggregating effects.
Fig. 4.
a Rho inhibitor CT04 was pretreated for 2 h before UDP stimulation at the indicated concentrations. After 3 min of UDP stimulation, the cells were lysed and subjected to Western blot analysis. CT04 suppressed VASP phosphorylation in a dose-dependent manner. b The cells were pretreated with CT04 (0.5 μg/ml) for 2 h and then stimulated with UDP. After 3 min of UDP stimulation, the cells were fixed and stained with phalloidin (red) and DAPI (blue). UDP-induced actin aggregation (indicated by arrow heads) was decreased by CT04 pretreatment. c The lentivirus expressing p115-RGS or the mutant form of p115-RGS (mut-RGS) were added to the mixed cell culture and incubated for 1 week. Microglial cells were isolated and stimulated by UDP for 3 min. Expression of p115-RGS in microglia, but not mutant form of p115-RGS, suppressed UDP-induced VASP phosphorylation
UDP-induced VASP phosphorylation was independent of P2Y12 receptor activation
Recently, it was reported that the phosphorylation of VASP was induced by P2Y12 receptor activation [11]. We investigated the involvement of P2Y12 receptor activation in UDP-induced VASP phosphorylation. ADP stimulation slightly up-regulated VASP phosphorylation, and this effect was suppressed by the P2Y12 receptor antagonist AR-C69931MX (ARC). However, UDP-induced VASP phosphorylation was not inhibited by ARC treatment (Fig. 5). These results show that UDP-induced VASP phosphorylation is initiated by a distinct pathway from the downstream signaling of P2Y12 receptor activation.
Fig. 5.
The cells were pretreated with P2Y12 receptor antagonist AR-C69931MX (ARC) (0.1–1 μM) for 10 min and stimulated with UDP (100 μM) or ADP (10 μM) for 3 min. After the reaction, the cells were lysed and subjected to Western blot analysis. Both UDP and ADP induced VASP phosphorylation, but only ADP-mediated phosphorylation of VASP was inhibited by ARC pretreatment. UDP-induced VASP phosphorylation was not affected by ARC at any concentrations (0.1–1 μM)
Discussion
UDP rapidly induced actin aggregation and P2Y6 receptor-dependent VASP phosphorylation
In this study, we found that UDP stimulation rapidly (within 3 min) induced transient actin aggregation at the microglial cell membrane. This event was well correlated with the phosphorylation and membrane accumulation of the actin binding protein VASP after UDP stimulation, indicating the close involvement of VASP in UDP-induced actin aggregation. In addition, the phosphorylation of VASP was inhibited by Suramin and RB2 and also induced by 3-P-UDP. This responsiveness was consistent with that of previously reported pharmacological profile of P2Y6 receptors [24, 25].
UDP-induced VASP phosphorylation at Ser157 is mediated by PKC
VASP is known to be regulated by phosphorylation [14]. Three phosphorylation sites are identified: Ser157 is phosphorylated by PKA, Ser239 is phosphorylated by PKG [26] and Thr278 is a less favored site for both PKA and PKG and reported to be phosphorylated by AMP-activated kinase [27]. In our experiments, we found that UDP stimulation rapidly induced transient phosphorylation of VASP at Ser157. All three PKC inhibitors abolished the phosphorylation of VASP. We did not detect any phosphorylation at Ser239 after UDP stimulation (data not shown), and phosphorylation at Thr278 could not be assessed because of lack of commercially available antibodies. There was only one report indicating that the P2Y6 receptor couples with PLA activity [28], but it is known as a canonical pathway that P2Y6 receptor activation leads to PKC activation [17]. Our results indicate that phosphorylation at Ser157 of VASP is mediated by PKC. Similar results were reported in the experiment using serum-induced VASP phosphorylation in vascular smooth cells [16]. Consistent with the pharmacological results, PKC inhibition suppressed UDP-induced actin aggregation. It is known that PKC is involved in cell spreading, migration and neurite out-growth by regulating integrin signaling and interacting with a number of PKC substrates which are directly associated with actin filaments [29]. We observed that UDP-induced cellular movements of microglia may be regulated by PKC associated with VASP phosphorylation.
PKC subtypes involved in VASP phosphorylation
The PKC family has different subtypes; conventional, novel and atypical PKC which are activated by different pathways. Conventional PKC subtypes (α, β, γ) are activated by Ca2+, DAG and phosphatidyl serine; novel subtypes (δ, ε, η, θ) are activated by DAG and atypical subtypes (ζ, λ/ι) are activated by a Ca2+/DAG-independent pathway. In our experiments, UDP-induced VASP phosphorylation was suppressed by all three PKC inhibitors, which have different selectivity for each PKC isoform; Bisindolylmaleimide I inhibits PKCα, βI, δ and ε; Gö6976 inhibits PKCα, βI, and μ and Gö6983 inhibits PKCα, β, δ, and ζ [30–32]. Based on the common characteristics exhibited by the inhibitors, we concluded that PKCβI may be the first candidate involved in UDP-induced VASP phosphorylation in microglia. Previous studies on protein expression revealed that, among classical PKC subtypes, only PKCβII was abundantly expressed in rat microglial culture but PKCα was not detectable. Although the expression of PKCβI in microglia was less abundant, phorbol 12-myristate 13-acetate (PMA) treatment transiently up-regulated the expression of PKCβI in a non-cytosolic fraction [33].
UDP-induced VASP phosphorylation at Ser157 is mediated by Rho activation
It was reported that P2Y6 receptor coupled with Gα12/13 signaling in cardiomyocytes [23] and human umbilical vein endothelial cells, phosphorylation of VASP was mediated by Gα13 [19] and Rho activation was mediated by Gα12/13 signals [34–36]. It has been established that Rho activation regulates cytoskeletal arrangements [37]. We observed partial inhibitory effects on VASP phosphorylation by the Rho inhibitor CT04 and Gα12/13 signal inhibition by p115-RGS. The suppressive effect of CT04 on actin aggregation indicates at least partial involvement of Rho activation. Since some Gq coupled receptors have been reported to also interact with G12/13 signaling [38–41], it is conceivable that G12/13 signaling is activated following P2Y6 receptor stimulation. It was also shown that Rho signaling activated PKCs [18, 42, 43]. These findings suggest that the activation of Rho and PKC is induced by P2Y6 receptor activation and regulates F-actin aggregation.
UDP-dependent VASP phosphorylation was not mediated by P2Y12R activation
There are no reports about the regulation of actin cytoskeleton dynamics by P2Y6 receptor activation. Some reports stated that the activation of P2Y12 receptors leads to altered cellular morphology and motility in microglia. ATP/ADP act on their cognate receptor P2Y12, leading to actin aggregation, microglial chemotaxis and process extension [5, 6, 8, 9, 44]. In a recent study, it was reported that the phosphorylation of VASP is involved in ADP-induced ruffling and protrusion formation in the BV2 microglial cell line [11]. In BV2 cells, the intracellular concentration of cAMP was up-regulated through P2Y12 receptor after ADP stimulation, resulting in PKA activation and subsequent phosphorylation of VASP by PKA. This puzzling phenomenon may be due to the isoform of adenylyl cyclase (AC). It is known that only AC 5 and AC 6 are sensitive to inhibition by Gαi, and Gβγ can be either stimulatory, as for AC 2, AC 4, and AC 7, or inhibitory, as for AC 1 and AC 8. Microglia may express AC isoforms insensitive to Gαi and activated by Gβγ. We also observed ADP-induced VASP phosphorylation which was suppressed by the P2Y12 receptor inhibitor AR-C69931MX. The phosphorylation of VASP induced by UDP stimulation was not suppressed by the P2Y12 receptor antagonist (Fig. 5). These data indicate that UDP-induced VASP phosphorylation is independent from P2Y12 receptor mediated VASP phosphorylation.
The function of phosphorylated VASP in UDP-stimulated microglia
VASP is a member of the Ena/VASP proteins and directly regulates assembly of the actin-filament network, modulates the morphology and behavior of membrane protrusion structures (such as filopodia and lamellipodia) and influences cell motility [45–47]. The phosphorylation of VASP negatively regulates actin nucleation/G-actin binding and interaction with F-actin and some cell adhesion regulators [48–50]. On the other hand, VASP binding to focal adhesion protein and profilin, a regulator of actin polymerization, are independent of the VASP phosphorylation status [48, 51]. Additionally, the phosphorylation at Ser157 of VASP influenced VASP localization, but little impact on F-actin assembly [14]. In our experiment, inhibition of PKC-mediated VASP phosphorylation resulted in suppression of local actin aggregation, which indicate that the phosphorylation of VASP is required for UDP-induced actin aggregation.
Additionally, VASP has been observed at the site of the phagocytic cup after binding of sheep red blood cells (SRBC) to the macrophage. It is required for phagocytic cup formation and SRBC internalization [52]. This research also showed that the accumulation of VASP at the phagocytic cup was abolished by inhibition of Rho-GTPase. VASP phosphorylation induced by the UDP-activated P2Y6 receptor may serve as the driving force for the dynamic processes of movement in microglial phagocytosis.
Acknowledgements
We thank Prof. Hitoshi Kurose for providing the plasmid coding for the RGS domain of p115-RhoGEF (p115-RGS), the mutant of the RGS domain of p115-RhoGEF (mut-RGS). This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to H.S.-T., M.T. and K.I.).
References
- 1.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446(7139):1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 3.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 4.Farber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Arch. 2006;452(5):615–621. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- 5.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21(6):1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nasu-Tada K, Koizumi S, Inoue K. Involvement of beta1 integrin in microglial chemotaxis and proliferation on fibronectin: different regulations by ADP through PKA. Glia. 2005;52(2):98–107. doi: 10.1002/glia.20224. [DOI] [PubMed] [Google Scholar]
- 7.Irino Y, Nakamura Y, Inoue K, Kohsaka S, Ohsawa K. Akt activation is involved in P2Y12 receptor-mediated chemotaxis of microglia. J Neurosci Res. 2008;86(7):1511–1519. doi: 10.1002/jnr.21610. [DOI] [PubMed] [Google Scholar]
- 8.Ohsawa K, Irino Y, Sanagi T, Nakamura Y, Suzuki E, Inoue K, Kohsaka S. P2Y12 receptor-mediated integrin-beta1 activation regulates microglial process extension induced by ATP. Glia 58(7):790–801. doi:10.1002/glia.20963 [DOI] [PubMed]
- 9.Haynes SE, Hollopeter G, Yang G, Kurpius D, Dailey ME, Gan WB, Julius D. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9(12):1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 10.Bear JE, Gertler FB. Ena/VASP: towards resolving a pointed controversy at the barbed end. J Cell Sci. 2009;122(Pt 12):1947–1953. doi: 10.1242/jcs.038125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Chung CY. Role of VASP phosphorylation for the regulation of microglia chemotaxis via the regulation of focal adhesion formation/maturation. Mol Cell Neurosci. 2009;42(4):382–390. doi: 10.1016/j.mcn.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima K, Shimojo M, Hamanoue M, Ishiura S, Sugita H, Kohsaka S. Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem. 1992;58(4):1401–1408. doi: 10.1111/j.1471-4159.1992.tb11356.x. [DOI] [PubMed] [Google Scholar]
- 13.Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424(6950):778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 14.Benz PM, Blume C, Seifert S, Wilhelm S, Waschke J, Schuh K, Gertler F, Munzel T, Renne T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J Cell Sci. 2009;122(Pt 21):3954–3965. doi: 10.1242/jcs.044537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 16.Chitaley K, Chen L, Galler A, Walter U, Daum G, Clowes AW. Vasodilator-stimulated phosphoprotein is a substrate for protein kinase C. FEBS Lett. 2004;556(1–3):211–215. doi: 10.1016/S0014-5793(03)01435-2. [DOI] [PubMed] [Google Scholar]
- 17.Kugelgen I, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn Schmiedebergs Arch Pharmacol. 2000;362(4–5):310–323. doi: 10.1007/s002100000310. [DOI] [PubMed] [Google Scholar]
- 18.Slater SJ, Seiz JL, Stagliano BA, Stubbs CD. Interaction of protein kinase C isozymes with Rho GTPases. Biochemistry. 2001;40(14):4437–4445. doi: 10.1021/bi001654n. [DOI] [PubMed] [Google Scholar]
- 19.Profirovic J, Gorovoy M, Niu J, Pavlovic S, Voyno-Yasenetskaya T. A novel mechanism of G protein-dependent phosphorylation of vasodilator-stimulated phosphoprotein. J Biol Chem. 2005;280(38):32866–32876. doi: 10.1074/jbc.M501361200. [DOI] [PubMed] [Google Scholar]
- 20.Maruyama Y, Nishida M, Sugimoto Y, Tanabe S, Turner JH, Kozasa T, Wada T, Nagao T, Kurose H. Galpha(12/13) mediates alpha(1)-adrenergic receptor-induced cardiac hypertrophy. Circ Res. 2002;91(10):961–969. doi: 10.1161/01.RES.0000043282.39776.7C. [DOI] [PubMed] [Google Scholar]
- 21.Arai K, Maruyama Y, Nishida M, Tanabe S, Takagahara S, Kozasa T, Mori Y, Nagao T, Kurose H. Differential requirement of G alpha12, G alpha13, G alphaq, and G beta gamma for endothelin-1-induced c-Jun NH2-terminal kinase and extracellular signal-regulated kinase activation. Mol Pharmacol. 2003;63(3):478–488. doi: 10.1124/mol.63.3.478. [DOI] [PubMed] [Google Scholar]
- 22.Nishida M, Tanabe S, Maruyama Y, Mangmool S, Urayama K, Nagamatsu Y, Takagahara S, Turner JH, Kozasa T, Kobayashi H, Sato Y, Kawanishi T, Inoue R, Nagao T, Kurose H. G alpha 12/13- and reactive oxygen species-dependent activation of c-Jun NH2-terminal kinase and p38 mitogen-activated protein kinase by angiotensin receptor stimulation in rat neonatal cardiomyocytes. J Biol Chem. 2005;280(18):18434–18441. doi: 10.1074/jbc.M409710200. [DOI] [PubMed] [Google Scholar]
- 23.Nishida M, Sato Y, Uemura A, Narita Y, Tozaki-Saitoh H, Nakaya M, Ide T, Suzuki K, Inoue K, Nagao T, Kurose H. P2Y6 receptor-Galpha12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. 2008;27(23):3104–3115. doi: 10.1038/emboj.2008.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110(3):415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 25.El-Tayeb A, Qi A, Muller CE. Synthesis and structure–activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2006;49(24):7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- 26.Halbrugge M, Friedrich C, Eigenthaler M, Schanzenbacher P, Walter U. Stoichiometric and reversible phosphorylation of a 46-kDa protein in human platelets in response to cGMP- and cAMP-elevating vasodilators. J Biol Chem. 1990;265(6):3088–3093. [PubMed] [Google Scholar]
- 27.Blume C, Benz PM, Walter U, Ha J, Kemp BE, Renne T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem. 2007;282(7):4601–4612. doi: 10.1074/jbc.M608866200. [DOI] [PubMed] [Google Scholar]
- 28.Wong AM, Chow AW, Au SC, Wong CC, Ko WH. Apical versus basolateral P2Y(6) receptor-mediated Cl(−) secretion in immortalized bronchial epithelia. Am J Respir Cell Mol Biol. 2009;40(6):733–745. doi: 10.1165/rcmb.2008-0020OC. [DOI] [PubMed] [Google Scholar]
- 29.Larsson C. Protein kinase C and the regulation of the actin cytoskeleton. Cell Signal. 2006;18(3):276–284. doi: 10.1016/j.cellsig.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268(13):9194–9197. [PubMed] [Google Scholar]
- 31.Gschwendt M, Dieterich S, Rennecke J, Kittstein W, Mueller HJ, Johannes FJ. Inhibition of protein kinase C mu by various inhibitors. Differentiation from protein kinase c isoenzymes. FEBS Lett. 1996;392(2):77–80. doi: 10.1016/0014-5793(96)00785-5. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann J. The potential for isoenzyme-selective modulation of protein kinase C. FASEB J. 1997;11(8):649–669. doi: 10.1096/fasebj.11.8.9240967. [DOI] [PubMed] [Google Scholar]
- 33.Slepko N, Patrizio M, Levi G. Expression and translocation of protein kinase C isoforms in rat microglial and astroglial cultures. J Neurosci Res. 1999;57(1):33–38. doi: 10.1002/(SICI)1097-4547(19990701)57:1<33::AID-JNR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 34.Hart MJ, Jiang X, Kozasa T, Roscoe W, Singer WD, Gilman AG, Sternweis PC, Bollag G. Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science. 1998;280(5372):2112–2114. doi: 10.1126/science.280.5372.2112. [DOI] [PubMed] [Google Scholar]
- 35.Fukuhara S, Murga C, Zohar M, Igishi T, Gutkind JS. A novel PDZ domain containing guanine nucleotide exchange factor links heterotrimeric G proteins to Rho. J Biol Chem. 1999;274(9):5868–5879. doi: 10.1074/jbc.274.9.5868. [DOI] [PubMed] [Google Scholar]
- 36.Fukuhara S, Chikumi H, Gutkind JS. Leukemia-associated Rho guanine nucleotide exchange factor (LARG) links heterotrimeric G proteins of the G(12) family to Rho. FEBS Lett. 2000;485(2–3):183–188. doi: 10.1016/S0014-5793(00)02224-9. [DOI] [PubMed] [Google Scholar]
- 37.Ridley AJ. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006;16(10):522–529. doi: 10.1016/j.tcb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Klages B, Brandt U, Simon MI, Schultz G, Offermanns S. Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets. J Cell Biol. 1999;144(4):745–754. doi: 10.1083/jcb.144.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan J, Slice LW, Rozengurt E. Activation of protein kinase D by signaling through Rho and the alpha subunit of the heterotrimeric G protein G13. J Biol Chem. 2001;276(42):38619–38627. doi: 10.1074/jbc.M105530200. [DOI] [PubMed] [Google Scholar]
- 40.Xie Z, Ho WT, Spellman R, Cai S, Exton JH. Mechanisms of regulation of phospholipase D1 and D2 by the heterotrimeric G proteins G13 and Gq. J Biol Chem. 2002;277(14):11979–11986. doi: 10.1074/jbc.M109751200. [DOI] [PubMed] [Google Scholar]
- 41.Sinnett-Smith J, Santiskulvong C, Duque J, Rozengurt E. [d-Arg(1), d-Trp(5,7,9), Leu(11)]Substance P inhibits bombesin-induced mitogenic signal transduction mediated by both G(q) and G(12) in Swiss 3T3cells. J Biol Chem. 2000;275(39):30644–30652. doi: 10.1074/jbc.M003702200. [DOI] [PubMed] [Google Scholar]
- 42.Nonaka H, Tanaka K, Hirano H, Fujiwara T, Kohno H, Umikawa M, Mino A, Takai Y. A downstream target of RHO1 small GTP-binding protein is PKC1, a homolog of protein kinase C, which leads to activation of the MAP kinase cascade in Saccharomyces cerevisiae. EMBO J. 1995;14(23):5931–5938. doi: 10.1002/j.1460-2075.1995.tb00281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hippenstiel S, Kratz T, Krull M, Seybold J, Eichel-Streiber C, Suttorp N. Rho protein inhibition blocks protein kinase C translocation and activation. Biochem Biophys Res Commun. 1998;245(3):830–834. doi: 10.1006/bbrc.1998.8525. [DOI] [PubMed] [Google Scholar]
- 44.Ohsawa K, Irino Y, Nakamura Y, Akazawa C, Inoue K, Kohsaka S. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55(6):604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 45.Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109(4):509–521. doi: 10.1016/S0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 46.Gertler FB, Niebuhr K, Reinhard M, Wehland J, Soriano P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell. 1996;87(2):227–239. doi: 10.1016/S0092-8674(00)81341-0. [DOI] [PubMed] [Google Scholar]
- 47.Lacayo CI, Pincus Z, VanDuijn MM, Wilson CA, Fletcher DA, Gertler FB, Mogilner A, Theriot JA. Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLoS Biol. 2007;5(9):e233. doi: 10.1371/journal.pbio.0050233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harbeck B, Huttelmaier S, Schluter K, Jockusch BM, Illenberger S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J Biol Chem. 2000;275(40):30817–30825. doi: 10.1074/jbc.M005066200. [DOI] [PubMed] [Google Scholar]
- 49.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, Walter U, Feller SM, Renne T. Cytoskeleton assembly at endothelial cell–cell contacts is regulated by alphaII-spectrin-VASP complexes. J Cell Biol. 2008;180(1):205–219. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lambrechts A, Kwiatkowski AV, Lanier LM, Bear JE, Vandekerckhove J, Ampe C, Gertler FB. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J Biol Chem. 2000;275(46):36143–36151. doi: 10.1074/jbc.M006274200. [DOI] [PubMed] [Google Scholar]
- 51.Ferron F, Rebowski G, Lee SH, Dominguez R. Structural basis for the recruitment of profilin–actin complexes during filament elongation by Ena/VASP. EMBO J. 2007;26(21):4597–4606. doi: 10.1038/sj.emboj.7601874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coppolino MG, Krause M, Hagendorff P, Monner DA, Trimble W, Grinstein S, Wehland J, Sechi AS. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J Cell Sci. 2001;114(Pt 23):4307–4318. doi: 10.1242/jcs.114.23.4307. [DOI] [PubMed] [Google Scholar]